ABSTRACT
Rhesus macaques are used to model human immunodeficiency virus type 1 (HIV-1) infections, but they are not natural hosts of HIV-1 or any simian immunodeficiency virus (SIV). Rather, they became infected with SIV through cross-species transfer from sooty mangabeys in captivity. It has been shown that HIV-1 utilizes rhesus CD4 less efficiently than human CD4. However, the relative ability of SIV envelope glycoproteins to bind or utilize these CD4 orthologs has not been reported. Here we show that several SIV isolates, including SIVmac239, are more efficiently neutralized by human CD4-Ig (huCD4-Ig) than by the same molecule bearing rhesus CD4 domains 1 and 2 (rhCD4-Ig). An I39N mutation in CD4 domain 1, present in human and sooty mangabey CD4 orthologs, largely restored rhCD4-Ig neutralization of SIVmac239 and other SIV isolates. We further observed that SIVmac316, a derivative of SIVmac239, bound to and was neutralized by huCD4-Ig and rhCD4-Ig with nearly identical efficiencies. Introduction of two SIVmac316 CD4-binding site residues (G382R and H442Y) into the SIVmac239 envelope glycoprotein (Env) markedly increased its neutralization sensitivity to rhesus CD4-Ig without altering neutralization by human CD4-Ig, SIV neutralizing antibodies, or sera from SIV-infected macaques. These changes also allowed SIVmac239 Env to bind rhCD4-Ig more efficiently than huCD4-Ig. The variant with G382R and H442Y (G382R/H442Y variant) also infected cells expressing rhesus CD4 with markedly greater efficiency than did unaltered SIVmac239 Env. We propose that infections of rhesus macaques with SIVmac239 G382R/H442Y might better model some aspects of human infections.
IMPORTANCE Rhesus macaque infection with simian immunodeficiency virus (SIV) has served as an important model of human HIV-1 infection. However, differences between this model and the human case have complicated the development of vaccines and therapies. Here we report the surprising observation that SIVmac239, a commonly used model virus, more efficiently utilizes human CD4 than the CD4 of rhesus macaques, whereas the closely related virus SIVmac316 uses both CD4 orthologs equally well. We used this insight to generate a form of SIVmac239 envelope glycoprotein (Env) that utilized rhesus CD4 more efficiently, while retaining its resistance to antibodies and sera from infected macaques. This Env can be used to make the rhesus model more similar in some ways to human infection, for example by facilitating infection of cells with low levels of CD4. This property may be especially important to efforts to eradicate latently infected cells.
KEYWORDS: CD4, SIVmac239, SIVmac316, human immunodeficiency virus, rhesus macaques, simian immunodeficiency virus
INTRODUCTION
Although human immunodeficiency virus type 1 (HIV-1) can replicate in humans and chimpanzees, it cannot replicate efficiently in nonhuman primates commonly used to model human disease (1). The discovery of simian immunodeficiency viruses (SIVs) that caused AIDS-like disease in rhesus macaques provided an alternative model for HIV-1 infection (1–4). SIVs were originally identified in macaques, but in general, these viruses originated from sooty mangabeys and were later transferred to macaques in captivity (2). SIVmac239 is a widely studied molecular clone from one such virus passaged extensively in rhesus macaques (4, 5). Unlike many HIV-1 isolates, it does not replicate efficiently in macrophages or cause significant disease in the central nervous system (6, 7). It is also notable because its Env protein is highly resistant to antibody neutralization (8). In contrast, SIVmac316, isolated from alveolar macrophages from an SIVmac239-infected macaque, replicates efficiently in rhesus macrophages and is neutralization sensitive (7, 9).
SIVmac239 is also the basis of most simian-human immunodeficiency viruses (SHIVs) (10, 11). These chimeric viruses include the HIV-1 env and vpu genes and were developed to study the properties of HIV-1 envelope glycoprotein (Env) in the macaque model. Only a subset of SHIVs replicate efficiently in macaques, a consequence in part of the inefficiency with which most HIV-1 Envs utilize rhesus CD4 (12, 13). An isoleucine at rhesus CD4 residue 39 has been identified as a major determinant of this inefficiency (13). Substitution of this isoleucine with an asparagine (I39N) present in human CD4 significantly increases infection by most SHIVs. Recently, efforts have been made to modify the CD4-binding sites of these SHIVs so they might better replicate in rhesus macaques (12). Nonetheless, these adapted Envs still bind and utilize human CD4 much more efficiently than rhesus CD4. As a consequence, cells expressing low levels of CD4, including macrophages in the brain and periphery, are less efficiently infected in SHIV models of infection (8, 14). In contrast, it has been presumed that most SIV isolates, including the commonly studied SIVmac239, are well adapted to their rhesus macaque host and utilize rhesus CD4 efficiently.
Here we observe that several SIV variants, including SIVmac239, SIVmac251, SIVsmE543-3, and SIVmac1A11, are more efficiently neutralized by the CD4-Ig entry inhibitor bearing human CD4 (huCD4-Ig) than by the same molecule with rhesus CD4 (rhCD4-Ig). Introduction of the I39N mutation into rhCD4-Ig (rhCD4-IgI39N) largely restored its ability to neutralize these SIVs. Surprisingly, SIVmac316 was neutralized with equal efficiencies by huCD4-Ig, rhCD4-Ig, and rhCD4-IgI39N. An SIVmac239 Env variant with two SIVmac316-derived changes in its CD4-binding site bound rhCD4-Ig more efficiently, became markedly more neutralization sensitive to rhCD4-Ig, and more efficiently infected cells expressing rhesus CD4. Nonetheless, this SIVmac239 variant retained its neutralization resistance to SIV neutralizing antibodies and to the sera of SIVmac239-infected macaques. Thus, SIVmac239 can be modified to bind and use rhesus CD4 more efficiently without making it more susceptible to antibody neutralization.
RESULTS
Most SIV isolates are more efficiently neutralized by huCD4-Ig and rhCD4-IgI39N than by rhCD4-Ig.
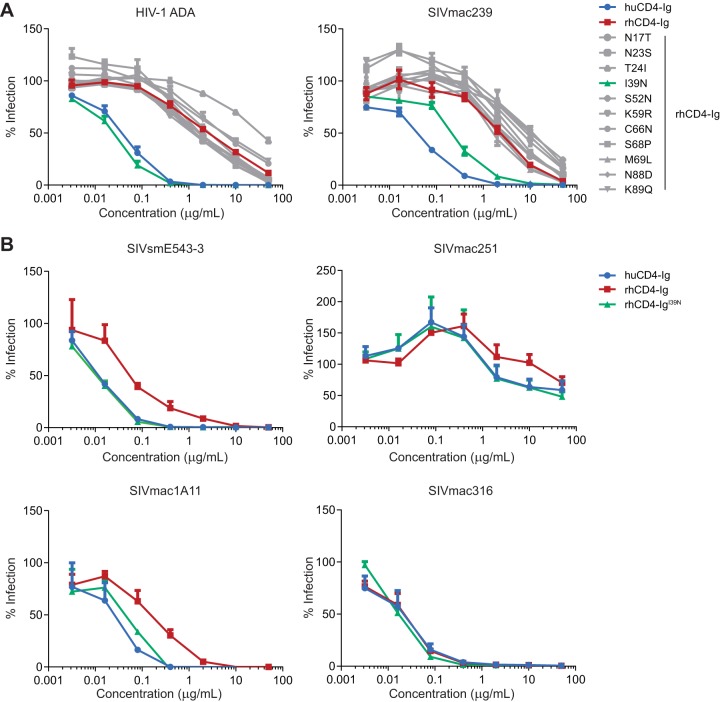
We have previously observed that huCD4-Ig more efficiently neutralizes various HIV-1 Envs than does a CD4-Ig variant with rhesus CD4 and Fc domains (15). As suggested by Humes et al. (13), we also observed that introduction of human CD4 asparagine 39 into this rhesus CD4-Ig construct neutralized HIV-1 isolates with efficiencies similar to huCD4-Ig (15). To focus our current studies on the CD4 domains of these constructs, here we used a common human IgG1 Fc domain for huCD4-Ig, rhCD4-Ig, and rhCD4-Ig variants. As expected, huCD4-Ig and rhCD4-IgI39N neutralized pseudovirus expressing the HIV-1 ADA Env markedly more efficiently than rhCD4-Ig (Fig. 1A). We also explored the roles of 10 other CD4 domain 1 differences between huCD4-Ig and rhCD4-Ig. None of these other changes improved neutralization of ADA pseudovirus. Surprisingly, huCD4-Ig also neutralized SIVmac239 more efficiently than did rhCD4-Ig. Again, among the rhCD4-Ig variants, only rhCD4-IgI39N neutralized SIVmac239 more efficiently than rhCD4-Ig, albeit in this case less efficiently than huCD4-Ig. We then investigated whether other SIV strains were similarly more sensitive to huCD4-Ig than rhCD4-Ig (Fig. 1B). SIVsmE543-3, SIVmac251, and SIVmac1A11 were each more efficiently neutralized by huCD4-Ig and rhCD4-IgI39N than by rhCD4-Ig. In contrast, SIVmac316 was neutralized by huCD4-Ig, rhCD4-Ig, and rhCD4-IgI39N with equivalent efficiencies.
FIG 1.
Neutralization of HIV-1 and SIV by huCD4, rhCD4-Ig, and rhCD4-Ig variants. (A) HIV-1 pseudotyped with the Env protein of the ADA isolate or wild-type SIVmac239 was preincubated for 1 h with human CD4-Ig, rhesus CD4-Ig, or rhesus CD4-Ig with the indicated amino acid substitution. TZM-bl cells were then added and incubated for 48 h. Luciferase expression was measured and normalized to expression in the absence of any CD4-Ig variant. (B) Experiment similar to that shown in panel A except that the SIVs (SIVmac1A11, SIVmac251, SIVsmE543-3, and SIVmac316) were preincubated with human CD4-Ig, rhesus CD4-Ig, or rhesus CD4-IgI39N. The results of each experiment are representative of at least two experiments with similar results. Error bars represent standard errors of the means (SEM).
Two changes in the SIVmac239 Env increase its binding to and neutralization by rhCD4-Ig but not huCD4-Ig.
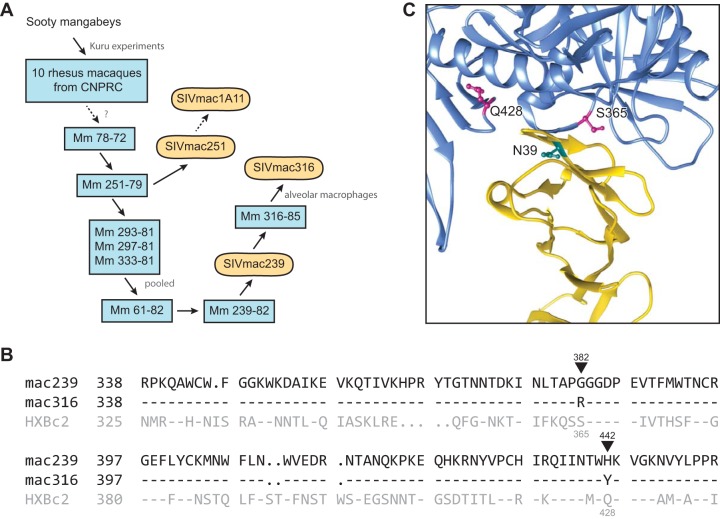
SIVmac316 was isolated from culture supernatants of alveolar macrophages from rhesus macaque 316-85, which had previously been infected with SIVmac239 (Fig. 2A) (2, 7). Consistent with its origin, SIVmac316 Env differs from that of SIVmac239 at only seven ectodomain residues, two of which reside in its CD4-binding site proximal to CD4 residue 39 (Fig. 2B and C), at positions 382 and 442 (SIVmac239 numbering). We introduced these CD4-binding site changes into SIVmac239 Env, individually or together, and compared the sensitivity of the resulting Envs to huCD4-Ig, rhCD4-Ig, and rhCD4-IgI39N (Fig. 3A). We observed that introduction of either G382R or H442Y into the SIVmac239 Env markedly increased its sensitivity to rhCD4-Ig without significantly altering its neutralization by huCD4-Ig or rhCD4-IgI39N. When both changes were introduced, the resulting Env (G382R/H442Y) was neutralized by rhCD4-Ig with a 50% inhibitory concentration (IC50) 20-fold lower than that observed with the wild-type SIVmac239 Env. In contrast, this variant was slightly more resistant to huCD4-Ig. These neutralization sensitivities were also reflected in the efficiencies with which each Env bound these CD4-Ig variants. Specifically, SIVmac239 Env bound huCD4-Ig and rhCD4-IgI39N much more efficiently than rhCD4-Ig (Fig. 3B). In contrast, SIVmac316 bound each CD4-Ig construct with nearly equal efficiencies (Fig. 3C), and the SIVmac239 Env variant (G382R/H442Y) bound rhCD4-Ig and rhCD4-IgI39N more efficiently than huCD4-Ig (Fig. 3D). We conclude that two CD4-binding site residues are largely responsible for the ability of the SIVmac316 Env, but not the SIVmac239 Env, to bind rhCD4-Ig efficiently.
FIG 2.
Origins of and CD4-binding site differences between SIVmac239 and SIVmac316. (A) Derivation of some commonly studied SIVmac isolates. Blue rectangles represent rhesus macaques in which virus derived from sooty mangabey was passaged. Rounded rectangles represent uncloned biological isolates or infectious molecular clones. Solid arrows indicate direct isolation steps or viral transmission, and dotted arrows represent passaging and/or cloning steps omitted for simplicity. CNPRC, California National Primate Research Center (1, 44). (B) Sequence alignment of regions of the SIVmac239, SIVmac316, and HXBc2 Env protein participating in CD4 binding. Residues conserved with SIVmac239 are indicated by dashes. Black triangles indicate the two differences between SIVmac239 and SIVmac316 Envs in their CD4-binding sites. SIVmac239 numbering (black) and HXBc2 numbering (gray) is also provided. (C) The crystal structure (PDB accession no. 1G9M [45]) of CD4 (yellow) in complex with HXBc2 gp120 (blue) is shown. HXBc2 CD4-binding site residues S365 and Q428, corresponding to SIV residues 382 and 442, respectively, are indicated (magenta). The location of N39 of human CD4 is also indicated (cyan). Note the proximity of CD4 residue 39 to the CD4-binding site differences between SIVmac239 and SIVmac316.
FIG 3.
Neutralization and binding studies of CD4-Ig variants with the Envs of SIVmac316 and SIVmac239 variants. (A) IC50s were determined with experiments similar to those of Fig. 1 except that pseudoviruses expressing the Env proteins of SIVmac239, SIVmac239 G382R, SIVmac239 H442Y, or SIVmac239 G382R/H442Y were preincubated with various concentrations of the indicated CD4-Ig variants. IC50s are the means ± SEM from two or three independent experiments. (B to D) 293T cells were transfected to express envelope glycoproteins of SIVmac239 (B), SIVmac316 (C), or SIVmac239 G382/H442Y (D). Cells were preincubated with the indicated concentrations of CD4-Ig variants, washed, incubated with APC-conjugated anti-human antibody, and analyzed by flow cytometry. Mean fluorescence intensities (MFI) ± SEM from three replicate samples are indicated. Experimental results depicted are representative of two experiments with similar results.
The G382R/H442Y Env variant mediates more efficient infection of cells expressing rhesus CD4.
We next compared the abilities of SIVmac239 Env and its G382R/H442Y variant to infect two cell lines, each expressing roughly equivalent levels of rhesus CD4, human CD4, or rhesus CD4 bearing the I39N change (rhesus CD4I39N; Fig. 4A and C). We observed that SIVmac239 Env infects cells expressing human CD4 and rhesus CD4I39N more efficiently than rhesus CD4. Further, the G382R/H442Y variant more efficiently infects cells expressing rhesus CD4 than does wild-type SIVmac239 (Fig. 4B and D), consistent with its greater neutralization sensitivity to and binding affinity for rhCD4-Ig. We concluded that SIVmac239 utilizes rhesus CD4 inefficiently despite passage through multiple rhesus macaques.
FIG 4.
Infection of cell lines stably expressing CD4 variants. (A) Flow cytometric analyses of expression of rhesus CD4, rhesus CD4I39N, or human CD4 on the surfaces of stably transduced Cf2Th-CCR5 cells using an antibody cross-reactive to human and rhesus CD4. HOS.CCR5 cell lines transduced with vector alone are represented in black. Numbers indicate mean fluorescence intensities (MFI). (B) Infection of rhesus CD4 and rhesus CD4I39N cells with luciferase pseudoviruses expressing SIVmac239 or SIVmac239 G382R/H442Y Env and normalized to infection observed with human CD4. (C) An experiment similar to that in panel A except that HOS.CCR5 cells stably expressing CD4 variants were analyzed. (D) An infection experiment similar to that in panel B except that the cells used in panel C were assayed. Data in panels A and C are representative of two experiments with nearly identical results. Data in panels B and D are representative of three experiments with similar results. Error bars indicate SEM.
The G382R/H442Y variant remains as neutralization resistant as wild-type SIVmac239.
Despite their similarity and common origin, SIVmac316 is in general much more neutralization sensitive than SIVmac239 (7, 16). We sought to determine whether introduction of two SIVmac316 residues into the SIVmac239 Env altered the sensitivity of this Env to neutralizing antibodies (Fig. 5A) and to sera from SIVmac239-infected macaques (Fig. 5B). We observed that the G382R/H442Y variant remained as resistant to the SIV neutralizing antibodies 4L6 and 5L7 (16, 17) as wild-type SIVmac239 Env up to concentrations of 50 μg/ml, whereas pseudovirus expressing the SIVmac316 Env was efficiently neutralized at the lowest concentration assayed (0.0032 μg/ml). Similarly, both the SIVmac239 Env and the G382R/H442Y variant remained more than 400× more resistant to sera from two infected macaques than SIVmac316. We conclude that two mutations that increased the ability of SIVmac239 to bind and infect cells expressing rhesus CD4 nonetheless did not alter the overall neutralization resistance of the Env. Our data further suggest that the ability of SIVmac316 to bind and utilize rhesus CD4 efficiently can be dissociated from its high neutralization sensitivity.
FIG 5.
Neutralization of SIVmac239, SIVmac316, and SIVmac239 G382R/H442Y by anti-SIVmac239 antibodies and SIV-positive sera. (A) Experiments similar to those shown in Fig. 1 except that HIV-1 pseudotyped with the indicated Env was preincubated with various concentrations of the SIV-neutralizing antibody 4L6 IgG1 or 5L7 IgG1. (B) Experiments similar to those of panel A except that pseudoviruses were incubated with various dilutions of sera from rhesus macaques infected with SIVmac239.
DISCUSSION
In this study, we first make the surprising observation that at least four SIV variants—SIVmac239, SIVsmE543-3, SIVmac251, and SIVmac1A11—are more efficiently neutralized by huCD4-Ig than by rhCD4-Ig and that SIVmac239 utilizes human CD4 more efficiently than rhesus CD4. We further show that a key determinant of the greater neutralization by human CD4-Ig is residue 39, an asparagine in human CD4 but an isoleucine in the rhesus ortholog. This observation is consistent with previous reports demonstrating that this CD4 residue can also account for the ability of various SHIVs to utilize human CD4 more efficiently than rhesus CD4 (13, 15). While it is not unexpected that SHIVs, whose Envs are derived from HIV-1, would utilize human CD4 more efficiently, SIV's preference for human CD4 requires greater explanation. Although SIVs are usually studied in rhesus macaques, their natural host is the sooty mangabey (1, 2). The sooty mangabey CD4, like human CD4, bears an asparagine at position 39. Thus, efficient utilization of human CD4 is likely to be coincidental, although passage on human cells may also have helped maintain this property. Nonetheless, our data indicate that despite passage through a number of rhesus macaques, most SIVs have not evolved to utilize rhesus CD4 more efficiently. Previous studies suggest that, in the absence of CD4, the SIVmac239 Env binds rhesus chemokine (C-C motif) receptor 5 (CCR5) with higher affinity than do most HIV-1 Envs (18, 19). This greater affinity for CCR5 may compensate in part for inefficient binding to rhesus CD4.
Our data highlight a limitation of the rhesus macaque model. Namely, most SIVs and SHIVs studied in this model bind rhesus CD4 poorly and therefore may poorly infect cells with low CD4 expression, including macrophages. This problem extends to recently described SHIVs adapted to better utilize rhesus CD4 (12). Inefficient infection of macrophages or other cells with low levels of CD4 remains an important caveat to studies that use the rhesus model to study neurologic consequences of infection or that rely on immune clearance of infected cells to reduce the viral reservoir. Poor binding to rhesus CD4 may also tend to exaggerate the potential efficacy of some Env-based subunit vaccines, which might be sequestered or occluded in humans by binding to high-affinity CD4.
We also observed that, among the SIVs tested, SIVmac316 was unusual in that it bound to and was neutralized by rhCD4-Ig as efficiently as huCD4-Ig. At first glance, this is surprising, given its close relationship to SIVmac239, but SIVmac316 was isolated from alveolar macrophages from an SIVmac239-infected macaque and subsequently passaged on rhesus macrophages ex vivo (1, 2, 7). Thus, it was selected under conditions where rhesus CD4 was likely limiting. We further showed that two differences in the SIVmac316 CD4-binding site, namely, G382R and H442Y, largely account for its more efficient utilization of rhesus CD4. In addition to replicating in macrophages and binding rhesus CD4 more efficiently, SIVmac316 is markedly more sensitive to neutralizing antibodies and to sera from infected animals than SIVmac239 is (7, 16). We show here that SIVmac316's sensitivity to neutralization is partially independent of its ability to bind rhesus CD4 efficiently, because introduction of G382R and H442Y did not increase the neutralization sensitivity of SIVmac239. This stands in contrast to some HIV-1 and SIV Envs, which overcome the hurdle of infecting macrophages expressing low CD4 by enhancing their capacity to mediate fusion via structural changes in the Env trimer (6, 9, 20, 21). These changes, however, will often render the viruses more sensitive to neutralization (8, 22, 23).
We have thus identified an SIVmac239 Env that binds rhesus CD4 with an affinity that better reflects the binding affinities of most HIV-1 Envs for human CD4. This Env nonetheless remained highly resistant to antibody neutralization. A rhesus macaque infected with an SIVmac239 bearing these mutations may more accurately reflect the pathology of a human infection than macaques infected with wild-type SIVmac239 or with any current SHIV. Specifically, cells expressing low levels of CD4, including macrophages in the periphery and the brain, are more likely to be infected by this virus (24). These cells may contribute to a long-term viral reservoir, complicate clearance by natural killer or cytotoxic T cells, or perhaps mediate neurologic disease (14, 25–27). Future animal studies will be necessary to determine whether this virus does indeed cause more serious disease or is more difficult to eradicate than currently studied SIV or SHIV isolates.
MATERIALS AND METHODS
Cells, plasmids, and viruses.
HEK293T (human embryonic kidney; ATCC CRL-3216), TZM-bl, HOS.CCR5, and Cf2Th-CCR5 cell lines (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH) were grown in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) at 37°C. HOS CCR5+ cells were further supplemented with 1 μg/ml puromycin. Cf2Th-CCR5 cells were supplemented with 500 μg/ml G418, 500 μg/ml zeocin, and 3 μg/ml puromycin. TZM-bl cells were provided by John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. (28–32). HOS.CCR-5 and pNL4-3.Luc.R−E− were supplied by Nathaniel Landau (33–36), and Cf2Th-CCR5 cells were provided by Joseph Sodroski (37). Expi293 cells were grown in Expi293 expression medium (Thermo Fisher Scientific). Plasmids V132 and V82 for the expression of antibodies 4L6 and 5L7, respectively, were provided by Ronald Desrosiers, as were plasmids encoding SIVmac239 SpX, SIVmac316 open SpX, and SIVsmE543-3 full-length viral genomes, and SIVmac251 stock virus (5, 16, 17, 38). SIVmac1A11 infectious molecular clone, also from the NIH AIDS reagent program, was supplied by Paul Luciw (39). The ADA expression vector has been previously described (40, 41). SIVmac239 and SIVmac316 env genes were cloned from full-length virus plasmids into pcDNA 3.1(+) (Thermo Fisher Scientific) using Gibson Assembly (New England BioLabs). Nucleotide changes encoding the G382R and H442Y mutations were introduced into the SIVmac239 envelope glycoprotein using PCR site-directed mutagenesis. Plasmids expressing human and rhesus CD4-Ig have been previously described (15).
CD4-Ig and antibody production.
To produce huCD4-Ig, rhCD4-Ig, and rhCD4-Ig variants, Expi293 cells (Thermo Fisher Scientific) were grown to a density of 3 × 106 cells/ml in 250 ml Expi293 expression medium (Thermo Fisher Scientific). Vectors expressing CD4-Ig variants (140 μg) were transfected with ExpiFectamine (Thermo Fisher Scientific) according to the manufacturer's instructions. CD4-Ig was harvested after 5 days. To produce the SIV neutralizing antibodies 5L7 and 4L6, 293T cells seeded in T175 flasks (Falcon) were transfected with 80 μg of plasmid per flask using CalPhos mammalian transfection kit (TaKaRa Bio). Twelve to 16 h posttransfection, cells were washed with phosphate-buffered saline (PBS) and grown in FreeStyle 293 expression medium (Thermo Fisher Scientific). Antibodies were harvested 48 h after medium change. To harvest antibodies and CD4-Ig variants, medium was collected, centrifuged at 4,000 × g for 10 min, and filtered with a 0.45-μm filter flask (Millipore). Protein was isolated with HiTrap protein A or HiTrap MabSelect SuRe columns (GE Healthcare) and eluted with IgG elution buffer (Thermo Fisher Scientific) into 1 M Tris-HCl buffer (pH 9) (G-Biosciences). Buffer exchange was performed with Amicon Ultra-15 centrifugal filter units (Millipore) and PBS.
TZM-bl neutralization assays.
TZM-bl neutralization assays were performed as previously described (40, 42). Briefly, pseudotyped HIV-1 was produced in T175 flasks by transfecting 293T cells with a mixture of an HIV-1 expression vector lacking a functional env gene (45 μg DNA), a plasmid encoding the desired envelope glycoprotein (25 μg), a plasmid expressing the tat gene (5 μg), and a plasmid expressing the rev gene (5 μg). Viral supernatants were passed through a 0.45-μm syringe filter and stored at −80°C. For the assay, pseudoviruses were preincubated with titrated amounts of antibody, CD4-Ig variants, or serum in DMEM with 10% FBS for 1 h at 37°C. TZM-bl cells were detached by trypsin and diluted to 100,000 cells/ml with DMEM containing 10% FBS. Cells were then added to the pseudovirus-inhibitor mixture and incubated for 36 to 48 h at 37°C. Viral entry was determined using Britelite Plus (PerkinElmer), and luciferase was measured using a Victor X3 plate reader (PerkinElmer). Data were analyzed with Prism software (GraphPad).
Flow cytometric analysis of binding to cell-expressed envelope glycoproteins.
293T cells were transfected with plasmids expressing the envelope glycoproteins of SIVmac239, SIVmac239 variants, or SIVmac316, all lacking cytoplasmic residues 721 to 879 (SIVmac239 numbering) using a CalPhos mammalian transfection kit (TaKaRa Bio). The transfection medium was replaced after incubation overnight, and 48 h later, the cells were harvested using nonenzymatic cell dissociation solution (Sigma-Aldrich). Harvested cells were washed twice in PBS with 2% goat serum and 0.1% sodium azide (flow cytometry buffer) and then incubated with serially diluted CD4-Ig variants on ice for 1 h. Cells were subsequently washed twice and then incubated with allophycocyanin (APC)-labeled secondary antibodies recognizing human Fc (Jackson Immuno Research) for 30 to 60 min on ice. Cells were washed thrice and then resuspended in PBS with 1% paraformaldehyde. Binding was analyzed with an Accuri C6 flow cytometer (BD Biosciences), and data were analyzed with C6 software (BD Bioscience).
Generation of cells stably expressing CD4 receptor variants.
The expression plasmid encoding rhCD4 receptor has been previously described (15). Nucleotide changes encoding the I39N mutation were introduced by PCR site-directed mutagenesis. The open reading frame of CD4 was cloned into pBABE-hygro vector (Cell Biolabs) using restriction enzymes NotI and EcoRI (New England BioLabs). Virus-like particles were generated by cotransfecting the pBABEvector encoding CD4 variants, murine leukemia virus Gag and Pol, and a plasmid expressing the vesicular stomatitis virus G protein (Clontech) at a 2:2:1 ratio. Supernatants were harvested 60 h posttransfection, filtered through a 0.45-μm syringe filter, and stored at −80°C. Supernatant was added to HOS.CCR5 and Cf2Th-CCR5 cells, and after 48 h, medium was changed and supplemented with 0.5 μg/ml hygromycin (Life Technologies). Cells were harvested using nonenzymatic cell dissociation solution (Sigma-Aldrich) and washed twice in flow cytometry buffer. Cells were then incubated with anti-CD4 mouse antibody (Sigma-Aldrich) on ice for 1 h and again washed twice with flow cytometry buffer. Cells were subsequently incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Jackson Immuno Research) for 30 to 60 min on ice, washed thrice, and then resuspended in PBS with 1% paraformaldehyde. Binding was analyzed with an Accuri C6 flow cytometer (BD Biosciences), and data were analyzed with C6 software (BD Bioscience).
Infection assays.
HIV-1 pseudoviruses were generated similarly as described above except that pNL4-3.Luc.R−E− was used as the HIV-1 expression vector (43). HOS.CCR5 and Cf2Th-CCR5 cells stably expressing CD4 variant were harvested, diluted in DMEM with 10% FBS to 100,000 cells/ml, and incubated with virus for 4 h at 37°C. Viral entry was analyzed after 48 h using Britelite Plus (PerkinElmer), and luciferase was measured using a Victor X3 plate reader (PerkinElmer). Data were analyzed with Prism software (GraphPad).
ACKNOWLEDGMENTS
We are grateful to Ronald Desrosiers and Sebastian Fuchs for their helpful insights into SIV biology and history. We also thank Meredith Davis Gardner for her thoughtful comments and careful reading of the manuscript.
This research was funded by the following NIH awards to M.F.: R37 AI091476, P01 AI100263, and UM1 AI126623 (defeat HIV Delaney Cure Collaboratory).
REFERENCES
- 1.Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nat Rev Microbiol 10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansfield KG, Lerch NW, Gardner MB, Lackner AA. 1995. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J Med Primatol 24:116–122. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Daniel M, Letvin N, King N, Kannagi M, Sehgal P, Hung R. 1985. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 228:1201–1205. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 4.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 5.Regier DA, Desrosiers RC. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses 6:1221–1231. [DOI] [PubMed] [Google Scholar]
- 6.Mori K, Ringler DJ, Kodama T, Desrosiers RC. 1992. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol 66:2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desrosiers RC, Hansen-Moosa A, Mori K, Bouvier DP, King NW, Daniel MD, Ringler DJ. 1991. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol 139:29–35. [PMC free article] [PubMed] [Google Scholar]
- 8.Puffer BA, Pohlmann S, Edinger AL, Carlin D, Sanchez MD, Reitter J, Watry DD, Fox HS, Desrosiers RC, Doms RW. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J Virol 76:2595–2605. doi: 10.1128/JVI.76.6.2595-2605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori K, Rosenzweig M, Desrosiers RC. 2000. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J Virol 74:10852–10859. doi: 10.1128/JVI.74.22.10852-10859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Lord CI, Haseltine W, Letvin NL, Sodroski J. 1992. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr 5:639–646. [PubMed] [Google Scholar]
- 11.Luciw PA, Pratt-Lowe E, Shaw KE, Levy JA, Cheng-Mayer C. 1995. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV). Proc Natl Acad Sci U S A 92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Wang S, Kong R, Ding W, Lee FH, Parker Z, Kim E, Learn GH, Hahn P, Policicchio B, Brocca-Cofano E, Deleage C, Hao X, Chuang GY, Gorman J, Gardner M, Lewis MG, Hatziioannou T, Santra S, Apetrei C, Pandrea I, Alam SM, Liao HX, Shen X, Tomaras GD, Farzan M, Chertova E, Keele BF, Estes JD, Lifson JD, Doms RW, Montefiori DC, Haynes BF, Sodroski JG, Kwong PD, Hahn BH, Shaw GM. 2016. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A 113:E3413–E3422. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humes D, Emery S, Laws E, Overbaugh J. 2012. A species-specific amino acid difference in the macaque CD4 receptor restricts replication by global circulating HIV-1 variants representing viruses from recent infection. J Virol 86:12472–12483. doi: 10.1128/JVI.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho DD, Rota TR, Hirsch MS. 1986. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest 77:1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, Neale ES Jr, Fellinger CH, Joshi VR, Fuchs SP, Martinez-Navio JM, Quinlan BD, Yao AY, Mouquet H, Gorman J, Zhang B, Poignard P, Nussenzweig MC, Burton DR, Kwong PD, Piatak M Jr, Lifson JD, Gao G, Desrosiers RC, Evans DT, Hahn BH, Ploss A, Cannon PM, Seaman MS, Farzan M. 2015. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson WE, Sanford H, Schwall L, Burton DR, Parren PW, Robinson JE, Desrosiers RC. 2003. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J Virol 77:9993–10003. doi: 10.1128/JVI.77.18.9993-10003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Navio JM, Fuchs SP, Pedreno-Lopez S, Rakasz EG, Gao G, Desrosiers RC. 2016. Host anti-antibody responses following adeno-associated virus-mediated delivery of antibodies against HIV and SIV in rhesus monkeys. Mol Ther 24:76–86. doi: 10.1038/mt.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin KA, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard NP. 1997. CD4-independent binding of SIV gp120 to rhesus CCR5. Science 278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 19.Farzan M, Choe H, Vaca L, Martin K, Sun Y, Desjardins E, Ruffing N, Wu L, Wyatt R, Gerard N, Gerard C, Sodroski J. 1998. A tyrosine-rich region in the N terminus of CCR5 is important for human immunodeficiency virus type 1 entry and mediates an association between gp120 and CCR5. J Virol 72:1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorry PR, Taylor J, Holm GH, Mehle A, Morgan T, Cayabyab M, Farzan M, Wang H, Bell JE, Kunstman K, Moore JP, Wolinsky SM, Gabuzda D. 2002. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J Virol 76:6277–6292. doi: 10.1128/JVI.76.12.6277-6292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edinger AL, Blanpain C, Kunstman KJ, Wolinsky SM, Parmentier M, Doms RW. 1999. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J Virol 73:4062–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Means RE, Matthews T, Hoxie JA, Malim MH, Kodama T, Desrosiers RC. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J Virol 75:3903–3915. doi: 10.1128/JVI.75.8.3903-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunfee RL, Thomas ER, Gabuzda D. 2009. Enhanced macrophage tropism of HIV in brain and lymphoid tissues is associated with sensitivity to the broadly neutralizing CD4 binding site antibody b12. Retrovirology 6:69. doi: 10.1186/1742-4690-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bannert N, Schenten D, Craig S, Sodroski J. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J Virol 74:10984–10993. doi: 10.1128/JVI.74.23.10984-10993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexaki A, Liu Y, Wigdahl B. 2008. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res 6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. 2005. Pathogenesis of macrophage tropic HIV-1. Curr HIV Res 3:53–60. doi: 10.2174/1570162052772951. [DOI] [PubMed] [Google Scholar]
- 27.Dunfee R, Thomas ER, Gorry PR, Wang J, Ancuta P, Gabuzda D. 2006. Mechanisms of HIV-1 neurotropism. Curr HIV Res 4:267–278. doi: 10.2174/157016206777709500. [DOI] [PubMed] [Google Scholar]
- 28.Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. 2009. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J Virol 83:8289–8292. doi: 10.1128/JVI.00709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi Y, McClure MO, Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J Virol 82:12585–12588. doi: 10.1128/JVI.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol 74:8358–8367. doi: 10.1128/JVI.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 34.Landau NR, Littman DR. 1992. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol 66:5110–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 69:6705–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 37.Mirzabekov T, Bannert N, Farzan M, Hofmann W, Kolchinsky P, Wu L, Wyatt R, Sodroski J. 1999. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J Biol Chem 274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs SP, Martinez-Navio JM, Piatak M Jr, Lifson JD, Gao G, Desrosiers RC. 2015. AAV-delivered antibody mediates significant protective effects against SIVmac239 challenge in the absence of neutralizing activity. PLoS Pathog 11:e1005090. doi: 10.1371/journal.ppat.1005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luciw PA, Shaw KE, Unger RE, Planelles V, Stout MW, Lackner JE, Pratt-Lowe E, Leung NJ, Banapour B, Marthas ML. 1992. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res Hum Retroviruses 8:395–402. doi: 10.1089/aid.1992.8.395. [DOI] [PubMed] [Google Scholar]
- 40.Gardner MR, Fellinger CH, Prasad NR, Zhou AS, Kondur HR, Joshi VR, Quinlan BD, Farzan M. 2016. CD4-induced antibodies promote association of the HIV-1 envelope glycoprotein with CD4-binding site antibodies. J Virol 90:7822–7832. doi: 10.1128/JVI.00803-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Gerard C, Sodroski J, Choe H. 1999. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 96:667–676. doi: 10.1016/S0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choe H, Li W, Wright PL, Vasilieva N, Venturi M, Huang CC, Grundner C, Dorfman T, Zwick MB, Wang L, Rosenberg ES, Kwong PD, Burton DR, Robinson JE, Sodroski JG, Farzan M. 2003. Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell 114:161–170. doi: 10.1016/S0092-8674(03)00508-7. [DOI] [PubMed] [Google Scholar]
- 44.Schultz AM, Hu SL. 1993. Primate models for HIV vaccines. AIDS 7(Suppl 1):S161–S170. [PubMed] [Google Scholar]
- 45.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]