ABSTRACT
Invasive pulmonary aspergillosis (IPA) is an important cause of morbidity and mortality in immunocompromised patients. We hypothesized that simultaneous inhibition of biosynthesis of ergosterol in the fungal cell membrane and (1→3)-β-d-glucan in the cell wall, respectively, by the antifungal triazole isavuconazole (ISA) and the echinocandin micafungin (MFG) may result in improved outcomes in experimental IPA in persistently neutropenic rabbits. Treatments included ISA at 20 mg/kg of body weight/day (ISA20), 40 mg/kg/day (ISA40), and 60 mg/kg/day (ISA60); MFG at 2 mg/kg/day (MFG2); combinations of ISA20 and MFG2, ISA40 and MFG2, and ISA60 and MFG2; and no treatment (untreated controls [UC]). The galactomannan index (GMI) and (1→3)-β-d-glucan levels in serum were measured. The residual fungal burden (number of CFU per gram) was significantly reduced in ISA20-, ISA40-, ISA60-, ISA20-MFG2-, ISA40-MFG2-, and ISA60-MFG2-treated rabbits compared with that in MFG2-treated or UC rabbits (P < 0.01). Measures of organism-mediated pulmonary injury, lung weights, and pulmonary infarct score were lower in ISA40-MFG2-treated rabbits than in rabbits treated with ISA40 or MFG2 alone (P < 0.01). Survival was prolonged in ISA40-MFG2-treated rabbits in comparison to those treated with ISA40 or MFG2 alone (P < 0.01). These outcome variables correlated directly with significant declines in GMI and serum (1→3)-β-d-glucan levels during therapy. The GMI correlated with measures of organism-mediated pulmonary injury, lung weights (r = 0.764; P < 0.001), and pulmonary infarct score (r = 0.911; P < 0.001). In summary, rabbits receiving combination therapy with isavuconazole and micafungin demonstrated a significant dose-dependent reduction in the residual fungal burden, decreased pulmonary injury, prolonged survival, a lower GMI, and lower serum (1→3)-β-d-glucan levels in comparison to rabbits receiving isavuconazole or micafungin as a single agent.
KEYWORDS: (1→3)-β-d-glucan, Aspergillus fumigatus, isavuconazole, aspergillosis, combination therapy, galactomannan, micafungin, neutropenia, pharmacokinetics, rabbit
INTRODUCTION
Invasive pulmonary aspergillosis (IPA) is a life-threatening infection in immunosuppressed patients, particularly in those with severe and prolonged neutropenia as a consequence of aplastic anemia, in those receiving myelotoxic chemotherapy for treatment of acute leukemia, and in those receiving immunosuppressive medication for rejection prophylaxis after organ transplantation or treatment of graft-versus-host disease after allogeneic bone marrow transplantation (1–5). Despite advances in antifungal therapy, the rates of mortality and morbidity remain unacceptably high. New therapeutic strategies for IPA are clearly needed.
Our recent in vitro studies of the new extended-spectrum antifungal triazole isavuconazole (ISA) and the echinocandin micafungin (MFG) demonstrated that they exhibited a synergistic interaction against Aspergillus fumigatus, Aspergillus flavus, and Aspergillus terreus (6). We then hypothesized that simultaneous inhibition of the biosynthesis of ergosterol in the fungal cell membrane and (1→3)-β-d-glucan in the cell wall, respectively, by the antifungal triazole isavuconazole and the echinocandin micafungin may result in improved outcomes in experimental IPA in persistently neutropenic rabbits.
We therefore studied the efficacy of isavuconazole in combination with micafungin for the treatment of experimental IPA in persistently neutropenic rabbits. The data from this study will establish the foundation for further clinical evaluation.
RESULTS
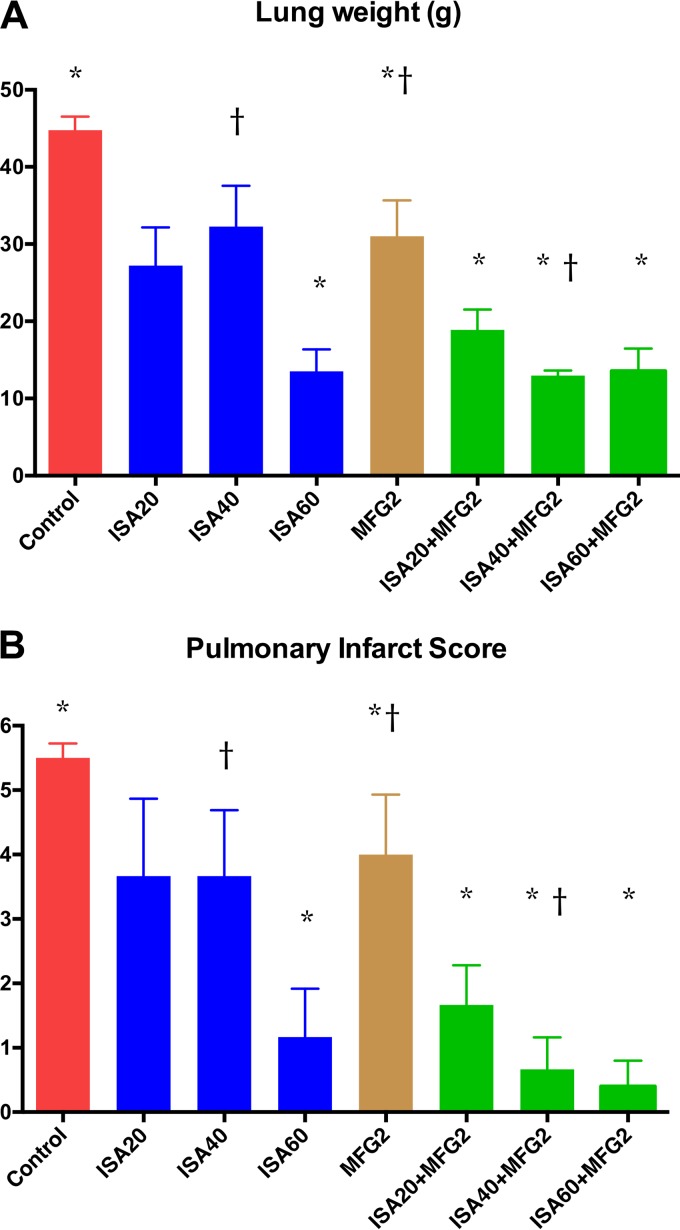
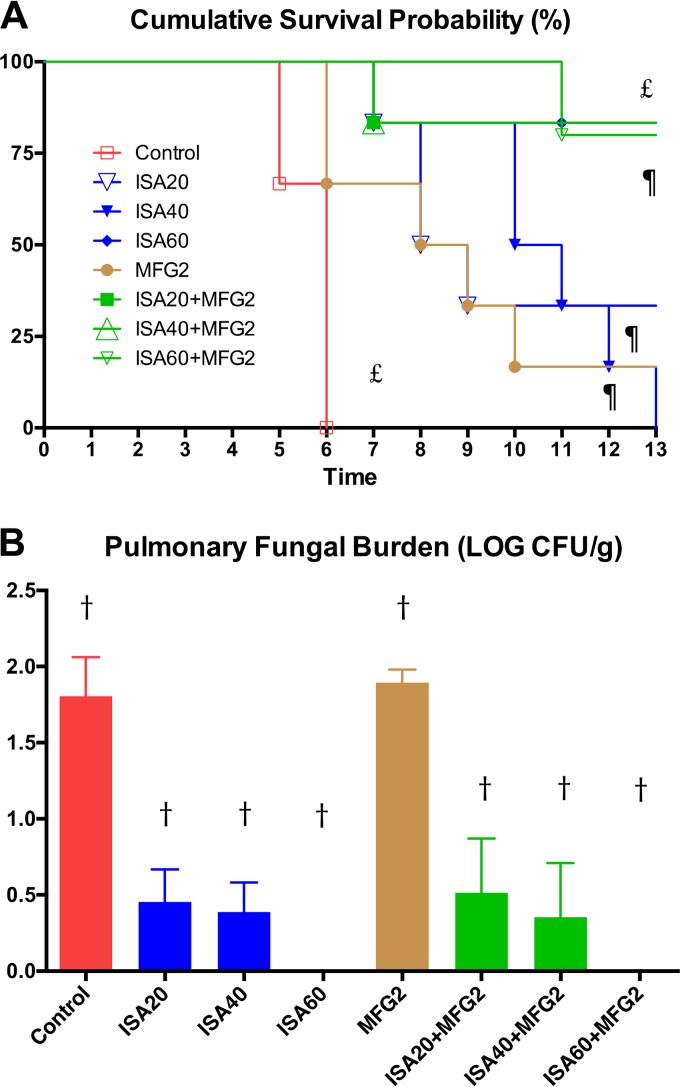
Organism-mediated pulmonary injury was measured by determination of total lung weights and the pulmonary infarct score. Total lung weights and pulmonary infarct scores were significantly lower in rabbits treated with ISA at 60 mg/kg of body weight/day (ISA60), ISA at 20 mg/kg/day (ISA20) plus MFG at 2 mg/kg/day (MFG2), ISA at 40 mg/kg/day (ISA40) plus MFG2, and ISA at 60 mg/kg/day (ISA60) plus MFG2 than in those treated with MFG2 and untreated control (UC) rabbits (P < 0.05) (Fig. 1A and B). In addition, rabbits treated with ISA40-MFG2 demonstrated significantly lower lung weights and pulmonary infarct scores than rabbits treated with ISA40 or MFG2 alone (P < 0.01). Rabbits treated with ISA20-MFG2, ISA40-MFG2, and ISA60-MFG2 had significantly prolonged survival in comparison to UC rabbits (P < 0.01) (Fig. 2A). Mortality was numerically greater in monotherapy regimens of ISA20, ISA40, and MCG2. ISA40-MFG2-treated rabbits demonstrated significantly prolonged survival in comparison to that for rabbits receiving a single therapy of ISA40 or MFG2 (P < 0.01) (Fig. 2A). There was a significant reduction in the residual fungal burden (numbers of CFU per gram) in ISA20-, ISA40-, ISA60-, ISA20-MFG2-, ISA40-MFG2-, and ISA60-MFG2-treated rabbits compared with that in MFG2-treated or UC rabbits (P < 0.01) (Fig. 2B).
FIG 1.
Response of primary pulmonary aspergillosis to antifungal therapy in persistently neutropenic untreated control (UC) rabbits and rabbits receiving oral isavuconazole (BAL4815), measured by mean lung weight (A) and mean pulmonary infarct score (B). Values are given as means ± SEMs. P values are indicated as follows: *, P < 0.05; †, P < 0.01. P values are for decreased lung weights and pulmonary infarct scores in ISA40-MFG2-treated rabbits in comparison to those in rabbits treated with ISA40 or MFG2 alone.
FIG 2.
Response of primary pulmonary aspergillosis to antifungal therapy in persistently neutropenic untreated control (UC) rabbits and persistently neutropenic rabbits receiving oral isavuconazole (BAL4815), measured by survival (A) and mean pulmonary tissue residual fungal burden (log number of CFU per gram) (B). Values are given as means ± SEMs. For the measure of survival, the values on the y axis are the probability of survival. Survival was plotted by Kaplan-Meier analysis. Differences in the rates of survival between the treatment groups and the untreated controls were analyzed by the log-rank test. P values are indicated as follows: †, P < 0.01 for decreased residual fungal burden in ISA20-, ISA40-, ISA60-, ISA20-MFG2-, ISA40-MFG2-, and ISA60-MFG2-treated rabbits versus MFG2-treated or UC rabbits; ¶, P < 0.01 for prolonged survival of ISA40-MFG2-treated rabbits versus rabbits treated with ISA40 or MFG2 alone; £, P < 0.01 for prolonged survival of rabbits treated with ISA20-MFG2, ISA40-MFG2, and ISA60-MFG2 versus UC rabbits.
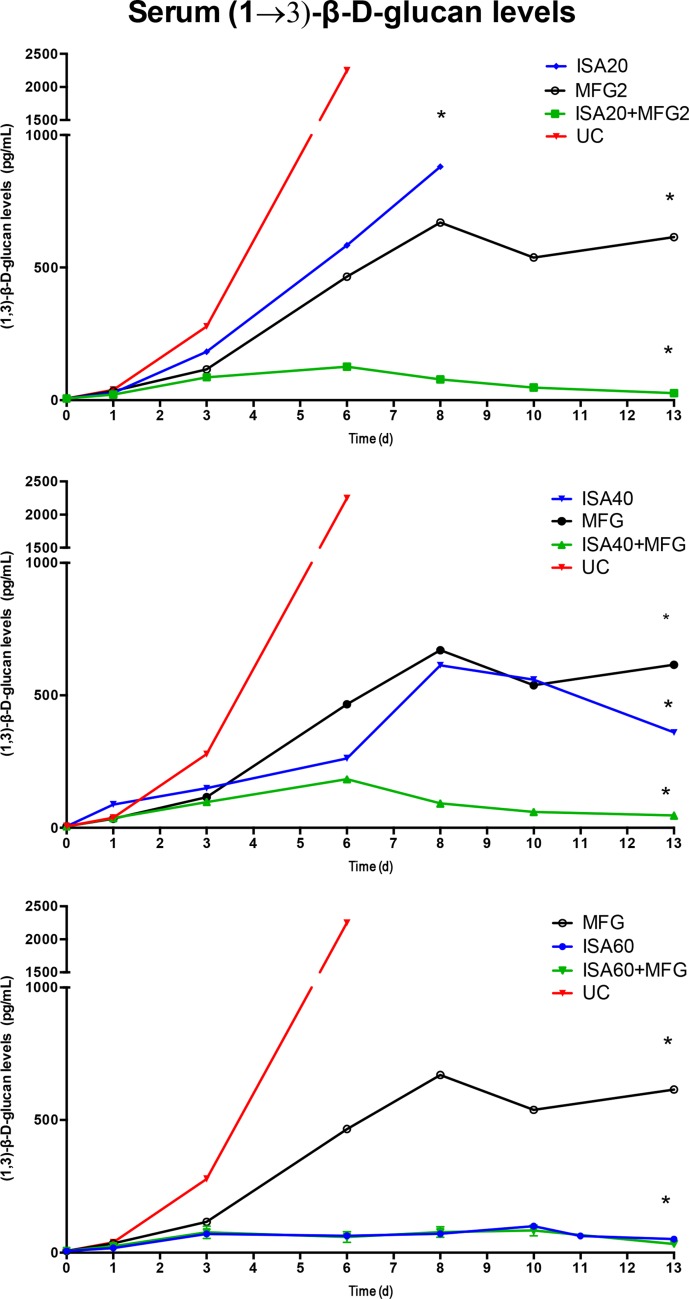
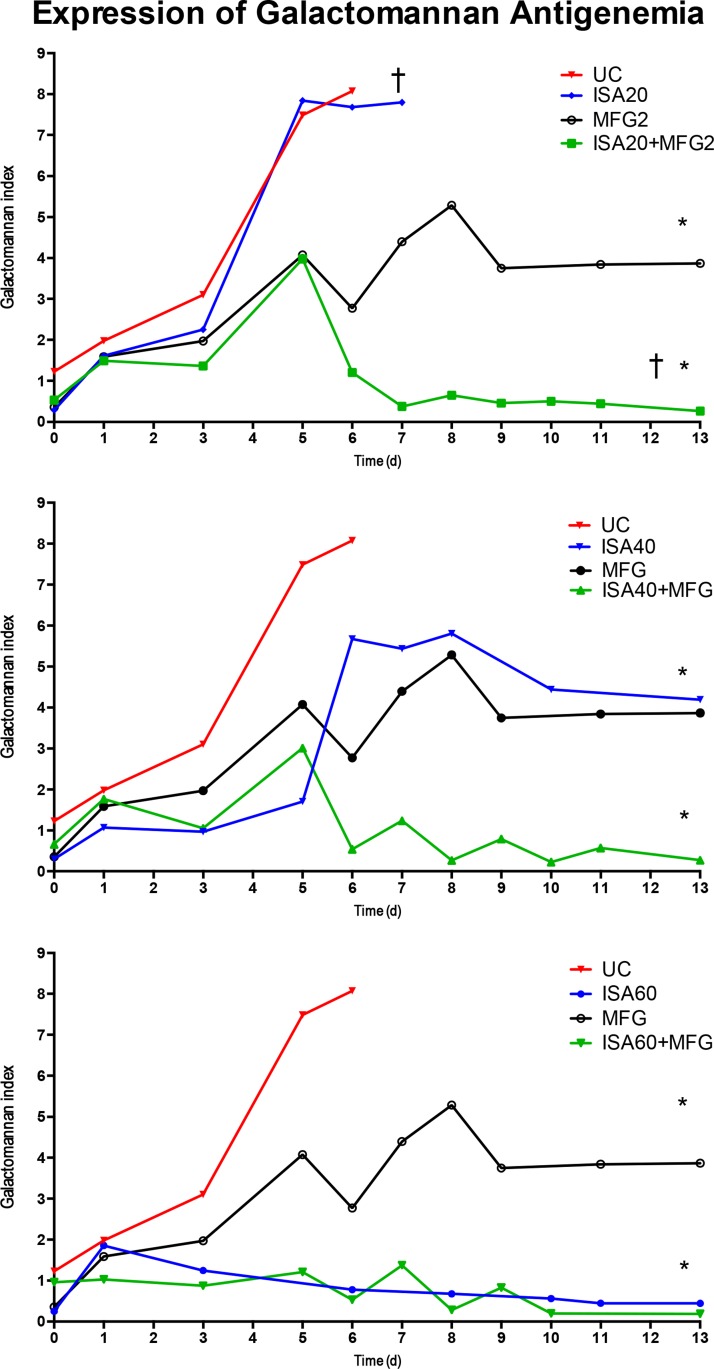
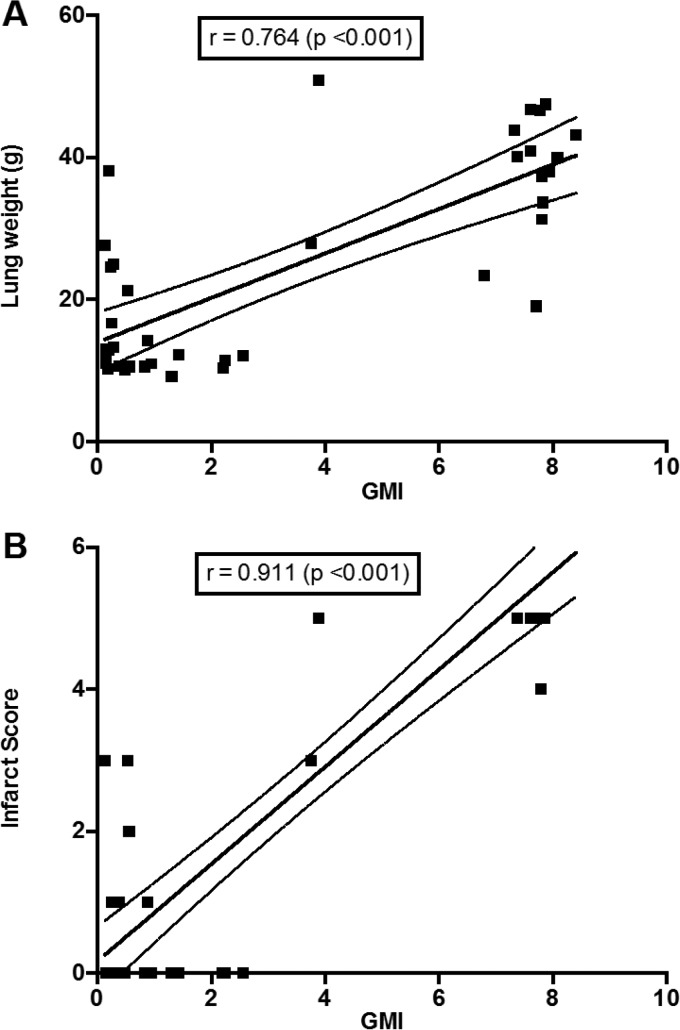
Serum (1→3)-β-d-glucan levels were significantly lower in all isavuconazole combination treatment groups than in the groups treated with isavuconazole or micafungin alone, with the exception of the group treated with ISA60 (P < 0.05) (Fig. 3). The serum galactomannan index (GMI) was significantly lower in all isavuconazole combination treatment groups than in the groups treated with isavuconazole or micafungin alone (P < 0.05) (Fig. 4). GMI strongly correlated with measures of organism-mediated pulmonary injury (total lung weights [r = 0.764; P < 0.001] and pulmonary infarct scores [r = 0.911; P < 0.001]) (Fig. 5).
FIG 3.
Serum (1→3)-β-d-glucan levels in persistently neutropenic untreated control (UC) rabbits and persistently neutropenic rabbits receiving oral doses of isavuconazole (BAL4815) in the model of experimental pulmonary aspergillosis. Values are given as (1→3)-β-d-glucan concentrations. P values are indicated as follows: *, P < 0.05 for the decrease in plasma (1→3)-β-d-glucan concentrations in ISA20-MFG2-, ISA40-MFG2-, and ISA60-MFG2-treated rabbits versus MFG2-treated or UC rabbits. d, days.
FIG 4.
Expression of galactomannan antigenemia in persistently neutropenic untreated control (UC) rabbits with pulmonary aspergillosis and persistently neutropenic rabbits with pulmonary aspergillosis receiving an oral dose of isavuconazole (BAL4815). Values are given as the mean GMI ± SEM. P values are indicated as follows: *, P < 0.05 for a lower GMI in ISA20-MFG2-, ISA40-MFG2-, and ISA60-MFG2-treated rabbits versus MFG2-treated or UC rabbits; †, P < 0.01 for a lower GMI in ISA20-MFG2-treated rabbits than MFG2-treated rabbits.
FIG 5.
Strong correlation between GMI and outcome variables. (A) Total lung weights (r = 0.764; P < 0.001); (B) infarct scores (r = 0.911; P < 0.001).
DISCUSSION
This study demonstrated that the combination of ISA40 and MFG2 was significantly more active than either isavuconazole at 40 mg/kg or micafungin as single agents in significantly reducing mortality, as well as the parameters of organism-mediated pulmonary injury (lung weights and pulmonary infarct score), when they were used to treat experimental pulmonary aspergillosis in persistently neutropenic rabbits. Moreover, the combinations ISA20-MFG2 and ISA40-MFG2 were more active than monotherapy in significantly reducing the serum GMI and circulating levels of (1→3)-β-d-glucan. GMI also correlated with measures of organism-mediated pulmonary injury (lung weights and pulmonary infarct score). Thus, rabbits receiving combination therapy consisting of isavuconazole with micafungin demonstrated a significant dose-dependent reduction in the residual fungal burden, decreased pulmonary injury, prolonged survival, a lower GMI, and lower serum (1→3)-β-d-glucan levels in comparison to rabbits treated with isavuconazole or micafungin as a single agent.
The study of combination antifungal therapy in experimental model systems of invasive aspergillosis is essential for understanding the basic pharmacology of the combination, as well for designing, implementing, and derisking clinical protocols for the treatment of invasive fungal infections. The model system described here investigated a series of endpoints that individually and collectively allowed a detailed understanding of the efficacies of the antifungals in relation to the distinctive properties of invasive pulmonary aspergillosis (7). Determination of the values of markers of organism-mediated pulmonary injury (lung weights and pulmonary infarct scores) is essential to understand the impact of an echinocandin-based regimen, in which the quantitative results for cultures may, paradoxically, be elevated. As patients succumb to pulmonary aspergillosis as the result of organism-mediated pulmonary injury, understanding the impact of a combination regimen yields insight into improving a key clinical outcome parameter. Figure 1 demonstrates significant reductions in the lung weights and pulmonary infarct scores with all three ISA-MFG2 combinations in comparison to those for the untreated controls and a greater numerical effect in comparison to that of monotherapy. While the difference between the effect of the combination of ISA40 and MFG2 versus that of ISA40 alone on reducing organism-mediated tissue injury achieved statistical significance, a similar pattern was observed for the effect of the combination of ISA20 and MFG2 versus that of monotherapy. Monotherapy with ISA60 was also as effective as the combination therapeutic regimens. However, in order to achieve the plasma exposure conferred by ISA60, the comparable human dose would be approximately 400 mg, which is double the licensed daily dose of 200 mg. By comparison, the plasma exposure achieved with the human dose of 200 mg is approximated by the ISA dosage range in rabbits of between 20 and 40 mg/kg/day.
The Kaplan-Meier plot of survival is consistent with the findings of the reduction of organism-mediated pulmonary injury, demonstrating that the greatest efficacy is observed with ISA-MFG combinations as well as with ISA60 monotherapy. The greater mortality observed with MFG2, ISA20, and ISA40 further supports the role of combination therapy in achieving improved outcomes of invasive aspergillosis in persistently neutropenic hosts. The antifungal effect is further reflected in the pulmonary fungal burden, in which complete clearance to the lower limit of quantitation was achieved by only the two regimens, ISA40-MFG2 and ISA60.
The temporal patterns of serial serum biomarkers for (1→3)-β-d-glucan and galactomannan also support the efficacy of the ISA and MFG combination. Consistent with other endpoint parameters, serum (1→3)-β-d-glucan levels in animals treated with ISA20, ISA40, or MFG2 monotherapy remained persistently elevated by the end of therapy. By comparison, combination therapy with ISA20-MFG2 or ISA40-MFG2 resulted in the resolution of the elevated serum (1→3)-β-d-glucan levels. As was observed with other parameters, the elevated serial (1→3)-β-d-glucan levels also resolved either with ISA60 monotherapy or with ISA60-MFG2 combination therapy. This correlation between the therapeutic response to combination therapy or triazole monotherapy and the temporal pattern of (1→3)-β-d-glucan levels has also been demonstrated in experimental and clinical invasive aspergillosis (8–13).
Similarly, serial GMI values for animals treated with ISA20, ISA40, or MFG2 monotherapy remained persistently elevated by the end of therapy, while the elevated GMI values for animals treated with ISA20-MFG2 or ISA40-MFG2 combination therapy were resolved. Recapitulating the findings for the parameters of organism-mediated injury, survival, pulmonary fungal burden, and serial (1→3)-β-d-glucan levels, animals treated with ISA60 or ISA60-MFG2 also demonstrated resolution of the elevated GMI in serum. The validity of GMI in reflecting the therapeutic response is buttressed by previous studies with animal models of pulmonary aspergillosis (8, 9, 13, 14) and patients with invasive aspergillosis (15–21).
Current treatment of IPA in immunosuppressed hosts relies on the administration of antifungal triazoles, particularly voriconazole, as primary therapy (22). Unfortunately, the overall rate of the response of invasive aspergillosis to voriconazole remains approximately 50% to 60%, with responses being as low as nearly 30% in hematopoietic stem cell transplantation recipients. Although voriconazole is an important therapeutic advance against IPA, the problems of visual hallucinations, cutaneous solar hypersensitivity, hepatotoxicity, drug interactions, and variable plasma pharmacokinetics and the need for therapeutic drug monitoring warrant the need for new antifungal agents active against Aspergillus spp. (23). Clearly, new strategies for the treatment of IPA are needed.
The antifungal triazoles inhibit fungal cell membrane biosynthesis through inhibition of ergosterol formation at the level of lanosterol 14-demethylase (24). Isavuconazole is a new broad-spectrum triazole antifungal agent that has recently been approved by the FDA for the primary treatment of invasive aspergillosis and mucormycosis (25–27). Isavuconazole in vitro demonstrates superior hyphal growth inhibition and MICs against A. fumigatus in comparison to the hyphal growth inhibition and MICs of voriconazole (28–31). The pharmacodynamics and efficacy of isavuconazole as a single agent were explored in a model of IPA in neutropenic mice by Lepak and colleagues (32), in a model of disseminated aspergillosis in immunocompetent mice by Seyedmousavi et al. (33), and in a model of invasive pulmonary aspergillosis in persistently neutropenic rabbits (14, 34).
Micafungin is a cyclic hexapeptide echinocandin that inhibits (1→3)-β-d-glucan synthase, an enzyme complex specific to fungi and essential for fungal cell wall biosynthesis. In vitro studies indicate that micafungin has broad-spectrum fungicidal activity against Candida spp. (including azole-resistant Candida albicans isolates) and fungistatic activity against Aspergillus spp. More recent studies have demonstrated that the combination of micafungin and the triazole isavuconazole achieves significant synergy against Aspergillus spp. (6).
The comparative pharmacodynamics of micafungin and isavuconazole merit discussion. Micafungin demonstrates an in vitro and in vivo concentration-dependent effect of paradoxically increasing the number of CFU as its mechanism of disrupting cell wall biosynthesis in Aspergillus fumigatus (35). The disruption of cell wall integrity results in truncated hyphal elements with a dose-dependent decrease in angioinvasion, a reduction of pulmonary infarcts, and an increase in survival. By comparison, isavuconazole causes an in vitro concentration-dependent and in vivo dose-dependent reduction of the number of CFU of A. fumigatus, which correlates with reduced organism-mediated pulmonary injury and increased survival (34). Moreover, using serum GMI as the dynamic pharmacodynamic variable, a mean plasma isavuconazole area under the concentration-time curve/MIC (50% effective concentration) ratio of 79.65 (95% confidence interval [CI], 32.2 to 127.1) produced a half-maximal effect in GMI suppression (14). By comparison, the serum GMI paradoxically increases during micafungin treatment of invasive aspergillosis as the result of the dispersal of cell wall fragments following inhibition of (1→3)-β-d-glucan synthesis (35). The dosage of micafungin used in this study is comparable to a level of plasma exposure of approximately 0.5 mg/kg/day in humans. The dosage of isavuconazole of 20 to 40 mg/kg/day in the rabbit model approximates the level of plasma exposure achieved by the human adult dose of 200 mg/day, while the 60-mg/kg dosage of isavuconazole in rabbits would correspond to approximately 400 mg/day in human adults.
We hypothesized that the simultaneous inhibition of ergosterol biosynthesis in the fungal cell membrane and (1→3)-β-d-glucan in the cell wall, respectively, by the antifungal triazole isavuconazole and the echinocandin micafungin may result in improved outcomes of experimental IPA in persistently neutropenic rabbits. Such findings provide a scientific foundation for the use of this combination for the treatment of proven and probable IPA in immunocompromised patients and build upon the findings of previous work with the triazole-echinocandin combination suggesting that combination therapy may be more effective than triazole therapy alone.
This study has several limitations. Although this study used only one isolate of A. fumigatus, in vitro assays have demonstrated similar properties of synergistic activity between isavuconazole and micafungin against other isolates, suggesting the applicability of the findings from this study. Further in vivo studies using additional strains of A. fumigatus with various MICs would provide valuable insight into the potential clinical utility of the combination of isavuconazole and micafungin. While additional dosages of the echinocandin may have been used, our earlier data demonstrated an optimal effect with 2 mg/kg/day of micafungin, allowing a more focused investigation of the range of dosages of isavuconazole.
Given the observations in this study that the effects of combination therapy with ISA40 and MFG2 and monotherapy with ISA60 are comparable, one must consider two options for clinical trials. The first option would be to combine isavuconazole at 200 mg/day with micafungin and to compare that regimen with isavuconazole at 400 mg/day as monotherapy. As the licensed dose of isavuconazole for adults, 200 mg/day, has been well studied and found to have a toxicity profile significantly more favorable than that of voriconazole (36), patient safety could be better ensured with a dose of 200 mg/day than one of 400 mg/day. A doubling of the dose of isavuconazole from 200 mg/day to 400 mg/day may incur dose-dependent intolerance and end organ toxicity. Thus, a study of combination therapy with isavuconazole plus micafungin would be the next logical step in harnessing these data for improved treatment of invasive aspergillosis.
Previously, the activity of the combination of the echinocandin anidulafungin and voriconazole against A. fumigatus was studied by an in vitro broth microdilution checkerboard assay based on the CLSI M-38A method (13). To quantify the concentration-effect relationships of anidulafungin and voriconazole alone, a sigmoid maximum effect model was fitted to the percent growth inhibition obtained at each concentration of the drugs alone for each replicate to allow Bliss independence-based drug interaction analysis. In parallel with these in vitro studies, the activities of anidulafungin and voriconazole for the treatment of experimental invasive pulmonary aspergillosis in persistently neutropenic rabbits were studied (8). These experiments demonstrated in vitro and in vivo concentration- and dose-dependent synergistic interactions between the echinocandin and the triazole by Bliss independence drug interaction analysis of microbiological, radiological, and antigenic endpoints. The decreases in the pulmonary infarct score, lung weight, residual fungal burden, and level of galactomannan antigenemia achieved with the combination of anidulafungin at 5 mg/kg/day and voriconazole were significant compared to those achieved with the monotherapies. Importantly, the magnitude of these interactions was similar for the in vitro and in vivo combination studies when they were analyzed by Bliss independence drug interaction analysis. These results, as well as those of subsequent animal and retrospective human studies showing a trend toward improved survival with combination therapy, laid the groundwork for prospective clinical trials of echinocandin-triazole combinations for the treatment of invasive aspergillosis in humans (37).
This randomized, double-blind, placebo-controlled, multicenter trial was performed to assess the safety and efficacy of voriconazole and anidulafungin compared with those of voriconazole monotherapy for the treatment of invasive aspergillosis (38). Among 277 patients with hematologic malignancy or hematopoietic stem cell transplantation in whom invasive aspergillosis was confirmed, mortality rates at 6 weeks were 19.3% for combination therapy and 27.5% for monotherapy (95% CI, −19.0 to 1.5%; P = 0.087). However, as invasive aspergillosis was diagnosed by radiographic findings and maximum galactomannan positivity in most patients (n = 218), a post hoc analysis of this subgroup was conducted. The rate of mortality at 6 weeks was significantly lower among those patients receiving combination therapy than among those receiving monotherapy (15.7% versus 27.3%; 95% CI, −22.7 to −0.4%; P = 0.037). Although there were limitations to the study design, this trial demonstrated the potential utility of combination antifungal therapy for the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancy or hematopoietic stem cell transplantation.
Further clinical trials of echinocandin-triazole combinations for the primary treatment of invasive aspergillosis are warranted. Our data show that rabbits treated with combination therapy with isavuconazole and micafungin demonstrated significant dose-dependent reductions in residual fungal burdens, decreased pulmonary injury, prolonged survival, lower GMI, and lower serum (1→3)-β-d-glucan levels in comparison to the findings achieved by treatment with isavuconazole or micafungin alone. These encouraging results, in conjunction with the favorable pharmacokinetic and pharmacodynamic profiles of these compounds, make them attractive agents for use in patients with invasive pulmonary aspergillosis. These data may help guide the design and interpretation of the results of studies of isavuconazole and micafungin in prospective clinical trials for the treatment of invasive aspergillosis.
MATERIALS AND METHODS
Isolate.
NIH Aspergillus fumigatus isolate 4215 (ATCC MYA-1163), which has been described previously (8) and which was obtained from a patient with a fatal case of pulmonary aspergillosis, was used in the study. The MICs of isavuconazole and the minimum effective concentration (MEC) of micafungin against A. fumigatus, determined according to CLSI standard microdilution methods (described in CLSI document M38-A2 [39]), were 1 μg/ml and 0.06 μg/ml, respectively. This isolate has been extensively used for more than 2 decades in studies of the activities of antifungal agents against invasive pulmonary aspergillosis.
Animals.
Healthy female New Zealand White rabbits (Covance Research Products, Inc., Denver, PA) weighing 2.6 to 3.5 kg at the time of endotracheal inoculation were used in replicate experiments. All rabbits were monitored and received humane care in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International according to the guidelines of the National Research Council (40) for the care and use of laboratory animals. This study was approved by the Institutional Animal Care and Use Committee (Weill Cornell Medicine of Cornell University). Rabbits were housed individually and were given water and standard rabbit feed ad libitum. Atraumatic vascular access was established by modified surgical placement of a silastic tunneled central venous catheter as previously described elsewhere (41). The silastic catheter permitted nontraumatic venous access for administration of parental agents and for repeated blood sampling for the study of plasma pharmacokinetics, serum galactomannan and (1→3)-β-d-glucan levels, and biochemical and hematological parameters.
Inoculum and inoculation.
For each experiment, an inoculum of 1 × 108 to 1.25 × 108 conidia of A. fumigatus was prepared in a volume of 250 μl to 350 μl. Inoculation was performed on day 2 of the experiments while the rabbits were under general anesthesia (0.5 to 0.6 ml of a 2:1 [vol/vol] mixture of ketamine at 100 mg/ml and xylazine at 20 mg/ml administered intravenously [i.v.]), as described previously (8).
Immunosuppression and maintenance of neutropenia.
Immunosuppression and profound persistent neutropenia (neutrophil concentration, <100 neutrophils/μl) were established and maintained using cytarabine (Ara-C; cytarabine injection; Zydus Hospira Oncology Private Ltd., Gujarat, India, for Hospira, Inc., Lake Forest, IL) and methylprednisolone (Solu-Medrol; Pfizer for Pharmacia & Upjohn Co., Division of Pfizer Inc., New York, NY), as described previously (8). Antibiotics (ceftazidime, gentamicin, vancomycin) were used for the prevention of opportunistic bacterial infections during neutropenia (8).
Antifungal compounds and treatment regimens.
The treatments included the prodrug isavuconazonium sulfate (BAL8557), which is equivalent to the active moiety isavuconazole (ISA; BAL4815), administered orally at 20 mg/kg/day (ISA20), 40 mg/kg/day (ISA40), and 60 mg/kg/day (ISA60); micafungin administered intravenously at 2 mg/kg/day (MFG2); the combination of ISA20 and MFG2, ISA40 and MFG2, or ISA60-MFG2; or no treatment (UC). Treatment started 24 h after endotracheal administration of the A. fumigatus inoculum and continued once daily for up to 12 days. The dosage of micafungin used in this study is comparable to the level of plasma exposure to approximately 0.5 mg/kg/day in humans. The dosage of isavuconazole of 20 to 40 mg/kg/day in the rabbit model approximates the level of plasma exposure achieved with the licensed human adult dose of 200 mg/day, while the dosage of 60 mg/kg/day produces an exposure approximating that achieved with 400 mg/day in human patients.
Outcome variables.
The following panel of outcome variables was used to assess antifungal efficacy: survival, pulmonary infarct score, lung weight, and residual fungal burden (log number of CFU per gram). The outcome variable panel was applied to all study rabbits when possible.
Pulmonary lesion scores, lung weights, and residual fungal burden.
The lungs were carefully resected at autopsy. Pulmonary lesion scores and lung weights were assessed and calculated as previously described (8). Lung tissue from each rabbit was sampled and cultured by standard excision of tissue from each lobe as previously described (8). The number of CFU of A. fumigatus was counted and recorded for each lobe, and the number of CFU per gram was calculated.
Survival.
The survival time (in days postinoculation) was recorded for each rabbit in each group. Following the achievement of humane endpoints, rabbits were euthanized by i.v. administration of pentobarbital (65 mg of pentobarbital sodium/kg of body weight; Beuthanasia-D Special [euthanasia solution]; Schering-Plough Animal Health Corp., Union, NJ) on day 13 postinoculation, 24 h after the last dose of study drug (41).
BAL.
Bronchoalveolar lavage (BAL) was performed as described previously (42) on each lung preparation by the instillation and subsequent withdrawal of 10 ml of sterile normal saline into the clamped trachea with a sterile 12-ml syringe. The instillations were repeated twice. The lavage fluid was then centrifuged for 10 min at 400 × g. Part of the supernatant was discarded, leaving 2 ml of pellet with supernatant, which was then vortexed. An aliquot of 100 μl of this fluid and 100 μl of a dilution (101) of this fluid were cultured on 5% Sabouraud glucose agar (SGA) plates.
Detection of galactomannan.
Serum samples were collected from each rabbit every other day and stored at −80°C before analysis. Galactomannan antigen levels in serial serum samples were determined, and BAL fluid obtained postmortem was analyzed by a one-stage immunoenzymatic sandwich microplate assay method (43) (Platelia Aspergillus enzyme immunoassay [EIA]; Bio-Rad, Marnes la Coquette, France) according to the manufacturer's instructions and as described elsewhere (42). Enzyme immunoassay data were expressed as a serum galactomannan index (GMI), which was plotted over time. The GMI for each test serum or BAL fluid sample was equal to the absorbance of a standard sample divided by the absorbance of a threshold serum sample provided by the manufacturer. A GMI of less than 0.5 was considered a negative result.
Detection of (1→3)-β-d-glucan.
Serum from each rabbit was collected every other day for determination of (1→3)-β-d-glucan levels by using a colorimetric assay (Fungitell; Associates of Cape Cod, Inc.) read at 405 nm (with subtraction of the 490-nm background reading), based upon para-nitroanilide absorption at that wavelength. The assay was performed according to the manufacturer's instructions and as described in detail elsewhere (44). The (1→3)-β-d-glucan levels were determined by taking the mean optical density for duplicate readings and comparing them with the values on a standard curve of predetermined concentrations. Interpretation of the results for the (1→3)-β-d-glucan levels was performed according to the manufacturer's instructions, as follows: <60 pg/ml, negative; 60 to 79 pg/ml, indeterminate; ≥80 pg/ml, positive. The median correlation coefficient (r) of the standard curves performed in these studies was ≥0.9992 (range, 0.9982 to 0.9998).
Statistical analysis.
Comparisons between the groups were performed by analysis of variance (ANOVA) with Bonferroni's correction for multiple comparisons or the Mann-Whitney U test, as appropriate. The central hypothesis of this analysis was based upon the response to isavuconazole in comparison to that to voriconazole and that of the untreated controls. A two-tailed P value of ≤0.05 was considered statistically significant. Survival was plotted by Kaplan-Meier analysis. Differences in the survival of the animals in the treatment groups and the untreated controls were analyzed by the log-rank test. Values are expressed as means ± standard errors of the means (SEMs). The values of the pharmacokinetic parameters were compared using ANOVA or Student's t test, as appropriate.
ACKNOWLEDGMENTS
This research was supported by an investigator-initiated grant from Astellas. T.J.W. is a scholar of the Henry Schueler Foundation and a scholar of Pediatric Infectious Diseases of the Sharp Family Foundation and receives support from the Save our Sick Kids Foundation, as well as research grants for experimental and clinical antimicrobial pharmacotherapeutics from Novartis, Merck, ContraFect, Pfizer, and Cubist.
T.J.W. has served as a consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. W.W.H. has acted as a consultant and/or received research support from Gilead, Astellas, Pfizer, and F2G. L.L.K. is an employee of Astellas Pharma Global Development, Inc. V.P., R.P., M.W.M., M.H.Z., K.H., N.S., B.B.W.M., and N.K.S. have no conflicts of interest to declare.
REFERENCES
- 1.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 2.Kosmidis C, Denning DW. 2015. The clinical spectrum of pulmonary aspergillosis. Thorax 70:270–277. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- 3.Patterson TF. 2009. Risk stratification for invasive aspergillosis: early assessment of host susceptibility. Med Mycol 47(Suppl 1):S255–S260. doi: 10.1080/13693780902718339. [DOI] [PubMed] [Google Scholar]
- 4.Rubio PM, Sevilla J, Gonzalez-Vicent M, Lassaletta A, Cuenca-Estrella M, Diaz MA, Riesco S, Madero L. 2009. Increasing incidence of invasive aspergillosis in pediatric hematology oncology patients over the last decade: a retrospective single centre study. J Pediatr Hematol Oncol 31:642–646. doi: 10.1097/MPH.0b013e3181acd956. [DOI] [PubMed] [Google Scholar]
- 5.Valdez JM, Scheinberg P, Young NS, Walsh TJ. 2009. Infections in patients with aplastic anemia. Semin Hematol 46:269–276. doi: 10.1053/j.seminhematol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Katragkou A, McCarthy M, Meletiadis J, Petraitis V, Moradi PW, Strauss GE, Fouant MM, Kovanda LL, Petraitiene R, Roilides E, Walsh TJ. 2014. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrob Agents Chemother 58:6934–6937. doi: 10.1128/AAC.03261-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petraitis V, Petraitiene R, Hope WW, Walsh TJ. 2017. Endpoint assessment in rabbit models of invasive pulmonary aspergillosis and mucormycosis. Methods Mol Biol 1625:259–277. doi: 10.1007/978-1-4939-7104-6_18. [DOI] [PubMed] [Google Scholar]
- 8.Petraitis V, Petraitiene R, Sarafandi AA, Kelaher AM, Lyman CA, Casler HE, Sein T, Groll AH, Bacher J, Avila NA, Walsh TJ. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J Infect Dis 187:1834–1843. doi: 10.1086/375420. [DOI] [PubMed] [Google Scholar]
- 9.Hope WW, Petraitis V, Petraitiene R, Aghamolla T, Bacher J, Walsh TJ. 2010. The initial 96 hours of invasive pulmonary aspergillosis: histopathology, comparative kinetics of galactomannan and (1→3) β-d-glucan and consequences of delayed antifungal therapy. Antimicrob Agents Chemother 54:4879–4886. doi: 10.1128/AAC.00673-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy MW, Petraitiene R, Walsh TJ. 2017. Translational development and application of (1→3)-beta-d-glucan for diagnosis and therapeutic monitoring of invasive mycoses. Int J Mol Sci 18:E1124. doi: 10.3390/ijms18061124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neofytos D, Railkar R, Mullane KM, Fredricks DN, Granwehr B, Marr KA, Almyroudis NG, Kontoyiannis DP, Maertens J, Fox R, Douglas C, Iannone R, Kauh E, Shire N. 2015. Correlation between circulating fungal biomarkers and clinical outcome in invasive aspergillosis. PLoS One 10:e0129022. doi: 10.1371/journal.pone.0129022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petraitiene R, Petraitis V, Bacher JD, Finkelman MA, Walsh TJ. 2015. Effects of host response and antifungal therapy on serum and BAL levels of galactomannan and (1→3)-beta-d-glucan in experimental invasive pulmonary aspergillosis. Med Mycol 53:558–568. doi: 10.1093/mmy/myv034. [DOI] [PubMed] [Google Scholar]
- 13.Petraitis V, Petraitiene R, Hope WW, Meletiadis J, Mickiene D, Hughes JE, Cotton MP, Stergiopoulou T, Kasai M, Francesconi A, Schaufele RL, Sein T, Avila NA, Bacher J, Walsh TJ. 2009. Combination therapy in treatment of experimental pulmonary aspergillosis: in vitro and in vivo correlations of the concentration- and dose-dependent interactions between anidulafungin and voriconazole by Bliss independence drug interaction analysis. Antimicrob Agents Chemother 53:2382–2391. doi: 10.1128/AAC.00329-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovanda LL, Petraitiene R, Petraitis V, Walsh TJ, Desai A, Bonate P, Hope WW. 2016. Pharmacodynamics of isavuconazole in experimental invasive pulmonary aspergillosis: implications for clinical breakpoints. J Antimicrob Chemother 71:1885–1891. doi: 10.1093/jac/dkw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TK, Groncy PK, Javahery R, Chai RY, Nagpala P, Finkelman M, Petraitiene R, Walsh TJ. 2017. Successful treatment of Aspergillus ventriculitis through voriconazole adaptive pharmacotherapy, immunomodulation, and therapeutic monitoring of cerebrospinal fluid (1→3)-beta-d-glucan. Med Mycol 55:109–117. doi: 10.1093/mmy/myw118. [DOI] [PubMed] [Google Scholar]
- 16.Kovanda LL, Desai AV, Hope WW. 2017. Prognostic value of galactomannan: current evidence for monitoring response to antifungal therapy in patients with invasive aspergillosis. J Pharmacokinet Pharmacodyn 44:143–151. doi: 10.1007/s10928-017-9509-1. [DOI] [PubMed] [Google Scholar]
- 17.Kovanda LL, Kolamunnage-Dona R, Neely M, Maertens J, Lee M, Hope WW. 2017. Pharmacodynamics of isavuconazole for invasive mold disease: role of galactomannan for real-time monitoring of therapeutic response. Clin Infect Dis 64:1557–1563. doi: 10.1093/cid/cix198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marr KA. 2008. Aspergillus galactomannan index: a surrogate end point to assess outcome of therapy? Clin Infect Dis 46:1423–1425. doi: 10.1086/528715. [DOI] [PubMed] [Google Scholar]
- 19.Miceli MH, Grazziutti ML, Woods G, Zhao W, Kocoglu MH, Barlogie B, Anaissie E. 2008. Strong correlation between serum Aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin Infect Dis 46:1412–1422. doi: 10.1086/528714. [DOI] [PubMed] [Google Scholar]
- 20.Nouer SA, Nucci M, Kumar NS, Grazziutti M, Barlogie B, Anaissie E. 2011. Earlier response assessment in invasive aspergillosis based on the kinetics of serum Aspergillus galactomannan: proposal for a new definition. Clin Infect Dis 53:671–676. doi: 10.1093/cid/cir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods G, Miceli MH, Grazziutti ML, Zhao W, Barlogie B, Anaissie E. 2007. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer 110:830–834. doi: 10.1002/cncr.22863. [DOI] [PubMed] [Google Scholar]
- 22.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer-Korting M. 2003. New systemic antifungal drugs: mechanisms of action, drug interactions and side effects. Mycoses 46(Suppl 1):28–31. (In German.) doi: 10.1111/j.1439-0507.2003.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 24.Podust LM, Poulos TL, Waterman MR. 2001. Crystal structure of cytochrome P450 14alpha-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc Natl Acad Sci U S A 98:3068–3073. doi: 10.1073/pnas.061562898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ananda-Rajah MR, Kontoyiannis D. 2015. Isavuconazole: a new extended spectrum triazole for invasive mold diseases. Future Microbiol 10:693–708. doi: 10.2217/fmb.15.34. [DOI] [PubMed] [Google Scholar]
- 26.Livermore J, Hope W. 2012. Evaluation of the pharmacokinetics and clinical utility of isavuconazole for treatment of invasive fungal infections. Expert Opin Drug Metab Toxicol 8:759–765. doi: 10.1517/17425255.2012.683859. [DOI] [PubMed] [Google Scholar]
- 27.Miceli MH, Kauffman CA. 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis 61:1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 28.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Guinea J, Hagen F, Meis JF, Thompson GR III, Turnidge J. 2015. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for the Cryptococcus neoformans-Cryptococcus gattii species complex using the CLSI M27-A3 broth microdilution method. Antimicrob Agents Chemother 59:666–668. doi: 10.1128/AAC.04055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Florl C, Martin-Mazuelos E, Meis J, Pelaez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob Agents Chemother 57:3823–3828. doi: 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2013. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J Clin Microbiol 51:2608–2616. doi: 10.1128/JCM.00863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller MA, Rhomberg PR, Messer SA, Jones RN, Castanheira M. 2015. Isavuconazole, micafungin, and 8 comparator antifungal agents' susceptibility profiles for common and uncommon opportunistic fungi collected in 2013: temporal analysis of antifungal drug resistance using CLSI species-specific clinical breakpoints and proposed epidemiological cutoff values. Diagn Microbiol Infect Dis 82:303–313. doi: 10.1016/j.diagmicrobio.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and cyp51 mutant isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 57:6284–6289. doi: 10.1128/AAC.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seyedmousavi S, Verweij PE, Mouton JW. 2015. Isavuconazole, a broad-spectrum triazole for the treatment of systemic fungal diseases. Expert Rev Anti Infect Ther 13:9–27. doi: 10.1586/14787210.2015.990382. [DOI] [PubMed] [Google Scholar]
- 34.Petraitis V, Petraitiene R, Moradi PW, Strauss GE, Katragkou A, Kovanda LL, Hope WW, Walsh TJ. 2016. Pharmacokinetics and concentration-dependent efficacy of isavuconazole for treatment of experimental invasive pulmonary aspergillosis. Antimicrob Agents Chemother 60:2718–2726. doi: 10.1128/AAC.02665-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petraitis V, Petraitiene R, Groll AH, Roussillon K, Hemmings M, Lyman CA, Sein T, Bacher J, Bekersky I, Walsh TJ. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother 46:1857–1869. doi: 10.1128/AAC.46.6.1857-1869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee DG, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR III, Lee M, Maher RM, Schmitt-Hoffmann AH, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Sun WK, Wu T, Chen F, Xu XY, Su X, Shi Y. 2014. Efficacy of combination therapy of triazole and echinocandin in treatment of invasive aspergillosis: a systematic review of animal and human studies. J Thorac Dis 6:99–108. doi: 10.3978/j.issn.2072-1439.2014.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee DG, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR, Maertens JA. 2015. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 40.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 41.Walsh TJ, Bacher J, Pizzo PA. 1988. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci 38:467–471. [PubMed] [Google Scholar]
- 42.Petraitiene R, Petraitis V, Groll AH, Sein T, Piscitelli S, Candelario M, Field-Ridley A, Avila N, Bacher J, Walsh TJ. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob Agents Chemother 45:857–869. doi: 10.1128/AAC.45.3.857-869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stynen D, Sarfati J, Goris A, Prevost MC, Lesourd M, Kamphuis H, Darras V, Latge JP. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun 60:2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petraitiene R, Petraitis V, Hope WW, Mickiene D, Kelaher AM, Murray HA, Mya-San C, Hughes JE, Cotton MP, Bacher J, Walsh TJ. 2008. Cerebrospinal fluid and plasma (1→3)-beta-d-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother 52:4121–4129. doi: 10.1128/AAC.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]