ABSTRACT
Differences of opinion regarding whether there may, or may not, have been protective efficacy in the RV144 vaccine trial have important societal implications.
KEYWORDS: HIV vaccine, efficacy trials
COMMENTARY
I am surprised that the leaders of our nation's effort toward a vaccine for HIV/AIDS continue to believe in the protective efficacy claimed for the RV144 vaccine trial (1). See, for example, Corey et al. (2) and a lecture given on 23 March 2015 at the Keystone Symposium (3).
The final statistical analyses, published by leading U.S. investigators of the RV144 trial subsequent to the original New England Journal of Medicine (NEJM) report, concluded that the chance for no efficacy in the trial was greater than or equal to 22%; i.e., there was a less than 78% chance that there was protective efficacy in the trial (4). The authors go on further to state that this number “reflects greater uncertainty than has much of the discussion about this trial.” Hardly a ringing endorsement for efficacy by the U.S. leaders of the trial.
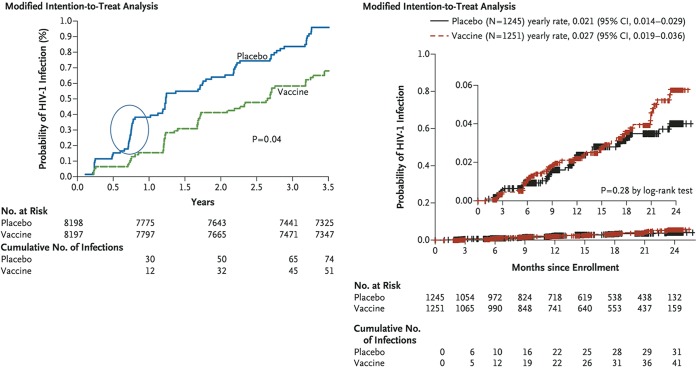
Next, it is important to examine closely the Kaplan-Meier HIV-1 acquisition curves in the original NEJM report on the RV144 trial (1). In all other efficacy trials of HIV vaccines, the acquisition curves have been nicely linear for both vaccine and placebo groups. In the RV144 trial, the acquisition curve is nonlinear for the placebo group, with a sudden nonlinear increase in acquisition in the placebo arm within the first year of the trial that accounts for most or all of the difference in acquisition compared to the vaccine arm (Fig. 1, left panel). Compare this with the linear acquisition curves from the HVTN505 trial in the report by Hammer et al. (5) (Fig. 1, right panel). Thus, the difference in acquisition does not appear to be due to protective effects of the vaccine but rather to an anomalous increase in acquisition in the placebo arm in the 6-to-12-month time frame. In addition, the “intent-to-treat” analysis and the “per-protocol” analysis revealed no significant differences in HIV acquisition; only with a “modified intention-to-treat” analysis in the initial NEJM publication was a marginally significant difference (P = 0.04) in acquisition observed (1). There was no lowering of viral load in vaccinated individuals who became infected.
FIG 1.
(Left panel) Kaplan-Meier acquisition curve from the RV144/Thai trial. Data are from the one analysis (the modified intention-to-treat group) of three that showed a P value of less than 0.05 with the tests used. (Reprinted from The New England Journal of Medicine with permission of the publisher [see Fig. 2C in reference 1].) The circled data represent the anomalous nonlinear increase in acquisition in the placebo group in the first year of the trial that was responsible for most or all of the differences in HIV-1 acquisition. (Right panel) Linear rates of HIV-1 acquisition in both the placebo and vaccine groups in the HVTN505 trial. CI, confidence interval. (Reprinted from The New England Journal of Medicine with permission of the publisher [see Fig. 2B in reference 5].)
A “sieving” effect on HIV sequences acquired in the vaccine arm has also been used as evidence for the protective effects in the RV144 trial (6). Certain amino acids present at positions 169 and 181 in the envelope protein were preferentially associated with HIV-1 acquisition in the vaccine arm compared to the placebo arm. The logic here is that immune responses to the sequences in the vaccine can preferentially select for the presence of different sequences at certain locations when HIV infection is acquired. Unfortunately, the amino acid at position 169 in the vaccine was lysine and it was lysine at this position that was preferentially acquired in the vaccine group compared to the placebo group. In addition, statistically significant sieving effects were observed for HIV gene products that were not even included in the vaccine (7). There is no logic to these observations; these sieving effects should not be used as an argument to support claims of protective efficacy in the RV144 trial.
An immune correlate of protection has also been presented in support of the claims of protective efficacy in the RV144 trial (8). Close inspection of the immune correlate in this publication reveals a median enzyme-linked immunosorbent assay (ELISA) binding value for V1V2 sequences in Env of approximately 0.36 among infected individuals in the vaccine arm versus 0.40 among uninfected individuals in the vaccine arm, with almost complete overlap in the scatter plots (Fig. 2). It is unclear to what extent a variety of technical issues could have influenced the underwhelming difference in median ELISA values.
FIG 2.
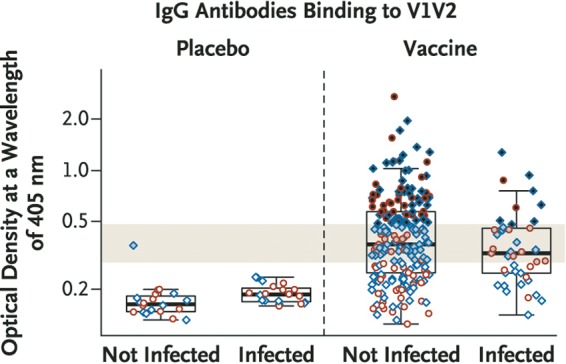
IgG antibodies as a correlate of immunity in the RV144/Thai trial. (Reprinted from The New England Journal of Medicine with permission of the publisher [see Fig. 2A in reference 8].)
Differences of opinion regarding the extent to which there may, or may not, have been protective efficacy in the RV144 trial are not without important societal implications. Those on one side of the fence have called for additional trials to confirm and extend the findings of RV144. In fact, such trials are already under way (9, 10). Quite clearly, efficacy trials are hugely expensive ventures. Those like myself on the other side of the fence see the need for more basic and preclinical research so that approaches can be discovered with a more realistic chance of actual efficacy and a more realistic chance of impacting the worldwide problem. There are certainly some very promising ideas out there that are in dire need of additional research funding.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH; MOPH-TAVEG Investigators. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Corey L, Gilbert PB, Tomaras GD, Haynes BF, Pantaleo G, Fauci AS. 2015. Immune correlates of vaccine protection against HIV-1 acquisition. Sci Transl Med 7:310rv7. doi: 10.1126/scitranslmed.aac7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci AS. 2017. Towards an HIV vaccine: a scientific journey. https://www.youtube.com/watch?v=8iHK3B-g7GI Accessed June 2017.
- 4.Gilbert PB, Berger JO, Stablein D, Becker S, Essex M, Hammer SM, Kim JH, Degruttola VG. 2011. Statistical interpretation of the RV144 HIV vaccine efficacy trial in Thailand: a case study for statistical issues in efficacy trials. J Infect Dis 203:969–975. doi: 10.1093/infdis/jiq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, DeJesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB; HVTN 505 Study Team. 2013. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edlefsen PT, Rolland M, Hertz T, Tovanabutra S, Gartland AJ, deCamp AC, Magaret CA, Ahmed H, Gottardo R, Juraska M, McCoy C, Larsen BB, Sanders-Buell E, Carrico C, Menis S, Bose M, RV144 Sequencing Team, Arroyo MA, O'Connell RJ, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Robb ML, Kirys T, Georgiev IS, Kwong PD, Scheffler K, Pond SL, Carlson JM, Michael NL, Schief WR, Mullins JI, Kim JH, Gilbert PB. 2015. Comprehensive sieve analysis of breakthrough HIV-1 sequences in the RV144 vaccine efficacy trial. PLoS Comput Biol 11:e1003973. doi: 10.1371/journal.pcbi.1003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Collaboration for AIDS Vaccine Discovery. 2016. Corey: Support for HVTN 702. https://www.cavd.org/grantees/Pages/Grantee-Corey.aspx Accessed 2 June 2017.
- 10.AVAC. 2016. HVTN 702. http://www.avac.org/trial/hvtn-702 Accessed 2 June 2017.