Abstract
Attention has become focused on resveratrol not only because of its role in grapevine fungal resistance but also because of its benefits in human health. This report describes the Chinese wild grapevine Vitis quinquangularis accession Danfeng-2 in relation to the high resveratrol content of its ripe berries. In this study, we used isobaric tags for relative and absolute quantification (iTRAQ) tandem mass spectrometry strategy to quantify and identify proteome changes, resulting in the detection of a total of 3,751 proteins produced under natural conditions. Among the proteins quantified, a total of 578 differentially expressed proteins were detected between Danfeng-2 and Cabernet Sauvignon during berry development. Differentially expressed proteins are involved in secondary metabolism, biotic stress, abiotic stress and transport activity and indicate novel biological processes in Chinese wild grapevine. Eleven proteins involved in phenylpropanoid metabolism and stilbene synthesis were differently expressed between Danfeng-2 and Cabernet Sauvignon at the veraison stage of berry development. These findings suggest that Chinese wild V. quinquangularis accession Danfeng-2 is an extremely important genetic resource for grape breeding and especially for increasing the resveratrol content of European grape cultivars for disease resistance and for improved human nutritional benefits.
Introduction
Grapes, Vitis spp, can be eaten fresh or processed into wine, raisins and a range of other foods. They are thus one of the world’s most widely-cultivated fruit crops and are the basis of an age-old industry. This industry is exceedingly large and has expanded very rapidly over the last 50 years. The European grapevine (Vitis vinifera L.) is the predominant species grown, mainly because it is highly valued for its winemaking properties. There are a very large number of named V. vinifera cultivars, some ancient and some modern. However, most of these V. vinifera cultivars are very susceptible to fungal pathogens, which cause major economic loss. Disease management comes with its costs. These costs are not only financial (loss of yield and expense of control) but also environmental (impacts on wildlife and heavy-metal residues), and so pose a risk both to this and to future generations1.
Intraspecific grape breeding for increased disease resistance is one solution, but disease resistance within V. vinifera is quite limited. Instead, interspecific breeding to produce hybrid cultivars offers a much more promising solution. Either way, disease-resistant germplasm is essential. In general, the wild Vitis species offer a very useful source of pathogen resistance2. China is one of three main centers of origin of Vitis, where a large number of wild Vitis species are conserved. The Chinese wild Vitis spp. represent an important part of the world Vitis germplasm resource with good pathogen resistance and high levels of resveratrol3, 4. Numerous Chinese wild grape accessions are highly disease-resistant, among these are V. pseudoreticulata ‘Baihe-35-1’ and V. quinquangularis ‘Danfeng-2’3.
Resveratrol, which is well known as a phytoalexin, is considered to be associated with the grapevine’s resistance to fungi5. In the past few years, increasing attention has been paid to grape germplasm that is high in resveratrol. In human medicine, resveratrol has been shown to possess anti-inflammatory, anti-platelet, anti-carcinogenic, antifungal and antibacterial activities6. Stilbene synthase is the key enzyme for resveratrol synthesis7. Stilbenes are strongly accumulated in grapevine when stilbene synthase is overexpressed and disease resistance of transgenic grapevines has been significantly increased6. Indeed, transformation of stilbene synthase genes in tobacco8, rice9, alfalfa10, kiwifruit11, wheat12, 13, apple14, papaya15, poplar16, Arabidopsis17, pea18 and hops19 can enhance disease resistance or raise the resveratrol content of the receptor plants.
Danfeng-2, a Chinese wild V. quinquangularis accession, has been confirmed in a number of studies to contain high levels of resveratrol20, 21. In a previous study, we have isolated 41 stilbene synthase genes from V. quinquangularis Danfeng-2 and compared these with the expression profiles of the stilbene synthase gene family in V. vinifera cv. Pinot Noir during Uncinula necator infection20. Soon after infection, a fruit-specific, highly-expressed stilbene synthase gene VqSTS6 from V. quinquangularis Danfeng-2 was transformed to V. vinifera cv. Thompson Seedless and high levels of Trans-resveratrol were detected in the leaves of the transgenic lines22. However, the mechanism for high levels of resveratrol in V. quinquangularis Danfeng-2 is still unknown.
A number of molecular biology tools have been developed to study a range of biological problems. Proteomics has been proved an effective tool to analyze the proteome changes in grape under abiotic and biotic stress as well as during berry development23–27. Dimensional electrophoresis followed by Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOFI-MS) has been used to characterize the differential expressed proteins in V. vinifera 28–31. However, isobaric tags for relative and absolute quantification (iTRAQ) can determine accurate protein level changes and more protein differences than gel-based proteomics. In recent years, more researchers have used iTRAQ followed by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) to carry out proteome study in Vitis and iTRAQ has proved to be an excellent method for studying biological problems in grapevine32–35.
In this study, the Chinese wild species V. quinquangularis Danfeng-2 and V. vinifera cv. Cabernet Sauvignon, a very well-known winemaking grape cultivar, were analyzed by iTRAQ to obtain specific protein identification and for future use in improving grapevine breeding properties. The aim was to obtain and identify specific proteins expressed from V. quinquangularis accession Danfeng-2 with high levels of resveratrol and disease resistance during the various stages of berry development. Additionally, differentially expressed proteins involved in secondary metabolism, biotic stress, abiotic stress and transport activity will be valuable for revealing the elite traits of high resveratrol content and disease resistance from Chinese wild Vitis quinquangularis Danfeng-2. The levels of selected proteins were correlated with their transcript levels by quantitative real-time polymerase chain reaction (RT-PCR) analysis. The novel findings of this study will provide new opportunities to explore the mechanisms involved in the high levels of resveratrol and disease resistance found in Chinese wild V. quinquangularis. This study also proposes candidate genetic resource for further grape breeding, designed to increase the resveratrol content of some of the more popular wine grape cultivars.
Results
iTRAQ analyses of proteins in berries of V. quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon at four developmental stages
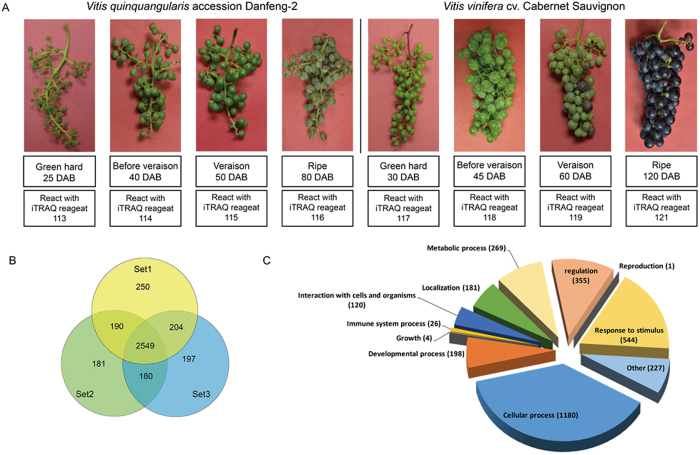
A flow chart for the iTRAQ analysis is shown in Fig. 1A. The samples were labeled with different iTRAQ reagents (Sigma-Aldrich, Germany) and mixed for MS/MS analysis. The raw data were then analyzed using MASCOT, and the proteins were quantified. For three iTRAQ 8 × plex experiments, totals of 3,193, 3,100 and 3,130 proteins were identified, respectively. Amongst these, 2549 of the proteins generated were common. A Venn diagram shows the details of the overlap, as well as the proteins that were unique to each replicate (Fig. 1B). A total of 3,751 proteins that were unique to a single replicate were identified and the GO terms (biological process) of the proteins identified were clustered (Fig. 1C). The majority of the proteins were involved in cellular processes (38%), responses to stress (18%), and regulation (11%). Details of the Uniport IDs, percent coverage, number of unique peptides, ratios of different plex, protein length, predicted molecular weights and isoelectric points are listed in Supplementary Tables S2 and S3. Of the proteins identified, we generated many that are involved in secondary metabolism and stress responses. Of the proteins related to secondary metabolism, phenylalanine ammonialyase, chalcone synthase, resveratrol glucosyltransferase (RSGT), flavonoid synthesis proteins, and anthocyanin synthesis proteins were identified and verified. Of the stress response proteins, we annotated alcohol dehydrogenase proteins, major latex proteins (MLP), ubiquitin proteins, the mildew resistance locus o (MLO) proteins, pathogenesis-related proteins, aquaporin, heat shock proteins, calcineurin B-like proteins, pectinesterases, dehydrins, EF-hand calcium binding proteins and phospholipases and others.
Figure 1.
Workflow and proteins identified in four developmental stages of berries of Vitis quinquangularis accession Danfeng-2 and control grapevine V. vinifera cv. Cabernet Sauvignon with iTRAQ. (A) iTRAQ 8-plex labeling of different developmental stages (green hard, before veraison, veraison and ripe). DAB, days after blooming; (B) Venn diagram showing proteins among the three iTRAQ data sets of Danfeng-2 and Cabernet Sauvignon; Set1, Set2 and Set3 represented three independent biological replicates. (C) GO term enriched broader functional classification of 3751 proteins identified during four developmental stages of berries of Danfeng-2 and Cabernet Sauvignon. Number of each GO term was next to the corresponding pie.
Contents of resveratrol, piceid, flavonoid and anthocyanin in berries of V. quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon
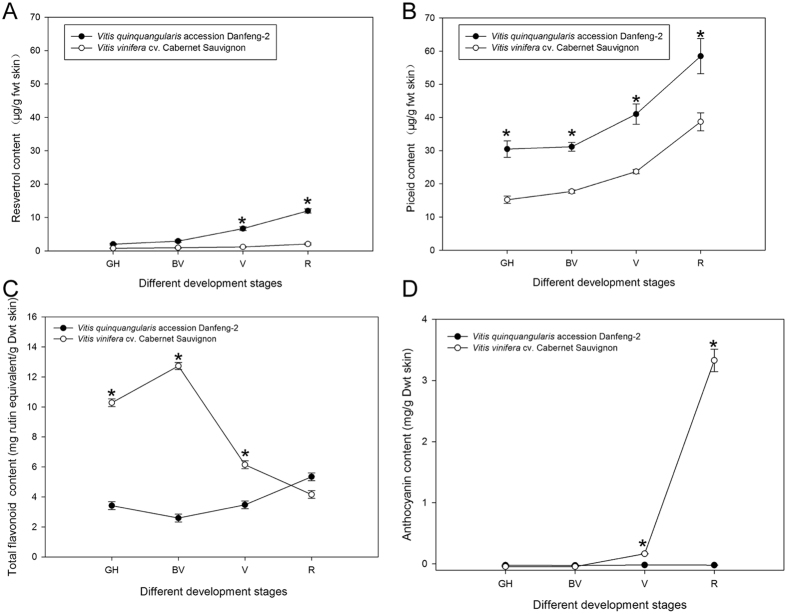
Danfeng-2 believed to contain high amounts of resveratrol. We determined the contents of resveratrol and piceid using HPLC and results indicate significant differences in trans-resveratrol and piceid between Danfeng-2 and Cabernet Sauvignon (Fig. 2A and B). The resveratrol content of Danfeng-2 berries increased continually during ripening and was 5.70-times greater than in Cabernet Sauvignon at stage of veraison and 5.79-times greater at stage of ripe. The content of piceid was at least 1.50-times higher in Danfeng-2 than in Cabernet Sauvignon. The main stilbene components in Danfeng-2 were significantly higher than in Cabernet Sauvignon. To compare the differences in flavonoid and anthocyanin accumulation between Danfeng-2 and Cabernet Sauvignon, we measured the contents of total flavonoid and total anthocyanins. Total flavonoids in Danfeng-2 berry skins first declined then increased (Fig. 2C). However, total flavonoids in Danfeng-2 were significantly less than in Cabernet Sauvignon during development except at ripe stage (Fig. 2C). Anthocyanin accumulation was not detected in the berry skins of Danfeng-2, but in Cabernet Sauvignon anthocyanin content began to rise at veraison stage (Fig. 2D).
Figure 2.
Determination of metabolites in berry skins of Vitis quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon at four different development stages. GH, green hard, BV, before veraison, V, veraison, and R, ripe. (A) Trans-resveratrol content; (B) Piceid content. (C) Total flavonoid content; (D) Anthocyanin content. The p-value level less than 0.05 was considered significantly different at each development stage between Vitis quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon and labeled with an asterisk.
Differentially expressed proteins between V. quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon during berry development
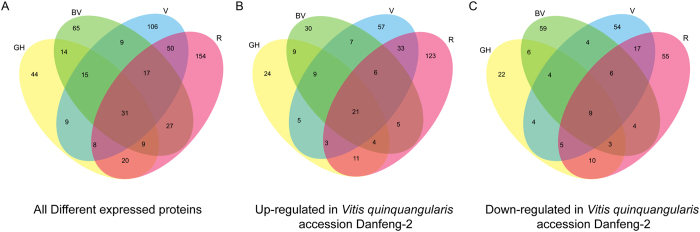
We identified proteins that were differentially expressed during development in the two grape genotypes. Comparing the four stages of berry development, proteins with expression level differences >1.5-fold were considered to have been up-regulated and those <0.67-fold to have been down-regulated. The differentially expressed proteins between Danfeng-2 and Cabernet Sauvignon at each of the four developmental stages were identified. There were 151 differentially expressed proteins identified for stage GH, 188 for stage BV, 246 for V and 317 for R (Fig. 3A, Supplementary Table S4). Up-regulated proteins in Danfeng-2 were 87 for GH, 92 for BV, 142 for V and 208 for R (Fig. 3B). The up-regulated proteins in Cabernet Sauvignon were 64 for GH, 96 BV, 104 for V and 109 for R (Fig. 3C). The differentially expressed proteins were involved in various cellular processes and annotation of differentially expressed proteins was carried out using the UniProt database.
Figure 3.
Overlaps of different expression proteins over the developmental stages between Vitis quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon. GH, green hard, BV, before veraison, V, veraison, and R, ripe. (A) Venn diagram illustrating overlaps of 578 differential proteins at all stages between Danfeng-2 and Cabernet Sauvignon. DEPs-differentially expressed proteins; (B) Venn diagram illustrating overlaps of proteins with higher expression profiles in Danfeng-2 at each stage; (C) Venn diagram illustrating overlaps of proteins with lower expression profiles in Danfeng-2 at each stage.
Proteomic analysis of the grape epicarp of Danfeng-2 with Cabernet Sauvignon as control, reveals distinct developmental processes
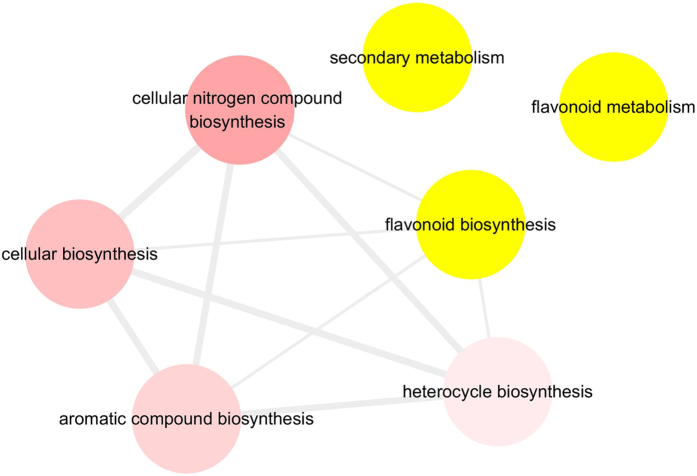
The iTRAQ analysis identified differentially expressed proteins in Danfeng-2 and Cabernet Sauvignon at each of the four developmental stages as described above. GO annotated was based on the annotation in the Uniport database. To visualize the differential proteins, the p-value of the GO terms were enriched in agriGO and displayed using Cytoscape (Fig. 4, Supplementary Table S5). Enriched GO terms were clustered, based on their functions. Most of the differentially expressed proteins were annotated to be cellular ketone metabolism and rRNA metabolism. However, differentially expressed proteins involved in secondary metabolic pathways were also identified. GO terms related to secondary metabolic were highlighted with yellow background (Fig. 4). These results indicated many differentially expressed proteins are involved in flavonoid metabolism. In addition, flavonoid biosynthesis was related with heterocyclic biosynthesis, aromatic compound biosynthesis, cellular biosynthesis, and cellular nitrogen compound biosynthesis (Fig. 4).
Figure 4.
Enriched GO terms of the different expression proteins related with secondary metabolism over the developmental stages of Vitis quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon.
Proteins involved in the phenylalanine metabolism pathway
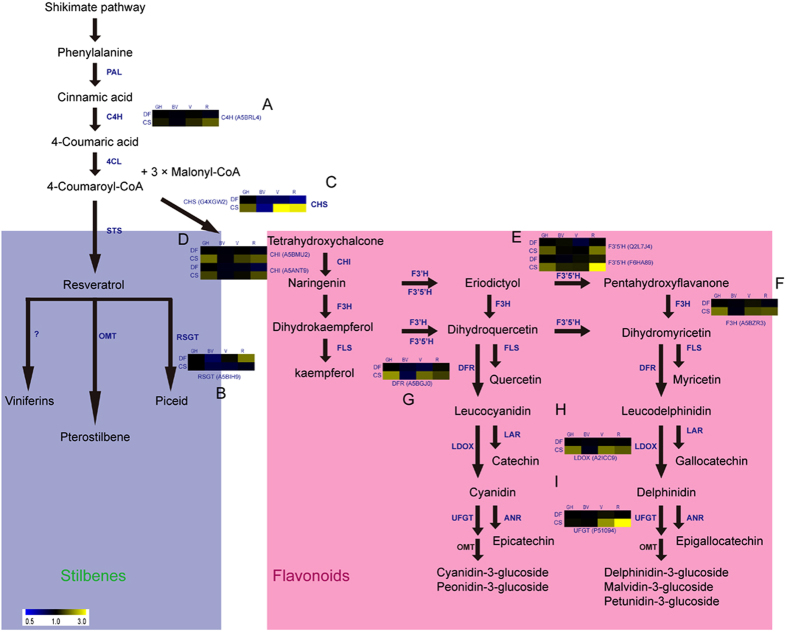
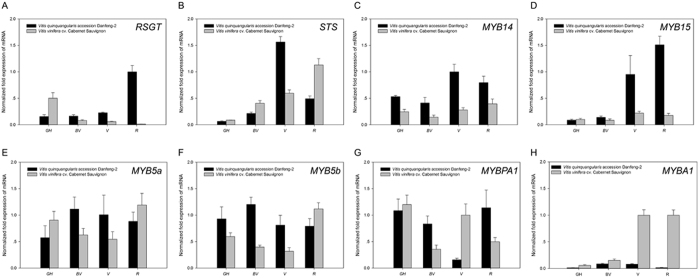
Differential expressed proteins involved in the phenylalanine metabolism pathway revealed specific proteins promoting stilbene accumulation in V. quinquangularis accession Danfeng-2. To generate a more complete picture of the dynamic changes in protein levels of enzymes involved in the phenylalanine metabolic pathway during development, their expression profiles were examined in more detail. The phenylalanine metabolic pathway is a fully characterized metabolic pathway in plants, including in grapevine. A specific STS branch has evolved that results in the accumulation of the polyphenol, resveratrol. Here, the expression profiles of several enzymes in this pathway showed significant differences in abundance in the two varieties, at the veraison and ripe stages. Cabernet Sauvignon berries began to accumulate color at the veraison stage and the enzymes in the CHS branch showed several-fold higher expression levels than the corresponding enzymes in Danfeng-2, while the distinction at the ripe stage was less obvious. The proteins identified with significant differences in expression in relation to the phenylalanine metabolic pathway are shown in Fig. 5. Proteins in the pathway downstream of CHS began to accumulate at the veraison stage in Cabernet Sauvignon, while no differences were observed in Danfeng-2. Trans-cinnamate 4-monooxyygenase (C4H) (A5BRL4), an enzyme downstream of phenylalnine ammonialyase, expressed more highly in Cabernet Sauvignon than in Danfeng-2 at the ripe stage (Fig. 5A). A resveratrol glucosyltransferase (RSGT) (A5BIH9) accumulated to high levels in Danfeng-2 at the ripe stage, which was not observed in Cabernet Sauvignon (Fig. 5B). However, the enzymes in the CHS branch in Cabernet Sauvignon showed significantly higher levels than that in Danfeng-2 including CHS (Fig. 5C), CHI (Fig. 5D), F3′5′H (Fig. 5E), F3H (Fig. 5F), DFR (Fig. 5G), LDOX (Fig. 5H), UFGT (Fig. 5I) at the ripe stage and/or at the veraison stage and green hard stage. These enzymes control the synthesis of flavonoid and anthocyanins. Next we used RT-PCR to gain support for the protein expression profiles. A possible explanation for the interesting resveratrol accumulation pattern in Cabernet Sauvignon is that STS and CHS compete during berry development for the common substrates, p-coumaroyl-CoA and malonyl-CoA. The expressions of several genes related to the pathway, such as RSGT, STS, MYB14, MYB15, MYB5a, MYB5b, MYBPA1 and MYBA1 were analyzed using qRT-PCR (Fig. 5A–H). The results demonstrate that these genes are expressed differently for same development stage. The expression of gene RSGT gradually increased during berry development in Danfeng-2 but decreased in Cabernet Sauvignon. Meanwhile, RSGT was highly expressed at ripe stage (Fig. 5A). The expression of STS reached a peak in Danfeng-2 at the veraison stage when it was 2.6-fold greater than in Cabernet Sauvignon (Fig. 5B). However, it was 2.3-fold more highly expressed in Cabernet Sauvignon than in Danfeng-2 at the ripe stage (Fig. 5B). The MYB14 was more highly expressed in Danfeng-2 at all four stages (Fig. 5C). The transcript levels of MYB15 in Danfeng-2 were 4.3-fold higher than in Cabernet Sauvignon at stage veraison and 8.5-fold higher at stage ripe (Fig. 5D). The genes MYB5a, MYB5b, MYBPA1 and MYBPA1 are related to anthocyanins and flavonoids, and regulate CHS, chalcone isomerase, leucoanthocyanidin dioxygenase and anthocyanin reductase in the phenylalanine metabolic pathway. Surprisingly, transcript expression levels of MYBPA1 were 6.4-fold higher in Cabernet Sauvignon than in Danfeng-2 at Stage veraison (Fig. 5G). MYBA1 was not expressed in Danfeng-2 and expressed highly in Cabernet Sauvignon at stages veraison and ripe (Fig. 5H).
Figure 5.
Differential expressed proteins involved in phenylalanine metabolism pathway revealed specific protein promoting stilbenes accumulation in V. quinquangularis accession Danfeng-2. (A) C4H, Resveratrol metabolism pathway, (B) RSGT, and flavonoid metabolism (C) CHS, (D) CHI, (E) F3′5′H, (F) F3H, (G) DFR, (H) LDOX, and (I) UFGT, were identified. Especially, a resveratrol glucosyltransferase (A5BIH9) was significantly up-regulated at stage Ripe in Danfeng-2. PAL, phenylalanine ammonia-lyase, C4H, trans-cinnamate 4-monooxyygenase, 4CL, 4-coumarate-CoA ligase, STS, stilbene synthase; RSGT, resveratrol glucosyltransferase, OMT, O-methyltransferase, CHS, chalcone synthase, CHI, chalcone isomerase, F3H, flavanone 3-hydroxylase, F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; DFR, dihydroflavonol-4-reductase; FLS, flavonol synthase, LDOX, leucoanthocyanidin dioxygenase, LAR, leucoanthocyanidin reductase, ANR, anthocyanidin reductase, UFGT, UDP-glucose:flavonoid 3-O-glucosyltransferase. Heat maps of proteins were constructed with iTRAQ-derived quantitative data. D-2, Danfeng-2, CS, Cabernet Sauvignon. GH, green hard, BV, before veraison, V, veraison, and R, ripe. The proteins that increased and decreased are displayed in yellow and blue, respectively.
Evaluation of the transcript levels corresponding to the differentially expressed proteins
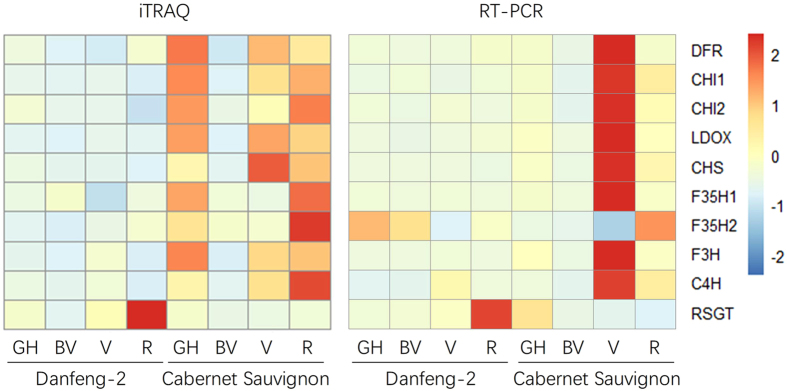
The details of the iTRAQ 8-plex data set and mRNA expression data are listed in Supplementary Table S6. Genes involved in the phenylalanine metabolic pathway were preferentially chosen for qRT-PCR analysis to complement the proteomic data. The targeted genes/proteins were a dihydroflavonol-4-reductase (A5BGJ0), a chalcone isomerase 1 (A5BMU2) and a chalcone isomerase 2 (A5ANT9), a leucoanthocyanin dioxygenase (A2ICC9), a CHS (G4XGW2), a flavonoid 3′,5′-hydroxylase 1 (Q2L7J4) and a flavonoid 3′,5′-hydroxylase 2 (F6HA89), a flavanone 3-hydroxylase (A5BZR3), a cinnamate-4-hydroxylase (A5BRL4), a resveratrol glucosyltransferase (A5BIH9), a ubiquitin conjugating enzyme 32 (A5BBF4), a leucine-rich repeat protein (A5BFQ1), a glycine rich protein (A5AI47), a plasma membrane intrinsic protein 2;3 (A3FA68), and a universal stress protein (D7TA35). The correlations between the detected proteins and their trancript levels were not strictly linear but generally showed similar trends. One notable discrepancy was the glycine rich protein (A5AI47), which displayed the highest trancript levels at stage V but the highest abundance of protein accumulation at stage R in Danfeng-2. Another was the mRNA levels of CHS (G4XGW2), which was up-regulated over 1,000-fold between stage BV and V but the protein levels increased only 5.4-fold. Stilbene and flavonoid biosynthesis related proteins were selected for correlation analysis with their responsive transcript levels (Fig. 6). The expression trends between protein and mRNA were similar although most proteins had highest expression profile in ripe stage while mRNA was highest in the veraison stage of Cabernet Sauvignon. The related coefficient of protein levels and transcript levels were 0.452 (Fig. 7).
Figure 6.
Relative expression levels of related transcripts. Data were normalized with the housekeeping gene GAPDH. Genes involved in stilbene synthesis are (A) RSGT; (B) STS; (C) MYB14; (D) MYB15; Genes involved in flavonoid and anthocyanin synthesis are (E) MYB5a; (F) MYB5b; (G) MYBPA1; (H) MYBA1. 2−ΔΔCt method was used to calculate the relative expression for each gene. Each value represents the means ± SE of three different experiments.
Figure 7.
Correlation of protein and mRNA levels of stilbene and flavonoid biosynthesis related members. C4H, trans-cinnamate 4-monooxyygenase, RSGT, resveratrol glucosyltransferase, CHS, chalcone synthase, CHI, chalcone isomerase, F3H, flavanone 3-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; DFR, dihydroflavonol-4-reductase; LDOX, leucoanthocyanidin dioxygenase. GH, green hard, BV, before veraison, V, veraison, and R, ripe. The proteins that increased and decreased are displayed in red and blue, respectively.
Discussion
Previous reports have made significant advances in understanding the proteome changes during grape berry ripening and the overall events in berry development are now generally understood23, 27, 36. Proteins related to photosynthesis and metabolism are highly expressed at the beginning of berry development23, 27, 36, while defense proteins are highly accumulated at harvest23. A comparative proteomic analysis based on cross-progeny populations of red and white V. vinifera grapes37 indicates many differences occur before veraison. However, comparison of proteomes between Chinese wild species and V. vinifera cultivars have rarely been reported. The mechanisms associated with the valuable traits of the wild species still need to be determined. The present study demonstrates the dynamic changes of proteins at different development stages between V. quinquangularis Danfeng-2 and V. vinifera cv. Cabernet Sauvignon. It also identifies candidate proteins related to resveratrol synthesis.
Stress response proteins are crucial for plant resistance to biotic and abiotic stresses38, 39. Of the differentially expressed stress response proteins between Danfeng-2 and Cabernet Sauvignon, we found a number of biological processes were involved. The up-regulated proteins in Danfeng-2 was mainly related to energy metabolism. Proteins involved in photosynthesis with high expression in Danfeng-2 suggests its berries collect more energy for production of down-stream products including secondary metabolites. Sedoheptulose-1,7-bisphosphatase can enhance photosynthesis and growth40. In addition, chlorophyll a-b binding proteins are responsible for harvesting light41. Other stress related proteins were also detected, such as dehydrins, heat shock proteins, and defensing. These indicate powerful stress-resistance in the Chinese wild grape. However, the up-regulated proteins in Cabernet Sauvignon belonged to a distinct category. The MLP-like proteins are believed to improve biotic and abiotic resistance in Arabidopsis42. Pathogenesis-related protein PR-4 and serine/threonine-protein kinase were also detected. The different categories of proteins between Danfeng-2 and Cabernet Sauvignon reveal different accumulations of stress proteins in Chinese wild grape during berry development. The chlorophyll concentrations in grape berries and transcripts of chlorophyll a-b binding proteins decrease during ripening43. This could explain the high level of disease resistance of the Chinese wild grape species.
The phenylalanine metabolic pathway, is one of the best characterized metabolic pathway in plants. It produces a variety of secondary metabolites including flavonoids, lignins and taxon-specific compounds, such as the stilbenes in grapevine44. Among the metabolites, flavonoids and stilbenes share the same precursors, namely, p-coumaroyl-CoA and malonyl-CoA45. In our search of the Uniprot KB database, the functions of thousands of proteins are annotated, However, we did not generate the expression of stilbene synthase. The reason may be that the protein level of stilbene synthase was below the minimum threshold of iTRAQ. Several previous studies have also failed to identify this protein26, 46. Fortunately, we identified a homolog (A5BIH9) of resveratrol glucosyltransferase (RSGT), a protein that makes the stilbenoid glucoside from resveratrol47. RSGT is up-regulated in Danfeng-2 at the R stage (Fig. 5B). The Q-RT PCR result for RSGT agreed with the iTRAQ one (Fig. 5A). This glucosyltransferase follows stilbene synthase and may control the carbon flow to stilbenes but this must still be confirmed. In V. vinifera cv. Muscat Hamburg berries, twelve STS genes and two phenylalanine ammonia lyase (PAL) genes have been observed to be up-regulated during berry ripening48. Also, the phenylpropanoid/stilbene biosynthetic genes PAL, CHS and STS genes have been reported to be highly expressed in ripe Shiraz berries49. So the transcript level of stilbene synthases and the positive regulation transcript factor of stilbene synthases MYB14 and MYB15 50, were also examined. STS showed the same expression profile as that described above for Cabernet Sauvignon, while its expression peaked at stage V in Danfeng-2 (Fig. 5B). The transcript levels of both MYB14 and MYB15 were higher in Danfeng-2 at stage V and R (Fig. 5C and D). Thus, high resveratrol content in grapevine seems to be related to these genes.
Major differences in the protein profiles involved in the flavonoid metabolic pathway show obvious increases from BV to V in Cabernet Sauvignon. CHS, CHI, F3H, F3′,5′H and UFGT, which control carbon flux along the CHS pathway, were identified. Our protein expression results could help explain the carbon flow when compared with the levels of total anthocyanin and flavonoid (Fig. 5). Transcript factors such as MYB5a, MYB5b and MYBPA1, control the expressions of enzymes in the flavonoid metabolic pathway51, however, the transcript levels of these were not significantly elevated in Cabernet Sauvignon (Fig. 5E and G). Although we detected the expression profile of these well studied transcript factors, our results indicated there are other regulators that control the flavonoid metabolic pathway. The high protein levels of enzymes in the flavonoid metabolic pathway (e.g. CHS, CHI, F3H, F3′5′H) (Supplementary Table S6), suggests complex processes exist in the berries between transcript factors and their target genes. MYBA1 which regulates the key enzyme UFGT for anthocyanins51, was not expressed at any developmental stage in Danfeng-2 but it was highly expressed in Cabernet Sauvignon at stages V and R (Fig. 5H). These results suggest there are huge differences between red and white grapevine cultivars when berries start to color.
Our earlier studies have systematically characterized stilbene synthase gene function in Chinese wild grape and their genetic transformation to V. vinifera 2, 22, 52. There were higher expression levels of stilbene synthase genes in the skins of berries of V. quinquangularis accession Danfeng-2 than in those of V. vinifera cv. Pinot Noir. Conversely, the resveratrol content of Danfeng-2 berries is higher than that of in Pinot Noir and Cabernet Sauvignon berries (Shi et al., 2014), which agrees with our results. Furthermore, the results for V. pseudoreticulata accession Baihe35-1 showed that the promoter activity of the stilbene synthase gene was strongly regulated by both pathogen and abiotic stresses52, 53. Also, transient expression of the stilbene synthase gene in tobacco increased the resveratrol content. When stilbene synthase genes were stably transformed into V. vinifera, the level of stilbenes and disease tolerance were both increased significantly22. Here, an enzyme RSGT, which glycosylates resveratrol into the more stable piceid, was highly expressed in Danfeng-2 (Fig. 5). Based on all the above results, we propose a potential mechanism to explain the high resveratrol content in the Chinese wild grapevines.
Our study provides a comparison of the proteomes of the Chinese wild grape V. quinquangularis and V. vinifera. Our results provide new information on the mechanisms involved in resveratrol accumulation and the defense systems of grapevines. Moreover, it becomes clear that Chinese wild grape germplasm will in time be able to contribute to the development of disease resistant cultivars of V. vinifera and hence benefit both the grape industry and the health benefits associated with grapes and grape products. This study analyzed specific proteins and elucidated the main features of the Danfeng-2 proteome during berry development. The two proteins, glucosyltransferase (RSGT) and stilbene synthase (STS), are involved in the phenylalanine metabolic pathway. These were found to be increased and in Chinese wild V. quinquangularis accession Danfeng-2 were apparently responsible for the accumulation of resveratrol and for known and significant changes in specific metabolites. The candidate proteins and transcription factor genes related to the high resveratrol content of Chinese wild V. quinquangularis are identified. The specific germplasm Chinese wild grape Danfeng-2 will likely be very useful for grape breeding to increase the resveratrol content of the European grapevine cultivars which will increase both the economics of grape production and the health benefits associated with human consumption of grape products.
Materials and Methods
Plant material
Grape berries of V. quinquangularis accession Danfeng-2 were sampled from the vineyard of Northwest A&F University, China in 2013 and V. vinifera cv. Cabernet Sauvignon was used as control. Both V. quinquangularis accession Danfeng-2 and V. vinifera cv. Cabernet Sauvignon were well watered and grown in a reasonable row spacing with no pest and disease occurred. Grape Cluster bagging after fruit set was conducted to avoid abiotic and biotic stresses. Berries were collected at stages spanning the developmental period from fruit set to harvest ripe. The berry stages for Cabernet Sauvignon berries were classified according to the system described by Lorenz et al.54. Danfeng-2 is one of most important wild grape germplasm resources. It comes from the mountainous regions of Danfeng county, Shannxi province, and has white berries and its phenophases (emergence of leaves, flowers and berries) are somewhat later than those of Cabernet Sauvignon20, 55. We classified Danfeng-2 berries on the basis of a previously-determined growth curve of fruit weight. The Danfeng-2 samples were collected from four developmental stages: green hard (GH), 25 days after bloom (DAB); before veraison (BF), 40 DAB; veraison (V), 50 DAB; ripe (R), 80 DAB. Meanwhile, the Cabernet Sauvignon samples were collected at the same developmental stages: GH, 30 DAB; BF, 45 DAB; V, 60 DAB; R, 110 DAB. Biological replicate berries at the same developmental stages were selected from different clusters. After detaching a berry, the epicarp was carefully separated using a sharp scalpel and forceps, and then frozen in liquid nitrogen and stored at −80 °C pending analysis21.
Protein extraction
Protein extraction used the method described by Kambiranda, et al.26 and Wang, et al.56 with some modification.
Danfeng-2 and Cabernet Sauvignon epicarp samples were used for protein extraction at each of the four developmental stages (GH, BV, V and R). The tissue was first finely ground and 1 g was then placed in a 10-ml tube containing 5 ml 10% m/v trichloroacetic acid in acetone, 5 mM DL-Dithiothreitol and 5 mM Phenylmethanesulfonyl fluoride, and the sample was held at −20 °C for 30 min. The sample was then centrifuged at 15,000 g at 4 °C for 30 min, and the pellet was washed three times in cold acetone and dried in a vacuum freeze drier (Labogene CS110-4). The dry pellet was then dissolved in extraction buffer57, thoroughly mixed by vortex and placed in a boiling water bath for 5 min and then in an ultra-sonicator (80 W) for 5 min56. A Bradford assay was used to determine the protein concentration58 and the quality of the protein samples was determined by SDS-PAGE gel electrophoresis59.
Protein digestion and isobaric peptide labeling
For each sample, 100 μg of protein was denatured by adding half the corresponding volume of 2% SDS and reducing with an equal volume of 50 mM Tris-(2-carboxyethyl)-phosphine. Methyl methanethiosulfonate was used to block cysteine residues, as described in the iTRAQ Reagents kit (AB Sciex). For the key developmental stages of both Vitis species, sequencing-grade trypsin was used to digest the proteins at 37 °C for 16 h and the resulting peptides were labeled following the instructions for the iTRAQ reagent kit (Fig. 1). Three biological replicates were analyzed. After labeling, the eight samples were mixed.
Peptide fractionation using strong cation exchange
Strong cation exchange (SCX) was used to fractionate the mixed labeled samples as previously described46. After separation, the 36 fractions collected were combined to yield 10 fractions. These were then vacuum-concentrated, re-suspended and desalted56.
Nanoflow LC-MS/MS
Nanoflow LC-MS/MS were conducted as previously described by Zhong, et al.60 and Wang, et al. 56.
Each fraction described above was applied to a split-free, nanoflow LC system (EASY-nLC, Thermo scientific) with an EASY column (75 μm × 100 mm, 3 μm-C18, Thermo scientific) and eluted with a 120 min gradient (0–35% B in 100 min, 35–100% B in 8 min, 10% B in 12 min, where A is 0.1% formic acid dissolved in HPLC grade H2O and B is 84% acetonitrile (ACN), 0.1% formic acid in HPLC grade H2O at a flow rate of 250 nl/min) modified from Zhong60. The eluent was sprayed directly into a Q Exactive mass spectrometer (Thermo Scientific). MS data were acquired as previously described56.
Sequence database retrieval
The MS/MS spectra were used to interrogate the proteins of Vitis in the Uniport database (http://www.uniprot.org/) (downloaded April 2014, 56,310 sequences) using MASCOT software (http://www.matrixscience.com). The MASCOT parameters were as previously described56. Peptide FDR ≤ 0.01 were generated for further analysis. We used Proteome Discoverer 1.4 (Thermo Scientific) for quantitative analysis with the following parameters: Protein Quantification: Use Only Unique Peptides, Experimental Bias: Normalize on Protein Median.
Functional annotation
The STRAP editor (structural alignments of proteins) was used for annotation of the proteins identified61. Differently expressed proteins were defined as those with a 1.5-fold quantitation ratio (a quantitation ratio ≤ 0.67 is defined as down-regulated and a quantitation ratio ≥ 1.5 as up-regulated) with p value < 0.05. The following subsets of differentially expressed proteins analyzed were divided into subparts: Danfeng-2 GH vs Cabernet Sauvignon GH, Danfeng-2 BV vs Cabernet Sauvignon BV, Danfeng-2 V vs Cabernet Sauvignon V, and Danfeng-2 R vs Cabernet Sauvignon R. Venn diagrams of the data were drawn using VennPainter (https://github.com/linguoliang/VennPainter). Gene ontology analyses for differentially expressed proteins were retrieved from the Uniport database (http://www.uniprot.org/). Cytoscape was used for plotting the GO annotation of the differentially expressed proteins.
Measurement of contents of resveratrol, piceid, anthocyanin and total flavonoid
The contents of resveratrol and piceid were determined using the method of Zhou, et al.21. Anthocyanin levels were measured as previously described62. The total flavonoid content was determined using ultraviolet spectroscopy63.
RNA extraction and qRT-PCR
Total RNA was extracted using the method of Reid64 and the primer sets for each gene were designed using Primer Premier 5.0 (Primer, Canada) (Supplementary Table S1). qRT-PCR was carried out as Xu et al.53.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31372039) and the Program for Innovative Research Team of Grape Germplasm Resource and Breeding (2013KCT-25). The authors thank Dr. Jocelyn Rose Professor, Plant Biology Section, School of Integrative Plant Science and Institute of Biotechnology, 412 Mann Library Building, Cornell University, Ithaca, New York, 14853 U.S.A. for editing revision and critical review of this manuscript. The authors thank Dr. Alexander (Sandy) Lang from RESCRIPT Co. (New Zealand) and Dr. Paul E. Read, Department of Agronomy and Horticulture, University of Nebraska, Lincoln, Nebraska, U.S.A. for useful comments and carefully language editing, which have greatly improved the manuscript.
Author Contributions
Y.W. and C.Z. supervised and designed the experiments and revised the manuscript. X.W., Y.X. and J.Z. revised the manuscript. R.L., X.X. and F.M. carried out the experiments and wrote the manuscript. R.L. and F.M. designed and carried out the computational analyses. D.W. and L.W. provided the metabolite data. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Ruimin Li, Xiaoqing Xie and Fuli Ma contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10171-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chaohong Zhang, Email: zhangchaohong@nwsuaf.edu.cn.
Yuejin Wang, Email: wangyj@nwsuaf.edu.cn.
References
- 1.Roubelakis-Angelakis, K. A. Grapevine molecular physiology and biotechnology[M]. Springer (2009).
- 2.Fan C, et al. Agrobacterium -mediated genetic transformation of grapevine (Vitis vinifera L.) with a novel stilbene synthase gene from Chinese wild Vitis pseudoreticulata. Plant Cell Tissue & Organ Culture. 2008;92:197–206. doi: 10.1007/s11240-007-9324-2. [DOI] [Google Scholar]
- 3.Wang Y, et al. Evaluation of foliar resistance to Uncinula necator in Chinese wild Vitis species. Vitis. 1995;34:159–164. [Google Scholar]
- 4.Wang Y, He P. Study on the inheritance of resistance to Powdery mildew in Chinese antive wild Vitis L.species. Zhongguo nongye kexue. 1996;30:19–25. [Google Scholar]
- 5.Hart JH. Role of Phytostilbenes in Decay and Disease Resistance. Phytopathology. 2003;19:437–458. doi: 10.1146/annurev.py.19.090181.002253. [DOI] [Google Scholar]
- 6.Jang M, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 7.Jeandet, P. & Adrian, M. In Resveratrol as an Antifungal Agent Oxidative Stress and Disease 475–497 (CRC Press, 2005).
- 8.Hain R, Bieseler B, Kindl H, Schröder G, Stöcker R. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Molecular Biology. 1990;15 doi: 10.1007/BF00036918. [DOI] [PubMed] [Google Scholar]
- 9.Starklorenzen P, Nelke B, Hanssler G, Muhlbach HP, Thomzik JE. Transfer of a grapevine stilbene synthase gene to rice (Oryza sativa L.) Plant Cell Reports. 1997;16:668–673. doi: 10.1007/s002990050299. [DOI] [PubMed] [Google Scholar]
- 10.Hipskind JD, Paiva NL. Constitutive Accumulation of a Resveratrol-Glucoside in Transgenic Alfalfa Increases Resistance to Phoma medicaginis. Molecular Plant-microbe Interactions. 2000;13:551–562. doi: 10.1094/MPMI.2000.13.5.551. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Ding CK, Nakamura Y, Nakajima I, Matsumoto R. Kiwifruits (Actinidia deliciosa) transformed with a Vitis stilbene synthase gene produce piceid (resveratrol-glucoside) Plant Cell Reports. 2000;19:904–910. doi: 10.1007/s002990000203. [DOI] [PubMed] [Google Scholar]
- 12.Liang H, et al. A transgenic wheat with a stilbene synthase gene resistant to powdery mildew obtained by biolistic method. Chinese Science Bulletin. 2000;45:634–638. doi: 10.1007/BF02886041. [DOI] [Google Scholar]
- 13.Serazetdinova L, Oldach KH, Lorz H. Expression of transgenic stilbene synthases in wheat causes the accumulation of unknown stilbene derivatives with antifungal activity. Journal of Plant Physiology. 2005;162:985–1002. doi: 10.1016/j.jplph.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Szankowski I, et al. Transformation of apple (Malus domestica Borkh.) with the stilbene synthase gene from grapevine (Vitis vinifera L.) and a PGIP gene from kiwi (Actinidia deliciosa) Plant Cell Reports. 2003;22:141–149. doi: 10.1007/s00299-003-0668-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, et al. An Arabidopsis homeodomain transcription factor gene, HOS9, mediates cold tolerance through a CBF-independent pathway. Proceedings of the National Academy of Sciences. 2004;101:9873–9878. doi: 10.1073/pnas.0403166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giorcelli A, et al. Expression of the stilbene synthase (StSy) gene from grapevine in transgenic white poplar results in high accumulation of the antioxidant resveratrol glucosides. Transgenic Research. 2004;13:203–214. doi: 10.1023/B:TRAG.0000034658.64990.7f. [DOI] [PubMed] [Google Scholar]
- 17.Yu CKY, et al. Constitutive Accumulation of cis-piceid in Transgenic Arabidopsis Overexpressing a Sorghum Stilbene Synthase Gene. Plant and Cell Physiology. 2006;47:1017–1021. doi: 10.1093/pcp/pcj061. [DOI] [PubMed] [Google Scholar]
- 18.Richter A, et al. Transgenic peas (Pisum sativum) expressing polygalacturonase inhibiting protein from raspberry (Rubus idaeus) and stilbene synthase from grape (Vitis vinifera) Plant Cell Reports. 2006;25:1166–1173. doi: 10.1007/s00299-006-0172-z. [DOI] [PubMed] [Google Scholar]
- 19.Schwekendiek A, et al. Constitutive expression of a grapevine stilbene synthase gene in transgenic hop (Humulus lupulus L.) yields resveratrol and its derivatives in substantial quantities. Journal of agricultural and food chemistry. 2007;55:7002–7009. doi: 10.1021/jf070509e. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, et al. The comparative analysis of the potential relationship between resveratrol and stilbene synthase gene family in the development stages of grapes (Vitis quinquangularis and Vitis vinifera) Plant Physiology and Biochemistry. 2014;74:24–32. doi: 10.1016/j.plaphy.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Q, et al. Resveratrol derivatives in four tissues of six wild Chinese grapevine species. New Zealand Journal of Crop and Horticultural Science. 2015;43:204–213. doi: 10.1080/01140671.2015.1010547. [DOI] [Google Scholar]
- 22.Cheng S, et al. Genetic transformation of a fruit-specific, highly expressed stilbene synthase gene from Chinese wild Vitis quinquangularis. Planta. 2016;243:1041–1053. doi: 10.1007/s00425-015-2459-1. [DOI] [PubMed] [Google Scholar]
- 23.Deytieux C, et al. Proteome analysis of grape skins during ripening. Journal of experimental botany. 2007;58:1851–1862. doi: 10.1093/jxb/erm049. [DOI] [PubMed] [Google Scholar]
- 24.Grimplet J, et al. Proteomic and selected metabolite analysis of grape berry tissues under well-watered and water-deficit stress conditions. Proteomics. 2009;9:2503–2528. doi: 10.1002/pmic.200800158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giribaldi M, Gény L, Delrot S, Schubert A. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. Journal of Experimental Botany. 2010;61:2447–2458. doi: 10.1093/jxb/erq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambiranda D, Katam R, Basha SM, Siebert S. iTRAQ-based quantitative proteomics of developing and ripening muscadine grape berry. Journal of proteome research. 2013;13:555–569. doi: 10.1021/pr400731p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghan R, et al. Five omic technologies are concordant in differentiating the biochemical characteristics of the berries of five grapevine (Vitis vinifera L.) cultivars. BMC genomics. 2015;16 doi: 10.1186/s12864-015-2115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarry JE, et al. Grape berry biochemistry revisited upon proteomic analysis of the mesocarp. Proteomics. 2004;4:201–215. doi: 10.1002/pmic.200300499. [DOI] [PubMed] [Google Scholar]
- 29.Sharathchandra RG, Stander C, Jacobson D, Ndimba B, Vivier MA. Proteomic analysis of grape berry cell cultures reveals that developmentally regulated ripening related processes can be studied using cultured cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0014708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, et al. Proteomic analysis of β-1, 3-glucanase in grape berry tissues. Acta physiologiae plantarum. 2009;31:597–604. doi: 10.1007/s11738-008-0269-9. [DOI] [Google Scholar]
- 31.Vincent D, et al. Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. Journal of Experimental Botany. 2007;58:1873–1892. doi: 10.1093/jxb/erm012. [DOI] [PubMed] [Google Scholar]
- 32.Lücker J, Laszczak M, Smith D, Lund ST. Generation of a predicted protein database from EST data and application to iTRAQ analyses in grape (Vitis vinifera cv. Cabernet Sauvignon) berries at ripening initiation. BMC genomics. 2009;10 doi: 10.1186/1471-2164-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh E, et al. Changes in protein abundance during powdery mildew infection of leaf tissues of Cabernet Sauvignon grapevine (Vitis vinifera L.) Proteomics. 2010;10:2057–2064. doi: 10.1002/pmic.200900712. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Esteso MJ, et al. iTRAQ-based profiling of grape berry exocarp proteins during ripening using a parallel mass spectrometric method. Molecular BioSystems. 2011;7:749–765. doi: 10.1039/C0MB00194E. [DOI] [PubMed] [Google Scholar]
- 35.Liu G-T, et al. Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. BMC plant biology. 2014;14 doi: 10.1186/1471-2229-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giribaldi M, Perugini I, Sauvage FX, Schubert A. Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics. 2007;7:3154–3170. doi: 10.1002/pmic.200600974. [DOI] [PubMed] [Google Scholar]
- 37.Niu N, Wu B, Yang P, Li S. Comparative analysis of the dynamic proteomic profiles in berry skin between red and white grapes (Vitis vinifera L.) during fruit coloration. Scientia Horticulturae. 2013;164:238–248. doi: 10.1016/j.scienta.2013.09.046. [DOI] [Google Scholar]
- 38.Chen F, Li Q, Sun L, He Z. The rice 14-3-3 gene family and its involvement in responses to biotic and abiotic stress. DNA research. 2006;13:53–63. doi: 10.1093/dnares/dsl001. [DOI] [PubMed] [Google Scholar]
- 39.Piffanelli P, et al. The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant physiology. 2002;129:1076–1085. doi: 10.1104/pp.010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefebvre S, et al. Increased sedoheptulose-1, 7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiology. 2005;138:451–460. doi: 10.1104/pp.104.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jansson S. The light-harvesting chlorophyll ab-binding proteins. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 42.Chen J-Y, Dai X-F. Cloning and characterization of the Gossypium hirsutum major latex protein gene and functional analysis in Arabidopsis thaliana. Planta. 2010;231:861–873. doi: 10.1007/s00425-009-1092-2. [DOI] [PubMed] [Google Scholar]
- 43.Deluc LG, et al. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC genomics. 2009;10 doi: 10.1186/1471-2164-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J-Y, et al. Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biology and Technology. 2006;40:64–72. doi: 10.1016/j.postharvbio.2005.12.017. [DOI] [Google Scholar]
- 45.Vannozzi A, Ian B, Fasoli M, Zenoni S, Lucchin M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. Bmc Plant Biology. 2012;12:1–22. doi: 10.1186/1471-2229-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martínez-Esteso MJ, Vilella-Antón MT, Pedreño MÁ, Valero ML, Bru-Martínez R. iTRAQ-based protein profiling provides insights into the central metabolism changes driving grape berry development and ripening. Bmc Plant Biology. 2013;13:167–167. doi: 10.1186/1471-2229-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall D, De Luca V. Mesocarp localization of a bi‐functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca) The Plant Journal. 2007;49:579–591. doi: 10.1111/j.1365-313X.2006.02987.x. [DOI] [PubMed] [Google Scholar]
- 48.Lijavetzky D, et al. Correction: Berry Flesh and Skin Ripening Features in Vitis vinifera as Assessed by Transcriptional Profiling. Plos One. 2012;7:1634–1634. doi: 10.1371/annotation/fd93800a-3b3c-484d-97a9-190043309e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sweetman C, Wong DC, Ford CM, Drew DP. Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics. 2012;13:105–105. doi: 10.1186/1471-2164-13-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Höll J, et al. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. The Plant Cell. 2013;25:4135–4149. doi: 10.1105/tpc.113.117127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czemmel S, Heppel SC, Bogs J. R2R3 MYB transcription factors: key regulators of the flavonoid biosynthetic pathway in grapevine. Protoplasma. 2012;249:109–118. doi: 10.1007/s00709-012-0380-z. [DOI] [PubMed] [Google Scholar]
- 52.Xu W, Yu Y, Ding J, Hua Z, Wang Y. Characterization of a novel stilbene synthase promoter involved in pathogen-and stress-inducible expression from Chinese wild Vitis pseudoreticulata. Planta. 2010;231:475–487. doi: 10.1007/s00425-009-1062-8. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, et al. Expression pattern, genomic structure, and promoter analysis of the gene encoding stilbene synthase from Chinese wild Vitis pseudoreticulata. Journal of experimental botany. 2011;62:2745–2761. doi: 10.1093/jxb/erq447. [DOI] [PubMed] [Google Scholar]
- 54.Lorenz DH, et al. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale†. Australian Journal of Grape and Wine Research. 1995;1:100–103. doi: 10.1111/j.1755-0238.1995.tb00085.x. [DOI] [Google Scholar]
- 55.Duan, C. Resveratrol Content in Berries of Chinese wild Vitis Species. [Master’s thesis]. Yangling: Northwest A&F University (2002).
- 56.Wang L, et al. Dynamics of chloroplast proteome in salt-stressed mangrove Kandelia candel (L.) Druce. Journal of Proteome Research. 2013;12:5124–5136. doi: 10.1021/pr4006469. [DOI] [PubMed] [Google Scholar]
- 57.Pan H-T, et al. Differential proteomic analysis of umbilical artery tissue from preeclampsia patients, using iTRAQ isobaric tags and 2D nano LC–MS/MS. Journal of proteomics. 2015;112:262–273. doi: 10.1016/j.jprot.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Hammond JBW, Kruger NJ. The Bradford Method for Protein Quantitation. Methods in Molecular Biology. 1988;3:25–32. doi: 10.1385/0-89603-126-8:25. [DOI] [PubMed] [Google Scholar]
- 59.Gallagher, S. R. One-dimensional SDS gel electrophoresis of proteins. Current protocols in cell biology, 6.1. 1–6.1. 38 (2007). [DOI] [PubMed]
- 60.Zhong W, Yang X, Tong W, Martin GE. Structural characterization of a novel degradant of the antifungal agent posaconazole. Journal of pharmaceutical and biomedical analysis. 2012;66:40–49. doi: 10.1016/j.jpba.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 61.Bhatia VN, Perlman DH, Costello CE, McComb ME. Software tool for researching annotations of proteins: open-source protein annotation software with data visualization. Analytical chemistry. 2009;81:9819–9823. doi: 10.1021/ac901335x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. Journal of AOAC international. 2005;88:1269–1278. [PubMed] [Google Scholar]
- 63.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC complementary and alternative medicine. 2012;12 doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reid K, Olsson N, Schlosser J, Peng F, Lund S. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biology. 2006;6:1–11. doi: 10.1186/1471-2229-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.