Abstract
Organoboron compounds play an irreplaceable role in synthetic chemistry and the related transformations based on the unique reactivity of C–B bond are potentially the most efficient methods for the synthesis of organic molecules. The synthetic importance of multiboron compounds in C–C bond formation and function transformation reactions is growing and the related borations of activated or nonactivated alkenes have been developed recently. However, introducing directly two boron moieties into the terminal sites of alkenes giving 1,1-diborylalkanes in a catalytic fashion has not been explored yet. Here we describe a synthetic strategy of 1,1-diborylalkanes via a Ni-catalyzed 1,1-diboration of readily available terminal alkenes. This methodology shows high level of chemoselectivity and regioselectivity and can be used to convert a large variety of terminal alkenes, such as vinylarenes, aliphatic alkenes and lower alkenes, to 1,1-diborylalkanes.
1,1-diborylalkanes are useful building blocks in synthetic chemistry. Here, the authors present a highly chemo- and regioselective Ni-catalyzed reaction for the synthesis of 1,1-diborylalkanes from a wide variety of readily available terminal alkenes.
Introduction
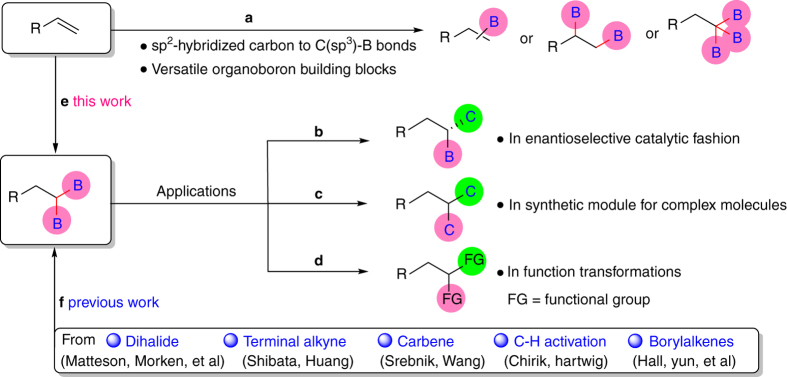
Organoboron compounds are recognized as versatile building blocks and fundamental intermediates in organic synthesis1–5. Particularly because these compounds are usually air and moisture stable and easy to handle compared with Grignard reagents, organolithium reagents, and also enable further transformations based on the unique reactivity of the C–B bond6. Transition-metal catalyzed conversion of sp2-hybridized carbon in alkenes to C(sp3)-B bond for the formation of organoboron building blocks is a more recently developed class7 (Fig. 1a). Several pioneer synthetic methods of momoborylalkanes, 1,2-diborylalkanes and 1,1,1-triborylalkanes from alkenes have been reported recently. Rh-catalyzed synthesis of 1,2-diborylalkanes from terminal vinylarenes was firstly reported by Westcott and co-workers, although the 1,2-diboration product was obtained in very low yield8. The enantioselective 1,2-diboration of terminal alkenes catalyzed by platinum catalyst, combined with Suzuki coupling reactions was achieved by Morken’s group9. Huang’s group also reported a Co-catalyzed protocol for the formation of 1,1,1-triborylalkanes, but the alkene substrates are restricted to vinylarenes10.
Fig. 1.
Borations of alkenes, synthetic methods and applications of 1,1-diborylalkanes. Transition metals catalyzed synthesis of monoboration (e.g., Rh23, Fe24–27, Co28, 29, Ir30, Ru31), 1,2-diboration (e.g., Rh32, 33, Pt34–36, Cu37, Ni38, Pd39 and metal-free40, 41) and 1,1,1-triboration10 of alkenes (a). Construction of chiral molecules utilizing racemic or nonracemic 1,1-diborylalkanes11–15, 42, 43 (b). Conversion C–B bonds to C–C bonds to afford complex molecules (c). Transformations of organoboron building blocks into a wide variety of functional groups44, 45 (d). Transition metal catalyzed synthesis of 1,1-diborylalkanes from terminal alkenes (e). Previous work was reported for preparation of 1,1-diborylalkanes from 1,1-dihalides11, 21, 46, terminal alkynes47–49, carbene insertion50, 51, hydroboration of borylalkenes42, 43 or C-H activation52–54 (f)
As an important class of organoboron compounds, it is undeniable that the 1,1-diborylalkanes show significant applications in organic synthesis. They can either be manipulated in enantioselective catalytic fashion11–15 (Fig. 1b) and or they can provide powerful synthetic module for concise synthesis of complex molecules through multiple C–C bond formation16–22 (Fig. 1c), function transformation reactions (Fig. 1d), or both. However, direct synthesis of 1,1-diborylalkanes from terminal alkenes has not been achieved yet. If successful, both synthesis of 1,1-diborylalkanes in synthetic chemistry and applications of alkenes from petrochemical industry will be greatly improved.
Results
Reaction discovery
As the major research focus of our group, efforts were devoted to discover new C–B bond forming reactions and alkenes functionalization reactions55–57. We now report the discovery of a synthetic method of 1,1-diborylalkanes, namely, Ni-catalyzed 1,1-diboration of terminal alkenes (Fig. 1e). This reaction provides a convenient strategy for the synthesis of 1,1-diborylalkanes from more stable and less expensive substrates (alkenes and boronic ester). The reaction shows high levels of chemo- and regio-selectivity. As reactions related to organoboron compounds have enjoyed great success in modern organic synthesis, the present reaction is expected to find important applications in synthetic chemistry.
Investigation of reaction conditions
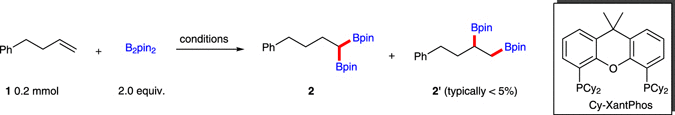
Various bases, ligands, solvents and Ni catalysts for the 1,1-diboration reaction of 4-phenyl-1-butene 1 with bis(pinacolato)diboron (B2pin2) were screened (Table 1 and Supplementary Tables 1–4). Initially, we tried tricyclohexyl phosphine, a monodentate phosphine ligand that worked well for boration reactions. To our delight, the desired product 2 was obtained in 39% GC yield and only trace amount of 1,2-diboration byproduct 2′ was detected. Some other bases except LiOMe have weakened the 1,1-diboration and only Cs2CO3 showed a little effect giving 2 in 22% yield (entry 2–5). We then moved our attention to a sample of 1-analogs and nitrogen heterocyclic carbene (NHC) ligands. To our disappointment, these ligands were found to be ineffective and only trace amount of 2 and 2′ were detected (entries 6–8). We were enlightened by the optimization results that the ligands bearing dicyclohexylphosphine moiety may be good for the 1,1-diboration reaction. Surprisingly, when Cy-XantPhos was used, 2 was increased to 62% yield (entry 9). Higher yield (52%) could also be obtained using THF as solvent (entry 10). Further optimization of the reaction system on the basis of entry 9 and entry 10, we used NEt3 as an additive, PhMe/THF (v/v 10:1) as solvent and this diboration reaction could proceed smoothly with lower catalyst (5%) and ligand loadings (5%) in 1 h, giving 2 in 78% yield (entry 11). The 1,1-diboration could not be proceeded in the absence of Ni catalyst (entry 12). Some other nickel sources were also tested, but they were not effective (entry 13, 14 and Supplementary Table 4).
Table 1.
Screening of conditions for the preparation of 1,1-diborylalkanes from alkenes
| |||||
|---|---|---|---|---|---|
| Entrya | Ni cat. | Ligand | Base | Solvent | Yield (%)b |
| 1 | Ni(COD)2 | PCy3 | LiOMe | PhMe | 39 |
| 2 | Ni(COD)2 | PCy3 | LiOBut | PhMe | <5 |
| 3 | Ni(COD)2 | PCy3 | Cs2CO3 | PhMe | 22 |
| 4 | Ni(COD)2 | PCy3 | NaOBut | PhMe | Trace |
| 5 | Ni(COD)2 | PCy3 | KOBut | PhMe | <5 |
| 6 | Ni(COD)2 | PCpent3 | LiOMe | PhMe | Trace |
| 7 | Ni(COD)2 | PCyPh2 | LiOMe | PhMe | Trace |
| 8 | Ni(COD)2 | IMes.HCl | LiOMe | PhMe | trace |
| 9 | Ni(COD)2 | Cy-XantPhos | LiOMe | PhMe | 62 |
| 10 | Ni(COD)2 | Cy-XantPhos | LiOMe | THF | 52 |
| 11c | Ni(COD)2 | Cy-XantPhos | LiOMe/NEt3 | PhMe/THF | 78 |
| 12 | – | Cy-XantPhos | LiOMe/NEt3 | PhMe/THF | 0 |
| 13 | Ni(PPh3)4 | PCy3 | LiOMe | PhMe | 0 |
| 14 | NiCl2(PCy3)2 | PCy3 | LiOMe | PhMe | 12 |
Standard reaction conditions: 5% Ni(COD)2, 5% Cy-XantPhos, 2.0 equiv. B2pin2, 1.0 equiv. LiOMe, 0.5 equiv. NEt3, 0.55 mL PhMe/THF(v:v/10:1) with Ar protection at 130 °C for 1 h
aFor entry 1–10 and 13–14, 10% Ni(COD)2 and 20% Ligand, at 130 °C for 12 h
bGC yield average of two runs using n-tetracosane as internal standard
cStandard reaction conditions
Scope of the methodology
The substrate scope for the 1,1-diboration of aliphatic alkenes was shown in Table 2. A variety of nonactivated terminal aliphatic alkenes could be readily converted to the desired products with modest to high yields (25–78%). Substrates with different chain lengths (2–7) could react smoothly. A host of different ethers, such as aryl ether (8–10), silyl ether (12) and benzyl ether (20) could participate well in the target reaction. Notably, methoxyl group amenable for Ni-catalyzed C-O cleavage did not compete with the efficacy of our 1,1-diboration event. As an important class of biologically active molecules, various nitrogen-containing moieties, such as indole (13), carbazole (14), and methyl benzylamine (16), amide (16, 17), all survived the 1,1-diboration. Interestingly, multiboron compounds (18, 19) could be smoothly synthesized from alkenes bearing Bpin moieties, thus providing an additional handle for further group transformations, cross-coupling reactions, or both. Furthermore, R-glyceraldehyde-acetonide derivative can be transformed into 20 in 78% yield. The diboration process is not affected by sterical hindrance of groups in mono-substituted alkenes (e.g., 21). Internal alkenes could be well accommodated in this reaction, albeit achieving 22 and 23 only in acceptable yield. In line with the expected reaction result, 1,1-disubstituted alkene 25 and internal alkene (e.g., 22) are ineffective substrates. Besides, B2pai2 can also be used as boron source and the desired product (24) was obtained in 56% yield. Underscoring the utility of our protocol, gram-scale diboration reactions of substrate 1-decene (5.61 g, 40.0 mmol) and 1-(hex-5-en-1-yl)-1H-indole (1.00 g, 5.0 mmol) were conducted under standard conditions respectively. Gratifyingly, there was very little lose in isolated yield of 6 (55%) and 13 (66%) (Table 2 and Supplementary Methods).
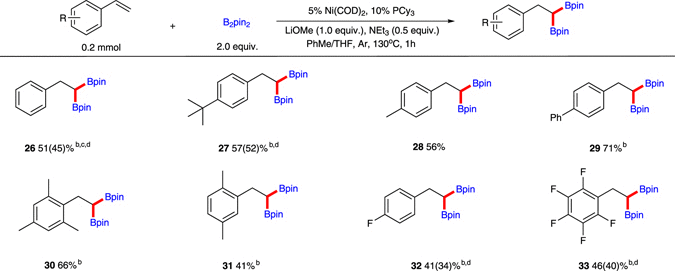
Table 3.
Substrate scope for 1,1-diboration of vinylarenesa
|
aThe reactions were conducted in 0.2 mmol scale. Isolated yield
b12 h
c140 °C
dYields were determined by 1H NMR using diphenylmethane as internal standard (isolated yields are in parentheses)
Next, we turned our attention to validate the generality of our 1,1-diboration protocol for vinylarenes (Table 3). To our disappointment, the optimized reaction conditions for terminal aliphatic alkenes have little effect on vinylarenes. However, fine-tuning of ligands on the basis of the reaction parameters, we found that tricyclohexyl phosphine could afford the desired product in satisfactory yields. Notably, similar results were obtained regardless of the electron-donating (e.g., 27, 29, 30) or electron-withdrawing (32, 33) groups on the substrates. Steric hindrance (e.g., 30) shows subtle influence on the yield of the desired products.
Table 2.
Substrate scope for 1,1-diboration of aliphatic alkenesa

|
aThe reactions were conducted in 0.2 mmol scale at 130 °C in 0.55 mL PhMe/THF (v/v = 10:1) for 1 h. Isolated yield
b5.61 g 1-decene (40.0 mmol) was used
c1.00 g 1-(hex-5-en-1-yl)-1H-indole (5.0 mmol) was used. Isolated yield of gram-scale is shown in a parenthesis
d10% Ni(COD)2 and 10% Cy-XantPhos, 4h
eYield was determined by 1H NMR using diphenyl methane as internal standard
f1.1 mL PhMe/THF (v/v = 10:1). TBDMS = dimethyl-tert-butylsilyl. Boc = t-butyloxy carbonyl. PMP = p-methoxybenzyl. B2pai2 = bis[(+)-pinanediolato]diboron
1,1-Diboration of sugar derivatives, LCs and lower alkenes
This 1,1-diboration reaction of terminal alkenes was utilized for the modification of sugar derivatives, liquid crystals (LCs) and even ethylene shown in Fig. 2. C-alkyl or O-alkyl glycosides are important bioactive candidates58. It would therefore be interesting to use our reaction to modify glycosides to form 1,1-diboron-containing bioactive synthons. C-allyl D-glucose derivative 35 was synthesized from tetrabenzyl-protected D-glucose 34 via fluorination and subsequent allylation. 1,1-Diboration of 35 under the optimal reaction conditions, the desired product 36 was obtained in 63% yield. Furthermore, the O-alkyl substrate 38 can be transformed into 39 in 69% yield with the protecting group acetonide well tolerated, which is a frequently used protecting group in sugar chemistry. Fluorinated Liquid crystals (LCs), because of their high birefringence and small losses in terahertzband and susceptibility for electric and magnetic field, are excellent materials to construct tunable optical devices (e.g., phase shifters, phase gratings lenses, filters and metamaterials)59. Commercial available LCs 40 possessing a side alkenyl chain was successfully modified using our protocol. The desired product 41 was obtained in 47% yield. The two newly formed C-B bonds made 41 possible to transform in a more enriched way to afford other possible LCs molecules via further C–B bond transformations. We notice that double bond in the side carbon chain of LCs is not a necessary functional group, and many other useful LCs molecules could be derived from this double bond59. To further highlight the practicality of our protocol in lower alkenes, propylene 42 was utilized and it could be successfully1,1-diborated. The desired product 43 was isolated in 82% yield. Performing diboration of another kind of lower alkenes ethylene 44 afforded the 1,1-diboration product 45 and 1,2-diboration undesired product 46 in 45% combined yield, and the ratio of 45/46 was 1:2 (Supplementary Fig. 56). Diboration results of 42 and 44 indicate that steric hindrance in alkenes plays a positive role in this site-selective matter. The above transformations demonstrated a vast application prospect of our developed reaction.
Fig. 2.
1,1-Diboration of sugar derivatives, LCs and lower alkenes. a (1) DAST (diethylaminotrifluorosulfur, 1.1 equiv.), THF, −30 °C. (2) Allyltrimetnylsilane (2.0 equiv.), 20% BF3 · Et2O, DCM. (3) 10% Ni(COD)2, 10% Cy-Xantphos. b (1) NaH, DMF, 5-bromopent-1-ene, 0 °C. (2) Standard conditions, 4 h. c Standard conditions. d Standard conditions, 12 h
Discussion
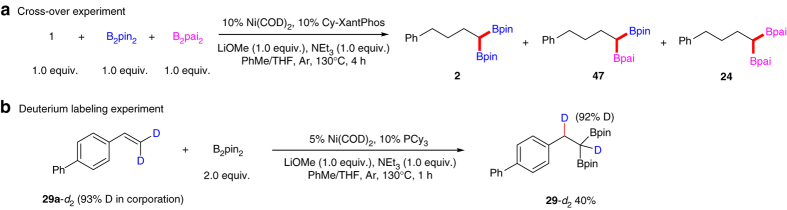
A set of experiments were executed as additional evidence in support of our proposed catalytic cycle (Supplementary Discussions). The cross-over experiment of 1 with B2pin2 and B2pai2 to yield 2, 47 and 24 indicates that the two boron motifs in the products were supplied by two molecular bisboronic ester (Fig. 3a). The result of cross-over experiment of 1 with B2pin2 and B2pin2-d 12 is also in accordance with the above observations (Supplementary Discussions and Supplementary Fig. 61 for more details). Deuterium labeling studies were conducted by using 4-(2,2-dideuterovinyl)-1,1′-biphenyl (29a-d 2) as the substrate (93% deuterium content) and one of benzylic hydrogen atoms in the product was 92% deuterated (Fig. 3b). This deuterium experiment illustrated that one of the benzylic hydrogen atoms is originated from hydrogen atoms bonded in terminal site of alkenes. Through nickel-catalyzed alkenes boration and subsequent hydroboration process, we realize the 1,1-selective diboration of alkenes. Diboration of alkenes was all proceeded in a 1,2-selective manner before this study.
Fig. 3.
Mechanism study experiments. a Cross-over experiments of 1 with B2pin2 and B2pai2 to afford 1,1-diboron compounds 2, 47 and 24 respectively. GC peak area ratio of 2, 47, 24 is 1/2.2/1.1 (Correction factor is not taken into account). b Deuterium labeling experiment was conducted using 29 a-d 2 as starting material
In summary, we have successfully developed a chemo- and regio-selective Ni-catalyzed 1,1-diboration reaction of terminal alkenes. Tremendous variety of terminal alkenes (vinylarenes, aliphatic alkenes and low alkenes) could react smoothly in this methodology. This reaction provides an effective and convenient way for the synthesis of 1,1-diborylalkanes. The obtained products provides an excellent platform for achieving complex structures and via C–C bond formation, function transformations and even enantioselective catalytic cross-coupling reactions. Novel reactions of 1,1-diborylalkanes are being explored in our laboratory.
Methods
General procedure for 1,1-diboration of aliphatic alkenes
In a glove box, a mixture of 5% Ni(COD)2 (2.8 mg, 0.01 mmol), 5% Cy-XantPhos (6.0 mg, 0.01 mmol), LiOMe (7.6 mg, 0.2 mmol, 1.0 equiv.) and B2pin2 (101.6 mg, 0.4 mmol, 2.0 equiv.) were added to a Schlenk tube equipped with a stir bar. The vessel was evacuated and filled with argon for three cycles. 0.55 mL PhMe/THF (v/v, 10:1), NEt3 (14 µL, 0.5 equiv.) and alkenes (0.2 mmol, 1.0 equiv.) were added respectively under a positive flow of argon. The reaction mixture was stirred at room temperature (r.t.) for seconds and then transferred to a pre-heated 130 °C oil bath. After 1 h stirring, the reaction mixture was cooled to r.t. and then diluted with DCM, filtered through silica gel with copious washings by EtOAc, concentrated, and purified by silica gel chromatography to afford the desired product.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures, see Supplementary Methods. For NMR, GC-MS and HRMS analysis of the compounds in this article, see Supplementary Figs 1–62. For X-ray data of compound 41 (CCDC No. 1525017), see Supplementary Table 5 and Supplementary Fig. 63. The crystal data can be obtained free of charge from the Cambridge Crystallographic Data Center (www.ccdc.cam.ac.uk).
Electronic supplementary material
Acknowledgements
We thank the support from the National Natural Science Foundation of China (21325208, 21472181, 21572212 and 21502184), Ministry of Science and Technology of China (2017YFA0303500), Chinese Academy of Science (XDB20000000), Youth Innovation Promotion Association of the Chinese Academy of Sciences (2015371), FRFCU and PCSIRT.
Author contributions
L.L. designed and carried out the experimental work. X.L. and T.G. helped to complete the experimental work. B.X. and Y.F. directed the project and wrote the manuscript.
Competing interests
The authors declare no competing financial interests
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-00363-4.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bin Xiao, Email: binxiao@ustc.edu.cn.
Yao Fu, Email: fuyao@ustc.edu.cn.
References
- 1.Ishiyama T, Miyaura N. Metal-catalyzed reactions of diborons for synthesis of organoboron compounds. Chem. Rec. 2004;3:271–280. doi: 10.1002/tcr.10068. [DOI] [PubMed] [Google Scholar]
- 2.Xu L, Zhang S, Li P. Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chem. Soc. Rev. 2015;44:8848–8858. doi: 10.1039/C5CS00338E. [DOI] [PubMed] [Google Scholar]
- 3.Burns M, et al. Assembly-line synthesis of organic molecules with tailored shapes. Nature. 2014;513:183–188. doi: 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonet A, Odachowski M, Leonori D, Essafi S, Aggarwal VK. Enantiospecific sp2–sp3 coupling of secondary and tertiary boronic esters. Nat. Chem. 2014;6:584–589. doi: 10.1038/nchem.1971. [DOI] [PubMed] [Google Scholar]
- 5.Mei TS, Patel HH, Sigman MS. Enantioselective construction of remote quaternary stereocentres. Nature. 2014;508:340–344. doi: 10.1038/nature13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neeve EC, Geier SJ, Mkhalid IA, Westcott SA, Marder TB. Diboron (4) compounds: from structural curiosity to synthetic workhorse. Chem. Rev. 2016;116:9091–9161. doi: 10.1021/acs.chemrev.6b00193. [DOI] [PubMed] [Google Scholar]
- 7.Takaya J, Iwasawa N. Catalytic, direct synthesis of bis(boronate) compounds. ACS Catal. 2012;2:1993–2006. doi: 10.1021/cs300320u. [DOI] [Google Scholar]
- 8.Baker RT, Nguyen P, Marder TB, Westcott SA. Transition metal catalyzed diboration of vinylarenes. Angew. Chem. Int. Ed. 1995;34:1336–1338. doi: 10.1002/anie.199513361. [DOI] [Google Scholar]
- 9.Mlynarski SN, Schuster CH, Morken JP. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature. 2014;505:386–390. doi: 10.1038/nature12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Huang Z. Synthesis of 1, 1, 1-tris(boronates) from vinylarenes by Co-catalyzed dehydrogenative borylations–hydroboration. J. Am. Chem. Soc. 2015;137:15600–15603. doi: 10.1021/jacs.5b11366. [DOI] [PubMed] [Google Scholar]
- 11.Sun C, Potter B, Morken JP. A catalytic enantiotopic-group-selective Suzuki reaction for the construction of chiral organoboronates. J. Am. Chem. Soc. 2014;136:6534–6537. doi: 10.1021/ja500029w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matteson DS. Boronic esters in stereodirected synthesis. Tetrahedron. 1989;45:1859–1885. doi: 10.1016/S0040-4020(01)80052-1. [DOI] [Google Scholar]
- 13.Joannou MV, Moyer BS, Meek SJ. Enantio- and diastereoselective synthesis of 1,2-hydroxyboronates through Cu-catalyzed additions of alkylboronates to aldehydes. J. Am. Chem. Soc. 2015;137:6176–6179. doi: 10.1021/jacs.5b03477. [DOI] [PubMed] [Google Scholar]
- 14.Murray SA, Green JC, Tailor SB, Meek SJ. Enantio- and diastereoselective 1,2-additions to α-ketoesters with diborylmethane and substituted 1,1-diborylalkanes. Angew. Chem. Int. Ed. 2016;55:9065–9069. doi: 10.1002/anie.201603465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Hoveyda AH. Catalytic SN2′- and enantioselective allylic substitution with a diborylmethane reagent and application in synthesis. Angew. Chem. Int. Ed. 2016;128:3516–3519. doi: 10.1002/ange.201600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo K, Ohkubo T, Hirokami M, Shibata T. Chemoselective and regiospecific Suzuki coupling on a multisubstituted sp3-carbon in 1,1-diborylalkanes at room temperature. J. Am. Chem. Soc. 2010;132:11033–11035. doi: 10.1021/ja105176v. [DOI] [PubMed] [Google Scholar]
- 17.Hong K, Liu X, Morken JP. Simple access to elusive α-boryl carbanions and their alkylation: an umpolung construction for organic synthesis. J. Am. Chem. Soc. 2014;136:10581–10584. doi: 10.1021/ja505455z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potter B, Szymaniak AA, Edelstein EK, Morken JP. Nonracemic allylic boronates through enantiotopic-group-selective cross-coupling of geminal bis(boronates) and vinyl halides. J. Am. Chem. Soc. 2014;136:17918–17921. doi: 10.1021/ja510266x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Park S, Park J, Cho SH. Synthesis of branched alkylboronates by copper-catalyzed allylic substitution reactions of allylic chlorides with 1,1-diborylalkanes. Angew. Chem. Int. Ed. 2016;55:1498–1501. doi: 10.1002/anie.201509840. [DOI] [PubMed] [Google Scholar]
- 20.Cui LC, Zhang ZQ, Lu X, Xiao B, Fu Y. Pd-catalyzed cross-coupling of 1,1-diborylalkanes with aryl triflates. RSC Adv. 2016;6:51932–51935. doi: 10.1039/C6RA09959A. [DOI] [Google Scholar]
- 21.Zhang ZQ, et al. Copper-catalyzed/promoted cross-coupling of gem-diborylalkanes with nonactivated primary alkyl halides: an alternative route to alkylboronic esters. Org. Lett. 2014;16:6342–6345. doi: 10.1021/ol503111h. [DOI] [PubMed] [Google Scholar]
- 22.Endo K, Ishioka T, Ohkubo T, Shibata T. One-pot synthesis of symmetrical and unsymmetrical diarylmethanes via diborylmethane. J. Org. Chem. 2012;77:7223–7231. doi: 10.1021/jo3015165. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto M, Miura T, Murakami M. Rhodium-catalyzed dehydrogenative borylation of aliphatic terminal alkenes with pinacolborane. Angew. Chem. Int. Ed. 2015;127:12850–12854. doi: 10.1002/ange.201506328. [DOI] [PubMed] [Google Scholar]
- 24.Tseng KNT, Kampf JW, Szymczak NK. Regulation of iron-catalyzed olefin hydroboration by ligand modifications at a remote Site. ACS Catal. 2014;5:411–415. doi: 10.1021/cs501820w. [DOI] [Google Scholar]
- 25.Zhang L, Peng D, Leng X, Huang Z. Iron-catalyzed, atom-economical, chemo- and regioselective alkene hydroboration with pinacolborane. Angew. Chem. Int. Ed. 2013;125:3764–3768. doi: 10.1002/ange.201210347. [DOI] [PubMed] [Google Scholar]
- 26.Greenhalgh MD, Thomas SP. Chemo-, regio-, and stereoselective iron-catalyzed hydroboration of alkenes and alkynes. Chem. Commun. 2013;49:11230–11232. doi: 10.1039/c3cc46727a. [DOI] [PubMed] [Google Scholar]
- 27.Obligacion JV, Chirik PJ. Highly selective bis(imino)pyridine iron-catalyzed alkene hydroboration. Org. Lett. 2013;15:2680–2683. doi: 10.1021/ol400990u. [DOI] [PubMed] [Google Scholar]
- 28.Obligacion JV, Chirik PJ. Bis(imino)pyridine cobalt-catalyzed alkene isomerization–hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 2013;135:19107–19110. doi: 10.1021/ja4108148. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Zuo Z, Wan X, Huang Z. Cobalt-catalyzed enantioselective hydroboration of 1,1-disubstituted aryl alkenes. J. Am. Chem. Soc. 2014;136:15501–15504. doi: 10.1021/ja5093908. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Fujikawa R, Umemoto T, Miyaura N. Iridium-catalyzed hydroboration of alkenes with pinacolborane. Tetrahedron. 2004;60:10695–10700. doi: 10.1016/j.tet.2004.09.014. [DOI] [Google Scholar]
- 31.Caballero A, Sabo-Etienne S. Ruthenium-catalyzed hydroboration and dehydrogenative borylation of linear and cyclic alkenes with pinacolborane. Organometallics. 2007;26:1191–1195. doi: 10.1021/om0610851. [DOI] [Google Scholar]
- 32.Morgan JB, Miller SP, Morken JP. Rhodium-catalyzed enantioselective diboration of simple alkenes. J. Am. Chem. Soc. 2003;125:8702–8703. doi: 10.1021/ja035851w. [DOI] [PubMed] [Google Scholar]
- 33.Trudeau S, Morgan JB, Shrestha M, Morken JP. Rh-catalyzed enantioselective diboration of simple alkenes: reaction development and substrate scope. J. Org. Chem. 2005;70:9538–9544. doi: 10.1021/jo051651m. [DOI] [PubMed] [Google Scholar]
- 34.Kliman LT, Mlynarski SN, Morken JP. Pt-Catalyzed enantioselective diboration of terminal alkenes with B2(pin)2. J. Am. Chem. Soc. 2009;131:13210–13211. doi: 10.1021/ja9047762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iverson CN, Smith MR. Efficient olefin diboration by a base-free platinum catalyst. Organometallics. 1997;16:2757–2759. doi: 10.1021/om970199x. [DOI] [Google Scholar]
- 36.Lillo V, Mata J, Ramírez J, Peris E, Fernández E. Catalytic diboration of unsaturated molecules with platinum(0)-NHC: selective synthesis of 1,2-dihydroxysulfones. Organometallics. 2006;25:5829–5831. doi: 10.1021/om060666n. [DOI] [Google Scholar]
- 37.Lillo V, et al. A valuable, inexpensive CuI/N‐heterocyclic carbene catalyst for the selective diboration of styrene. Chem. Eur. J. 2007;13:2614–2621. doi: 10.1002/chem.200601146. [DOI] [PubMed] [Google Scholar]
- 38.Ely RJ, Morken JP. Ni(0)-Catalyzed 1,4-selective diboration of conjugated dienes. Org. Lett. 2010;12:4348–4351. doi: 10.1021/ol101797f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodward AR, Burks HE, Chan LM, Morken JP. Concatenated catalytic asymmetric allene diboration/allylation/functionalization. Org. Lett. 2005;7:5505–5507. doi: 10.1021/ol052312i. [DOI] [PubMed] [Google Scholar]
- 40.Bonet A, Pubill‐Ulldemolins C, Bo C, Gulyás H, Fernández E. Transition‐metal‐free diboration reaction by activation of diboron compounds with simple Lewis bases. Angew. Chem. Int. Ed. 2011;50:7158–7161. doi: 10.1002/anie.201101941. [DOI] [PubMed] [Google Scholar]
- 41.Blaisdell TP, Caya TC, Zhang L, Sanz-Marco A, Morken JP. Hydroxyl-directed stereoselective diboration of alkenes. J. Am. Chem. Soc. 2014;136:9264–9267. doi: 10.1021/ja504228p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JCH, McDonald R, Hall DG. Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds. Nat. Chem. 2011;3:894–899. doi: 10.1038/nchem.1150. [DOI] [PubMed] [Google Scholar]
- 43.Feng X, Jeon H, Yun J. Regio- and enantioselective copper(I)-catalyzed hydroboration of borylalkenes: asymmetric synthesis of 1,1-diborylalkanes. Angew. Chem. Int. Ed. 2013;52:3989–3992. doi: 10.1002/anie.201208610. [DOI] [PubMed] [Google Scholar]
- 44.Lennox AJ, Lloyd-Jones GC. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014;43:412–443. doi: 10.1039/C3CS60197H. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt J, Choi J, Liu AT, Slusarczyk M, Fu GC. A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters. Science. 2016;354:1265–1269. doi: 10.1126/science.aai8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito H, Kubota K. Copper (I)-catalyzed boryl substitution of unactivated alkyl halides. Org. Lett. 2012;14:890–893. doi: 10.1021/ol203413w. [DOI] [PubMed] [Google Scholar]
- 47.Zuo Z, Huang Z. Synthesis of 1,1-diboronate esters by cobalt-catalyzed sequential hydroboration of terminal alkynes. Org. Chem. Front. 2016;3:434–438. doi: 10.1039/C5QO00426H. [DOI] [Google Scholar]
- 48.Lee S, Li D, Yun J. Copper-catalyzed synthesis of 1,1-diborylalkanes through regioselective dihydroboration of terminal alkynes. Chem. Asian J. 2014;9:2440–2443. doi: 10.1002/asia.201402458. [DOI] [PubMed] [Google Scholar]
- 49.Endo K, Hirokami M, Shibata T. Synthesis of 1,1-organodiboronates via Rh(I)Cl-catalyzed sequential regioselective hydroboration of 1-alkynes. Synlett. 2009;2009:1331–1335. doi: 10.1055/s-0028-1088131. [DOI] [Google Scholar]
- 50.Li H, Wang L, Zhang Y, Wang J. Transition-metal-free synthesis of pinacol alkylboronates from tosylhydrazones. Angew. Chem. Int. Ed. 2012;124:2997–3000. doi: 10.1002/ange.201108139. [DOI] [PubMed] [Google Scholar]
- 51.Abu Ali H, Goldberg I, Kaufmann D, Burmeister C, Srebnik M. Novel C1-bridged bisboronate derivatives by insertion of diazoalkanes into bis(pinacolato)diborane(4) Organometallics. 2002;21:1870–1876. doi: 10.1021/om011012b. [DOI] [Google Scholar]
- 52.Cho SH, Hartwig JF. Iridium-catalyzed diborylation of benzylic C–H bonds directed by a hydrosilyl group: synthesis of 1, 1-benzyldiboronate esters. Chem. Sci. 2014;5:694–698. doi: 10.1039/C3SC52824C. [DOI] [Google Scholar]
- 53.Palmer WN, Zarate C, Chirik PJ. Benzyltriboronates: building blocks for diastereoselective carbon-carbon bond formation. J. Am. Chem. Soc. 2017;139:2589–2592. doi: 10.1021/jacs.6b12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer WN, Obligacion JV, Pappas I, Chirik PJ. Cobalt-catalyzed benzylic borylation: enabling polyborylation and functionalization of remote, unactivated C(sp3)–H bonds. J. Am. Chem. Soc. 2016;138:766–769. doi: 10.1021/jacs.5b12249. [DOI] [PubMed] [Google Scholar]
- 55.Su W, et al. Ligand-controlled regiodivergent copper-catalyzed alkylboration of alkenes. Angew. Chem. Int. Ed. 2015;54:12957–12961. doi: 10.1002/anie.201506713. [DOI] [PubMed] [Google Scholar]
- 56.Lu X, et al. Practical carbon-carbon bond formation from olefins through nickel-catalyzed reductive olefin hydrocarbonation. Nat. Commun. 2016;7:11129–11136. doi: 10.1038/ncomms11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang CT, et al. Alkylboronic esters from copper-catalyzed borylation of primary and secondary alkyl halides and pseudohalides. Angew. Chem. Int. Ed. 2012;51:528–532. doi: 10.1002/anie.201106299. [DOI] [PubMed] [Google Scholar]
- 58.Regan BM, Hayes FN. 17- and 17a-Aza-D-homosteroids. J. Am. Chem. Soc. 1956;78:639–643. doi: 10.1021/ja01584a033. [DOI] [Google Scholar]
- 59.Kanie K, Muramatsu A. Organic-inorganic hybrid liquid crystals: thermotropic mesophases formed by hybridization of liquid-crystalline phosphates and monodispersed α-Fe2O3 particles. J. Am. Chem. Soc. 2005;127:11578–11579. doi: 10.1021/ja054232f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures, see Supplementary Methods. For NMR, GC-MS and HRMS analysis of the compounds in this article, see Supplementary Figs 1–62. For X-ray data of compound 41 (CCDC No. 1525017), see Supplementary Table 5 and Supplementary Fig. 63. The crystal data can be obtained free of charge from the Cambridge Crystallographic Data Center (www.ccdc.cam.ac.uk).