ABSTRACT
Diabetic foot ulcer treatment currently focuses on targeting bacterial biofilms, while dismissing fungi. To investigate this, we used an in vitro biofilm model containing bacteria and fungi, reflective of the wound environment, to test the impact of antimicrobials. Here we showed that while monotreatment approaches influenced biofilm composition, this had no discernible effect on overall quantity. Only by combining bacterium- and fungus-specific antibiotics were we able to decrease the biofilm bioburden, irrespective of composition.
KEYWORDS: biofilm, polymicrobial, chronic wound, antimicrobial, Candida
TEXT
Diabetic foot ulcers (DFU) are an increasing health care burden and cause excessive amounts of patient morbidity and mortality. It is known that infection both impairs healing and is linked to the recurrence of ulcers (1). It has been shown that chronic wounds often harbor pathogenic biofilms of a polymicrobial nature and are commonly recalcitrant to treatment (2–5). Guidelines suggest the initial use of oral antibiotics for empirical therapy with activity against Gram-positive organisms, especially penicillins such as flucloxacillin (6, 7). Further coverage is provided by other antibiotics, such as the fluoroquinolone ciprofloxacin (7). Despite these chemotherapeutic approaches, resolution of infection is often hindered because of the failure to account for coinfecting pathogenic fungi (8). Indeed, fungal infection in DFUs is underrecognized, although recently some studies have begun to shed light on the significant involvement of the wound mycobiome (9). While antifungal treatments have been shown to improve DFU outcome, they are only used routinely to treat superficial fungal infections in diabetics, such as onychomycosis (10, 11). Therefore, it is not a great leap to suspect fungal components as a key contributor to pathogenic DFU biofilms.
Characterized laboratory strains were used to create biofilms in standard 96-well plates, including the bacteria Pseudomonas aeruginosa PA14 and Staphylococcus aureus ATCC 13420 and the yeast Candida albicans SC5314 (12). Biofilm antimicrobial susceptibility testing was carried out as described previously by our group to determine the sessile MICs (13). Briefly, monospecies and coculture biofilms were grown in Mueller-Hinton broth (bacterial cultures) or RPMI broth (fungal cultures) before being treated with the flucloxacillin, ciprofloxacin, or fluconazole (Sigma, Dorset, United Kingdom), which are common clinically at a range of concentrations (0.125 to 128 mg/liter) (6, 7, 11). Viability posttreatment was assessed using alamarBlue metabolic dye. Here it was shown that an increase in species diversity leads to elevated viability following treatment (Fig. 1; P < 0.01), as evidenced by S. aureus coculture and triadic biofilms after ciprofloxacin therapy. A similar, but less dramatic, effect was observed with flucloxacillin, although for fluconazole, no discernible effect was observed (see Fig. S1 in the supplemental material). This indicates that the increased complexity of a biofilm community and physical structure provided by C. albicans lead to enhanced resistance, as has been shown elsewhere (14, 15).
FIG 1.

Increased microbial complexity of biofilms leads to reduced susceptibility to antimicrobial agents. Biofilm (sessile) MIC values were calculated using the alamarBlue viability test. All tests were carried out in quadruplicates on three separate occasions as described in the text. Monoculture (S. aureus [Sa]) biofilms were compared to coculture biofilms (S. aureus plus C. albicans [Sa + Ca] or S. aureus plus P. aeruginosa [Sa + Pa]) and triadic biofilms (S. aureus plus C. albicans plus P. aeruginosa [Sa + Ca + Pa]). Data were analyzed using a two-way analysis of variance (ANOVA) with Tukey's multiple-comparison test to compare each mono- or coculture at each antimicrobial (ciprofloxacin) concentration (in micrograms per milliliter).
Our group has recently created and described an in vitro interkingdom biofilm model that reflects a chronic wound environment, a model formed within a three-dimensional cellulose matrix (12). We therefore aimed to use this model to characterize in vitro responses to antibiotic pressure in a triadic interkingdom biofilm model that is reflective of the chronic wound environment. Triadic biofilms in the cellulose model were created by standardizing all three microorganisms to 1 × 106 CFU/ml in phosphate-buffered saline (PBS) and incubating them with 1.25-cm2 sections of cellulose matrix for 2 h at 37°C with agitation. The matrix was then placed on top of a 50% serum hydrogel surface and incubated at 37°C for 24 h (12). Following biofilm development, these were treated with 128 mg/ml flucloxacillin, ciprofloxacin, and fluconazole, either alone or in combination, for a further 24 h at 37°C alongside untreated controls. All testing was carried out in triplicate on three separate occasions. To differentiate between live and dead cells, a quantitative PCR (qPCR)-based assay (16–19) was used to assess the viable composition of the biofilms with species-specific primers (12, 19). This assay utilizes propidium monoazide, a DNA intercalating dye, which binds to DNA in cells with a compromised membrane preventing this DNA from being amplified in downstream PCR. Therefore, only DNA from viable cells with intact membranes is detected. MasterPure yeast DNA extraction kits were used as per the manufacturer's instruction (Epicentre, Cambio, Cambridge, United Kingdom) to process all samples. For qPCR, a Fast SYBR green master mix (Life Technologies, Paisley, United Kingdom) was used with the primer sequences and thermal profile previously defined (12). Each sample was analyzed in duplicate using a Step One real-time PCR system and software (Life Technologies, Paisley, United Kingdom). Samples were quantified to calculate the colony-forming equivalents (CFE) based upon a standard curve per reaction performed. Results were also confirmed by CFU counts using the Miles and Misra technique (20).
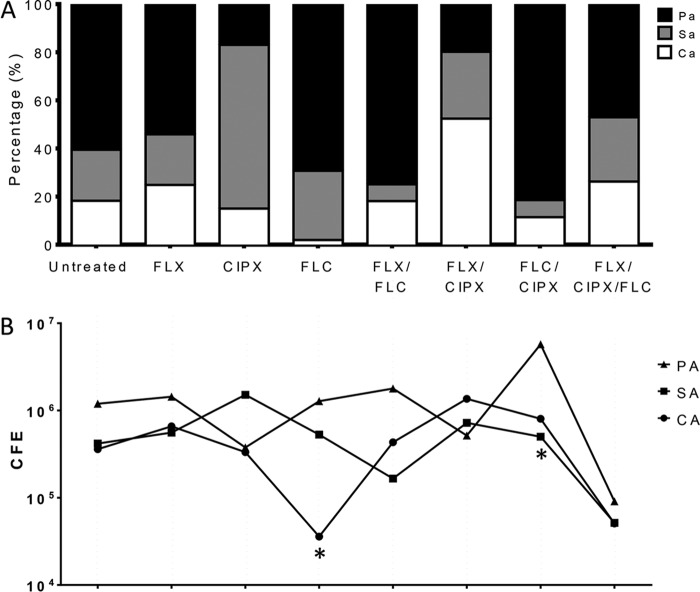
The cellulose matrix model has already been shown to create more resilient biofilms (12), and when treated with elevated levels of antibiotics (128 mg/liter), there was little impact on the viable cells within the matrix, although compositionally the biofilms were affected (Fig. 2A). This implies that elimination of one microbial component of the biofilm through targeted therapy creates a niche for the other species to thrive. For example, fluconazole treatment significantly decreased C. albicans by approximately 1 × log10 (P < 0.05). In contrast, a combined treatment with flucloxacillin-ciprofloxacin resulted in a 3-fold increase in C. albicans (Fig. 2B). In addition to consideration of the biofilm composition, the biovolume is equally important, given that a C. albicans cell is approximately 10 times the size of an S. aureus cell. Consequently, even in biofilms where C. albicans is in low abundance, the yeast and hyphal cells still provide physical structure and support to biofilm through its spatial dominance. The only treatment observed to cause a substantial decrease in all three microbial components and an overall reduction in viable cell composition was the combination of all three antimicrobials (Fig. 3). Hierarchical clustering analysis also shows that the C. albicans population is very closely linked to the total CFE present, suggesting it is a key driving force within the biofilm community (Fig. 3).
FIG 2.
Triadic biofilm composition is influenced by antimicrobial treatment. The antibiotics flucloxacillin (FLX) and ciprofloxacin (CIPX) and the antifungal fluconazole (FLC) were used to treat the biofilms, either alone or in combination as described in the text. Biofilm percentage composition is shown in the bar graphs (A), while absolute numbers of viable colony-forming equivalents (CFE) present are shown below (B). Treated biofilms were compared to untreated controls using a two-tailed unpaired t test (*, P < 0.05). Pa/PA, P. aeruginosa; Sa/SA, S. aureus; Ca/CA, C. albicans.
FIG 3.

Triple antimicrobial treatment elicits the largest impact on biofilms. The heat map shows a fold change increase in viable CFE (red), decrease (blue), or no change (white) after treatment with flucloxacillin (FLX), ciprofloxacin (CIPX), and fluconazole (FLC), either alone or in combination. Hierarchical clustering analysis (left) shows that the total bioburden is closely related to C. albicans (CA), suggesting this is the component of the biofilm that is integral to infection. SA, S. aureus; PA, P. aeruginosa.
The data from this investigation suggest that antifungal drugs should be included in empirical therapy options alongside antibiotics. It has been recently shown that the hyphal structure and extracellular matrix of C. albicans mycofilms support bacterial growth, leading to more resilient biofilms in terms of antibiotics and antifungals (14, 15). These key findings indicate that disrupting and/or impeding this supportive mycofilm structure could lead to a physical collapse of the polymicrobial community. Fungi are increasingly recognized as a key contributor to biofilms in DFUs, and we have shown here that C. albicans appears to be an important element behind the recalcitrant nature of these biofilms. Therefore, it is imperative to consider a treatment covering these major pathogens, rather than considering them as having a supporting role. Inclusion of antifungals in routine treatment strategies could allow for easier disruption of the biofilm, decreasing microbial load in DFUs and ultimately improving patient outcomes.
Supplementary Material
ACKNOWLEDGMENT
We thank Ian Davies (IPS Converters) for supplying the cellulose matrix used in the three-dimensional model.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00672-17.
REFERENCES
- 1.Dubský M, Jirkovská A, Bem R, Fejfarová V, Skibová J, Schaper NC, Lipsky BA. 2013. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J 10:555–561. doi: 10.1111/j.1742-481X.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James GA, Swogger E, Wolcott R, Secor P, Sestrich J, Costerton JW, Stewart PS. 2008. Biofilms in chronic wounds. Wound Repair Regen 16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 3.Neut D, Tijdens-Creusen EJ, Bulstra SK, van der Mei HC, Busscher HJ. 2011. Biofilms in chronic diabetic foot ulcers—a study of 2 cases. Acta Orthop 82:383–385. doi: 10.3109/17453674.2011.581265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith K, Collier A, Townsend EM, O'Donnell LE, Bal AM, Butcher J, Mackay WG, Ramage G, Williams C. 2016. One step closer to understanding the role of bacteria in diabetic foot ulcers: characterising the microbiome of ulcers. BMC Microbiol 16:54. doi: 10.1186/s12866-016-0665-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramage G, Rajendran R, Sherry L, Williams C. 2012. Fungal biofilm resistance. Int J Microbiol 2012:528521. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. 2015. Diabetic foot problems: prevention and management. National Institute for Health and Care Excellence, London, United Kingdom. [Google Scholar]
- 7.Edmonds M. 2006. Diabetic foot ulcers. Drugs 66:913–929. doi: 10.2165/00003495-200666070-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chellan G, Shivaprakash S, Ramaiyar SK, Varma AK, Varma N, Sukumaran MT, Vasukutty JR, Bal A, Kumar H. 2010. Spectrum and prevalence of fungi infecting deep tissues of lower-limb wounds in patients with type 2 diabetes. J Clin Microbiol 48:2097–2102. doi: 10.1128/JCM.02035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalan L, Loesche M, Hodkinson BP, Heilmann K, Ruthel G, Gardner SE, Grice EA. 2016. Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing. mBio 7:e01058-16. doi: 10.1128/mBio.01058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chellan G, Neethu K, Varma A, Mangalanandan T, Shashikala S, Dinesh K, Sundaram K, Varma N, Jayakumar R, Bal A. 2012. Targeted treatment of invasive fungal infections accelerates healing of foot wounds in patients with type 2 diabetes. Diabetic Med 29:e255–e262. doi: 10.1111/j.1464-5491.2012.03574.x. [DOI] [PubMed] [Google Scholar]
- 11.Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. 2006. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg 117:193S–209S. doi: 10.1097/01.prs.0000225459.93750.29. [DOI] [PubMed] [Google Scholar]
- 12.Townsend EM, Sherry L, Rajendran R, Hansom D, Butcher J, Mackay WG, Williams C, Ramage G. 2016. Development and characterisation of a novel three-dimensional inter-kingdom wound biofilm model. Biofouling 32:1259–1270. doi: 10.1080/08927014.2016.1252337. [DOI] [PubMed] [Google Scholar]
- 13.Sherry L, Millhouse E, Lappin DF, Murray C, Culshaw S, Nile CJ, Ramage G. 2013. Investigating the biological properties of carbohydrate derived fulvic acid (CHD-FA) as a potential novel therapy for the management of oral biofilm infections. BMC Oral Health 13:47. doi: 10.1186/1472-6831-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kean R, Rajendran R, Haggarty J, Townsend EM, Short B, Burgess KE, Lang S, Millington O, Mackay W, Williams C. 2017. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front Microbiol 8:258. doi: 10.3389/fmicb.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong EF, Tsui C, Kucharíková S, Andes D, Van Dijck P, Jabra-Rizk MA. 2016. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 7:e01365-16. doi: 10.1128/mBio.01365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez G, Gonzalez M, Isabal S, Blanc V, Leon R. 2013. Method to quantify live and dead cells in multi-species oral biofilm by real-time PCR with propidium monoazide. AMB Express 3:1. doi: 10.1186/2191-0855-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez MC, Marin MJ, Figuero E, Llama-Palacios A, Leon R, Blanc V, Herrera D, Sanz M. 2014. Quantitative real-time PCR combined with propidium monoazide for the selective quantification of viable periodontal pathogens in an in vitro subgingival biofilm model. J Periodont Res 49:20–28. doi: 10.1111/jre.12073. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez MC, Marin MJ, Figuero E, Llama-Palacios A, Herrera D, Sanz M. 2013. Analysis of viable vs. dead Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis using selective quantitative real-time PCR with propidium monoazide. J Periodont Res 48:213–220. doi: 10.1111/j.1600-0765.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- 19.Sherry L, Lappin G, O'Donnell L, Millhouse E, Millington OR, Bradshaw D, Axe A, Williams C, Nile CJ, Ramage G. 2016. Viable compositional analysis of an eleven species oral polymicrobial biofilm. Front Microbiol 7:912. doi: 10.3389/fmicb.2016.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miles A, Misra S, Irwin J. 1938. The estimation of the bactericidal power of the blood. J Hyg 38:732–749. doi: 10.1017/S002217240001158X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.