ABSTRACT
Porcine reproductive and respiratory syndrome, caused by porcine reproductive and respiratory syndrome virus (PRRSV), is a panzootic disease that is one of the most economically costly diseases to the swine industry. A key aspect of PRRSV virulence is that the virus suppresses the innate immune response and induces persistent infection, although the underlying mechanisms are not well understood. The dendritic cell (DC) marker CD83 belongs to the immunoglobulin superfamily and is associated with DC activation and immunosuppression of T cell proliferation when expressed as soluble CD83 (sCD83). In this study, we show that PRRSV infection strongly stimulates CD83 expression in porcine monocyte-derived DCs (MoDCs) and that the nucleocapsid (N) protein and nonstructural protein 10 (nsp10) of PRRSV enhance CD83 promoter activity via the NF-κB and Sp1 signaling pathways. R43A and K44A amino acid substitution mutants of the N protein suppress the N protein-mediated increase of CD83 promoter activity. Similarly, P192-5A and G214-3A mutants of nsp10 (with 5 and 3 alanine substitutions beginning at residues P192 and G214, respectively) abolish the nsp10-mediated induction of the CD83 promoter. Using reverse genetics, four mutant viruses (rR43A, rK44A, rP192-5A, and rG214-3A) and four revertants [rR43A(R), rK44A(R), rP192-5A(R), and rG214-3A(R)] were generated. Decreased induction of CD83 in MoDCs was observed after infection by mutants rR43A, rK44A, rP192-5A, and rG214-3A, in contrast to the results obtained using rR43A(R), rK44A(R), rP192-5A(R), and rG214-3A(R). These findings suggest that PRRSV N and nsp10 play important roles in modulating CD83 signaling and shed light on the mechanism by which PRRSV modulates host immunity.
IMPORTANCE Porcine reproductive and respiratory syndrome virus (PRRSV) is one of the most economically costly pathogens affecting the swine industry. It is unclear how PRRSV inhibits the host's immune response and induces persistent infection. The dendritic cell (DC) marker CD83 belongs to the immunoglobulin superfamily and has previously been associated with DC activation and immunosuppression of T cell proliferation and differentiation when expressed as soluble CD83 (sCD83). In this study, we found that PRRSV infection induces sCD83 expression in porcine MoDCs via the NF-κB and Sp1 signaling pathways. The viral nucleocapsid protein, nonstructural protein 1 (nsp1), and nsp10 were shown to enhance CD83 promoter activity. Amino acids R43 and K44 of the N protein, as well as residues 192 to 196 (P192-5) and 214 to 216 (G214-3) of nsp10, play important roles in CD83 promoter activation. These findings provide new insights into the molecular mechanism of immune suppression by PRRSV.
KEYWORDS: CD83, NF-κB, PRRSV, Sp1, nonstructural protein 10, nucleocapsid
INTRODUCTION
Over the last 3 decades, porcine reproductive and respiratory syndrome virus (PRRSV) has become a serious threat to the swine industry worldwide (1, 2). Current vaccines do not provide complete protection against this disease (3–5), and it is unclear how PRRSV inhibits the host's immune response and induces persistent infection.
PRRSV is a positive-strand RNA virus within the family Arteriviridae. The 15-kb viral RNA genome consists of seven open reading frames (ORFs). ORF1 comprises about 80% of the genome and encodes 14 nonstructural proteins (nsp's) with protease, replicase, and regulatory functions. The smaller, overlapping ORFs 2 through 7 encode five minor (GP2a, GP3, GP4, 5a, and E) and three major (GP5, M, and N) structural proteins (6–8). Accumulating evidence suggests that PRRSV nsp's, particularly nsp1α/β, nsp2, nsp4, and nsp11, are associated with the immunomodulation capability of the virus (9–12). nsp1, nsp2, nsp5, nsp7, and nsp9 to -11 have been implicated in the induction of gamma interferon (IFN-γ) and are likely to be involved in the development of the cell-mediated immune response (13–18).
The N protein is the sole component of the viral capsid and plays a fundamental role in viral infection and pathogenesis. Five immune reactivity domains are located within amino acid (aa) residues 30 to 52, 37 to 52, 69 to 112, and 112 to 123. NLS-1 (or the cryptic nuclear localization signal [NLS]) and NLS-2 (or the functional NLS) are located at aa residues 10 to 13 and 41 to 47 of the N protein, respectively (8). An SH3 binding motif within the N protein interacts with the host cellular signaling proteins (19). The N protein modulates IFN-β and interleukin-10 (IL-10) production, influencing cell cycle progression and the host response to PRRSV (20–22).
The helicase domain of PRRSV nsp10 participates in multiple processes during virus replication (23). Cys25 and His32 in nsp10 are critical for nucleic acid binding and unwinding, and Ala227 plays an important role in helicase activity (24). nsp10 also contains a putative zinc finger region which is implicated in subgenomic mRNA synthesis and genome replication. nsp10 expression may reduce ORF1ab transcription and is hypothesized to negatively regulate expression of other host and viral proteins (25, 26). nsp10 can induce apoptosis in peripheral blood mononuclear cells (PBMCs), whose function is dependent on the participation of both activated caspase-8 and Bid (27). nsp9 and nsp10 together contribute to the fatal effects of the highly pathogenic PRRSV (HP-PRRSV) now emerging in China (18, 28, 29).
CD83, a marker of mature dendritic cells (DCs), is a member of the immunoglobulin (Ig) superfamily and a type I transmembrane glycoprotein (30, 31). CD83 is upregulated by tumor necrosis factor alpha (TNF-α) in many cells, such as monocyte-derived DCs (MoDCs), monocytes, granulocyte precursor cells, and myelocytes (32). CD83 exists as two isoforms, namely, membrane-bound CD83 (mCD83) and soluble CD83 (sCD83) (33). mCD83, which has molecular masses of 40 to 45 kDa, functions as an essential enhancer during T cell activation and is important for the generation of thymocytes (32, 34). In contrast, sCD83 inhibits DC-mediated T cell stimulation and interferes with maturation of DCs and the cellular cytoskeleton (30–32). CD83 is also implicated in immune suppression in vivo, as sCD83 prevents cardiac allograft rejection and the development of experimental autoimmune encephalomyelitis (32, 35).
Some viruses, such as human cytomegalovirus (HCMV) (36, 37) and herpes simplex virus type 1 (HSV-1) (38, 39), regulate CD83 expression and prevent T cell activation. This suggests that viruses may target CD83 as part of the viral immune escape mechanism. In this study, we found that PRRSV infection upregulates CD83 expression, resulting in the shedding of sCD83 from MoDCs. Viral proteins were examined individually for the ability to affect CD83 promoter activity, revealing that N and nsp10 regulate this promoter. By constructing targeted mutations, we found that the amino acids R43 and K44 of the N protein, as well as P192 and G214 of nsp10, play important roles in inducing CD83 in MoDCs via the NF-κB and Sp1 signaling pathways. Our results provide new insights into the molecular mechanism underlying immune suppression by PRRSV.
RESULTS
CD83 is strongly induced in MoDCs by PRRSV infection.
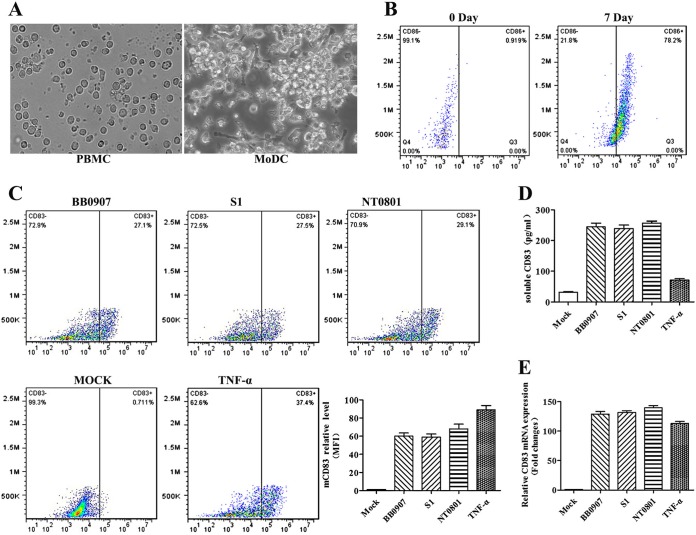
Porcine monocytes were cultured for 7 days in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 (40) and then harvested. Figure 1A shows that the morphology of MoDCs, as observed using an inverted microscope (×400), is significantly different from the morphology displayed by PBMCs. Figure 1B shows the results of an experiment in which CD86 on the cell surface was detected by fluorescence-activated cell sorter (FACS) analysis after cells were incubated with strains of different virulence levels (HP-PRRSV BB0907, classical PRRSV [C-PRRSV] S1, and low-pathogenicity PRRSV [LP-PRRSV] NT0801). mCD83, sCD83, and CD83 mRNA expression levels increased significantly as determined by FACS analysis, enzyme-linked immunosorbent assay (ELISA), and quantitative real-time reverse transcription-PCR (qRT-PCR). mRNA expression also increased significantly in the TNF-α-positive control (Fig. 1C to E). Because pandemic HP-PRRSV infection has caused immense economic damage in China, the highly virulent HP-PRRSV BB0907 strain was selected for subsequent experiments.
FIG 1.
CD83 is strongly induced in PRRSV-infected MoDCs. (A) Porcine monocytes (PBMC) were cultured for 0 and 7 days in the presence of GM-CSF and IL-4. The morphology of MoDCs was observed under an inverted microscope at 0 and 7 days. Magnification, ×400. (B) The cells were then harvested and analyzed for their expression of CD86 at the cell surface by use of a fluorescence-activated cell sorter (FACS). Pseudocolor dot plots represent staining with an isotype-matched control antibody. (C) HP-PRRSV BB0907, C-PRRSV S1, and LP-PRRSV NT0801 used at an MOI of 1. Mock-infected and TNF-α-treated MoDCs were used as negative and positive controls, respectively. Cells were collected for FACS analysis and RNA preparation. The pseudocolor dot plots represent results from FACS analysis for CD83 expression after treatment. The mean fluorescence intensity (MFI) (y axis) value is shown for each analyzed viral strain. (D) Culture supernatants were collected, and sCD83 was analyzed by ELISA. (E) RT-PCR analysis was conducted to measure CD83 mRNA levels, expressed as 2−ΔΔCT values. Actin was used as a reference gene, and the untreated sample was used for calibration. Data are expressed as means and standard errors of the means (SEM) for the fold changes with respect to expression levels in untreated cells. ELISA and qRT-PCR data are representative of one of three independent experiments.
PRRSV induces CD83 expression in a time- and dose-dependent manner.
MoDCs were inoculated with live or UV-inactivated HP-PRRSV BB0907 at a multiplicity of infection (MOI) of 1 and then harvested for CD83 analysis at 6, 12, 24, 36, and 48 h postinfection (hpi). Cells treated with TNF-α were used as a positive control. mCD83 and CD83 mRNA expression levels increased strongly as a result of TNF-α treatment and PRRSV infection over time, but UV inactivation of HP-PRRSV abolished this effect. sCD83 levels increased significantly only in cells infected with PRRSV (Fig. 2A to C). PRRSV titers in infected MoDCs peaked at 36 hpi (Fig. 2D), suggesting that CD83 induction is dependent on PRRSV replication.
FIG 2.
PRRSV increases CD83 expression in a time- and dose-dependent manner. MoDCs were inoculated with live or UV-inactivated HP-PRRSV BB0907 at an MOI of 1, and MoDCs were treated with PBS (1 mM) and TNF-α (50 ng/ml) as negative and positive controls, respectively. (A) At 6, 12, 24, 36, and 48 hpi, cells were collected, and surface CD83 (mCD83) expression was detected by flow cytometric analysis. Culture supernatants were collected, and sCD83 was analyzed by ELISA (B) and qRT-PCR (C). (D) PRRSV infection kinetics were measured in the supernatants of infected MoDCs by TCID50 assay. MoDCs were infected with live or UV-inactivated PRRSV at an MOI of 0, 0.1, 1, 2, or 5 for 36 h, and MoDCs were treated with PBS at 0, 0.1, 1, 2, and 5 mM as negative controls and with TNF-α at 0, 10, 50, 100, and 200 ng/ml as positive controls. CD83 expression levels were analyzed by flow cytometry (E), ELISA (F), and qRT-PCR (G).
In order to optimize viral infection and the effects of TNF-α treatment, MoDCs were incubated with live or UV-inactivated PRRSV at an MOI of 0, 0.1, 0.5, 1, or 2. Based on the results described above, samples were collected 36 h after PRRSV infection. Cells were treated with TNF-α at 0, 10, 50, 100, and 200 ng/ml. As shown in Fig. 2E to G, the levels of mCD83, sCD83, and CD83 mRNA expression were significantly higher in cells infected with PRRSV at an MOI of 1 than in those infected at an MOI of 0.1. These results indicate that mCD83 and CD83 mRNA expression increases in PRRSV-infected cells and that cells respond to TNF-α treatment in a dose-dependent manner. Finally, sCD83 was released from PRRSV-infected MoDCs but not from cells infected by UV-inactivated PRRSV or treated with TNF-α.
PRRSV infection and viral proteins affect the CD83 promoter.
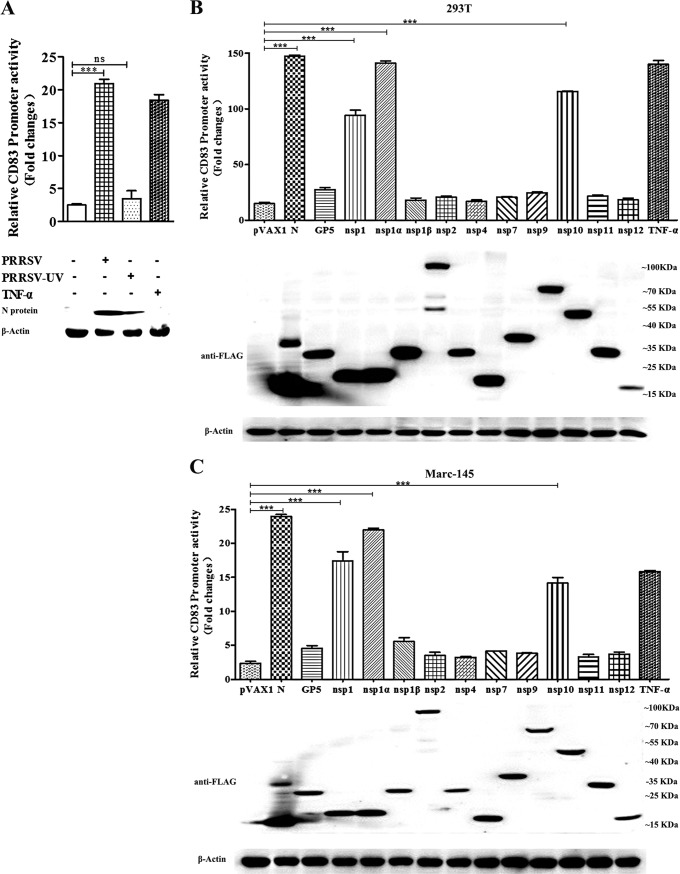
PRRSV can grow in cultured Marc-145 cells, which are derived from embryonic African green monkey kidney tissue. To examine the activity of the porcine CD83 promoter, Marc-145 cells were transfected with a reporter system consisting of the plasmids pCD83-luc and pRL-TK. Twenty-four hours later, the cells were incubated with live or UV-inactivated PRRSV or were treated with TNF-α as a positive control. PRRSV infection significantly stimulated promoter activity, as did TNF-α. In contrast, UV-inactivated PRRSV had no effect (Fig. 3A).
FIG 3.
PRRSV proteins increase the activity of the porcine CD83 promoter. (A) Marc-145 cells were cotransfected with pCD83-luc (1 μg) and the Renilla luciferase vector pRL-TK (0.1 μg). Twenty-four hours later, they were inoculated with live or UV-inactivated HP-PRRSV BB0907 at an MOI of 1. Lysates of TNF-α-treated and mock-infected Marc-145 cells were used as positive and negative controls, respectively. CD83 promoter activation was analyzed by luciferase reporter assay. (B and C) To identify viral proteins that induce CD83 promoter activity, 293T (B) and Marc-145 (C) cells were transfected with pCD83-luc (1 μg) along with plasmids expressing different viral proteins (1 μg) and pRL-TK (0.1 μg). TNF-α (50 ng/ml)-treated cells that were transfected with the pCD83 and pRL-TK plasmids were used as a positive control. Lysates of transfected cells were subjected to Western blotting with anti-Flag antibody to detect viral protein expression. Because the Flag tag was fused to the N terminus of nsp1, and nsp1 autocleaves to generate nsp1α and nsp1β, the molecular size of nsp1 is similar to that of nsp1α. All assays were repeated at least three times, with each experiment performed in triplicate. Bars represent means and SEM for three independent experiments. ***, significant difference between groups (P < 0.001); ns, not significant.
In order to identify viral proteins with the ability to stimulate the CD83 promoter, we constructed a series of recombinant plasmids by using the pVAX vector; these were named pVAX-GP2, pVAX-GP3, pVAX-GP4, pVAX-GP5, pVAX-M, pVAX-N, pVAX-nsp1, pVAX-nsp1α, pVAX-nsp1β, pVAX-nsp2, pVAX-nsp3, pVAX-nsp4, pVAX-nsp5, pVAX-nsp6, pVAX-nsp7, pVAX-nsp8, pVAX-nsp9, pVAX-nsp10, pVAX-nsp11, and pVAX-nsp12. However, efficient expression occurred only for the plasmids encoding GP5, N protein, nsp1, nsp2, nsp4, nsp7, and nsp9 to -12. HEK293T cells were cotransfected with pCD83-luc and pRL-TK together with a plasmid carrying an nsp, N, or GP5 gene. The results showed that N, nsp1α, and nsp10 strongly stimulated the porcine CD83 promoter. Because the Marc-145 cell line can be used to culture PRRSV, these cells were also used in this experiment. The results were consistent with those obtained using HEK293T cells (Fig. 3B and C).
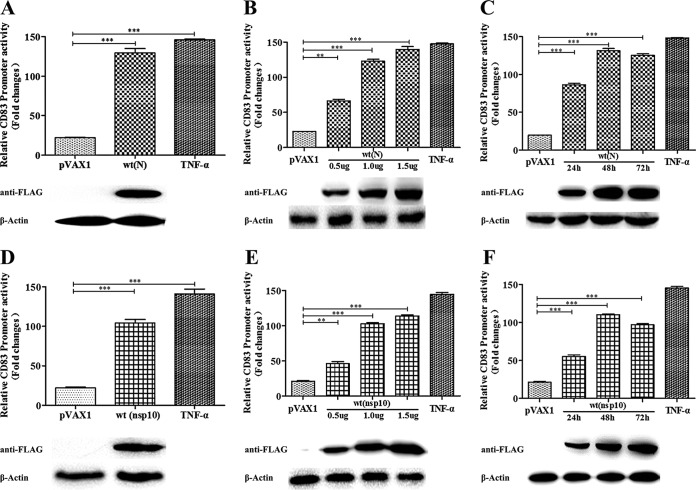
PRRSV N protein and nsp10 stimulate the CD83 promoter in a time- and dose-dependent manner.
To confirm that the N protein and nsp10 are involved in CD83 promoter activation, HEK293T cells were cotransfected with pCD83-luc and pRL-TK together with the pVAX-NP or pVAX-Nsp10 plasmid. The N or nsp10 construct was added at different concentrations and at different time points. The results showed that CD83 promoter activity varied directly with the N protein and nsp10 concentrations (P < 0.001) (Fig. 4A, B, D, and E). In addition, promoter activity increased from 24 to 48 hpi and was maintained for as long as 72 hpi (Fig. 4C and F).
FIG 4.
Relative CD83 promoter activity is increased by PRRSV N and nsp10. (A and D) HEK293T cells were cotransfected with the pVAX1 (negative control), pVAX-NP or pVAX-Nsp10, pCD83-luc, and pRL-TK plasmids. After incubation for 48 h, CD83 promoter activity was analyzed by dual-luciferase reporter assay. Transfected cells were also subjected to Western blotting to detect N and nsp10 protein expression. pVAX1 served as a negative control, and pVAX1-transfected cells stimulated with TNF-α 16 h prior to harvest served as a positive control. (B and E) The increase in CD83 promoter activity by pVAX-NP or pVAX-Nsp10 is dose dependent. (C and F) At different time points after transfection, pCD83 promoter activity was analyzed. All assays were repeated at least three times, with each experiment performed in triplicate. Bars represent means and SEM for three independent experiments. ***, significant difference between groups (P < 0.001).
Mutations in N protein can reduce CD83 promoter activity.
The truncated mutant ORF7t, which contains nucleotides 1 to 112, encoding the N terminus of the N protein, was derived from the intact gene by use of PCR. The product was inserted into a vector that generates a chimeric protein with a Flag tag at the C terminus. Ten single- or multinucleotide mutations were introduced into the putative functional region of the N protein, with the targeted amino acid residues being replaced by alanine, giving C23A, C75A, C90A, K10-4A, Q33-5A, K43-5A, P56-4A, E60-5A, H65-4A, and V112-4A mutants. The nomenclature of the mutants indicates the location and number of alanine substitutions, e.g., K10-4A indicates that four alanine substitutions were created beginning at position K10. HEK293T or Marc-145 cells were then transfected with pCD83-luc and pRL-TK together with pVAX-NP or an N-terminal construct. Luciferase assays showed that the ORF7t construct, containing nucleotides 1 to 112, and the C23, C75, C90, K10-4A, P56-4A, E60-5A, H65-4A, and V112-4A mutants were indistinguishable from the wild-type N protein. In contrast, promoter activity was significantly reduced in the Q33-5A, K43-5A N protein mutant. This result suggests that these amino acids encode two domains that can stimulate porcine CD83 promoter activity (Fig. 5A, B, G, and H).
FIG 5.
Characterization of N protein amino acid residues required for induction of the CD83 promoter. 293T cells (A to F) or Marc-145 cells (G to L) were transfected with pCD83-luc (1 μg) together with plasmids expressing wild-type N or a mutant protein (1 μg) and pRL-TK (0.1 μg). Cells transfected with pVAX1 served as negative controls, and cells transfected with pVAX1 and stimulated with TNF-α 16 h prior to harvest served as positive controls. Firefly luciferase activities were measured in cell lysates and normalized to Renilla luciferase activities. The immunoblot panels depict the levels of the corresponding viral proteins in transfected cells. The viral protein in cell lysates was detected by Western blotting using anti-N protein antibodies, with β-actin serving as a loading control. (A and G) Truncated N protein; (B and H) multiple alanine mutations at defined regions; (C and I) individual alanine substitutions at positions 33 to 37; (D and J) individual alanine substitutions at positions 43 to 47; (E and K) individual alanine substitutions at positions 65 to 68; (F and L) individual alanine substitutions at positions 112 to 115.
To identify individual amino acid residues within the functional domains that are necessary for inducing CD83 promoter activity, point mutations were constructed in the regions spanning amino acid residues 33 to 35 and 43 to 45. Mutations at Ser36, Arg43, and Lys44 significantly depressed CD83 promoter activity compared to that with the wild-type protein (Fig. 5C to F and I to L).
Mutations in nsp10 can reduce CD83 promoter stimulation.
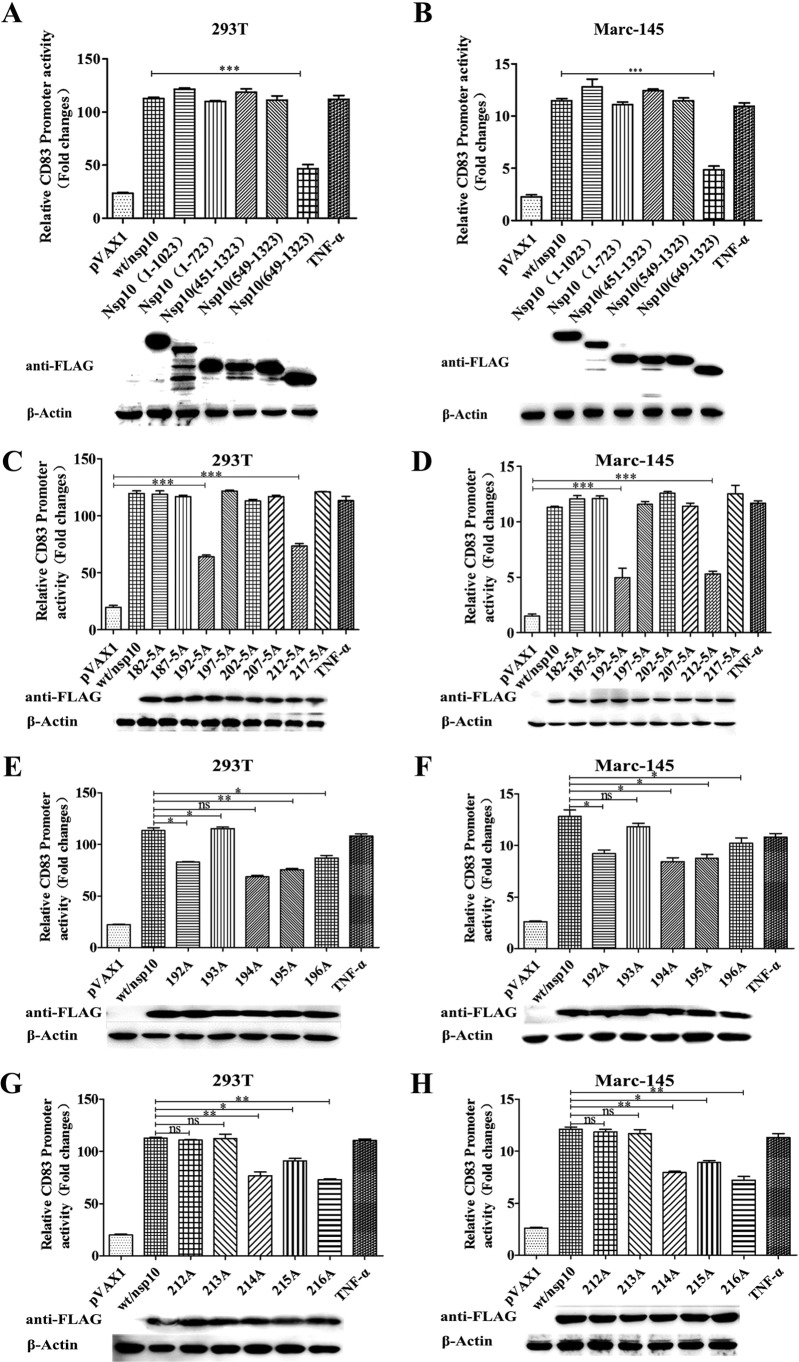
Truncated nsp10 mutants containing nucleotides 1 to 1023 and 1 to 723 (encoding the N terminus) and mutants containing nucleotides 451 to 1323, 549 to 1323, and 649 to 1323 (encoding the C terminus) were derived from the intact nsp10 gene by use of PCR. The amplified products were inserted into a vector that generates a chimeric polypeptide with a Flag tag at the C terminus. HEK293T or Marc-145 cells were then transfected with pCD83-luc and pRL-TK together with pVAX-Nsp10 or a truncated construct. Luciferase assays showed that several mutants were indistinguishable from wild-type nsp10, including the constructs containing nucleotides 1 to 1023, 1 to 723, 451 to 1323, and 549 to 1323. In contrast, promoter activity was significantly reduced in the nsp10 mutant containing nucleotides 649 to 1323. This result suggests that amino acids 182 through 221 in nsp10 contain a domain that can stimulate porcine CD83 promoter activity (Fig. 6A and B).
FIG 6.
Specific amino acid residues of nsp10 contribute to CD83 activation. HEK 293T (A and C) cells and Marc-145 (B and D) cells were transfected with pCD83-luc (1 μg) together with nsp10 wild-type or mutant expression plasmids (1 μg) and pRL-TK (0.1 μg). Firefly luciferase activities were measured in cell lysates and normalized to Renilla luciferase activities. Cells transfected with pVAX1 served as a negative control, and cells transfected with pVAX1 and stimulated with TNF-α 16 h prior to harvest served as a positive control. Protein expression in cell lysates was measured by Western blotting using anti-Flag antibody. β-Actin was used as a loading control. The blot images are shown under each graph. (A and B) Mutants with truncated nsp10 genes; (C and D) mutants with multiple alanine mutations at defined regions; (E and F) mutants with individual alanine substitutions at positions 192 to 196; (G and H) mutants with individual alanine substitutions at positions 212 to 216.
To refine these results, we constructed eight multipoint mutants with mutations in the putative functional region of nsp10, with the targeted amino acid residues being replaced by alanine, giving R182-5A, C187-5A, P192-5A, L197-5A, P202-5A, P207-5A, L212-5A, and C217-5A mutants. As shown in Fig. 6C and D, the P192-5A (Pro192-5A) and L212-5A (Leu212-5A) mutants did not activate the CD83 promoter as well as the wild-type nsp10 protein or any of the other multipoint mutants did. To identify individual amino acid residues within the functional domain that are necessary for inducing CD83 promoter activity, point mutations were introduced to the regions spanning amino acid residues 192 to 196 and 212 to 216. Mutations at Pro192, Gly194, Thr195, Thr196, Gly214, Thr215, and Thr216 in nsp10 obviously depressed CD83 promoter activity compared to that with wild-type nsp10 (Fig. 6E and F).
Roles of Sp1 and NF-κB in PRRSV-, N-, and nsp10-mediated activation of CD83.
To dissect the signaling pathways involved in PRRSV-mediated induction of the CD83 promoter, we examined the promoter for the presence of regulatory motifs associated with known signaling pathways. Putative transcription factor binding sites in the CD83 promoter region were identified by an online promoter scanning utility (http://www-bimas.cit.nih.gov/molbio/proscan/). As shown in Fig. 7A, three Sp1 binding sites and one NF-κB binding site are located in the TATA-less CD83 promoter, at coordinates −381 to −372, −222 to −113, and −62 to −50 relative to the +1 position.
FIG 7.
PRRSV mediates the expression of CD83 via Sp1 and NF-κB pathways. (A) Sp1 and NF-κB transcription factor binding sites in the porcine CD83 promoter region are underlined or shown in boxes. Numbers represent nucleotide positions relative to the transcription start site (ts; +1 position) in the swine sequence. (B and C) HEK293T and Marc-145 cells were transfected with 3 different siRNAs targeting the Sp1 gene or with a scrambled siRNA (NC). After incubation for 24 h, Sp1 mRNA levels were evaluated by qRT-PCR. (F, H, J, and L) To evaluate the involvement of the Sp1 and NF-κB pathways in CD83 promoter activation, 293T and Marc-145 cells were cotransfected with siRNA targeting Sp1, the luciferase vector, and pVAX-NP (F and H) or pVAX-nsp10 (J and L). CD83 promoter activity was analyzed as described in the other figure legends. (G, I, K, and M) Cells cotransfected with the plasmids described above were pretreated with an NF-κB inhibitor (BAY11-7082) at 0, 1, 5, or 10 μM for 12 h. After 24 h, CD83 promoter activity was analyzed. N (G and I), nsp10 (K and M), and β-actin were detected by Western blotting with anti-N protein, anti-Flag, and anti-β-actin antibodies. (D and E) Marc-145 cells were transfected with luciferase reporter plasmids together with siRNA targeted to Sp1 (D) or pretreated with BAY11-7082 (E), followed by infection with PRRSV at an MOI of 1. CD83 promoter activity was examined as described above. PRRSV N and β-actin proteins were detected by Western blotting.
To test the involvement of Sp1 in CD83 promoter activation, we evaluated three small interfering RNAs (siRNAs) that specifically target Sp1 (si-Sp1-633, si-Sp1-820, and si-Sp1-1595). All three markedly diminished the expression of exogenous and endogenous Sp1 mRNAs detected by qRT-PCR in HEK293T and Marc-145 cells (Fig. 7B and C). HEK293T and Marc-145 cells were then transfected with siRNAs targeting Sp1. After 12 h, the cells were cotransfected with pVAX-NP or pVAX-Nsp10 and the luciferase assay plasmids described earlier. Knockdown of Sp1 reduced CD83 promoter activation by both N (Fig. 7F and H) and nsp10 (Fig. 7J and L), strongly suggesting that Sp1 regulates N- and nsp10-mediated expression from this promoter. In related experiments, si-Sp1 siRNAs were used to knock down Sp1 expression in Marc-145 cells, which were then infected with PRRSV. Expression from the CD83 promoter was also inhibited by PRRSV infection (Fig. 7D).
To examine the potential role of NF-κB, HEK293T and Marc-145 cells were pretreated with an NF-κB inhibitor (BAY11-7082) at doses of 0, 1, 5, and 10 μM for 12 h and then cotransfected with the luciferase assay plasmid pVAX-NP or pVAX-Nsp10. The NF-κB inhibitor significantly inhibited activation of the CD83 promoter by both N (Fig. 7G and I) and nsp10 (Fig. 7K and M). In addition, treatment with the NF-κB inhibitor also decreased PRRSV-mediated activation of the CD83 promoter (Fig. 7E).
Construction and identification of recombinant N or nsp10 mutant and revertant viruses.
To confirm the identities of the viral amino acid residues that play critical roles in CD83 promoter induction, we used PRRSV infectious cDNA clones to construct recombinant PRRSV strains containing mutations in the N and nsp10 genes.
In the case of the N protein, attempts were made to obtain mutations affecting amino acid residues 36 to 39 and 43 to 47, as well as single point mutations in these regions, using the strategy shown in Fig. 8A. However, only two single point mutations (rR43A and rK44A) which caused cytopathic effects (CPEs) in Marc-145 cells were recovered. Two revertants, rR43A(R) and rK44A(R), were rescued from the corresponding rR43A and rK44A infectious cDNA clones. As shown in Fig. 8B, plaque formation levels by rR43A, rK44A, and the two corresponding revertants were similar in Marc-145 cells. In addition, rR43A, rK44A, rR43A(R), and rK44A(R) exhibited growth kinetics similar to those exhibited by the parental wild-type rBB/wt strain (Fig. 8C).
FIG 8.
Construction and identification of recombinant PRRSV strains containing N or nsp10 mutants. (A and D) Construction strategy for full-length cDNA clones and the 4 rescued PRRSV strains containing mutations in N (A) and nsp10 (D). (B and E) Viral plaque morphologies in Marc-145 cells. Cells in 12-well plates were infected with the PRRSV BB0907 wild type or mutants, cultured in DMEM with a 1% agarose overlay, fixed at 4 days postinfection, and stained with 1% crystal violet. (C and F) Multistep growth kinetics of PRRSV in Marc-145 cells after infection by the indicated viruses at an MOI of 0.1. Culture supernatants were collected at the indicated time points, and viral titration was performed. Results are expressed as TCID50. Titer data from three independent experiments are shown as means ± standard deviations (SD).
In the case of nsp10, attempts were made to obtain mutations affecting amino acid residues 192 to 196, 214 to 216, and 212 to 216, as well as single point mutations in these regions. Only two mutant viruses (rP192-5A and rG214-3A) were rescued that caused CPEs in Marc-145 cells. We rescued two revertants, rP192-5A(R) and rG214-3A(R), from the corresponding infectious cDNA mutant clones (Fig. 8D). As shown in Fig. 8E, plaque formation levels by rP192-5A, rG214-3A, rP192-5A(R), and rG214-3A(R) were similar in Marc-145 cells. In addition, rP192-5A, rG214-3A, rP192-5A(R), and rG214-3A(R) exhibited growth kinetics similar to those exhibited by the parental wild-type rBB/wt strain in Marc-145 cells (Fig. 8F).
Effects of mutant viruses on CD83 promoter activity and CD83 expression in MoDCs.
As shown in Fig. 9A and B, mutant viruses rR43A, rK44A, rP192-5A, and rG214-3A activated the CD83 promoter, though significantly less than the activation by rBB/wt (P < 0.001). However, the recovered viruses rR43A(R), rK44A(R), rP192-5A(R), and rG214-3A(R) exhibited nearly the same level of activation as that of rBB/wt. All virus infections were confirmed by Western blotting of cell lysates with an anti-N protein monoclonal antibody as a probe.
FIG 9.
Recombinant PRRSV strains containing N or nsp10 mutants decrease CD83 promoter activity and CD83 expression. (A) CD83 promoter activities in response to infection by recombinant PRRSV strains rBB/wt, rR43A, rK44A, rR43A(R), and rK44A(R). Results are expressed as relative luciferase activities. The panels below the bar graph show immunoblots of proteins from infected cells, probed with anti-N and β-actin antibodies. (B) CD83 promoter activities in response to infection by recombinant PRRSV strains rBB/wt, rP192-5A, rG214-3A, rP192-5A(R), and rG214-3A(R). Results are expressed as relative luciferase activities. (C) MoDCs were mock infected or infected with recombinant PRRSV [rBB/wt, rR43A, rK44A, rP192-5A, rG214-3A, rR43A(R), rK44A(R), rP192-5A(R), or rG214-3A(R)] at an MOI of 1. After incubation for 36 h, mCD83 on the cell surface was detected by flow cytometry. Pseudocolor dot plots represent the distribution of cells stained with an isotype-matched control antibody. (D) The MFI (y axis) value is shown for each analyzed recombinant. (E) sCD83 levels determined using sandwich ELISA. The optical density at 450 nm (OD450) was measured using an ELISA plate reader (Bio-Tek). Standard curves for estimation of the sCD83 concentration were generated using serial dilutions of CD83 Ig prepared in medium, and the results are expressed as protein concentrations based on the IgG1 standard. (F) Relative CD83 mRNA levels detected by qPCR. Levels were calculated relative to those of β-actin, and the fold change was calculated using the 2−ΔΔCT method. Data are presented as fold changes in CD83 mRNA expression, normalized to β-actin and relative to the mock-infected control level. All data are representative of one of three independent experiments. ***, significant difference between groups (P < 0.001).
Experiments were also performed to examine CD83 expression in MoDCs infected with the rBB/wt wild-type and nsp10 mutant viruses. After infection, CD83 surface expression was analyzed by flow cytometry. In parallel, cell culture medium was used to determine the concentration of sCD83 by ELISA and the CD83 mRNA level by qRT-PCR. The results showed that the mutant viruses rR43A, rK44A, rP192-5A, and rG214-3A significantly decreased the levels of mCD83, sCD83, and CD83 mRNA expression in MoDCs relative to those with the parental virus rBB/wt. However, the recovered mutants rR43A(R), rK44A(R), rP192-5A(R), and rG214-3A(R) induced mCD83, sCD83, and CD83 mRNA levels that were statistically indistinguishable from the levels induced by the wild-type virus rBB/wt (P < 0.05) (Fig. 9C to F).
Effects of mutant viruses on Sp1 and NF-κB in MoDCs.
To determine whether Sp1 and NF-κB are activated by PRRSV infection, MoDCs were inoculated with live or UV-inactivated HP-PRRSV BB0907 at an MOI of 0.1, 1, 2, or 5. After incubation for 36 h, Sp1 and NF-κB mRNA levels were measured by qRT-PCR. The results showed that PRRSV BB0907 infection significantly increased Sp1 and NF-κB mRNA expression in a dose-dependent manner (Fig. 10A and B).
FIG 10.
Sp1 and NF-κB signaling pathways are affected by PRRSV strains containing mutations in nsp10. (A and B) MoDCs were infected with PRRSV BB0907 at an MOI of 0, 0.1, 1, 2, or 5. After incubation for 36 h, Sp1 and NF-κB mRNA levels were determined by qPCR as described in the legend to Fig. 9. (C and D) MoDCs were infected with PRRSV rBB/wt, rR43A, rK44A, rP192-5A, and rG214-3A at an MOI of 1, and then Sp1 and NF-κB mRNA levels were determined by qPCR as described in the legend to Fig. 9. (E and F) Marc-145 cells were transfected with luciferase reporter plasmids together with siRNA targeting Sp1 (E) or treated with BAY11-7082 at 5 μM (F), followed by infection with PRRSV rBB/wt, rR43A, rK44A, rP192-5A, or rG214-3A at an MOI of 1. CD83 promoter activity was analyzed by dual-luciferase assay. PRRSV infection was confirmed by Western blotting with anti-N protein and β-actin antibodies (blot images are shown under panels E and F). The blots are representative of one of three independent experiments. ***, significant difference between groups (P < 0.001).
MoDCs were also inoculated with rBB/wt, rR43A, rK44A, rP192-5A, and rG214-3A at an MOI of 1. qRT-PCR analysis showed that rR43A, rK44A, rP192-5A, and rG214-3A decreased mRNA levels for Sp1 and NF-κB compared to the levels induced by the wild-type virus rBB/wt (Fig. 10C and D).
Finally, Marc-145 cells were cotransfected with pRL-TK and pCD83 along with siRNA targeting Sp1 or were treated with an NF-κB inhibitor (BAY11-7082). The cells were then infected with PRRSV rBB/wt, rR43A, rK44A, r192-5A, and rG214-3A at an MOI of 1. CD83 promoter activation by the mutant viruses rR43A, rK44A, rP192-5A, and rG214-3A was similar to the activation induced by rBB/wt treated with Sp1-targeted siRNA or the NF-κB inhibitor (Fig. 10E and F). This result indicates that the rR43A, rK44A, rP192-5A, and rG214-3A mutant viruses affected Sp1- and NF-κB-dependent transcriptional activities in transient-transfection assays.
DISCUSSION
Dendritic cells are the most potent antigen-presenting cells (APCs) of the immune system. The maturation of DCs is accompanied by physiological and phenotypic changes that ultimately result in the activation of T cells and B cells. Taking advantage of the central role played by DCs, some pathogens avoid activation of the immune system and override host defense mechanisms by influencing DC function (41, 42). HSV-1 induces the degradation of CD83, impairing the ability of infected DCs to stimulate allogeneic T cells in mixed-leukocyte reaction mixtures (38, 39, 43). Human cytomegalovirus (HCMV) decreases the expression of CD83 late in fibroblast infection and inhibits the maturation of MoDCs. Transforming growth factor beta (TGF-β), a soluble factor secreted by late HCMV-infected fibroblasts, is one of the mediators responsible for regulating CD83 expression and the stimulatory function of DCs (36, 38, 44). The hijacking of DC function by HIV-1 enables virus diffusion. Early-stage DCs capture HIV-1 particles, migrate to secondary lymphoid tissues, and then transmit the virus to T cells (45, 46). PRRSV has evolved mechanisms that specifically target DCs to circumvent the host's immune responses, making it easier for the virus to persist in the host. PRRSV productively infects MoDCs and impairs their normal antigen presentation ability by inducing cell death, downregulating the expression of major histocompatibility complex (MHC) class I and class II molecules, CD11b/c, and CD14, and inducing minimal Th1 cytokines (47). PRRSV infection inhibits the induction of IFN-α in MoDCs by as yet undefined posttranscriptional mechanisms (48), and it also affects the expression of swine leukocyte antigen DR (SLA-DR) and CD80/86 (49). In this study, our results show that PRRSV infection strongly stimulates the secretion of immunosuppressive CD83 in porcine MoDCs in a time- and dose-dependent manner. Furthermore, the N and nsp10 proteins play important roles in enhancing CD83 production via the NF-κB and Sp1 signaling pathways (Fig. 11).
FIG 11.
PRRSV-mediated increase of CD83 production in MoDCs. The model shows a possible mechanism by which PRRSV induces CD83 production in MoDCs. Following viral infection, the intracellular accumulation of the viral proteins N and nsp10 activates Sp1 and NF-κB, allowing them to bind to the promoter of the CD83 gene and induce CD83 expression. The net results are increases in CD83 mRNA levels and in membrane-bound and secreted CD83 levels. Mutations in N (rR43A and rK44A) and nsp10 (rP192-5A and rG214-3A) abolish this response.
CD83 was first described for Langerhans cells and activated lymphocytes. It is a conserved member of the immunoglobulin superfamily and is also known as one of the most characteristic cell surface markers for fully mature DCs in peripheral circulation (31, 50, 51). Although CD83 plays essential roles in both the central and peripheral immune systems, the underlying mechanisms by which CD83 regulates immune responses remain enigmatic. Two different protein isoforms of CD83 have been reported in vivo: a membrane-bound form (mCD83) and a soluble form (sCD83). Enhancement of CD83 expression inhibits the surface expression of other molecules and influences DC function by dramatically reducing T cell activation, underlining and confirming the critical function of mCD83 (51). sCD83 is a potent inhibitor of anti-CD3-induced proliferation of T cells and production of IL-2 and IFN-γ by PBMCs, but the molecular mechanism by which CD83 suppresses the immune system is not understood (52–54). Virus-mediated suppression of CD83 expression reduces the stimulatory capacity of human DCs (55).
In the study presented here, we found that PRRSV stimulates secretion of CD83 and increases CD83 mRNA levels. In order to identify viral proteins with the ability to stimulate the CD83 promoter, we constructed a series of recombinant plasmids by using an expression vector. However, efficient expression was obtained only from plasmids encoding the GP5, N, nsp1, nsp2, nsp4, nsp7, and nsp9 to -12 proteins. Of these, only N, nsp1, and nsp10 enhance CD83 promoter activity. Since N and nsp10 play fundamental roles in immune regulation and pathogenesis (19–23), we examined their genes more closely. Using domain-specific mutations, we determined that the 36-37, 43-47, and 112-123 domains in the N protein are important for the upregulation of CD83 gene promoter activity. Four amino acid residues (S36, R43, and K44 in the N protein and V112 in nsp10) were identified as essential sites for CD83 promoter activation. Two mutant viruses with mutations in the N protein (R43A and K44A) and two revertant viruses, rBB/R43A(R) and rBB/K44A(R), were rescued using cDNA infectious clones. Analysis indicated that the R43 and K44 sites in the N protein are crucial for regulating CD83 expression. In addition, we demonstrated that residues 192 to 196 (P192-5 domain) and 214 to 216 (G214-3 domain) of nsp10 play important roles in the stimulation of CD83 promoter activity and that the P192, G194–196, and G214–216 amino acids are essential for this function. Two mutant viruses with mutations in nsp10 (P192-5A and G214-3A) were rescued using reverse genetics, confirming that the amino acids at these positions are necessary for CD83 transcription.
TNF-α induces the human CD83 promoter (32, 40), which is strictly dependent on a functional NF-κB element. Latent membrane protein 1 (LMP1) of Epstein-Barr virus induces CD83 surface expression on B cells via the NF-κB pathway (56). Using an online promoter scanning utility (http://www-bimas.cit.nih.gov/molbio/proscan/), we found that putative binding motifs for NF-κB (TTGAAATTCCCCC) and Sp1 (AGTCAG) are also present in the porcine CD83 promoter, similar to the arrangement in the human and mouse promoters (57). AP-1, Sp1, and NF-κB are stimulated by PRRSV infection (58–60). Sp1 is a regulator of signal transduction and is critically regulated by posttranslational modifications (PTMs), including phosphorylation, methylation, and glycosylation (61). It plays important roles in regulating cell survival, growth, and tumor development through its ability to bind to GC-rich sequences associated with many cellular and viral genes (62). Sp1 is a ubiquitous transcription factor that mediates gene expression and responds to various extra- and intracellular signals to modulate gene expression (63, 64). Surprisingly, we found that an NF-κB inhibitor and Sp1 siRNA reduce the PRRSV-mediated activation of the CD83 promoter and that PRRSV infection significantly stimulates expression of Sp1 and NF-κB mRNAs in MoDCs. This suggests that the Sp1 and NF-κB signaling pathways are activated in MoDCs by PRRSV infection. In addition, the rR43A, rK44A, rP192-5A, and rG214-3A mutant viruses exhibited altered Sp1-dependent transcriptional activity in transient-transfection assays. Sp1 mRNA levels in cells infected by these mutants did not recover to wild-type levels. CD83 activation by these mutants did not decrease in response to treatment with siRNA targeted to Sp1 or with the NF-κB inhibitor. Sp1 and NF-κB therefore appear to be positive modulators of the PRRSV-triggered CD83 expression signaling pathway, probably mediated by binding via domains defined by amino acids R43 and K44 in the N protein and via the P192-5 and G214-3 domains in nsp10.
PRRSV can grow in primary pulmonary alveolar macrophages (PAMs), MoDCs, and the Marc-145 cell line (65), but not in HEK293 cells, in vitro. To examine the response of the porcine CD83 promoter to viral genes by use of a dual-luciferase reporter assay, the three cell types were transfected with the plasmids pCD83-luc and pRL-TK together with the plasmids containing viral genes. PAMs and MoDCs could not be transfected effectively, and we detected no fluorescence signal from these cells (data not shown). Viral gene stimulation of CD83 promoter activity in Marc-145 cells was lower than that in HEK293T cells, probably because 293T and Marc-145 cells differ in transfection efficiency, but the results are nonetheless consistent in these cells.
PRRSV nsp1α inhibits the LUBAC-mediated ubiquitination of NEMO, thereby suppressing NF-κB signal transduction (66). The nsp1 subunits are synthesized early after PRRSV infection, presumably because they are needed to inhibit innate cytokines, such as TNF-α and type I interferon (60). We found that CD83 can also be induced by PRRSV nsp1α. The role that this plays during PRRSV infection is potentially interesting. HSV-1 can disarm myeloid DCs by decreasing CD83 expression (38). CD83 is able to increase prostaglandin E2 (PGE2) expression in monocytes, which in turn suppress T cell proliferation and IL-2 and IFN-γ production by T cells. Antigenic stimulation of T lymphocytes by immature or CD83-treated APCs can result in the inhibition of T cell proliferation (54). Therefore, we hypothesize that interactions between APCs and N, nsp10, and/or virally induced CD83 may alter APC functions and influence PRRSV-specific T cell proliferation and differentiation.
In summary, we have shown that PRRSV infection induces CD83 expression in porcine MoDCs through the NF-κB and Sp1 signaling pathways. Viral N, nsp10, and nsp1 have the ability to stimulate CD83 promoter activity. Amino acids R43 and K44 in the N protein and the P192-5 and G214-3 domains in nsp10 play important roles in CD83 induction. These results offer new insights into the molecular mechanism underlying immune suppression by PRRSV.
MATERIALS AND METHODS
Cells and virus.
Marc-145 cells, derived from African green monkey kidney cells, were incubated in Dulbecco's modified essential medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco) containing 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C with 5% CO2. HEK293T cells were cultured in DMEM with the same supplements as those used for Marc-145 cells.
HP-PRRSV isolate BB0907 (GenBank accession number HQ315835) was passaged 10 times through Marc-145 cells and is designated BB in this study. The virus was isolated in 2009 in Guangxi Province, China, and has been maintained as a stock in our laboratory. Classical PRRSV (C-PRRSV) strain S1 (GenBank accession number AF090173) was isolated from pigs with clinical signs of PRRS in Jiangsu Province in 1997. The low-pathogenicity PRRSV (LP-PRRSV) isolate NT0801 (GenBank accession number HQ315836) was isolated in Jiangsu Province, China, in 2008. Recombinant viruses (rBB/wt, rR43A, rK44A, rP192-5A, and rG214-3A) were rescued from infectious clone pCMV-BB0907 (constructed in our laboratory) and were propagated in Marc-145 cells upon recovery. UV-inactivated PRRSV was generated by irradiating the HP-PRRSV isolate BB0907 with short-wave UV light (254 nm) for 1 h.
Plasmid construction.
Genes encoding PRRSV nsp1, nsp1α, nsp1β, nsp2, nsp3, nsp4, nsp5, nsp6, nsp7, nsp8, nsp9, nsp10, nsp11, nsp12, N, M, GP2, GP3, GP4, and GP5 were amplified from HP-PRRSV strain BB0907 by reverse transcription-PCR (RT-PCR) using the primers listed in Table 1. The PCR fragments were ligated into the pVAX1 vector (Invitrogen), generating an N-terminal Flag tag fusion.
TABLE 1.
Primers used to generate individual viral protein-expressing plasmids
| Primer | Sequence (5′–3′)a |
|---|---|
| GP2F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGAAATGGGGTCTATGCAAAGCCT |
| GP2R | TATCTCGAGTCACCATGAGTTCAAAAGAAAAGTT |
| GP3F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGCTAATAGCTGTACATTCCTCC |
| GP3R | TATCTCGAGCTATCGCCGTGCGGCACTGAGAACT |
| GP4F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGCTGCGCCCTTTCTTTTCCTCT |
| GP4R | TATCTCGAGTCAAATTGCCAGTAGGATGGCAAAA |
| GP5F | TAAGGTACCATGGATTACAAGGATGACGACGATAAGATGTTGGGGAAGTGCTTGACCGCGT |
| GP5R | CACTCTAGACTAGAGACGACCCCATTGTTCCGCT |
| MF | CCGCTCGAGATGGATTACAAGGATGACGACGATAAGATGGGGTCGTCTCTAGACGACTTCT |
| MR | CGCGCGGCCGCTTATTTGGCATATTTAACAAGGTTT |
| NF | GCGAAGCTTATGGATTACAAGGATGACGACGATAAGCCAAATAATAACGGCAAGCAGC |
| NR | GCGGAATTCTCATGCTGAGGGTGATGCTGTGGCG |
| Nsp1F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGTCTGGGATACTTGATCGGTGCA |
| Nsp1R | TATCTCGAGGTACCACTTATGACTGCCAAACCGG |
| Nsp1αF | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGTCTGGGATACTTGATCGGTGCA |
| Nsp1αR | TATCTCGAGCTGCGGGAGCGGCAGGTTGGTCAAC |
| Nsp1βF | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGAGGCCCAAACCTGAGGACTTTTGCC |
| Nsp1βR | TATCTCGAGGTACCACTTATGACTGCCAAACCGG |
| Nsp2F | TAAGGTACCATGGATTACAAGGATGACGACGATAAGGGTGCTGGAAAGAGAGCAAGAAGAA |
| Nsp2R | CACTCTAGAGCCCAGTAACCTGCCAAGAATGGCA |
| Nsp3F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGGGGCACGCTACATCTGGCACTTTT |
| Nsp3R | TATCTCGAGCTCAAGGAGGGACCCGAGCTGAGAC |
| Nsp4F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGGCGCTTTCAGAACTCAAAAGCCCT |
| Nsp4R | TATCTCGAGTTCCAGTTCGGGTTTGGCAGCAAGC |
| Nsp5F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGGAGGCCTTTCCACAGTTCAACTTC |
| Nsp5R | CACTCTAGACTCGGCAAAGTATCGCAAGAAGAAA |
| Nsp6F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGGAAAGTTGAGGGAAGGGGTGTCGC |
| Nsp6R | CACTCTAGACTCATGACTCATCCCGCAGGATTGCGACACCCCTT |
| Nsp7F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGTCGCTGACTGGTGCCCTCGCCATGA |
| Nsp7R | CACTCTAGATTCCCACTGAGCTCTTCTATTCTCG |
| Nsp8F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGCCGCCAAGCTTTCCGTGGAGCAAG |
| Nsp8R | CACTCTAGAGCAGTTTAAACACTGCTCCTTAGTC |
| Nsp9F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGTTTAAACTGCTAGCCGCCAGCGGC |
| Nsp9R | TATCTCGAGCTCATGATTGGACCTGAGTTTTTCC |
| Nsp10F | CCGCTCGAGATGGATTACAAGGATGACGACGATAAGGGGAAGAAGTCCAGAATGTGCGGGT |
| Nsp10R | CGCGCGGCCGCTCATTCCAGGTCTGCGCAAAT |
| Nsp11F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGGGTCGAGCTCCCCGCTCCCCAAGG |
| Nsp11R | TATCTCGAGTTTCAAGTTGAAAATAGGCCGTCTTG |
| Nsp12F | ACTAAGCTTATGGATTACAAGGATGACGACGATAAGGGCCGCCATTTTACCTGGTATCAAC |
| Nsp12R | TATCTCGAGATTCAGGCCTAAAGTTGGTTCAATG |
The genomic positions of the primers were based on the sequence under GenBank accession number HQ315835. The restriction enzyme sites used for cloning are underlined. The N-terminal Flag tag sequence in the “F” primers is shown in bold.
A truncated version of the N protein was subcloned from the pVAX-NP(wt) plasmid (including the Flag tag at the N terminus) and designated pVAX-NP(1-112) (ORF7t). In addition, truncated versions of nsp10 were subcloned from pVAX-Nsp10 (including the Flag tag at the N terminus) and designated pVAX-Nsp10(1-1023), pVAX-Nsp10(1-723), pVAX-Nsp10(451-1323), pVAX-Nsp10(549-1323), and pVAX-Nsp10(649-1323). Alanine substitution mutations in the N protein and nsp10 genes were generated using QuikChange II XL site-directed mutagenesis kits (Stratagene).
The porcine CD83 promoter sequence (GenBank accession number XM_001928655), spanning coordinates −800 to +50 relative to the translation initiation site (+1), was amplified from porcine MoDC genomic DNA, and the PCR product was verified by sequencing. The luciferase reporter plasmid pCD83-Luc was constructed by cloning the CD83 promoter fragment into the pGL3-Basic vector (Promega), which contains a modified firefly luciferase gene. The pRL-TK plasmid (Promega), containing the Renilla luciferase reporter, served as an internal control. All primers are shown in Table 2.
TABLE 2.
Primers used for construction of the CD83 promoter, N protein, and nsp10 mutants
| Primer | Sequence (5′–3′)a |
|---|---|
| pCD83F | TAAGGTACCCTCATATCTTTGATTTAATAGGAGA |
| pCD83R | ACTAAGCTTAGGGCAAGTCCACAGCCTCCGAACA |
| ORF7SF | GCGAAGCTTCACCATGGATTACAAGGATGACGACGATAAGATGGGGTCGTCTCTAG |
| ORF7SR | GCGGAATTCCATCATGCTGAGGGTGATGCT |
| ORF7tSR | GCGGAATTCCATCACACAGTATGATGCGTCGGCAAACT |
| C23ASF | CAGCCAGTCAATCAGCTGGCCCAAATGCTGGGTAAGATC |
| C23ASR | GATCTTACCCAGCATTTGGGCCAGCTGATTGACTGGCTG |
| C75ASF | CCTAGTGAGCGGCAATTGGCTCTGTCGTCGATCCAGACT |
| C75ASR | AGTCTGGATCGACGACAGAGCCAATTGCCGCTCACTAGG |
| C90ASF | AATCAGGGCGCTGGAACTGCTACCCTGTCAGATTCAGGG |
| C90ASR | CCCTGAATCTGACAGGGTAGCAGTTCCAGCGCCCTGATT |
| K10-4ASF | GGCAAGCAGCAAGCGGCAGCGGCGGGGAATGGCCAGCCA |
| K10-4ASR | TGGCTGGCCATTCCCCGCCGCTGCCGCTTGCTGCTTGCC |
| Q33-5ASF | GTAAGATCATCGCTCAAGCAGCCGCGGCCGCAGGCAAGGGACCGGGG |
| Q33-5ASR | CCCCGGTCCCTTGCCTCTGGCCGCGGCTGCTTGAGCGATGATCTTAC |
| K43-5ASF | GGCAAGGGACCGGGGGCGGCGGCTGCGGCGACAAAACCGGA |
| K43-5ASR | TCCGGTTTTGTCGCCGCAGCCGCCGCCCCCGGTCCCTTGCC |
| P56-4ASF | GGAGAAGCCCCATTTCGCTGCAGCGGCTGAAGATGACGTCAG |
| P56-4ASR | CTGACGTCATCTTCAGCCGCTGCAGCGAAATGGGGCTTCTCC |
| E60-5ASF | CATTTCCCTCTAGCGACTGCAGCTGCCGCCGCGCATCACTTTACCCCT |
| E60-5ASR | AGGGGTAAAGTGATGCGCGGCGGCAGCTGCAGTCGCTAGAGGGAAATG |
| H65-4ASF | ACTGAAGATGACGTCAGGGCTGCCGCTGCCCCTAGTGAGCGGCAATTG |
| H65-4ASR | CAATTGCCGCTCACTAGGGGCAGCGGCAGCCCTGACGTCATCTTCAGT |
| V112-4ASF | TTGCCGACGCATCATACTGCGGCTGCGGCCCGCGCCACAGCATCACCCT |
| V112-4ASR | AGGGTGATGCTGTGGCGCGGGCCGCAGCCGCAGTATGATGCGTCGGCAA |
| Q33ASF | AAGATCATCGCTCAAGCAAACCAGTCCAGAGG |
| Q33ASR | CCTCTGGACTGGTTTGCTTGAGCGATGATCTT |
| N34ASF | ATCATCGCTCAACAAGCCCAGTCCAGAGGCAA |
| N34ASR | TTGCCTCTGGACTGGGCTTGTTGAGCGATGAT |
| Q35ASF | ATCGCTCAACAAAACGCGTCCAGAGGCAAGGG |
| Q35ASR | CCCTTGCCTCTGGACGCGTTTTGTTGAGCGAT |
| S36ASF | GCTCAACAAAACCAGGCCAGAGGCAAGGGAC |
| S36ASR | GTCCCTTGCCTCTGGCCTGGTTTTGTTGAGC |
| R37ASF | GCTCAACAAAACCAGTCCGCAGGCAAGGGACCGGG |
| R37ASR | CCCCGGTCCCTTGCCTGCGGACTGGTTTTGTTGAGC |
| 43ASF | GGCAAGGGACCGGGGGCGAAAAATAGGAAGACA |
| 43ASR | TGTCTTCCTATTTTTCGCCCCCGGTCCCTTGCC |
| 44ASF | AAGGGACCGGGGAAGGCAAATAGGAAGACAAAA |
| 44ASR | TTT TGT CTT CCT ATT TGC CTT CCC CGG TCC CTT |
| 45ASF | GGACCGGGGAAGAAAGCTAGGAAGACAAAACCG |
| 45ASR | CGGTTTTGTCTTCCTAGCTTTCTTCCCCGGTCC |
| 46ASF | CCGGGGAAGAAAAATGCGAAGACAAAACCGGAG |
| 46ASR | CTCCGGTTTTGTCTTCGCATTTTTCTTCCCCGG |
| 47ASF | GGGAAGAAAAATAGGGCGACAAAACCGGAGAAG |
| 47ASR | CTTCTCCGGTTTTGTCGCCCTATTTTTCTTCCC |
| H65ASF | GAAGATGACGTCAGGGCTCACTTTACCCCTAG |
| H65ASR | CTAGGGGTAAAGTGAGCCCTGACGTCATCTTC |
| H66ASF | GATGACGTCAGGCATGCCTTTACCCCTAGTGA |
| H66ASR | TCACTAGGGGTAAAGGCATGCCTGACGTCATC |
| F67ASF | GACGTCAGGCATCACGCTACCCCTAGTGAGCG |
| F67ASR | CGCTCACTAGGGGTAGCGTGATGCCTGACGTC |
| T68ASF | GTCAGGCATCACTTTGCCCCTAGTGAGCGGC |
| T68ASR | GCCGCTCACTAGGGGCAAAGTGATGCCTGAC |
| V112ASF | CGACGCATCATACTGCGCGTCTGATCCGCGC |
| V112ASR | GCGCGGATCAGACGCGCAGTATGATGCGTCG |
| R113ASF | ACGCATCATACTGTGGCTCTGATCCGCGCCAC |
| R113ASR | GTGGCGCGGATCAGAGCCACAGTATGATGCGT |
| L114ASF | CATCATACTGTGCGTGCGATCCGCGCCACAGC |
| L114ASR | GCTGTGGCGCGGATCGCACGCACAGTATGATG |
| I115ASF | CATACTGTGCGTCTGGCCCGCGCCACAGCATC |
| I115ASR | GATGCTGTGGCGCGGGCCAGACGCACAGTATG |
| Nsp10F | CCGCTCGAGATGGGGAAGAAGTCCAGAATGTGCGGGT |
| Nsp10R | CGCGCGGCCGCTCACTTATCGTCGTCATCCTTGTAATCTTCCAGGTCTGCGCAAAT |
| Nsp10-1023R | CGCGCGGCCGCTCACTTATCGTCGTCATCCTTGTAATCCACATCAAATGTGGCGCC |
| Nsp10-723R | CGCGCGGCCGCTCACTTATCGTCGTCATCCTTGTAATCTTTACTGAGAAGCCTCAA |
| Nsp10-451F | CCGCTCGAGATGCCCGGTGCTGGGAAAACACACTGGC |
| Nsp10-549F | CCGCTCGAGATGTAGGGCTTTGGGGACGTGCCGGTTT |
| Nsp10-649F | CCGCTCGAGATGTGTCCTGGCAAGAACTCCTTCCTGG |
| R182-5ASF | ATGCTCGACATGATTGCGGCTGCGGCGGCGTGCCGGTTTAACGTT |
| R182-5ASR | AACGTTAAACCGGCACGCCGCCGCAGCCGCAATCATGTCGAGCAT |
| C187-5ASF | AGGGCTTTGGGGACGGCCGCGGCTGCCGCTCCAGCAGGTACAACG |
| C187-5ASR | CGTTGTACCTGCTGGAGCGGCAGCCGCGGCCGTCCCCAAAGCCCT |
| P192-5ASF | TGCCGGTTTAACGTTGCAGCAGCTGCAGCGCTGCAATTCCCTGCC |
| P192-5ASR | GGCAGGGAATTGCAGCGCTGCAGCTGCTGCAACGTTAAACCGGCA |
| L197-5ASF | CCAGCAGGTACAACGGCGGCAGCCGCTGCCCCCTCCCGTACCGGC |
| L197-5ASR | GCCGGTACGGGAGGGGGCAGCGGCTGCCGCCGTTGTACCTGCTGG |
| P202-5ASF | CTGCAATTCCCTGCCGCCGCCGCTGCCGCCCCATGGGTTCGCATC |
| P202-5ASR | GATGCGAACCCATGGGGCGGCAGCGGCGGCGGCAGGGAATTGCA |
| P207-5ASF | CCCTCCCGTACCGGCGCAGCGGCTGCCGCCTTGGCCGGCGGTTGG |
| P207-5ASR | CCAACCGCCGGCCAAGGCGGCAGCCGCTGCGCCGGTACGGGAGGG |
| L212-5ASF | CCATGGGTTCGCATCGCGGCCGCCGCTGCGTGTCCTGGCAAGAAC |
| L212-5ASR | GTTCTTGCCAGGACACGCAGCGGCGGCCGCGATGCGAACCCATGG |
| C217-5ASF | TTGGCCGGCGGTTGGGCTGCTGCCGCGGCCTCCTTCCTGGATGAA |
| C217-5ASR | TTCATCCAGGAAGGAGGCCGCGGCAGCAGCCCAACCGCCGGCCAA |
| P192ASF | TGCCGGTTTAACGTTGCAGCAGGTACAACGCTG |
| P192ASR | CAGCGTTGTACCTGCTGCAACGTTAAACCGGCA |
| A193ASF | CGGTTTAACGTTCCAGCAGGTACAACGCTGCAA |
| A193ASR | TTGCAGCGTTGTACCTGCTGGAACGTTAAACCG |
| G194ASF | TTTAACGTTCCAGCAGCTACAACGCTGCAATTC |
| G194ASR | GAATTGCAGCGTTGTAGCTGCTGGAACGTTAAA |
| T195ASF | AACGTTCCAGCAGGTGCAACGCTGCAATTCCCT |
| T195ASR | AGGGAATTGCAGCGTTGCACCTGCTGGAACGTT |
| T196ASF | TTCCAGCAGGTACAGCGCTGCAATTCCCTGCC |
| T196ASR | GGCAGGGAATTGCAGCGCTGTACCTGCTGGAAC |
| L212ASF | CCATGGGTTCGCATCGCGGCCGGCGGTTGGTGT |
| L212ASR | ACACCAACCGCCGGCCGCGATGCGAACCCATGG |
| A213ASF | TGGGTTCGCATCTTGGCCGGCGGTTGGTGTCCT |
| A213ASR | AGGACACCAACCGCCGGCCAAGATGCGAACCCA |
| G214ASF | GTTCGCATCTTGGCCGCCGGTTGGTGTCCTGGC |
| G214ASR | GCCAGGACACCAACCGGCGGCCAAGATGCGAAC |
| G215ASF | CGCATCTTGGCCGGCGCTTGGTGTCCTGGCAAG |
| G215ASR | CTTGCCAGGACACCAAGCGCCGGCCAAGATGCG |
| W216ASF | ATCTTGGCCGGCGGTGCGTGTCCTGGCAAGAAC |
| W216ASR | GTTCTTGCCAGGACACGCACCGCCGGCCAAGAT |
The genomic positions of the primers were based on the sequence under GenBank accession number XM_001928655. The restriction enzyme sites used for cloning are underlined. The N- or C-terminal Flag tag sequence in the “R” primers is indicated by double underlining. Locations of mutations are indicated in bold.
Generation of MoDCs.
Peripheral blood mononuclear cells (PBMCs) that were free of PRRSV, porcine circovirus type 2, classical swine fever virus, and pseudorabies virus were isolated from healthy piglets by sedimentation with Lymphoprep (Nycomed Pharma AS, Oslo, Norway) and cultured in RPMI 1640 medium (BioWhittaker, Verviers, Belgium) supplemented with 10 mM HEPES (pH 7.5; Sigma-Aldrich, Deisenhofen, Germany), 2 mM l-glutamine (Cambrex BioScience, Verviers, Belgium), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma). The cells were seeded into wells of a six-well plate at a concentration of 5 × 107 cells per well and incubated at 37°C in a 5% CO2 incubator for 5 h. Adherent cells were washed 4 times with RPMI 1640 medium and then cultured in RPMI 1640 medium supplemented with 10 mM HEPES, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 800 U/ml granulocyte-macrophage colony-stimulating factor (rpGM-CSF; R&D Systems), and 250 U/ml interleukin-4 (rpIL-4; R&D Systems). The monocytes were cultured for 5 additional days to allow differentiation of the porcine MoDCs (67). To confirm differentiation, MoDCs were tested for the expression of CD86 by use of mouse anti-human CD86–phycoerythrin (PE) (BD Bioscience). This antibody cross-reacts with pig CD86.
Sandwich ELISA.
sCD83 levels were determined using a sandwich ELISA (36, 39, 55, 68). Ninety-six-well plates were precoated with a polyclonal rabbit antibody to the CD83 C terminus (3 μg/ml; Abcam, Cambridge, United Kingdom) at 4°C overnight and then blocked with 10% nonfat dry milk in phosphate-buffered saline (PBS) prior to sample application. Following overnight incubation at 4°C, goat anti-CD83(K14) antibody (5 μg/ml; Santa Cruz Biotech, CA, USA) was added, and plates were incubated for 2 h at 37°C. Anti-goat–horseradish peroxidase (HRP) (1:1,000 dilution; Santa Cruz Biotech, CA, USA) was then added in 1% bovine serum albumin (BSA) in PBS and incubated for 1 h at 37°C. Between steps, the plates were thoroughly washed with 0.1% Tween 20 in PBS. The ELISA results were collected at 450 nm by using an ELISA plate reader (Bio-Tek).
MoDCs were resuspended in complete medium, transferred to 12-well plates at a concentration of 1 × 106 cells ml−1, and then inoculated with PRRSV or UV-inactivated PRRSV at a multiplicity of infection (MOI) of 2 for 6, 12, 24, 36, or 48 h. MoDCs were also treated with TNF-α (50 ng/ml; Hoffman-La Roche, Basel, Switzerland) or Marc-145 cell lysate (the same volume as that of PRRSV) as positive and negative controls, respectively. Cell supernatants were harvested for measurement of sCD83 protein levels by use of a sandwich ELISA.
FACS analysis.
Changes in the expression of CD83 at the cell surface were analyzed by FACS analysis. Cells were washed with 0.1% BSA, 0.02% NaN3 in PBS (40, 69, 70) and then incubated with goat anti-CD83(K14) antibody (1:100 dilution; Santa Cruz Biotech, CA, USA) followed by donkey anti-goat–fluorescein isothiocyanate (FITC) (1:500 dilution; Santa Cruz Biotech, CA, USA). Relative CD83 surface expression was evaluated by flow cytometry (BD FACSCanto II), and data were analyzed using FlowJo, version 7.6.1.
Luciferase reporter assay.
Marc-145 cells were seeded in 12-well plates and transfected with pCD83-luc or pGL3 (negative control), together with 0.1 μg pRL-TK, by use of Lipofectamine 3000 reagent (Invitrogen). Subsequently, cells were treated with PRRSV, recombinant plasmids, or TNF-α (50 ng/ml; Hoffman-La Roche, Basel, Switzerland). The cells were lysed in passive lysis buffer (Promega), and luciferase activity was measured with a dual-luciferase assay system (Promega) following the manufacturer's instructions. Results are expressed as fold changes of luciferase activities relative to those of mock-treated cells. All assays were repeated at least three times, with each experiment performed in triplicate.
Inhibition of signal transduction pathways.
Marc-145 cells or HEK293T cells seeded onto 12-well plates were pretreated with an NF-κB inhibitor (BAY11-7082; Enzo Life Sciences) at 1, 5, or 10 mM for 12 h. Cells were then transfected by use of Lipofectamine 3000 reagent (Invitrogen) with pCD83-luc plus pRL-TK and a PRRSV nsp10 vector or pVAX1 vector (1 μg for each plasmid) in the presence or absence of the inhibitor for 48 h. Cells were then harvested for luciferase analysis.
Marc-145 cells were transfected by use of Lipofectamine 3000 reagent (Invitrogen) with pCD83-luc and then pretreated with DMSO or the NF-κB inhibitor (BAY11-7082) at 1, 5, or 10 mM. After 12 h, cells were infected with PRRSV at an MOI of 1 in the presence of inhibitors for 36 h. Cells were then harvested for luciferase analysis.
RNA interference.
Small interfering RNAs (siRNAs) targeting Sp1 protein position 633 (si-Sp1-633), 820 (si-Sp1-820), or 1595 (si-Sp1-1595) and a negative-control siRNA (NC) were designed and synthesized by Invitrogen (Shanghai, China). In gene-silencing experiments, HEK293T or Marc-145 cells were transfected with 50 nM Sp1 siRNA or control siRNA by use of Lipofectamine 3000 (Invitrogen, CA, USA). Twenty-four hours later, cells were collected and analyzed by quantitative real-time reverse transcription-PCR (qRT-PCR) to determine Sp1 mRNA levels. Primer sequences are shown in Table 3.
TABLE 3.
Sequences of siRNAs targeting Sp1 and primers used for quantitative real-time PCR
| siRNA or primer name | Sequence (5′–3′) |
|---|---|
| Sp1 siRNA_633 | CCAACAGAUCAUCACAAAUTT |
| AUUUGUGAUGAUCUGUUGGTT | |
| Sp1 siRNA_820 | GCAGCUACCUUGACUCCUATT |
| UAGGAGUCAAGGUAGCUGCTT | |
| Sp1 siRNA_1595 | CCAUUAACCUCAGUGCAUUTT |
| AAUGCACUGAGGUUAAUGGTT | |
| NF-κB-F | GCCTCCACAAGGCAGCAAATA |
| NF-κB-R | CACCACTGGTCAGAGACTCGGTAA |
| β-Actin-F | TGGCACCCAGCACAATGAA |
| β-Actin-R | CTAAGTCATAGTCCGCCTAGAAGCA |
| Sp1-F | GCTATGCCAAACCTACTCC |
| Sp1-R | CTGTAGCCCACTGACCT |
Marc-145 cells or HEK293T cells at 80% confluence were transfected with 50 nM Sp1 siRNA (si-Sp1-633, si-Sp1-820, or si-Sp1-1595) and negative-control siRNA (NC) by use of Lipofectamine 3000 transfection reagent (Invitrogen) for 12 h. Cells were then transfected with pCD83-luc and a PRRSV nsp10 vector or pVAX1 vector (1 μg for each plasmid) for 36 h and then harvested for luciferase analysis.
Marc-145 cells at 80% confluence were transfected with 50 nM Sp1 siRNA (si-Sp1-633, si-Sp1-820, or si-Sp1-1595) and negative-control siRNA (NC) by use of Lipofectamine 3000 transfection reagent (Invitrogen) for 12 h. Cells were then transfected with pCD83-luc. Twelve hours later, cells were infected with PRRSV at an MOI of 1. Cells were harvested after 36 h for luciferase analysis.
Western blotting.
Marc-145 or HEK293T cells were lysed, cellular proteins were separated by 12% SDS-PAGE, and the resolved proteins were electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). Membranes were blocked for 2 h in Tris-buffered saline containing 10% nonfat dry milk and 0.1% Tween 20 and then probed at 37°C for 1 h with a monoclonal antibody (MAb) for N (1:100; prepared in our laboratory) or mouse anti-FLAG (1:800; Cell Signaling Technology) as the primary antibody. After rinsing with PBS, the membrane was treated with goat anti-mouse IgG–HRP (Boster, China) as the secondary antibody. Bound proteins were visualized with a Tanon 5200 chemiluminescence imaging system (Tanon, China). β-Actin was detected with an anti-β-actin mouse monoclonal antibody (1:1,000; Santa Cruz Biotech, CA, USA) to demonstrate equal protein sample loading.
RNA isolation and real-time PCR.
Total RNA was prepared from MoDCs by use of a Qiagen RNeasy kit following the manufacturer's instructions. Recovered RNA was treated with DNase I, and 1 μg was used for cDNA synthesis by reverse transcription, using an RT-PCR kit (TaKaRa). PCR was performed using SYBR Premix Ex Taq (TaKaRa) according to the manufacturer's protocol. All samples were analyzed in triplicate on the same plate, and β-actin was utilized as the reference gene. Gene expression levels were calculated relative to the β-actin gene expression level, and fold changes were calculated using the 2−ΔΔCT method. Data are presented as fold changes in gene expression, normalized to β-actin and relative to that of the mock-infected control. Primer sequences are shown in Table 3.
Construction of infectious cDNA clones of PRRSV.
The full-length PRRSV genome was amplified using the five primer pairs listed in Table 4. A recombinant plasmid (pCMV-BB) containing a full-length cDNA copy of the virus was constructed as shown in Fig. 8 and designated pCMV-BB (71, 72). It was used as the backbone to construct N protein and nsp10 mutants in a PRRSV BB0907 background.
TABLE 4.
Primers used for construction of an infectious cDNA clone of strain BB0907
| Primer | Sequence (5′–3′)a |
|---|---|
| A-BB0907-Fwd | GCGTTAATTAAACCGTCATGACGTATAGGTGTTG |
| A-BB0907-Rev | TGTCTCGAGAATCATCTTTGGGAGAAACC |
| B-BB0907-Fwd | TTCTTAATTAAATGATTCTCGAGACACCGCC |
| B-BB0907-Rev | GTGCTTAAGTTCATTACCACCTGTAACGGAT |
| C1-BB0907-Fwd | GCGTTAATTAAAATGAACTTAAGCACCTATGCC |
| C1-BB0907-Rev | TTGACACAGAGGTAATCGGGTCGCCAGAC |
| C2-BB0907-Fwd | GTCTGGCGACCCGATTACCTCTGTGTCAA |
| C2-BB0907-Rev | CGGGGGAAAATGAAACCTCATGCTGGT |
| D1-BB0907-Fwd | TCGTTAATTAAGTTTCGGGCGCGCCAGAAAGGG |
| D-BB0907-Rev(SwaI) | TTCGGCTTGGGATTTAAATATGCATTTTTTTTTTTTTTTTTTTTT |
| D-BB0907-Rev(SpeI) | CTCACTAGTAACGGCCGCCAGTGTGCTGGAATTCGGCTTGGGATTT |
| XhoI-BB0907-Fwd | GGTACCATGGCCAAACTCGAGGCTTTTGCCGATACC |
| XhoI-BB0907-Rev | GGTATCGGCAAAAGCCTCGAGTTTGGCCATGGTACC |
The genomic positions of the primers were based on the sequence under GenBank accession number HQ315835. The restriction enzyme sites used for cloning are underlined.
Mutagenesis of the N protein was performed on the D fragment of pCMV-BB. The MluI/SpeI fragment was amplified by PCR, using pCMV-BB as the template, and cloned into the pVAX1 vector. The deletion and site-directed mutations were prepared using pVAX-NP as the template. Briefly, oligonucleotide primers were designed such that a foreign insertion sequence was incorporated at the 5′ end or in the middle of the primer, leaving at least 10 nucleotides at the 3′ end that matched the template sequence. PCRs were conducted using circular plasmid DNA as the template, following the instructions provided with the QuikChange mutagenesis kit. The plasmid template was eliminated by DpnI digestion (New England BioLabs), followed by transformation of the digested PCR mixtures into Top10 competent cells (Invitrogen). The pVAX-BB-D (NPm) constructs containing N protein mutations were screened and verified by restriction enzyme mapping and nucleotide sequencing. The MluI/SpeI fragment of pCMV-BB was replaced by the corresponding region derived from the pVAX-BB-D (NPm) plasmid. The final full-length clone containing the mutation was designated pCMV-BB/NPm (Fig. 8A). To construct revertants, D-NPm(R) was first obtained by site-directed mutagenesis using pCMV-BB/NPm as the template. The MluI/SpeI fragment of pCMV-BB was replaced with D-NPm(R), generating pCMV-BB/NPm(R). All full-length mutant clones were verified by nucleotide sequencing. The infectious cDNA plasmid clones were isolated using a QIAprep spin miniprep kit (Qiagen). All recombinant viruses and amino acid substitution primers are listed in Table 2.
Mutagenesis of nsp10 was performed on the C fragment of pCMV-BB. Fragments spanning bp 6648 to 8979 and 8951 to 11945 were amplified by using pCMV-BB as the template and cloned into the pEASY-Blunt Simple vector (Beijing TransGen Biotech). The constructs were designated pEASY-BB-C1 and pEASY-BB-C2, respectively. Site-directed mutations were prepared using pEASY-BB-C2 as the template. The intermediate plasmids were screened and verified by restriction enzyme mapping and nucleotide sequencing. Splicing overlap extension PCR was then conducted using pEASY-BB-C1 and pEASY-BB-C2 (Nsp10m) to construct the pEASY-BB-C (Nsp10m) plasmid, containing an nsp10 mutation. The AflII/AscI fragment of pCMV-BB was replaced by the corresponding region derived from the pEASY-BB-C (Nsp10m) plasmid. The final full-length clone containing the mutation was designated pCMV-BB/Nsp10m (Fig. 8D). To construct revertants, C-Nsp10m(R) was first obtained by site-directed mutagenesis using C-Nsp10m as the template. The AflII/AscI fragment of pCMV-BB was replaced with C-Nsp10m(R), generating pCMV-BB/Nsp10m(R).
To rescue chimeric viruses, Marc-145 cells were transfected with the full-length cDNA clone plasmids by use of Lipofectamine 3000 reagent according to the manufacturer's protocol. When about 80% of cells exhibited cytopathic effects (CPEs), the supernatants were harvested and serially passaged four times in Marc-145 cells (65, 71, 72). Finally, the P2 to P5 virus stocks were prepared in the same manner. Rescued viruses were confirmed by whole-genome sequencing. The rescued viruses were designated rBB/wt, rR43A, rK44A, rR43A(R), rK44A(R), rP192-5A, rG214-3A, rP192-5A(R), and rG214-3A(R).
Plaque assay.
PRRSV plaque assays were performed on monolayers of Marc-145 cells seeded in 12-well plates. Cells were inoculated with 10-fold dilutions of stock virus and incubated for 1 h at 37°C. The cells were overlaid with DMEM containing 2% heat-inactivated FBS and 1% low-melting-point agarose (Sigma-Aldrich) and then incubated for about 72 h, until plaques were visible. Plaques were stained with 1% crystal violet in methanol.
Viral growth curves.
To determine viral one-step growth curves, the PRRSV mutants (106 50% tissue culture infective doses [TCID50]) were used to inoculate subconfluent Marc-145 cells in 24-well plates. One hundred microliters of infected cell supernatant was collected from each culture and replaced with the same volume of fresh medium at 12, 24, 36, 48, and 72 h postinfection (hpi). Supernatants were stored at −70°C for virus titration. The virus titers at each time point were determined by TCID50 assay.
Statistical analysis.
The significance of the variability among groups was determined with one-way or two-way analysis of variance (ANOVA), using GraphPad Prism (version 5.0; GraphPad Software). Differences for which the P value was <0.05 were considered to be statistically significant. Differences for which the P value was <0.001 are indicated in figures where appropriate.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation (grants 31230071 and 31672565 for PRRSV immunology), a grant from the Ministry of Agriculture (grant CARS-36) for swine disease control, and the Priority Academic Program Development (PAPD) program of Jiangsu institutions of higher education.
REFERENCES
- 1.Li H, Yang J, Bao D, Hou J, Zhi Y, Yang Y, Ji P, Zhou E, Qiao S, Zhang G. 4 January 2017. Development of an immunochromatographic strip for the detection of antibodies against porcine reproductive and respiratory syndrome virus. J Vet Sci http://www.vetsci.org/journal/view.html?uid=1246&vmd=Full&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu JK, Zhou X, Zhai JQ, Li B, Wei CH, Dai AL, Yang XY, Luo ML. 14 February 2017. Emergence of a novel highly pathogenic porcine reproductive and respiratory syndrome virus in China. Transbound Emerg Dis doi: 10.1111/tbed.12617. [DOI] [PubMed] [Google Scholar]
- 3.Sirisereewan C, Nedumpun T, Kesdangsakonwut S, Woonwong Y, Kedkovid R, Arunorat J, Thanawongnuwech R, Suradhat S. 2017. Positive immunomodulatory effects of heterologous DNA vaccine-modified live vaccine, prime-boost immunization, against the highly-pathogenic PRRSV infection. Vet Immunol Immunopathol 183:7–15. doi: 10.1016/j.vetimm.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Jeong J, Park C, Choi K, Chae C. 2017. Evaluation of the new commercial recombinant chimeric subunit vaccine PRRSFREE in challenge with heterologous types 1 and 2 porcine reproductive and respiratory syndrome virus. Can J Vet Res 81:12–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Mu G, Dang R, Yang Z. 2016. Up-regulation of IL-10 upon PRRSV vaccination impacts on the immune response against CSFV. Vet Microbiol 197:68–71. doi: 10.1016/j.vetmic.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Liu JK, Wei CH, Dai AL, Fan KW, Yang BH, Huang CF, Li XH, Yang XY, Luo ML. 2017. Complete genomic characterization of two European-genotype porcine reproductive and respiratory syndrome virus isolates in Fujian province of China. Arch Virol 162:823–833. doi: 10.1007/s00705-016-3136-9. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, Li Z, Zhang G, Niu J, Zeng X, Sun B, Ma J. 4 January 2017. Genetic diversity and phylogenetic analysis of porcine reproductive and respiratory syndrome virus in southern China from 2007 to 2014. J Vet Sci Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Music N, Gagnon CA. 2010. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim Health Res Rev 11:135–163. doi: 10.1017/S1466252310000034. [DOI] [PubMed] [Google Scholar]
- 9.Rascon-Castelo E, Burgara-Estrella A, Mateu E, Hernandez J. 2015. Immunological features of the non-structural proteins of porcine reproductive and respiratory syndrome virus. Viruses 7:873–886. doi: 10.3390/v7030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loving CL, Osorio FA, Murtaugh MP, Zuckermann FA. 2015. Innate and adaptive immunity against porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 167:1–14. doi: 10.1016/j.vetimm.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler JE, Lager KM, Golde W, Faaberg KS, Sinkora M, Loving C, Zhang YI. 2014. Porcine reproductive and respiratory syndrome (PRRS): an immune dysregulatory pandemic. Immunol Res 59:81–108. doi: 10.1007/s12026-014-8549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correas I, Osorio FA, Steffen D, Pattnaik AK, Vu HL. 2017. Cross reactivity of immune responses to porcine reproductive and respiratory syndrome virus infection. Vaccine 35:782–788. doi: 10.1016/j.vaccine.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Wang R, Ma Z, Xiao Y, Nan Y, Wang Y, Lin S, Zhang YJ. 2017. Porcine reproductive and respiratory syndrome virus antagonizes JAK/STAT3 signaling via nsp5, which induces STAT3 degradation. J Virol 91:e02087-16. doi: 10.1128/JVI.02087-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi P, Liu K, Wei J, Li Y, Li B, Shao D, Wu Z, Shi Y, Tong G, Qiu Y, Ma Z. 2017. Nonstructural protein 4 of porcine reproductive and respiratory syndrome virus modulates cell surface swine leukocyte antigen class I expression by downregulating β2-microglobulin transcription. J Virol 91:e01755-16. doi: 10.1128/JVI.01755-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DU, Yoo SJ, Kwon T, Je SH, Shin JY, Byun JJ, Kim MH, Lyoo YS. 2017. Genetic diversity of ORF 4-6 of type 1 porcine reproductive and respiratory syndrome virus in naturally infected pigs. Vet Microbiol 199:54–61. doi: 10.1016/j.vetmic.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Ke H, Han M, Chen N, Fang W, Yoo D. 2016. Nonstructural protein 11 of porcine reproductive and respiratory syndrome virus suppresses both MAVS and RIG-I expression as one of the mechanisms to antagonize type I interferon production. PLoS One 11:e168314. doi: 10.1371/journal.pone.0168314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overend CC, Cui J, Grubman MJ, Garmendia AE. 2017. The activation of the IFNbeta induction/signaling pathway in porcine alveolar macrophages by porcine reproductive and respiratory syndrome virus is variable. Vet Res Commun 41:15–22. doi: 10.1007/s11259-016-9665-6. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Wang Y, Duan H, Zhang A, Liang C, Gao J, Zhang C, Huang B, Li Q, Li N, Xiao S, Zhou EM. 2015. An intracellularly expressed Nsp9-specific nanobody in MARC-145 cells inhibits porcine reproductive and respiratory syndrome virus replication. Vet Microbiol 181:252–260. doi: 10.1016/j.vetmic.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Kenney SP, Meng X. 2015. An SH3 binding motif within the nucleocapsid protein of porcine reproductive and respiratory syndrome virus interacts with the host cellular signaling proteins STAMI, TXK, Fyn, Hck, and cortactin. Virus Res 204:31–39. doi: 10.1016/j.virusres.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Wongyanin P, Buranapraditkul S, Yoo D, Thanawongnuwech R, Roth JA, Suradhat S. 2012. Role of porcine reproductive and respiratory syndrome virus nucleocapsid protein in induction of interleukin-10 and regulatory T-lymphocytes (Treg). J Gen Virol 93:1236–1246. doi: 10.1099/vir.0.040287-0. [DOI] [PubMed] [Google Scholar]
- 21.Luo R, Fang L, Jiang Y, Jin H, Wang Y, Wang D, Chen H, Xiao S. 2011. Activation of NF-kappaB by nucleocapsid protein of the porcine reproductive and respiratory syndrome virus. Virus Genes 42:76–81. doi: 10.1007/s11262-010-0548-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Shi X, Zhang X, Wang A, Wang L, Yang Y, Deng R, Zhang G. 2017. MicroRNA 373 facilitates the replication of porcine reproductive and respiratory syndrome virus by its negative regulation of type I interferon induction. J Virol 91:e1311-16. doi: 10.1128/JVI.01311-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bautista EM, Faaberg KS, Mickelson D, McGruder ED. 2002. Functional properties of the predicted helicase of porcine reproductive and respiratory syndrome virus. Virology 298:258–270. doi: 10.1006/viro.2002.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Li H, Peng G, Zhang Y, Gao X, Xiao S, Cao S, Chen H, Song Y. 2015. Mutational analysis of the functional sites in porcine reproductive and respiratory syndrome virus non-structural protein 10. J Gen Virol 96:547–552. doi: 10.1099/jgv.0.000004. [DOI] [PubMed] [Google Scholar]
- 25.Fang Y, Snijder EJ. 2010. The PRRSV replicase: exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res 154:61–76. doi: 10.1016/j.virusres.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parida R, Choi IS, Peterson DA, Pattnaik AK, Laegreid W, Zuckermann FA, Osorio FA. 2012. Location of T-cell epitopes in nonstructural proteins 9 and 10 of type-II porcine reproductive and respiratory syndrome virus. Virus Res 169:13–21. doi: 10.1016/j.virusres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Yuan S, Zhang N, Xu L, Zhou L, Ge X, Guo X, Yang H. 2016. Induction of apoptosis by the nonstructural protein 4 and 10 of porcine reproductive and respiratory syndrome virus. PLoS One 11:e156518. doi: 10.1371/journal.pone.0156518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Zhou L, Zhang J, Ge X, Zhou R, Zheng H, Geng G, Guo X, Yang H. 2014. Nsp9 and Nsp10 contribute to the fatal virulence of highly pathogenic porcine reproductive and respiratory syndrome virus emerging in China. PLoS Pathog 10:e1004216. doi: 10.1371/journal.ppat.1004216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowland RR, Lunney J, Dekkers J. 2012. Control of porcine reproductive and respiratory syndrome (PRRS) through genetic improvements in disease resistance and tolerance. Front Genet 3:260. doi: 10.3389/fgene.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Jain R, Guan J, Vuong V, Ishido S, La Gruta NL, Gray DH, Villadangos JA, Mintern JD. 2016. Ubiquitin ligase MARCH 8 cooperates with CD83 to control surface MHC II expression in thymic epithelium and CD4 T cell selection. J Exp Med 213:1695–1703. doi: 10.1084/jem.20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krzyzak L, Seitz C, Urbat A, Hutzler S, Ostalecki C, Glasner J, Hiergeist A, Gessner A, Winkler TH, Steinkasserer A, Nitschke L. 2016. CD83 modulates B cell activation and germinal center responses. J Immunol 196:3581–3594. doi: 10.4049/jimmunol.1502163. [DOI] [PubMed] [Google Scholar]
- 32.Kristensen AM, Stengaard-Pedersen K, Hetland ML, Horslev-Petersen K, Junker P, Ostergaard M, Hollsberg P, Deleuran B, Hvid M. 2017. Expression of soluble CD83 in plasma from early-stage rheumatoid arthritis patients is not modified by anti-TNF-alpha therapy. Cytokine 96:1–7. doi: 10.1016/j.cyto.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Heilingloh CS, Grosche L, Kummer M, Muhl-Zurbes P, Kamm L, Scherer M, Latzko M, Stamminger T, Steinkasserer A. 2017. The major immediate-early protein IE2 of human cytomegalovirus is sufficient to induce proteasomal degradation of CD83 on mature dendritic cells. Front Microbiol 8:119. doi: 10.3389/fmicb.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seldon TA, Pryor R, Palkova A, Jones ML, Verma ND, Findova M, Braet K, Sheng Y, Fan Y, Zhou EY, Marks JD, Munro T, Mahler SM, Barnard RT, Fromm PD, Silveira PA, Elgundi Z, Ju X, Clark GJ, Bradstock KF, Munster DJ, Hart DN. 2016. Immunosuppressive human anti-CD83 monoclonal antibody depletion of activated dendritic cells in transplantation. Leukemia 30:692–700. doi: 10.1038/leu.2015.231. [DOI] [PubMed] [Google Scholar]
- 35.Horvatinovich JM, Grogan EW, Norris M, Steinkasserer A, Lemos H, Mellor AL, Tcherepanova IY, Nicolette CA, DeBenedette MA. 2017. Soluble CD83 inhibits T cell activation by binding to the TLR4/MD-2 complex on CD14+ monocytes. J Immunol 198:2286–2301. doi: 10.4049/jimmunol.1600802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oreshkova N, Wichgers SP, Spel L, Vloet RP, Moormann RJ, Boes M, Kortekaas J. 2015. Nonspreading Rift Valley fever virus infection of human dendritic cells results in downregulation of CD83 and full maturation of bystander cells. PLoS One 10:e142670. doi: 10.1371/journal.pone.0142670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Wei MQ, Liu X. 2013. Targeting CD83 for the treatment of graft-versus-host disease. Exp Ther Med 5:1545–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilingloh CS, Kummer M, Muhl-Zurbes P, Drassner C, Daniel C, Klewer M, Steinkasserer A. 2015. L particles transmit viral proteins from herpes simplex virus 1-infected mature dendritic cells to uninfected bystander cells, inducing CD83 downmodulation. J Virol 89:11046–11055. doi: 10.1128/JVI.01517-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heilingloh CS, Muhl-Zurbes P, Steinkasserer A, Kummer M. 2014. Herpes simplex virus type 1 ICP0 induces CD83 degradation in mature dendritic cells independent of its E3 ubiquitin ligase function. J Gen Virol 95:1366–1375. doi: 10.1099/vir.0.062810-0. [DOI] [PubMed] [Google Scholar]
- 40.Oudijk EJ, Lo TLA, Langereis JD, Ulfman LH, Koenderman L. 2008. Functional antagonism by GM-CSF on TNF-alpha-induced CD83 expression in human neutrophils. Mol Immunol 46:91–96. doi: 10.1016/j.molimm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. 2003. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol 170:4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 42.Guan X, Peng JR, Yuan L, Wang H, Wei YH, Leng XS. 2004. A novel, rapid strategy to form dendritomas from human dendritic cells and hepatocellular carcinoma cell line HCCLM3 cells using mature dendritic cells derived from human peripheral blood CD14+ monocytes within 48 hours of in vitro culture. World J Gastroenterol 10:3564–3568. doi: 10.3748/wjg.v10.i24.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clement C, Bhattacharjee PS, Kaufman HE, Hill JM. 2009. Heat-induced reactivation of HSV-1 in latent mice: upregulation in the TG of CD83 and other immune response genes and their LAT-ICP0 locus. Invest Ophthalmol Vis Sci 50:2855–2861. doi: 10.1167/iovs.08-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka Y, Mizuguchi M, Takahashi Y, Fujii H, Tanaka R, Fukushima T, Tomoyose T, Ansari AA, Nakamura M. 2015. Human T-cell leukemia virus type-I Tax induces the expression of CD83 on T cells. Retrovirology 12:56. doi: 10.1186/s12977-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutler CW, Jotwani R. 2006. Oral mucosal expression of HIV-1 receptors, co-receptors, and alpha-defensins: tableau of resistance or susceptibility to HIV infection? Adv Dent Res 19:49–51. doi: 10.1177/154407370601900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundell AC, Andersson K, Josefsson E, Steinkasserer A, Rudin A. 2007. Soluble CD14 and CD83 from human neonatal antigen-presenting cells are inducible by commensal bacteria and suppress allergen-induced human neonatal Th2 differentiation. Infect Immun 75:4097–4104. doi: 10.1128/IAI.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Eaton M, Mayer M, Li H, He D, Nelson E, Christopher-Hennings J. 2007. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch Virol 152:289–303. doi: 10.1007/s00705-006-0857-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Guo X, Nelson E, Christopher-Hennings J, Wang X. 2012. Porcine reproductive and respiratory syndrome virus activates the transcription of interferon alpha/beta (IFN-alpha/beta) in monocyte-derived dendritic cells (Mo-DC). Vet Microbiol 159:494–498. doi: 10.1016/j.vetmic.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Gomez IM, Kaser T, Gomez-Laguna J, Lamp B, Sinn L, Rumenapf T, Carrasco L, Saalmuller A, Gerner W. 2015. PRRSV-infected monocyte-derived dendritic cells express high levels of SLA-DR and CD80/86 but do not stimulate PRRSV-naive regulatory T cells to proliferate. Vet Res 46:54. doi: 10.1186/s13567-015-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L, Guan S, Zhou Q, Sheng S, Zhong F, Wang Q. 2015. Continuous expression of CD83 on activated human CD4(+) T cells is correlated with their differentiation into induced regulatory T cells. Mol Med Rep 12:3309–3314. doi: 10.3892/mmr.2015.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bates JM, Flanagan K, Mo L, Ota N, Ding J, Ho S, Liu S, Roose-Girma M, Warming S, Diehl L. 2015. Dendritic cell CD83 homotypic interactions regulate inflammation and promote mucosal homeostasis. Mucosal Immunol 8:414–428. doi: 10.1038/mi.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obendorf J, Renner VP, Fehlings M, Klotz C, Aebischer T, Ignatius R. 2013. Increased expression of CD25, CD83, and CD86, and secretion of IL-12, IL-23, and IL-10 by human dendritic cells incubated in the presence of Toll-like receptor 2 ligands and Giardia duodenalis. Parasit Vectors 6:317. doi: 10.1186/1756-3305-6-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tze LE, Horikawa K, Domaschenz H, Howard DR, Roots CM, Rigby RJ, Way DA, Ohmura-Hoshino M, Ishido S, Andoniou CE, Degli-Esposti MA, Goodnow CC. 2011. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J Exp Med 208:149–165. doi: 10.1084/jem.20092203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen L, Zhu Y, Zhang G, Gao C, Zhong W, Zhang X. 2011. CD83-stimulated monocytes suppress T-cell immune responses through production of prostaglandin E2. Proc Natl Acad Sci U S A 108:18778–18783. doi: 10.1073/pnas.1018994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin H, Liang S, Zhong Z, Wen J, Li W, Wang L, Xu J, Zhong F, Li X. 2014. Soluble CD83 inhibits human monocyte differentiation into dendritic cells in vitro. Cell Immunol 292:25–31. doi: 10.1016/j.cellimm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Dudziak D, Kieser A, Dirmeier U, Nimmerjahn F, Berchtold S, Steinkasserer A, Marschall G, Hammerschmidt W, Laux G, Bornkamm GW. 2003. Latent membrane protein 1 of Epstein-Barr virus induces CD83 by the NF-kappaB signaling pathway. J Virol 77:8290–8298. doi: 10.1128/JVI.77.15.8290-8298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berchtold S, Muhl-Zurbes P, Maczek E, Golka A, Schuler G, Steinkasserer A. 2002. Cloning and characterization of the promoter region of the human CD83 gene. Immunobiology 205:231–246. doi: 10.1078/0171-2985-00128. [DOI] [PubMed] [Google Scholar]
- 58.Yu Z, Huang C, Zhang Q, Feng WH. 2016. Porcine reproductive and respiratory syndrome virus (PRRSV) induces IL-12p40 production through JNK-AP-1 and NF-kappaB signaling pathways. Virus Res 225:73–81. doi: 10.1016/j.virusres.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Fu Y, Quan R, Zhang H, Hou J, Tang J, Feng WH. 2012. Porcine reproductive and respiratory syndrome virus induces interleukin-15 through the NF-kappaB signaling pathway. J Virol 86:7625–7636. doi: 10.1128/JVI.00177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramaniam S, Kwon B, Beura LK, Kuszynski CA, Pattnaik AK, Osorio FA. 2010. Porcine reproductive and respiratory syndrome virus non-structural protein 1 suppresses tumor necrosis factor-alpha promoter activation by inhibiting NF-kappaB and Sp1. Virology 406:270–279. doi: 10.1016/j.virol.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Chu S. 2012. Transcriptional regulation by post-transcriptional modification—role of phosphorylation in Sp1 transcriptional activity. Gene 508:1–8. doi: 10.1016/j.gene.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 62.Qi F, Liu X, Wu H, Yu X, Wei C, Huang X, Ji G, Nie F, Wang K. 2017. Long noncoding AGAP2-AS1 is activated by SP1 and promotes cell proliferation and invasion in gastric cancer. J Hematol Oncol 10:48. doi: 10.1186/s13045-017-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu K, Wang X, Zhang Q, Liang A, Zhu H, Huang D, Li C, Ye W. 2016. Sp1 downregulates proinflammatory cytokine-induced catabolic gene expression in nucleus pulposus cells. Mol Med Rep 14:3961–3968. doi: 10.3892/mmr.2016.5730. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Du S, Sheng X, Liu J, Cen B, Huang F, He Y. 2016. MicroRNA-29b inhibits endometrial fibrosis by regulating the Sp1-TGF-beta1/Smad-CTGF axis in a rat model. Reprod Sci 23:386–394. doi: 10.1177/1933719115602768. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Fan B, Bai J, Wang H, Li Y, Jiang P. 2015. The N-N non-covalent domain of the nucleocapsid protein of type 2 porcine reproductive and respiratory syndrome virus enhances induction of IL-10 expression. J Gen Virol 96:1276–1286. doi: 10.1099/vir.0.000061. [DOI] [PubMed] [Google Scholar]
- 66.Jing H, Fang L, Ding Z, Wang D, Hao W, Gao L, Ke W, Chen H, Xiao S. 2017. Porcine reproductive and respiratory syndrome virus nsp1α inhibits NF-κB activation by targeting the linear ubiquitin chain assembly complex. J Virol 91:e1911-16. doi: 10.1128/JVI.01911-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan B, Liu X, Bai J, Li Y, Zhang Q, Jiang P. 2015. The 15N and 46R residues of highly pathogenic porcine reproductive and respiratory syndrome virus nucleocapsid protein enhance regulatory T lymphocytes proliferation. PLoS One 10:e138772. doi: 10.1371/journal.pone.0138772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein MF, Blume K, Heilingloh CS, Kummer M, Biesinger B, Sticht H, Steinkasserer A. 2015. CD83 and GRASP55 interact in human dendritic cells. Biochem Biophys Res Commun 459:42–48. doi: 10.1016/j.bbrc.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 69.Ju X, Silveira PA, Hsu WH, Elgundi Z, Alingcastre R, Verma ND, Fromm PD, Hsu JL, Bryant C, Li Z, Kupresanin F, Lo TH, Clarke C, Lee K, McGuire H, Fazekas DSGB, Larsen SR, Gibson J, Bradstock KF, Clark GJ, Hart DN. 2016. The analysis of CD83 expression on human immune cells identifies a unique CD83+-activated T cell population. J Immunol 197:4613–4625. doi: 10.4049/jimmunol.1600339. [DOI] [PubMed] [Google Scholar]
- 70.Park MC, Kim D, Lee Y, Kwon HJ. 2013. CD83 expression induced by CpG-DNA stimulation in a macrophage cell line RAW 264.7. BMB Rep 46:448–453. doi: 10.5483/BMBRep.2013.46.9.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan B, Liu X, Bai J, Zhang T, Zhang Q, Jiang P. 2016. Influence of the amino acid residues at 70 in M protein of porcine reproductive and respiratory syndrome virus on viral neutralization susceptibility to the serum antibody. Virol J 13:51. doi: 10.1186/s12985-016-0505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan B, Liu X, Bai J, Zhang T, Zhang Q, Jiang P. 2015. The amino acid residues at 102 and 104 in GP5 of porcine reproductive and respiratory syndrome virus regulate viral neutralization susceptibility to the porcine serum neutralizing antibody. Virus Res 204:21–30. doi: 10.1016/j.virusres.2015.04.015. [DOI] [PubMed] [Google Scholar]