Abstract
Chloroplasts evolved from a free-living cyanobacterium acquired by the ancestor of all photosynthetic eukaryotes, including algae and plants, through a single endosymbiotic event. During endosymbiotic conversion, the majority of genes in the endosymbiont were transferred to the host nucleus and many of the proteins encoded by these genes must therefore be transported into the chloroplast after translation in the cytosol. Chloroplast-targeted proteins contain a targeting signal, named the transit peptide (TP), at the N-terminus. However, the evolution of TPs is not well understood. In this study, TPs from RbcS (rubisco small subunit) were compared between lower and higher eukaryotes. Chlamydomonas reinhardtii RbcS (CrRbcS) TP was non-functional in Arabidopsis. However, inclusion of a critical sequence motif, FP-RK, from Arabidopsis thaliana RbcS (AtRbcS) TP allowed CrRbcS TP to deliver proteins into plant chloroplasts. The position of the FP-RK motif in CrRbcS TP was critical for function. The QMMVW sequence motif in CrRbcS TP was crucial for its transport activity in plants. CrRbcS TPs containing additional plant motifs remained functional in C. reinhardtii. These results suggest that TPs evolved by acquiring additional sequence motifs to support protein targeting to chloroplasts during evolution of land plants from algae.
Introduction
Chloroplasts are found in a diverse range of eukaryotes, from single-celled organisms such as algae to multicellular higher plants. However, all chloroplasts are derived from an endosymbiotic organelle that evolved from a free-living cyanobacterium after a single endosymbiotic event with a cyanobacterium approximately 1 billion years ago1. In endosymbiotic conversion, one earliest evolutionary event to occur after endosymbiosis was transfer of genes from the endosymbiont to the nucleus of the host. Subsequently, proteins encoded by the transferred genes were provided to the endosymbiont after transcription and translation in the host cytosol. Thus, establishing protein import mechanisms by which the endosymbiont undergoing organellogenesis could obtain proteins from the host can be considered the most crucial event that gave rise to chloroplast evolution. The successful import of proteins from the host cytosol to the endosymbiont might have made possible for further transfer of genes from the endosymbiont to the host. In addition, the import of the encoded proteins from the host into the endosymbiont over evolutionary time might have led to the conversion of a free-living bacterium to an organelle. Chloroplasts in present-day higher plants contain less than 100 genes in their genomes2 and import approximately 3000 proteins that are encoded by nuclear genes and translated in the cytosol3–5.
Host cell transcription and translation mechanisms were exploited to produce proteins for the nascent chloroplast. However, sorting of chloroplast proteins in the cytosol and translocation through the outer and inner envelope membranes originated from double membrane layer in cyanobacteria must have required the establishment of new mechanisms during endosymbiotic conversion of the cyanobacterium. Currently, an N-terminal transit peptide (TP), defined by the sequence composed of N-terminal region (cTP) cleaved off by SPP (stromal processing peptidase) and some part of mature region, is both necessary and sufficient for targeting proteins to the chloroplast6–11. However, it is not fully understood how chloroplast proteins acquired TPs, and whether and how TPs were modified over evolutionary time. TPs are recognized by molecular machinery localized at chloroplast envelope membranes, and one possible scenario is that TPs coevolved with the molecular components involved in the import process.
Photosynthetic eukaryotes share common translocons for import of chloroplast proteins. Small sequence motifs found throughout the TP play key roles in import into chloroplasts, probably by acting as binding or recognition motifs for various translocon components in Toc/Tic complexes6–9, 12. However, TP sequences are not highly conserved in length or amino acid sequence13. The factors underlying the differences among different types of chloroplast proteins are not fully understood.
RbcS, which is involved in CO2 fixation and is one of the most important chloroplast proteins, is encoded by nuclear gene(s). In addition, RbcS TP has been used as a model to study how TPs can support specific protein import into chloroplasts6–12, 14. In this study, RbcS TPs from Chlamydomonas and Arabidopsis were used to examine TP development during the evolution of land plants from algae. TP capacity to deliver proteins into chloroplasts was compared and used to model TP evolutionary development. Wild-type TP from Chlamydomonas reinhardtii RbcS (CrRbcS) was unable to deliver proteins to chloroplasts in Arabidopsis. However, a small critical sequence motif in the TP of Arabidopsis thaliana RbcS (AtRbcS) was able to complement the defect and allow CrRbcS TP to function in Arabidopsis. These results strongly suggest that plant TPs evolved from corresponding algal TPs by acquisition of new sequence motifs during evolution.
Results
CrRbcS TP is not functional in Arabidopsis
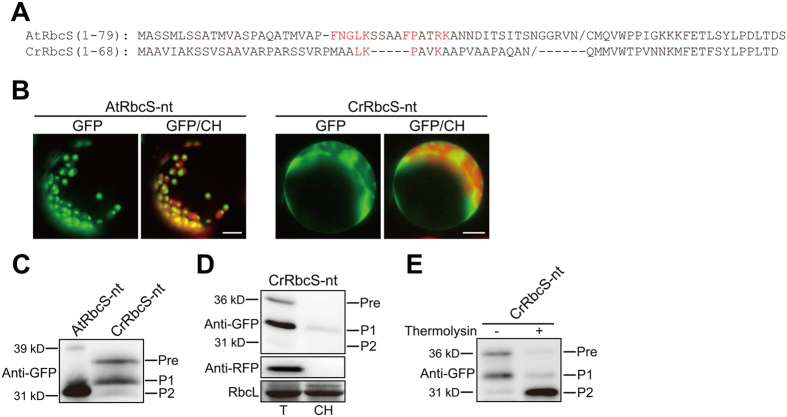
To gain insights into TP evolution, initially, sequence similarities were examined between 83 plant and 33 algal RbcS TPs (Table S1). Alignment of TP sequences from RbcS in algae and higher land plants revealed that RbcS TPs showed a high degree of similarity among higher land plant TPs and also among algal TPs but not between plant and algal TPs (Fig. S1). Moreover, phylogenetic analysis indicates that all algal RbcS TPs were grouped into a clade to which RbcS TPs from land plants do not belong (Fig. S2). The proposed Toc34-interacting motifs FGLK and FP-RK (highlighted in yellow) were not clearly defined in algal RbcS TPs8, 12. Moreover, the fifth 10-amino-acid (T5) segment (DITSITSNGG)6 in plant TPs (highlighted in green) was almost absent in algal TPs, and algal RbcS TPs were thus shorter in length than plant RbcS TPs (Fig. S1).
Next, we examined whether sequence differences between algal and plant TPs reflect any functional differences using TPs of CrRbcS and AtRbcS as a model system (Fig. 1A). Previous studies showed that an N-terminal segment containing 60 to 80 amino acid residues is sufficient to efficiently deliver GFP into chloroplasts6, 7, 10, 11. An N-terminal 68-amino-acid segment of CrRbcS was fused to GFP, and the resulting construct, CrRbcS-nt:GFP, was introduced into Arabidopsis protoplasts. AtRbcS-nt:GFP, which consists of an N-terminal 79-amino-acid segment of AtRbcS fused to GFP6, was used as a control. As reported previously6, AtRbcS-nt:GFP exhibited a strong chloroplast localization pattern (Fig. 1B). By contrast, GFP expression was observed only in the cytosol, and not in chloroplasts, indicating that CrRbcS-nt:GFP was not imported into chloroplasts in Arabidopsis protoplasts (Fig. 1B). To confirm this, protein extracts from protoplasts were analyzed by western blotting using anti-GFP antibody. Consistent with the microscopy analysis, AtRbcS-nt:GFP was largely processed to a mature form with only a minimal amount of precursors. However, CrRbcS:GFP produced a different band pattern; the top band at the position of precursors (Pre) was equal with an intermediate (P1) in intensity alongside a minor processed form (P2) at the position of the mature form (Fig. 1C). Cellular localization of the two processed forms, P1 and P2, was analyzed by purifying chloroplasts. No P2 and only a small amount of P1 was copurified with chloroplasts, indicating that the processed forms were not imported into chloroplasts. Co-transformed cytosolic RFP was not detected in the chloroplast fraction, confirming successful fractionation (Fig. 1D). Furthermore, gently lysed protoplast extracts were treated with thermolysin, a protease that can degrade proteins in the cytosol and outer envelope proteins exposed to the cytosolic face. Both precursor and P1 were sensitive to thermolysin, confirming that they were not imported into chloroplasts (Fig. 1E). A previous study showed that properly folded GFP is resistant to thermolysin, which is the reason why a protein band almost identical to the P2 band in size was generated6 (Fig. 1E). These results strongly suggest that CrRbcS TP is not functional in Arabidopsis.
Figure 1.
CrRbcS TP is not functional in Arabidopsis. (A) Sequences of RbcS TPs from Arabidopsis and C. reinhardtii. All constructs were fused to GFP. The cleavage sites predicted by ChloroP are indicated by ‘/’. (B) Localization of reporter proteins. Protoplasts from Arabidopsis plants were transformed with the indicated constructs, and GFP patterns were observed 12 h after transformation. Green, red, and yellow signals represent GFP, chlorophyll autofluorescence, and the overlap between green and red signals, respectively. Scale bar = 20 μm. (C) Western analysis of reporter proteins. Total protein extracts from transformed protoplasts were analyzed by western blotting with anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2. (D) Isolation of chloroplasts from transformed protoplasts. Chloroplasts were isolated from protoplasts transformed with CrRbcS-nt:GFP. At 12 h after transformation, protoplasts were gently lysed and chloroplasts were isolated using a Percoll gradient. Total and chloroplast fractions were analyzed by western blotting with anti-GFP and anti-RFP antibodies. RFP was used as a control for cytosolic proteins. Rubisco complex large subunit (RbcL) stained with Coomassie brilliant blue was used as a loading control. T, total protein; CH, chloroplast fraction; Pre, precursor form; P1, processed form 1; P2, processed form 2. (E) Thermolysin sensitivity of CrRbcS-nt:GFP. Protoplasts transformed with CrRbcS-nt:GFP were gently lysed and treated with thermolysin. Protein extracts were analyzed by western blotting using anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2.
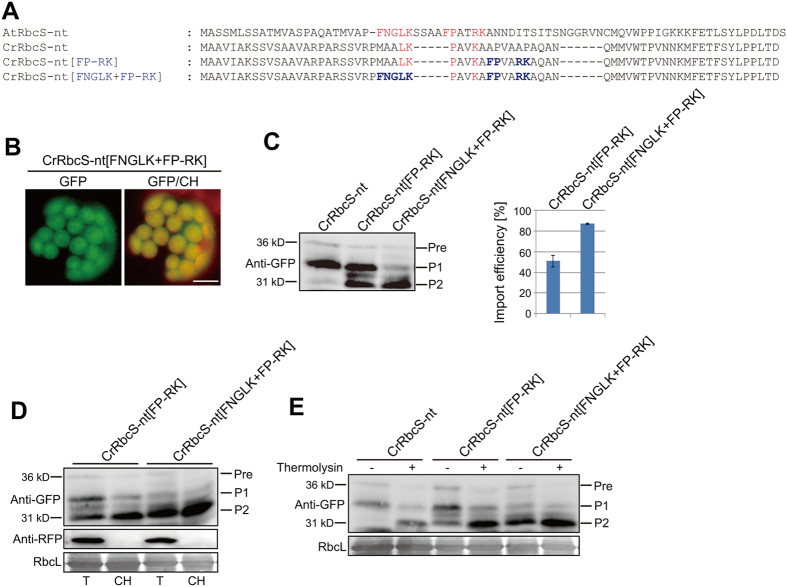
The FP-RK motif of AtRbcS-nt rescues the defect of CrRbcS-nt in protein import into plant chloroplasts
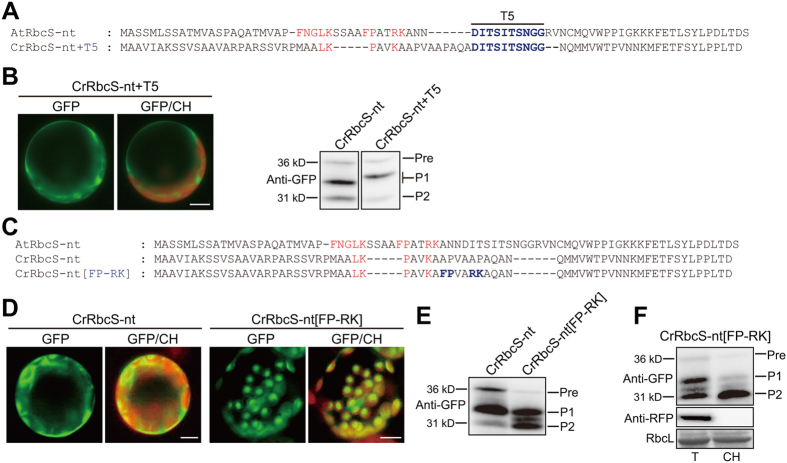
CrRbcS-nt was unable to deliver proteins into chloroplasts in plant cells, and we wished to determine what underlies this deficiency. The most conspicuous difference between the plant and algal TPs was that CrRbcS TP was shorter than AtRbcS TP. A previous study noted that internal deletions were detrimental to the activity of AtRbcS TP6. Here, we inserted the T5 segment of AtRbcS-nt into the corresponding region of CrRbcS to produce CrRbcS[1–68] + T5 because this segment was clearly absent in algal RbcS TP (Figs 2A and S1)6. The resulting construct was fused to GFP and introduced into protoplasts. CrRbcS[1–68] + T5 did not deliver proteins into chloroplasts (Fig. 2B), indicating that the elongated CrRbcS TP did not function as a TP in plant cells. Sequence alignment suggested that CrRbcS TP lacked some sequence motifs, such as FNGLK and FP-RK, which were crucial for protein import into chloroplasts in Arabidopsis (Fig. S1)8, 12. We asked whether introduction of these motifs to CrRbcS TP could rescue its activity and allow protein import into chloroplasts in Arabidopsis. We incorporated the sequence motif FP-RK, one of the most important Arabidopsis AtRbcS TP sequence motifs, at position 35 in CrRbcS-nt and examined the ability of GFP-fused CrRbcS[FP-RK] to deliver proteins into chloroplasts in Arabidopsis protoplasts (Fig. 2C). CrRbcS-nt[FP-RK]:GFP showed strong GFP signals in chloroplasts (Fig. 2D), indicating that the FP-RK motif greatly improved the protein delivery efficiency of CrRbcS TP in Arabidopsis. Protein import was assessed by western analysis using anti-GFP antibody. As with CrRbcS-nt:GFP, three protein forms (two processed and one precursor) were seen with CrRbcS-nt[FP-RK]:GFP. However, the band intensities seen with CrRbcS-nt[FP-RK]:GFP were markedly different. The intensity of the P2 band increased and concomitantly the intensity of the precursor band decreased in CrRbcS-nt[FP-RK]:GFP relative to CrRbcS-nt:GFP (Fig. 2E), indicating that protein delivery to chloroplasts was improved by inclusion of the FP-RK motif.
Figure 2.
The FP-RK motif of AtRbcS-nt rescues the defect of CrRbcS-nt in protein import into plant chloroplasts. (A and C) Sequences of AtRbcS TP, CrRbcS TP, and modified CrRbcS TP. All constructs were fused to GFP. (B and D) Localization of reporter proteins. Protoplasts from Arabidopsis plants were transformed with the indicated constructs, and GFP patterns were observed 12 h after transformation. Green, red, and yellow signals represent GFP, chlorophyll autofluorescence, and the overlap between green and red signals, respectively. Scale bar = 20 μm. In the western blot image of Fig. 2B, both lanes were cropped from the different parts of the same gel (Fig. S3). (E) Western analysis of reporter proteins. Total protein extracts from transformed protoplasts were analyzed by western blotting with anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2. (F) Isolation of chloroplasts from transformed protoplasts. Chloroplasts were isolated from protoplasts transformed with CrRbcS-nt[FP-RK]:GFP. At 12 h after transformation, protoplasts were gently lysed and chloroplasts were isolated using a Percoll gradient. Total and chloroplast fractions were analyzed by western blotting using anti-GFP and anti-RFP antibodies. RFP was used as a control for cytosolic proteins. Rubisco complex large subunit (RbcL) stained with Coomassie brilliant blue was used as a loading control. T, total protein; CH, chloroplast fraction; Pre, precursor form; P1, processed form 1; P2, processed form 2.
To further examine this, chloroplasts were isolated from protoplasts and protein extracts from the chloroplast fraction were analyzed by western blotting using anti-GFP antibody. Most of P2, and a small proportion of P1, copurified with chloroplasts, confirming that P2 was the stromal form of CrRbcS-[FP-RK]:GFP (Fig. 2F). P1 might be generated through proteolysis of the precursor form in the cytosol and be weakly associated with chloroplasts. These results confirmed that the FP/RK motif rescued the ability of CrRbcS-nt to import proteins into plant chloroplasts. RFP was included in transformations as a control for inadvertent copurification of cytosolic proteins. No RFP was detected in the chloroplast fraction, confirming that copurification was effective. RbcL was used as a control for equal loading and chloroplast isolation (Fig. 2F). Together, these results indicate that 1) CrRbcS-nt contains some, but not all, of the critical sequence motifs required for protein import into plant chloroplasts, and that 2) the FP-RK motif can complement missing motifs in CrRbcS-nt and allow protein import to occur.
The position of the FP-RK motif in CrRbcS TP is critical for its function in protein import into plant chloroplasts
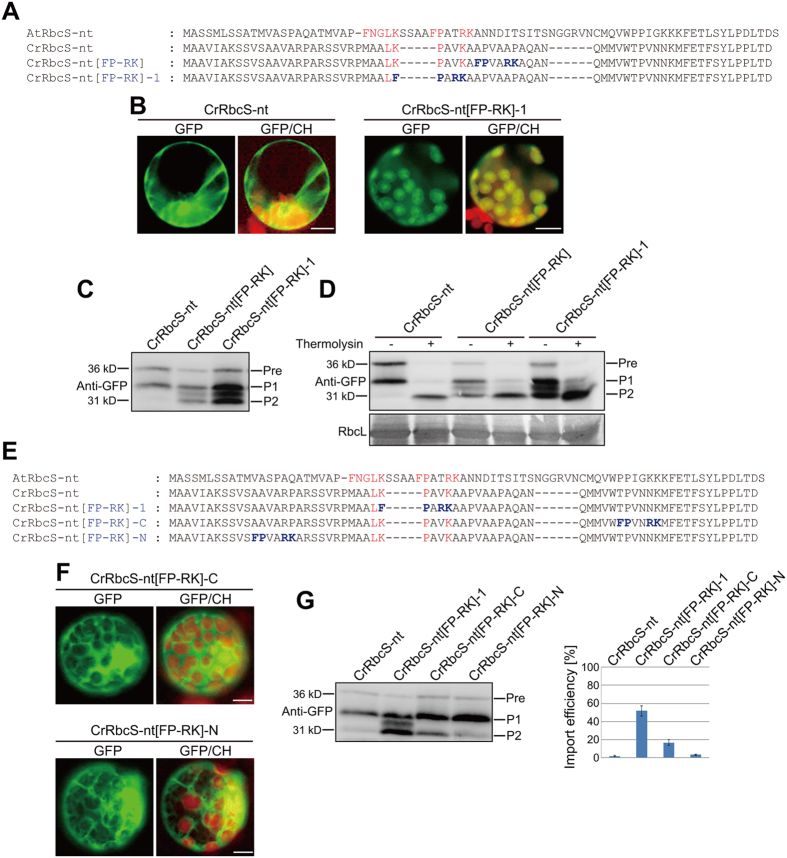
The FP-RK motif is predominantly located in the middle of RbcS TPs (Fig. S1). Thus, we tested whether FP-RK function was dependent on position within the TP. First, an alternative FP-RK construct (CrRbcS-nt[FP-RK]-1) was created in which the FP-RK motif was moved six amino acids towards the N-terminus relative to its position in CrRbcS-nt[FP-RK] (Fig. 3A). In this position, CrRbcS-nt contained P and R residues equivalent to the second and third residues, respectively, of the FP-RK motif. The construct was fused to GFP and introduced into Arabidopsis protoplasts. CrRbcS-nt[FP-RK]-1 also efficiently delivered GFP into chloroplasts (Fig. 3B), indicating that the minor change in the location of FP-RK in the TP did not affect its protein delivery function. Protein extracts from protoplasts were again analyzed by western blotting using anti-GFP antibody. As with CrRbcS-nt[FP-RK], two processed protein forms and a small proportion of precursor were observed with CrRbcS-nt[FP-RK]-1 (Fig. 3C). Import of P2 into chloroplasts was also assessed by treating gently lysed protoplasts with thermolysin. P2 was protected from thermolysin, whereas the precursor and P1 were largely subjected to proteolytic degradation, confirming that P2 was imported into chloroplasts (Fig. 3D).
Figure 3.
The position of the FP-RK motif in CrRbcS TP is critical for its function in protein import into plant chloroplasts. (A and E) Sequences of AtRbcS TP, CrRbcS TP, and modified CrRbcS TPs. All constructs were fused to GFP. (B and F) Localization of reporter proteins. Protoplasts from Arabidopsis plants were transformed with the indicated constructs, and GFP patterns were observed 12 h after transformation. Green, red, and yellow signals represent GFP, autofluorescence of chlorophyll, and the overlap between green and red signals, respectively. Scale bar = 20 μm. (C and G) Western analysis of reporter proteins. Total protein extracts from transformed protoplasts were analyzed by western blotting with anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2. Signal intensity of protein bands was measured using LAS3000 imager (FUJI FILM) software, and import efficiency was defined as the amount of P2 relative to the total amount of expressed protein. Three independent transformation experiments were performed, and the data represent means with standard deviation (SD). (D) Thermolysin sensitivity of reporter proteins. Protoplasts transformed with the indicated constructs were gently lysed and treated with thermolysin. Protein extracts were analyzed by western blotting using anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2.
Two additional constructs were generated, namely, CrRbcS-nt[FP-RK]-N and CrRbcS-nt[FP-RK]-C, with insertions of the FP-RK motif at positions 12 and 50 of CrRbcS-nt, respectively (Fig. 3E). The resulting TPs were fused to GFP and introduced into protoplasts. Severe defects in chloroplast targeting were seen with both constructs, and cytosolic GFP was observed (Fig. 3F). Protein extracts from protoplasts were analyzed by western blotting using anti-GFP antibody. By contrast with CrRbcS-nt[FP-RK], both CrRbcS-nt[FP-RK]-N and CrRbcS-nt[FP-RK]-C produced largely the P1 protein form and only small proportions of the precursor and P2 (Fig. 3G). Import efficiencies, as expressed by the amount of P2 relative to total expressed proteins, were only 4% and 18% for CrRbcS-nt[FP-RK]-N and CrRbcS-nt[FP-RK]-C, respectively, compared with 50% for CrRbcS-nt[FP-RK]. This indicated that the position of the FP-RK motif was critical for protein import function.
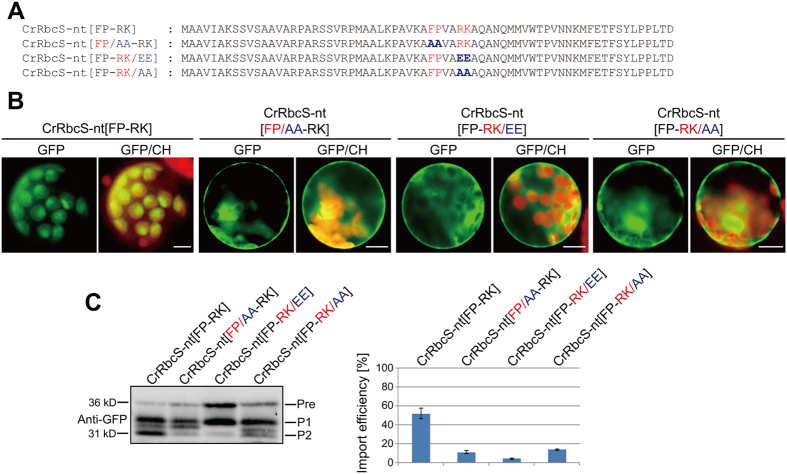
The results shown in Fig. 3 strongly suggested that the FP-RK motif in the middle position complemented the defect of CrRbcS-nt in delivering protein import into chloroplasts in Arabidopsis. However, other possibilities could not be excluded, such as improvement of CrRbcS-nt import efficiency by inserting additional amino acids at that position. The FP-RK motif contains the aromatic amino acid phenylalanine (F) and positively charged amino acids arginine (R) and lysine (K), which are found in most TPs. To examine the importance of the conserved FP-RK motif in chloroplast import, the FP or RK residues of the FP-RK motif were substituted with alanine or glutamic acid and import efficiency was assessed (Fig. 4A). First, FP or RK was substituted with two alanines to produce CrRbcS-nt[FP/AA-RK] and CrRbcS-nt[FP-RK/AA], respectively. The resulting constructs were introduced into protoplasts after fusion to GFP. The alanine-substituted mutants exhibited strong GFP signals in the cytosol with concomitant decreases in GFP signals in chloroplasts, indicating that both FP and RK residues in CrRbcS-nt[FP-RK] were crucial for protein import into chloroplasts (Fig. 4B). Next, the positively charged R and K residues were replaced with the negatively charged residue glutamic acid (E), which is under-represented in TPs. CrRbcS-nt[FP-RK/EE] also showed a strong cytosolic GFP signal (Fig. 4B).
Figure 4.
The FP-RK motif is critical in CrRbcS-nt[FP-RK] for protein import into chloroplasts. (A) Sequences of CrRbcS-nt[FP-RK] and its substitution mutants. All constructs were fused to GFP. (B) Localization of reporter proteins. Protoplasts from Arabidopsis plants were transformed with the indicated constructs, and GFP patterns were observed 12 h after transformation. Green, red, and yellow signals represent GFP, chlorophyll autofluorescence, and the overlap between green and red signals, respectively. Scale bar = 20 μm. (C) Western analysis of the reporter proteins. Total protein extracts from transformed protoplasts were analyzed by western blotting using anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2. Signal intensity of protein bands was measured using LAS3000 imager (FUJI FILM) software, and import efficiency was defined as described in Fig. 3G. Error bar = SD (n = 3).
Protein extracts from protoplasts were analyzed by western blotting using anti-GFP antibody. In contrast with CrRbcS-nt[FP-RK], alanine- or glutamic acid-substituted mutants showed significant decreases in P2 (Fig. 4C). The import efficiencies of CrRbcS-nt[FP/AA-RK], CrRbcS-nt[FP-RK/AA], and CrRbcS-nt[FP-RK/EE] were approximately 15%, 18%, and 5%, respectively, confirming that the specific FP-RK motif was critical for the chloroplast protein import function of CrRbcS-nt[FP-RK].
Two critical AtRbcS sequence motifs, FNGLK and FR-PK, improve the chloroplast protein import ability of CrRbcS-nt
The sequence motif FNGLK is also critical for chloroplast protein import mediated by AtRbcS TP8, 12. This motif was tested for its ability to improve transport by CrRbcS-nt in Arabidopsis. FNGLK was introduced into CrRbcS-nt[FP-RK] to give CrRbcS-nt[FNGLK + FP-RK] (Fig. 5A). The resulting construct was fused to GFP and introduced into protoplasts, and GFP signals were strongly detected in the chloroplasts (Fig. 5B). Protoplast proteins were analyzed by western blotting using anti-GFP antibody. CrRbcS-nt[FNGLK + FP-RK] produced the same three protein bands as were seen with CrRbcS-nt[FP-RK], but band pattern and intensity differed. CrRbcS-nt[FNGLK + FP-RK] largely produced the P2 protein, with only small proportions of P1 and the precursor, indicating that the FNGLK motif further increased import efficiency compared with the FP-RK motif alone. Import efficiency of CrRbcS-nt[FNGLK + FP-RK] was close to 90%, very similar to that of AtRbcS-nt in Arabidopsis protoplasts (Fig. 5C). To confirm import, extracts of purified chloroplasts and protoplast extracts that had been treated with thermolysin were analyzed by western blotting using anti-GFP antibody. As before, P2 copurified with chloroplasts and was resistant to thermolysin treatment, confirming that P2 was imported into chloroplasts (Fig. 5D and E).
Figure 5.
Two sequence motifs, FNGLK and FR-PK, of AtRbcS TP further improve the ability of CrRbcS-nt to import proteins into plant chloroplasts. (A) Sequences of AtRbcS TP, CrRbcS TP, and modified CrRbcS TPs. All constructs were fused to GFP. (B) Localization of reporter proteins. Protoplasts from Arabidopsis plants were transformed with the indicated constructs, and GFP patterns were observed 12 h after transformation. Green, red, and yellow signals represent GFP, chlorophyll autofluorescence, and the overlap between green and red signals, respectively. Scale bar = 20 μm. (C) Western analysis of the reporter proteins. Total protein extracts from transformed protoplasts were analyzed by western blotting using anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2. Signal intensity of protein bands was measured using LAS3000 imager (FUJI FILM) software, and import efficiency was defined as described in Fig. 3G. Error bar = SD (n = 3). (D) Isolation of chloroplasts from transformed protoplasts. Chloroplasts were isolated from the protoplasts transformed with the indicated constructs. At 12 h after transformation, protoplasts were gently lysed and chloroplasts were isolated using a Percoll gradient. Total and chloroplast fractions were analyzed by western blotting using anti-GFP and anti-RFP antibodies. RFP was used as a control for cytosolic proteins. Rubisco complex large subunit (RbcL) stained with Coomassie brilliant blue was used as a loading control. T, total protein; CH, chloroplast fraction; Pre, precursor form; P1, processed form 1; P2, processed form 2. (E) Thermolysin sensitivity of reporter proteins. Protoplasts transformed with the indicated constructs were gently lysed and treated with thermolysin. Protein extracts were analyzed by western blotting using anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2.
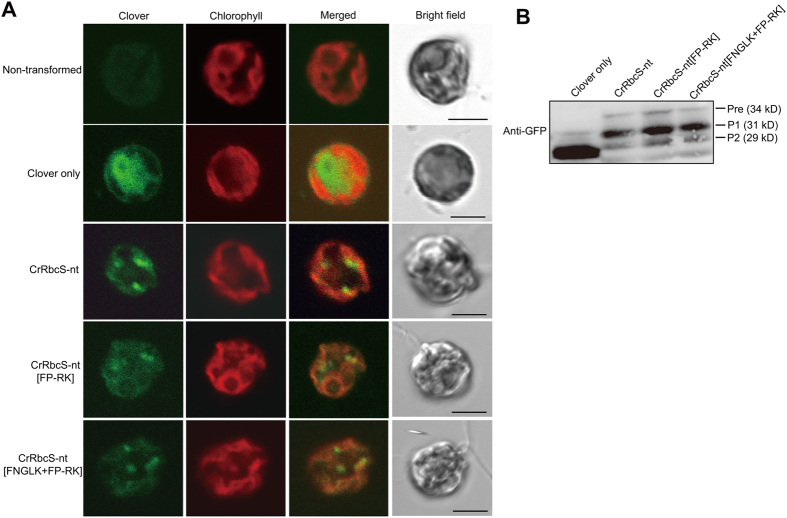
CrRbcS-nt[FP-RK] and CrRbcS-nt[FNGLK + FP-RK] efficiently deliver proteins into C. reinhardtii chloroplasts
Next, the behavior of modified CrRbcS-nt constructs was examined in C. reinhardtii. First, the CrRbcS(1–68):Clover (a GFP variant) construct was generated and transformed into C. reinhardtii (Fig. 6A). Transgenic C. reinhardtii expressing Clover only was also generated as a control. Transgenic algae were examined using a laser scanning confocal microscope. Transgenic C. reinhardtii transformed with Clover only exhibited Clover signal in the cytosol (Fig. 6A), but CrRbcS-nt:Clover distribution differed to that of Clover only. CrRbcS-nt:Clover was observed as punctate as well as diffuse signals that overlapped the chloroplast, indicating that CrRbcS-nt:Clover was targeted to chloroplasts.
Figure 6.
CrRbcS-nt[FP-RK] and CrRbcS-nt[FNGLK + FP-RK] efficiently deliver protein into the chloroplast in C. reinhardtii. (A) Localization of reporter proteins. C. reinhardtii (wild-type strain CC-503 cw92 mt+) was transformed with the indicated constructs, and Clover patterns were examined using a confocal laser scanning microscope. Green, red, and yellow signals represent Clover, chlorophyll autofluorescence, and the overlap between green and red signals, respectively. Scale bar = 5 μm. (B) Western analysis of the reporter proteins. Total protein extracts from transformed C. reinhardtii were analyzed by western blotting using anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2.
CrRbcS-nt[FP-RK]:Clover and CrRbcS-nt[FNGLK + FP-RK]:Clover were then generated and transformed into C. reinhardtii (Fig. 6A). Multiple transgenic C. reinhardtii lines were isolated and used for examination of Clover distribution. Both CrRbcS-nt[FP-RK]:Clover and CrRbcS-nt[FNGLK + FP-RK] were imported into chloroplasts (Fig. 6A). These results indicated that insertion of the FP-RK motif alone or together with the FNGLK motif did not adversely affect the ability of CrRbcS-nt to deliver proteins into the chloroplast in C. reinhardtii.
Protein import was confirmed by western analysis of protein extracts from transgenic lines of C. reinhardtii using an anti-GFP antibody. The three transgenic lines harboring wild-type or hybrid CrRbcS-nt constructs all exhibited a strong band at 31 kDa alongside two minor bands at 29 and 34 kDa. The 34 kDa and 31 kDa forms corresponded to precursor and processed mature forms, respectively, and the 29 kDa band may have represented a further processed form. By contrast, transgenic lines containing Clover alone produced a single band at 29 kDa, the predicted molecular weight of Clover (Fig. 6B). These results confirmed that CrRbcS-nt with additional motifs from AtRbcS-nt was efficiently imported into chloroplasts in C. reinhardtii.
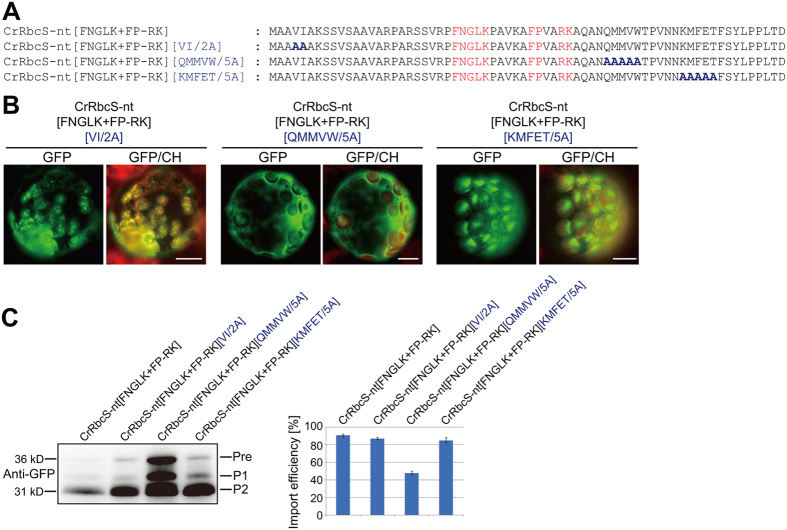
CrRbcS-nt contains a sequence motif that is functional for protein import into plant chloroplasts
Multiple sequence motifs are required for efficient protein import into chloroplasts in plants. The results showing that CrRbcS-nt[FP-RK] could deliver proteins into plant chloroplasts prompted us to examine whether CrRbcS-nt contained additional sequence motifs other than FNGLK and FP-RK that contributed to delivering proteins to plant chloroplasts. We selected three regions in the CrRbcS-nt[FNGLK + FP-RK] and substituted with alanines (Fig. 7A). The corresponding regions in AtRbcS TP contain functional sequence motifs6. Of these mutants, VI/2 A and KKFET/5 A showed only minor or no defects in protein import (Fig. 7B and C). However, protein import into chloroplasts was greatly impaired with alanine substitution of QMMVW (Fig. 7B and C), indicating that the QMMVW motif of CrRbcS-nt was functional in plants6.
Figure 7.
CrRbcS-nt contains a sequence motif that is functional in protein import into plant chloroplasts. (A) Sequences of CrRbcS-nt[FNGLK + FP-RK] and substitution mutants. All constructs were fused to GFP. (B) Localization of reporter proteins. Protoplasts from Arabidopsis plants were transformed with the indicated constructs, and GFP patterns were observed 12 h after transformation. Green, red, and yellow signals represent GFP, chlorophyll autofluorescence, and the overlap between green and red signals, respectively. Scale bar = 20 μm. (C) Western analysis of the reporter proteins. Total protein extracts from transformed protoplasts were analyzed by western blotting using anti-GFP antibody. Pre, precursor form; P1, processed form 1; P2, processed form 2. Signal intensity of protein bands was measured using LAS3000 imager (FUJI FILM) software, and import efficiency was defined as described in Fig. 3G. Error bar = SD (n = 3).
Discussion
In this study, we showed that CrRbcS TP did not support protein import into chloroplasts in Arabidopsis. This is in contrast to the observation that the low-CO2-inducible C. reinhardtii proteins LCIA, LCIB and LCIC were successfully imported into chloroplasts when expressed in tobacco15. One possible explanation for the difference in capacity of RbcS TPs between Arabidopsis and C. reinhardtii in delivering protein into chloroplasts in plants is that RbcS TPs gained additional motifs for protein import in plant cells during evolution. In fact, plant TPs are longer than those in algae. The central region appears to be inserted between conserved N- and C-terminal regions of algal TPs to give rise to plant TPs. Consistent with this idea, critical sequence motifs were identified in the central region of AtRbcS TP6, 7, 12. Moreover, when the FP-RK motif, one of the sequence motifs in the central region, was introduced into CrRbcS-nt, the mutant TP, CrRbcS-nt[FP-RK], was able to support protein import into chloroplasts in Arabidopsis. Furthermore, the FNGLK motif, another sequence motif in the central region, further improved the protein import efficiency of CrRbcS TP. These results suggest that these plant motifs function together with sequence motifs in CrRbcS TP to efficiently deliver protein into chloroplasts in plants. One of the CrRbcS motifs that function as a critical sequence motif in plants was the QMMVW motif at the C-terminal region of CrRbcS-nt. The CrRbcS QMMVW motif resembles the CMQVW motif of AtRbcS TP6. Thus, these results support gaining of sequence motifs in plant RbcS TPs during evolution. Although we favor this idea, we cannot exclude the possibility that CrRbcS TPs might have lost these sequence motifs from the unknown ancestral RbcS TP because they were not essential in protein import into C. reinhardtii. Consistent with this idea, the presence and absence of the sequence motifs, FP-RK and FNGLK, did not affect not only protein import into chloroplasts in C. reinhardtii but also preprotein processing.
Another important finding in this study was that the critical sequence motifs needed to be placed in the center of the CrRbcS TP to be functional. This corresponded with the original source location of the two sequence motifs in the center of the RbcS TP. These results suggest that the critical sequence motifs require correct placement to function, consistent with results from previous studies8, 10, 16. First, Lee et al.8 showed that hybrid TPs generated by swapping domains between two different types of TPs (RbcS and Cab TPs) could support protein import into chloroplasts as efficiently as the wild-type TPs when the interchanged domains were appropriately fused to one another. Correspondingly, the sequence motifs become non-functional when positioned inappropriately in the domain-swapping mutants. Second, Rolland et al.10 showed that both cTP and MPL (membrane protein leader) located between cTP and the first TMD (transmembrane domain) are required for targeting to inner envelope membrane of chloroplasts. Interestingly, MPL was interchangeable between different inner envelope proteins. Moreover, when the primary sequence of MPL possessing asymmetric charge distribution was inverted, the functionality of TP was lost, suggesting that the position of charged residues is critical for chloroplast targeting. The factors underlying the positional dependency of TP sequence motifs remain to be determined, but may be linked to motif functions. The entire import process may include multiple distinct steps including cytosolic sorting, binding to receptors at the chloroplast surface, translocation through the two envelope membranes, and pulling the protein into the stroma3, 5, 17. Thus, the sequence motifs in TPs are likely recognized by factors in the cytosol, components of translocons at the outer and inner membranes, and chaperones in the stroma during protein import into the chloroplasts5, 17.
Although almost all the translocon components of the translocon machinery are conserved between C. reinhardtii and Arabidopsis18, 19, the composition of Toc/Tic components may differ between algae and plants. Toc/Tic translocons may be more complex in higher plants than in C. reinhardtii: multiple isoforms of Toc/Tic components are present in Arabidopsis, whereas the C. reinhardtii translocon contains relatively few isoforms of simpler structure19. Another possibility is that low sequence homology between Arabidopsis and Chlamydomonas Toc/Tic complexes affects the binding affinity for TPs. AtRbcS TP contains the FGLK motif that binds to Toc33/Toc3412. The FP/RK motif is closely related to the FGLK motif and may also bind to Toc33/Toc34. CrRbcS TP does not contain the FP/RK motif and may not bind efficiently to Arabidopsis Toc33/Toc34, which would underlie the failure of CrRbcS TP in delivering protein into Arabidopsis chloroplasts. However, in CrRbcS TP, the sequence motif for Toc33/34-binding may differ from the Arabidopsis motif. Indeed, sequence motifs are highly flexible and can be replaced by other motifs of completely different amino acid sequence8. For example, RbcS TP contains functionally redundant motifs that have no sequence similarity6, and CrRbcS TP may thus contain an alternative motif for binding to Toc33/Toc34 in C. reinhardtii. Alternative possibilities are that a specific motif is not required for CrRbcS TP binding to Toc33/Toc34, or that binding to Toc33/Toc34 is less critical for protein import into chloroplasts in C. reinhardtii than in higher plants because in the alga, the chloroplast occupies most of the cellular space.
What would have been the driving force underpinning acquisition of new sequence motifs in TPs for delivery of proteins to chloroplasts in higher plants? One prominent change that occurred during plant evolution was the increase in cell size. TPs of chloroplast proteins would need to bind to chloroplast surface receptors more efficiently to facilitate effective protein import in a larger cellular environment. This might have been achieved by acquiring additional sequence motifs in TPs and additional import receptors at the surface of chloroplasts. The FGLK motif found in plant TPs binds to Toc34 and possibly other Toc components of the Toc/Tic complex12. In the case of Toc34, two isoforms (Toc33 and Toc34) are present in Arabidopsis20. Likewise, multiple Toc159 isoforms including Toc159, Toc132, Toc120, and Toc90 are found in plants21–23. Consistent with this, GFP fused to CrRbcS TP largely remained in the cytosol in Arabidopsis protoplasts, indicating inefficient binding to chloroplast receptors. Although this hypothesis remains to be tested, multiple receptors and multiple binding motifs in TPs might have facilitated highly efficient binding and protein import into chloroplasts despite the larger relative size of the cell.
Methods
Plant Materials and Cultivation
Arabidopsis thaliana (Columbia-0 ecotype) was grown in a growth chamber at 22–23 °C with a 16 h light/8 h dark cycle on Gamborg B5 agar plates. Leaf tissues were harvested from 2 to 3-week-old plants.
Plasmid Construction
A DNA fragment encoding the N-terminal 68-amino-acid fragment of CrRbcS, termed CrRbcS-nt, was generated by three successive PCR reactions with three forward primers in which GC contents were modified (Primer 1, Primer 2, and then Primer 3) and a reverse primer (nosT-B) with AtRbcS-nt:GFP as template (Table S2). Substitution mutants used in this study were generated by PCR as described previously6. Primer sequences used for generation of mutant constructs are shown in Table S2.
PEG-Mediated Transformation of Arabidopsis Protoplasts
Plasmid DNA used for PEG (polyethylene glycol)-mediated transformation was purified using a Qiagen MIDI kit (Qiagen). Plasmid DNA was transformed into Arabidopsis protoplasts by the PEG-mediated transformation method as described in detail previously24, 25. Briefly, Arabidopsis leaf tissues were incubated in the enzyme solution containing cellulose and macerozyme with gentle agitation overnight. Protoplast solution was passed through the 100-μm mesh to remove debris. Harvested protoplasts were loaded onto 21% sucrose solution followed by centrifugation at 730 rpm (98 × g) for 10 min. The intact protoplasts were isolated from the top and interface fractions and used for PEG-mediated transformation. Plasmid DNA (10 μg) was transformed into protoplasts by PEG-mediated transformation.
Fluorescence Microscopy and Immunoblotting
Images of subcellular localization in transformed protoplasts were acquired as described previously25. Images were obtained using a cooled CCD camera and a Zeiss Axioplan-fluorescence microscope at 400 x magnification. The filter sets used were XF116 (exciter, 474AF20; dichroic, 500DRLP; emitter, 510AF23) and XF137 (exciter, 540AF30; dichroic, 570DRLP; emitter, 585ALP) (Omega, Inc., Brattleboro, VT) for green fluorescent protein and autofluorescence of chlorophyll, respectively. Adobe Photoshop software was used to equally balance images for brightness and contrast and to process image data. Protoplasts were resuspended in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 1 × protease inhibitor cocktail) and lysed by brief sonication. Cell lysates were centrifuged at 3,000 × g at 4 °C for 10 min to remove cell debris. Protein extracts were separated by SDS-PAGE and analyzed by western blotting using appropriate antibodies. Anti-GFP antibody was purchased from Clontech (Cat. #632381). Proteins were visualized using enhanced chemiluminescence (ECL kit; Amersham Pharmacia Biotech), and images were obtained using a LAS 3000 image capture system (FUJIFILM). Immunoblots were quantified by measuring the intensity of protein bands using LAS 3000 imager software (FUJIFILM).
To estimate chloroplast import efficiency, the intensity of the P2 form was divided by the sum of total expressed proteins. Three independent transformation experiments were performed. Calculations were performed using Microsoft Excel software.
Chloroplast Isolation
Chloroplasts were isolated from protoplasts using a Percoll gradient7. Briefly, transformed protoplasts were gently lysed in ice-cold HMS buffer. Lysed protoplasts were overlaid on top of step-wise gradient consisting of 20% and 80% of percoll, and centrifuged at 3000 rpm for 5 min. The intact chloroplasts at the interface were harvested as a chloroplast (CH) fraction.
C. reinhardtii Strains and Culture Conditions
C. reinhardtii wild-type strain CC-503 cw92 mt+ was obtained from the Chlamydomonas Resource Center (USA). Cells were cultured in a 500 ml Erlenmeyer flask containing 200 ml Tris-acetate-phosphate (TAP) medium at 23 °C under continuous light (75 µmol photons/m2/s) in a shaker.
Generation of Transgenic C. reinhardtii and Subcellular Localization of CrRbcS TP in C. reinhardtii
CrRbcS-nt was inserted into the pOpt_Clover_Paro vector26, 27 using NsiI and BglII sites to produce pOpt-CrRbcS-Clover-Paro. C. reinhardtii wild-type strain CC-503 cw92 mt+ was transformed with pOpt-CrRbcS-Clover-Paro by electroporation28. Briefly, Chlamydomonas cells grown to mid-log phase in TAP medium were subject to centrifugation at 1,800 × g at 16 °C for 5 min. Chlamydomonas cells resuspended with TAP medium containing 60 mM sucrose were transformed with 1 μg of plasmid DNA by electroporation, followed by recovery in TAP medium containing 60 mM sucrose for 16 h. Transformed Chlamydomonas cells were subjected to centrifugation at 1,800 × g at 16 °C for 5 min, followed by selection on the paromomycin-containing medium.
Transformed cells were observed using a laser scanning confocal microscope (Olympus Fluo View™FV1000). Transformed cells were excited at 488 nm. Clover fluorescence was simultaneously collected at 500–530 nm, and chlorophyll fluorescence was collected at 650–700 nm.
Protein Extraction from C. reinhardtii and Immunoblotting
Transformed Chlamydomonas cultures were grown in 5 ml TAP medium until late log phase, collected by centrifugation at 1,800 × g, and resuspended in a buffer containing 50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1% SDS, and 1 × protease inhibitor cocktail. Cells were lysed by sonication and centrifuged at 3,000 × g for 10 min. Protein extracts were collected, and protein concentration was measured using a Bradford assay (Bio-Rad, Hercules, CA USA). Proteins (10 µg per sample) were separated by 10% SDS-PAGE and analyzed by western blotting using an anti-GFP antibody.
Data availability statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016R1E1A1A02922014). This research was supported by a grant from the Collaborative Genome Program (20140428) funded by the Ministry of Oceans and Fisheries, Korea. Dong Wook Lee was supported by grant NRF-2017R1C1B1006784 from the National Research Foundation, Ministry of Science, Technology and Future Planning, Korea.
Author Contributions
M.A.R., D.W.L. and I.H. designed research. M.A.R. and D.W.L. performed all the experiments. Y.J.Y. performed the phylogenetic analysis. M.A.R., D.W.L. and I.H. wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Md. Abdur Razzak and Dong Wook Lee contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09473-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zimorski V, Ku C1, Martin WF, Gould SB. Endosymbiotic theory for organelle origins. Curr. Opin. Microbiol. 2014;22:38–48. doi: 10.1016/j.mib.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Martin W, et al. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc. Natl. Acad. Sci. USA. 2002;99:12246–12251. doi: 10.1073/pnas.182432999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DW, Jung C, Hwang I. Cytosolic events involved in chloroplast protein targeting. Biochim. Biophys. Acta. 2013;1833:245–252. doi: 10.1016/j.bbamcr.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis P. Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol. 2008;179:257–285. doi: 10.1111/j.1469-8137.2008.02452.x. [DOI] [PubMed] [Google Scholar]
- 5.Li HM, Chiu CC. Protein transport into chloroplasts. Annu. Rev. Plant Biol. 2010;61:157–180. doi: 10.1146/annurev-arplant-042809-112222. [DOI] [PubMed] [Google Scholar]
- 6.Lee DW, et al. Functional characterization of sequence motifs in the transit peptide of Arabidopsis small subunit of Rubisco. Plant. Physiol. 2006;140:466–483. doi: 10.1104/pp.105.074575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, et al. Arabidopsis nuclear-encoded plastid transit peptides contain multiple sequence subgroups with distinctive chloroplast-targeting sequence motifs. Plant Cell. 2008;20:1603–1622. doi: 10.1105/tpc.108.060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Woo S, Geem KR, Hwang I. Sequence motifs in transit peptides act as independent functional units and can be transferred to new sequence contexts. Plant Physiol. 2015;169:471–484. doi: 10.1104/pp.15.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Lee S, Oh YJ, Hwang I. Multiple sequence motifs in the rubisco small subunit transit peptide independently contribute to Toc159-dependent import of proteins into chloroplasts. Plant Physiol. 2009;151:129–141. doi: 10.1104/pp.109.140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolland V, Badger MR, Price GD. Redirecting the Cyanobacterial Bicarbonate Transporters BicA and SbtA to the Chloroplast Envelope: Soluble and Membrane Cargos Need Different Chloroplast Targeting Signals in Plants. Front Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bionda T, et al. Chloroplast import signals: the length requirement for translocation in vitro and in vivo. J Mol Biol. 2010;402(3):510–23. doi: 10.1016/j.jmb.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 12.Holbrook K, et al. Functional analysis of semi-conserved transit peptide motifs and mechanistic implications in precursor targeting and recognition. Mol. Plant. 2016;9:1286–1301. doi: 10.1016/j.molp.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Li HM, Teng YS. Transit peptide design and plastid import regulation. Trends Plant. Sci. 2013;18:360–366. doi: 10.1016/j.tplants.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Comai L, et al. Chloroplast transport of a ribulose bisphosphate carboxylase small subunit-5-enolpyruvyl 3-phosphoshikimate synthase chimeric protein requires part of the mature small subunit in addition to the transit peptide. J Biol Chem. 1988;263(29):15104–9. [PubMed] [Google Scholar]
- 15.Atkinson N, et al. Introducing an algal carbon-concentrating mechanism into higher plants: location and incorporation of key components. Plant Biotechnol. J. 2016;14:1302–1315. doi: 10.1111/pbi.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chotewutmontri P, et al. Differential transit peptide recognition during preprotein binding and translocation into flowering plant plastids. Plant Cell. 2012;24:3040–3059. doi: 10.1105/tpc.112.098327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paila YD, Richardson LG, Schnell DJ. New insights into the mechanism of chloroplast protein import and its integration with protein quality control, organelle biogenesis and development. J Mol. Biol. 2015;427:1038–1060. doi: 10.1016/j.jmb.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalanon M, McFadden GI. The chloroplast protein translocation complexes of Chlamydomonas reinhardtii: a bioinformatic comparison of toc and tic components in plants, green algae and red algae. Genetics. 2008;179:95–112. doi: 10.1534/genetics.107.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi LX, Theg SM. The chloroplast protein import system: from algae to trees. Biochim. Biophys. Acta. 2013;1833:314–331. doi: 10.1016/j.bbamcr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Constan D, Patel R, Keegstra K, Jarvis P. An outer envelope membrane component of the plastid protein import apparatus plays an essential role in Arabidopsis. Plant J. 2004;38:93–106. doi: 10.1111/j.1365-313X.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- 21.Kubis S, et al. Functional specialization amongst the Arabidopsis Toc159 family of chloroplast protein import receptors. Plant Cell. 2004;16:2059–2077. doi: 10.1105/tpc.104.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova Y, Smith MD, Chen K, Schnell DJ. Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol. Biol. Cell. 2004;15:3379–3392. doi: 10.1091/mbc.E03-12-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Infanger S, et al. The chloroplast import receptor Toc90 partially restores the accumulation of Toc159 client proteins in the Arabidopsis thaliana ppi2 mutant. Mol. Plant. 2011;4:252–263. doi: 10.1093/mp/ssq071. [DOI] [PubMed] [Google Scholar]
- 24.Lee DW, Hwang I. Transient expression and analysis of chloroplast proteins in Arabidopsis protoplasts. Methods Mol. Biol. 2011;774:59–71. doi: 10.1007/978-1-61779-234-2_4. [DOI] [PubMed] [Google Scholar]
- 25.Jin JB, et al. A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell. 2001;13:1511–1526. doi: 10.1105/tpc.13.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauersen KJ, Kruse O, Mussgnug JH. Targeted expression of nuclear transgenes in Chlamydomonas reinhardtii with a versatile, modular vector toolkit. Appl. Microbiol. Biotechnol. 2015;99:3491–503. doi: 10.1007/s00253-014-6354-7. [DOI] [PubMed] [Google Scholar]
- 27.Lam AJ, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 2012;9(10):1005–12. doi: 10.1038/nmeth.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaoka Y, et al. Identification of a Chlamydomonas plastidial 2-lysophosphatidic acid acyltransferase and its use to engineer microalgae with increased oil content. Plant Biotechnol J. 2016;14:2158–2167. doi: 10.1111/pbi.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).