ABSTRACT
Hospital-associated methicillin-resistant Staphylococcus aureus (MRSA) strains typically express high-level, homogeneous (HoR) β-lactam resistance, whereas community-associated MRSA (CA-MRSA) more commonly express low-level heterogeneous (HeR) resistance. Expression of the HoR phenotype typically requires both increased expression of the mecA gene, carried on the staphylococcal cassette chromosome mec element (SCCmec), and additional mutational event(s) elsewhere on the chromosome. Here the oxacillin concentration in a chemostat culture of the CA-MRSA strain USA300 was increased from 8 μg/ml to 130 μg/ml over 13 days to isolate highly oxacillin-resistant derivatives. A stable, small-colony variant, designated HoR34, which had become established in the chemostat culture was found to have acquired mutations in gdpP, clpX, guaA, and camS. Closer inspection of the genome sequence data further revealed that reads covering SCCmec were ∼10 times overrepresented compared to other parts of the chromosome. Quantitative PCR (qPCR) confirmed >10-fold-higher levels of mecA DNA on the HoR34 chromosome, and MinION genome sequencing verified the presence of 10 tandem repeats of the SCCmec element. qPCR further demonstrated that subculture of HoR34 in various concentrations of oxacillin (0 to 100 μg/ml) was accompanied by accordion-like contraction and amplification of the SCCmec element. Although slower growing than strain USA300, HoR34 outcompeted the parent strain in the presence of subinhibitory oxacillin. These data identify tandem amplification of the SCCmec element as a new mechanism of high-level methicillin resistance in MRSA, which may provide a competitive advantage for MRSA under antibiotic selection.
KEYWORDS: continuous culture, SCCmec, Staphylococcus, antibiotic resistance, beta-lactams, mecA beta-lactam, antibiotic, resistance, mechanism
INTRODUCTION
In recent decades, the overall incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections has greatly increased due to the emergence of community-associated MRSA (CA-MRSA), which are increasingly displacing hospital-associated-MRSA (HA-MRSA) strains in health care settings (1). Methicillin resistance is mediated by the mecA-encoded low-affinity penicillin binding protein 2a carried on the mobile staphylococcal cassette chromosome mec element (SCCmec). Heterogeneity is a feature of S. aureus methicillin resistance (2). In general, clinical CA-MRSA isolates exhibit low-level, heterogeneous methicillin resistance (HeR) under laboratory growth conditions, whereas HA-MRSA isolates can exhibit high-level, homogeneous methicillin resistance (HoR). HeR strains can express a HoR phenotype after selection on elevated concentrations of β-lactam antibiotics, via mechanism(s) involving the stringent response and altered cyclic di-AMP (c-di-AMP) signaling (2).
In general, the capacity of pathogens like MRSA to become resistant to new drugs only becomes apparent months or years after their introduction into clinical practice, during which time exposure of the pathogen to new drugs gradually increases, as does the likelihood that endogenous resistance will emerge. This clinical scenario can be mimicked in the laboratory using standard, batch culture techniques to isolate bacterial mutants exhibiting resistance to an antimicrobial drug. However, such artificial culture conditions can mask the impact of acquired antimicrobial resistance (AMR) on bacterial fitness (3, 4), a phenomenon that plays a significant role in determining maintenance and spread of the AMR genotype in natural bacterial populations, and affects the disease-causing capacity of the pathogen. Here we used a continuous-growth chemostat to address this limitation by creating a more dynamic and competitive environment from which to isolate physiologically relevant β-lactam-resistant mutants. An S. aureus USA300 culture was exposed to increasing concentrations of oxacillin (8 to 130 μg/ml) over a 13-day period. Among the hyperresistant mutants isolated was a stable small-colony variant in which the tandem amplification of the SCCmec element was identified as a new mechanism of high-level β-lactam resistance in MRSA.
RESULTS AND DISCUSSION
Isolation of S. aureus USA300 oxacillin-hyperresistant mutants.
An S. aureus USA300 nutrient broth culture was grown in a chemostat for 13 days. A sub-MIC of oxacillin was used at the start of the chemostat culture and increased on an incremental, daily basis up to 130 μg/ml (equivalent to 800 μg/ml on Mueller-Hinton, brain heart infusion [BHI], or nutrient agar), as described in Materials and Methods. Isolated hyperresistant mutants were readily differentiated into (i) white-colored small-colony variants and (ii) regular-sized, pigmented colonies (Fig. 1A). Using population analysis profiling as described previously (5), all the mutants were shown to be homogeneously resistant (HoR) (data not shown) and exhibited oxacillin MICs of 800 μg/ml. Further analysis revealed that the small-colony mutants appeared to be phenotypically similar, exhibiting the same biofilm-forming capacity and repressed beta-hemolysis (data not shown). In contrast, the faster-growing HoR mutants appeared to be heterogeneous, exhibiting different levels of biofilm-forming capacity and beta-hemolytic activity on sheep blood agar (data not shown). Whole-genome sequencing further revealed a variety of different mutations in nine HoR mutants recovered from the chemostat (Table 1). These included four mutants with Ser67Lys amino acid substitutions in DacA, the diadenylate cyclase responsible for synthesis of c-di-AMP, which has previously been implicated in the HoR phenotype (2, 6, 7), four mutants with five different mutations in genes encoding predicted lipoproteins, and one mutant with a Glu227Gln substitution in a predicted ABC transporter designated abcA (8). Mutation of the abcA gene has previously been shown to increase β-lactam resistance and is associated with upregulation of the adjacent pbpD gene, which encodes penicillin binding protein 4 (8).
FIG 1.
Growth and cell morphology phenotypes of MRSA strains USA300 and HoR34. (A) Small-colony variants and other isolates recovered from the chemostat culture after 13 days at a final oxacillin concentration of 130 mg/liter grown on BHI agar for 24 h. (B) Growth curve of USA300 and HoR34 grown for 20 h in BHI medium at 37°C with vigorous aeration. The number of CFU per milliliter in culture samples removed at regular intervals was determined by plating on BHI agar. (C) Cell morphology of USA300 and HoR34 imaged using transmission electron microscopy (TEM) at a magnification of ×8,000. (D) Cell wall thickness of USA300 and HoR34 determined using TEM at a magnification of ×100,000 and AMT v.542 imaging software.
TABLE 1.
Genetic alterations in MRSA USA300 oxacillin-hyperresistant mutants from the chemostat culture
| Growth characteristic and isolate(s) | Genome position(s) | Nucleotide change(s) | Amino acid change(s) | Locus tag/gene(s) (protein encoded by gene [reference]) |
|---|---|---|---|---|
| Fast-growing isolates | ||||
| HoR20 | 703854 | G to C | Glu227Gln | RS03375/abcA |
| HoR18, HoR21, HoR27, and HoR36 | 110748, 110752,111618, 111630, and 111648 | Multiple | Multiple | RS00520 to RS00525/uncharacterized lipoprotein genes |
| HoR33, HoR41, HoR43, and HoR46 | 2288896 | G to A | Ser67Lys | RS11640/dacA |
| Slow-growing HoR34 isolate | 19122 | A to C | Thr260Pro | gdpP (c-di-AMP phosphodiesterase [14]) |
| 44078 | C to T | Ala314Val | guaA (GMP synthetase [9]) | |
| 441379 | G to T | Glu511Asp | guaA | |
| 1775825 | C to A | Glu37STOP | clpX (chaperone with ClpP-dependent role in protein degradation and ClpP-independent role in protein folding [10]) | |
| 2046530 | G to A | Gln305STOP | camS (membrane lipoprotein involved in sex pheromone biosynthesis [11]) |
In addition to a small colony size (Fig. 1A), impaired growth (Fig. 1B), and expression of hyperresistance to oxacillin, a representative small-colony variant (SCV) HoR, designated HoR34, also exhibited altered cell morphology, including defective septum formation (Fig. 1C) and an approximately twofold increase in cell wall thickness (18.6 ± 1.8 nm in strain USA300 versus 36.1 ± 4.2 nm in strain HoR34) (Fig. 1D). Whole-genome sequence analysis of HoR34 and the parent USA300 strain compared to the publically available USA300 FPR3757 genome revealed that plasmid pUSA02 (which carries tetracycline resistance) had been lost and identified nonsynonymous mutations in the gdpP (c-di-AMP phosphodiesterase [2, 7]), guaA (GMP synthetase [9]), clpX (chaperone protein [10]), and camS (membrane lipoprotein [11]) genes (Table 1). GdpP is an c-di-AMP phosphodiesterase responsible for turnover of c-di-AMP synthesized by DacA, and it has previously been implicated in the HoR phenotype (2, 7) but not a small-colony phenotype, which is clinically important in persistent infections (12). Therefore, to determine whether the guaA, clpX, and/or camS mutation(s) (alone or in combination) was involved in the small colony size of HoR34, the mutant was subjected to daily subculture in the absence of antibiotic selection for 2 weeks in an effort to isolate fast-growing revertants. The SCV phenotype of HoR34 was stable, and no fast-growing revertants were isolated even after repeated attempts. However, the oxacillin MIC of the passaged HoR34 strain, designated HoR34p, was reduced from 800 μg/ml to 300 μg/ml, indicating that although the strain continued to be hyperresistant, oxacillin resistance levels in this strain can be regulated.
To further tease out the contributions of the guaA, clpX, camS, and gdpP mutations to the HoR34 phenotypes, wild-type alleles of the four genes, including their upstream promoter sequences, were cloned on the medium-copy-number Escherichia coli-Staphylococcus shuttle plasmid pLI50 and introduced into strain HoR34. The multicopy clpX plasmid was unstable in HoR34 and rapidly lost in the absence of antibiotic selection. Furthermore, imposition of continuous antibiotic selection for the pclpX plasmid in HoR34 appeared to be accompanied by the selection of compensatory mutations, as evidenced by the rapid emergence of fast-growing colonies among the HoR34 small-colony variants. Although we were unable to carry out this complementation experiment further, two previous studies have shown that mutation of clpX is associated with increased resistance to β-lactam antibiotics (albeit not to the levels measured in HoR34) (10, 13), suggesting that the clpX mutation may contribute in part to increased oxacillin resistance in HoR34. The remaining complementation experiments revealed that neither gdpP nor the camS and guaA genes had any significant effect on the colony morphology (data not shown) or oxacillin MIC of HoR34, as measured by Etest (Fig. 2A) and agar dilutions (data not shown). Furthermore, the doubling times for HoR34 (31.9 min), HoR34 pguaA (33.0 min), HoR34 pgdpP (31.7 min), and HoR34 pcamS (28.9 min) were all substantially lower than the doubling times for USA300 (22.6 min) and HoR34 grown in 0.5 μg of oxacillin per ml (32.70 min) but not significantly different from each other, indicating that the guaA, camS, and gdpP mutations alone did not affect growth rate. Because GdpP and c-di-AMP signaling also contributes to the regulation of autolytic activity (2, 14), we further investigated this phenotype. Consistent with previous studies, the gdpP mutation in HoR34 was associated with increased autolytic activity that was successfully complemented only by gdpP and not by camS or guaA (Fig. 2B). The potential roles of the identified mutations in guaA, clpX, and camS in the HoR phenotype remain unclear, but they may have emerged initially to support growth or maintain fitness at relatively lower oxacillin concentrations during the early stages of growth in the chemostat. It seems unlikely that the mutations in camS, clpX, or guaA are accompanied by any gain of function; the clpX and camS genes contain mutations introducing stop codons (Table 1), while predicted loss-of-function mutations in guaA have previously been implicated in the HoR phenotype (7). Taken together, these data suggest that the mutations in guaA, clpX, camS, and gdpP, at least on their own, are not responsible for the HoR34 oxacillin hyperresistance phenotype and raised the possibility that other genomic rearrangements were responsible for this phenotype.
FIG 2.
Oxacillin susceptibility and autolysis phenotypes of strains USA300 and HoR34. (A) Oxacillin MICs of strains USA300, HoR34, and HoR34 carrying plasmids pLI50 (control), pgdpP, pguaA, and pcamS determined using Etests. (B) Autolytic activity in USA300 and HoR34. Strains USA300, HoR34, and HoR34 carrying plasmids pLI50 (control), pgdpP, pguaA, and pcamS, and a USA300 JE2 atl mutant (negative control) were grown to early exponential phase in BHI at 37°C, washed in phosphate-buffered saline (PBS), and adjusted to an A600 of 1.0 in 0.01% Triton X-100. The A600 was measured initially and at 15-min intervals thereafter with shaking incubation at 37°C. Autolytic activity is expressed as a percentage of the initial A600. Average results from three independent experiments are shown.
Chromosomal amplification of the SCCmec element in strain HoR34.
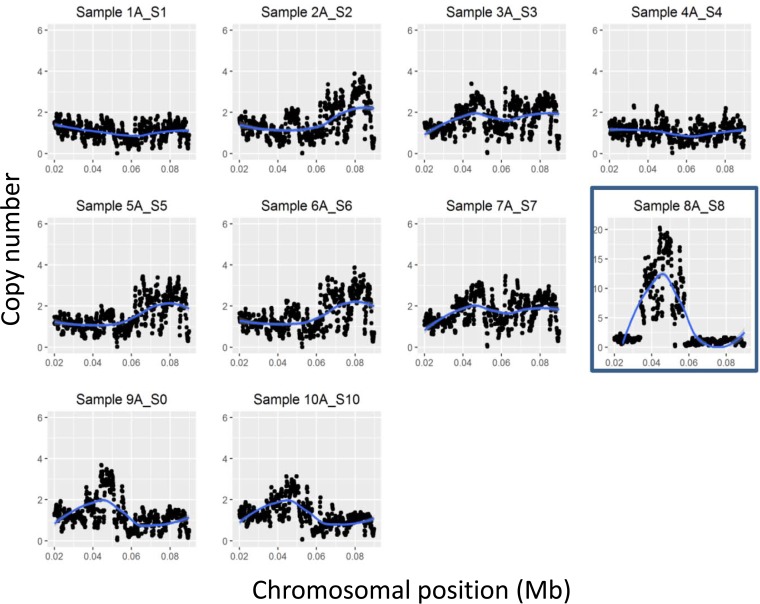
A number of recent studies have indicated that large regions of the S. aureus chromosome can undergo duplication and amplification events (15, 16). To investigate whether such genomic rearrangements had taken place in strain HoR34, read coverage across the genome was analyzed. Illumina sequence reads covering the SCCmec element were >10 times overrepresented compared to other parts of the chromosome (Fig. 3). LightCycler quantitative PCR (qPCR) confirmed 10-fold-higher levels of mecA in HoR34 genomic DNA samples compared to USA300 (data not shown). To determine whether the SCCmec element had amplified on the chromosome or excised and reintegrated at multiple sites around the chromosome, we attempted to assemble the Illumina sequence reads corresponding to the SCCmec element into contigs. However, these efforts were hampered by the short reads. To address this, we resequenced the HoR34 genome using MinION technology, which generates sequence reads of 10 kb onto which the Illumina sequence reads were mapped. The combined MinION/Illumina sequence data revealed the presence of 10 tandem SCCmec element repeats on the HoR34 chromosome (Fig. 4). All 10 copies of SCCmec were completely intact, and no additional DNA sequences were identified at the join sites. Oligonucleotide primers designed to span the join sites of tandem SCCmec elements amplified PCR products of the predicted size from HoR34 but not USA300, whereas control primers targeting mecA amplified PCR products of the predicted size from both HoR34 and USA300 (Fig. 5A).
FIG 3.
Copy number as determined by Illumina sequence read coverage across SCCmec for strain USA300 (Sample 1A_S1), HoR34 (Sample 8A_S8; outlined by a blue box) and eight other isolates from the chemostat culture. The position on the chromosome is indicated, with SCCmec coordinates between 0.034 and 0.057 Mb. The blue lines in the graphs depict locally weighted scatterplot smoothing (lowess) applied to the black data points. Note that the y axis for HoR34 differs from those for the other samples.
FIG 4.
Chromosomal organization of strain HoR34 depicting expansion of the SCCmec element and locations of gdpP, clpX, camS, and guaA mutations. On the circular map, the innermost track shows the copy number of 10-kb nonoverlapping loci across the genome with loci that had copy numbers greater than two shown in red and those with copy numbers less than two shown in blue. The next track shows black blocks illustrating different regions on the genome, e.g., SCCmec and arginine catabolic mobile element (ACME). Single nucleotide polymorphisms are shown on the third track. Missense mutations are labeled in green, whereas stop gain mutations are labeled in blue. Genes are shown on the outermost tracks. Genes transcribed in the forward (5′→3′) direction are labeled in green and are in the outside track, whereas those transcribed in the reverse direction are labeled in red.
FIG 5.
Chromosomal amplification of SCCmec can drive high-level oxacillin resistance. (A) PCR amplification across the SCCmec junctions in strain HoR34. Amplification of the mecA gene in both strains USA300 and HoR34 was used as a control. The positions of molecular size markers (MSM) (in kilobases) are shown to the right of the gel. (B) Comparison of relative mecA abundance by LightCycler qPCR in USA300 and HoR34 grown for 24 h in BHI supplemented with 0, 0.5, 64, or 100 mg/ml oxacillin (OX). The data presented are means plus standard deviations (SD) (error bars) (***, P < 0.001; ****, P < 0.0001) from three experiments. Statistical evaluation was performed using a paired two-tailed t test. (C) Comparison of relative PBP2a expression by Western blotting in USA300 and HoR34 grown in BHI alone and BHI supplemented with 0, 0.5, 64, or 100 mg/ml oxacillin. (D) Competitive growth of USA300 and HoR34 over 48 h in BHI alone and BHI supplemented with oxacillin (0.5 mg/ml). The CFU of each strain was enumerated on BHI agar to count all bacteria and on BHI oxacillin (30 mg/ml) to count HoR34. The ratio of the two strains in each culture is shown.
Stability of the SCCmec amplification event.
The reduction in oxacillin MIC in the HoR34 strain passaged in BHI medium (from 800 to 300 μg/ml) indicated that the amplified SCCmec elements may be unstable in the absence of antibiotic selection. To determine the SCCmec copy number, LightCycler qPCR was used to compare the relative abundance of mecA in HoR34 grown in the presence and absence of oxacillin. These experiments revealed that the mecA copy number in the HoR34p strain that had been passaged daily in antibiotic-free BHI medium for 2 weeks (oxacillin MIC of 300 μg/ml) was only threefold higher than the copy number in strain USA300 (Fig. 5B), indicating that up to seven of the amplified SCCmec elements were excised/lost from the original chemostat isolate during this time. Interestingly, the doubling time of HoR34p (32.70 min) was not significantly different from that of HoR34 (31.9 min), indicating that a reduction in the number of amplified SCCmec elements was not sufficient to alleviate the growth defect. However, further passage of HoR34p in 0.5, 64, and 100 μg/ml oxacillin was accompanied by a significant, concentration-dependent increase in mecA copy number (up to 17-fold compared to USA300) (Fig. 5B) and an increase in MIC to ≥800 μg/ml. PCR amplification and sequencing were used to confirm that the identified mutations in the guaA, gdpP, clpX, and camS genes of HoR34 had not reverted to the wild type following passage in antibiotic-free media (data not shown). These data suggest that recombination between the tandem SCCmec elements in HoR34 facilitates accordion-like contraction and expansion in response to oxacillin exposure. Consistent with these qPCR data, Western blot analysis of HoR34 grown in 0, 0.5, 64, and 100 μg/ml oxacillin also revealed concentration-dependent increases in PBP2a expression (Fig. 5C).
To investigate why a small-colony variant may have been selected and maintained in the chemostat, we performed competition experiments between the USA300 and HoR34 strains. Predictably, USA300 outcompeted the slower-growing HoR34 in the absence of antibiotic selection (Fig. 5D). However, in the presence of subinhibitory oxacillin (0.5 μg/ml), HoR34 strongly outcompeted the wild type (Fig. 5D). Collectively, these data identify tandem amplification of the SCCmec element as a new mechanism of high-level methicillin resistance in MRSA, which may provide a competitive advantage for MRSA under antibiotic selection.
Concluding remarks.
Several genetic mechanisms may have contributed alone or in combination to the SCCmec amplification event in strain HoR34. Expression of the ccr recombinase genes that excise SCCmec (17) can be increased by β-lactams and vancomycin (18), potentially generating multiple, extrachromosomal copies of SCCmec capable of subsequent reintegration. This possibility is supported by a recent study that identified a replication initiator gene upstream of the ccr recombinase genes, suggesting that the element may be replicative (19). Other mechanisms that may have contributed to the SCCmec amplification, alone or in combination with Ccr-mediated excision, include RecA-dependent nonequal homologous recombination or RecA-independent mechanisms such as recombination between single-stranded repetitive sequence on sister chromatids at the replication fork (20). The absence of repeat sequences flanking the SCCmec amplification may also suggest that an initial double-strand break (DSB), followed by RecA-dependent DSB repair during rolling circle replication may drive the production of long tandem arrays in a single generation, which have previously been implicated in fast adaption to drug treatment (21). Following the initial SCCmec duplication/amplification, the long stretches of homology are likely to facilitate RecA-mediated expansion and contraction of the element in different concentrations of oxacillin, as recently observed in a Staphylococcus lugdunensis strain carrying an amplified isd locus (16). Recombination events leading to partial deletion of the SCCmec locus have been described previously. For instance, increased vancomycin resistance has been linked to site-specific insertion sequence-mediated excision of SCCmec (22), suggesting that distinct RecA-independent mechanisms may favor high or low copy numbers of mecA in environments with high concentrations of β-lactam or vancomycin, respectively.
Even though multiple copies of SCCmec were maintained by strain HoR34 following repeated subculture in the absence of oxacillin selection, no evidence for SCCmec amplification was found in a search of 404 MRSA genomes using read coverage of the mecA gene normalized with read coverage of three single-copy genes (data not shown). The clinical relevance of these data merits further investigation, particularly given that β-lactams are not typically part of the treatment regimen for MRSA infections. However, this may change in view of ongoing clinical trials showing the therapeutic value of combining flucloxacillin and vancomycin for the treatment of MRSA sepsis (23, 24). Our growth competition experiments revealed that the increased competitiveness of HoR34 in the presence of oxacillin was balanced by a significant loss of competitiveness in the absence of antibiotic selection, suggesting that MRSA strains carrying multiple SCCmec elements are unlikely to be maintained under physiological conditions or in clinical environments where exposure to antibiotics is sporadic. Taken together, our data identify chromosomal amplification of the SCCmec element as a new mechanism that may be used by MRSA to adapt to, and be more competitive in, high-oxacillin environments.
MATERIALS AND METHODS
Strains and culture conditions.
Bacterial strains used in this study are listed in Table 2 and were grown at 37°C in LB (Sigma), brain heart infusion (BHI) (Oxoid), Mueller-Hinton (Oxoid), or nutrient (Oxoid) broth supplemented with ampicillin (50 μg/ml), oxacillin (0.5, 64, 100, or 130 μg/ml), chloramphenicol (10 μg/ml), or erythromycin (10 μg/ml) as indicated. S. aureus strains were also grown on BHI agar medium plates supplemented with oxacillin concentrations up to 1,200 μg/ml.
TABLE 2.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a |
|---|---|
| S. aureus strains | |
| RN4220 | Restriction-deficient laboratory S. aureus |
| USA300 | CA-MRSA expressing heterogeneous resistance to oxacillin |
| HoR34 | USA300 derivative expressing high-level resistance to oxacillin |
| ATCC 29213 | MSSA strain for susceptibility testing |
| JE2 atl::erm | Transposon mutation in the major autolysin gene atl of strain JE2, a USA300 derivative used in the construction of the Nebraska Transposon mutant library (41). Exhibits impaired autolytic activity |
| E. coli TOP10 | E. coli TOP10 cloning strain |
| Plasmids | |
| pLI50 | E. coli-Staphylococcus shuttle vector; Apr (E. coli), Cmr (Staphylococcus) |
| pDrive | E. coli cloning vector |
MSSA, methicillin-sensitive S. aureus.
Measurement of oxacillin MIC.
The oxacillin MIC for the S. aureus strains used in this study was determined in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines and using Etest strips from bioMérieux on Mueller-Hinton agar (Oxoid) containing 2% NaCl.
Isolation of USA300 oxacillin-hyperresistant mutants using chemostat system.
The community-associated CA-MRSA strain, USA300 FPR3757, which expresses a HeR phenotype with an oxacillin MIC on brain heart infusion or nutrient agar of 32 μg/ml was used in this study. A 580-ml-capacity laboratory reactor containing 500 ml of nutrient broth (Oxoid) was used as described previously (25). Strain USA300 was inoculated into the chemostat and allowed to grow to stationary phase for 2 days at 37°C in the absence of any antibiotic selection or medium replacement. A growth medium reservoir containing 20 liters of nutrient broth was then connected to the chemostat and fed to the chemostat using a peristaltic pump at a flow rate of 100 ml/h, replacing the entire nutrient broth volume of the chemostat every 5 h. After 24-h continuous culture growth in the absence of antibiotic selection, the nutrient broth in the feeding tank was supplemented with oxacillin at a concentration of 8 mg/liter. Thereafter, the oxacillin concentration in the growth medium reservoir was increased in a stepwise manner every day, reaching a final concentration of 130 mg/liter on day 12. Culture samples were collected aseptically from the chemostat after 24-h culture at each oxacillin concentration before being serially diluted and inoculated onto BHI agar supplemented with 100 μg of oxacillin per ml. The MICs of colonies recovered from these plates were determined on BHI agar supplemented with oxacillin ranging from 100 to 1,000 μg/ml. All isolates examined were hyperresistant and capable of robust growth on BHI agar supplemented with 800 μg/ml oxacillin. Phenotypic and whole-genome sequence analyses of the hyperresistant mutants are described in the supplemental methods.
Hemolysis, biofilm, and autolysis assays.
Beta-hemolysis was assessed on BHI agar supplemented with 5% sheep blood following overnight growth at 37°C and a further 24 h at 4°C. Semiquantitative measurements of biofilm formation were determined under static conditions using Nunclon hydrophilic, tissue culture-treated, 96-well polystyrene plates (Nunc, Denmark) as described previously (26). Triton X-100-induced autolysis was measured essentially as described previously (27). Each experiment was repeated at least three times, and average data are presented.
TEM.
Overnight BHI cultures were diluted 1:200 in fresh BHI and grown at 37°C to an A600 of 1.0. Culture aliquots (10-ml aliquots) were subjected to centrifugation at 8,000 × g, and the cell pellets were resuspended in fixation solution (2.5% glutaraldehyde in 0.1 M cacodylate buffer [pH 7.4]) and incubated overnight at 4°C. The fixed cells were further treated with 2% osmium tetroxide, followed by 0.25% uranyl acetate for contrast enhancement. The pellets were then dehydrated in increasing concentrations of ethanol as described above for the transmission electron microscopy (TEM) cell preparation, followed by pure propylene oxide, and transferred to a series of resin and propylene oxide mixtures (50:50, 75:25, pure resin) before being embedded in Epon resin. Thin sections were cut on an ultramicrotome. Images were analyzed using AMT v.542 software using a Hitachi H7000 instrument. At least three to five measurements of cell wall thickness were performed on each cell, and 88 cells were measured for each sample.
PCR and quantitative PCR.
Amplification of the mecA gene and the SCCmec junctions in strain HoR34 was achieved using the following primers (Table 3): primers mecA_Fwd (Fwd stands for forward) and mecA_Rev (Rev stands for reverse) for mecA and primers SCCmecJnFwd (Jn stands for junction) and SCCmecJnRev for the SCCmec junctions. Quantitative PCR (qPCR) for mecA was performed on the Roche LightCycler 480 instrument using the LightCycler 480 Sybr green kit (Roche) and the following primers: mecA1_Fwd and mecA2_Rev. Cycling conditions were 95°C for 5 min, followed by 45 cycles, with 1 cycle consisting of 95°C for 10 s, 58°C for 20 s, and 72°C for 20 s. Melt curve analysis was performed at 95°C for 5 s, followed by 65°C for 1 min up to 97°C at a ramp rate of 0.11°C/sec with five readings taken for every degree of temperature increase. The gyrB gene was used as an internal standard for all reactions using previously described primers (2). For each reaction, the ratio of mecA and gyrB transcript number was calculated as follows: 2CT gyrB − CT mecA where CT gyrB is the threshold cycle of the gyrB gene. Each qPCR experiment was performed at least three times, and average data and standard errors are presented.
TABLE 3.
Oligonucleotide primers used in this study
| Target gene | Primer | Primer sequence (5′–3′) |
|---|---|---|
| gdpP | gdpP_Fwd | GCCGAATGCAGTAACGATTT |
| gdpP_Rev | TTGTTGGCGTTCTTGTTTTG | |
| guaA | guaA_Fwd | AGAGGACAAAGCGCCTAAGA |
| guaA_Rev | CCTTACCCCTTTTCCGTCCT | |
| clpX | clpX_Fwd | AACGCAAAGTTCGTTGAAGG |
| clpX_Rev | TGAGCGTCAACTTTGATTGG | |
| camS | camS_Fwd | GCTGGTGAAGATGCAGGTTC |
| camS_Rev | CCTGGTGCATTTGTTGAAACTG | |
| mecA | mecA_Fwd | CATATCGTGAGCAATGAACTGA |
| mecA_Rev | CATCGTTACGGATTGCTTCA | |
| SCCmec junction | SCCmecJn_Fwd | CTTGCTGGGTGCTATTTGA |
| SCCmecJn_Rev | CGCTGTCTTCCTGTATTTCG | |
| mecA | mecA1_Fwd | TGCTCAATATAAAATTAAAACAAACTACGGTAAC |
| mecA1_Rev | GAATAATGACGCTATGATCCCAA | |
| gyrB | gyrB_Fwd | CCAGGTAAATTAGCCGATTGC |
| gyrB_Rev | AAATCGCCTGCGTTCTAGAG |
Analysis of PBP2a expression.
Total cell protein preparations were prepared from overnight cultures grown in 0, 0.5, 64, or 100 μg/ml oxacillin. Cell pellets were resuspended in distilled water containing 5 μg/ml lysostaphin, 10 U of DNase I, and 50 μl of 10% SDS before being incubated at 37°C for 30 min. Insoluble material was pelleted by centrifugation, and the supernatant was used for Western blotting. Protein concentration was assessed using the Pierce BCA protein assay kit (Thermo Scientific). Protein samples were separated on a 10% SDS gel (Thermo Scientific) and transferred to nitrocellulose membranes (Thermo Scientific) using a TE 70 semidry transfer unit (Amersham). Anti-PBP2a antibodies (Abnova) were used at a 1:2,000 dilution. A 1:200 dilution of protein G-horseradish peroxidase (HRP) conjugate (Sigma) was used to detect bound antibody, and visualization was achieved using a colorimetric detection system (Bio-Rad).
Complementation of strain HoR34 with gdpP, guaA, camS, and clpX.
The gdpP, guaA, camS, and clpX genes were amplified from USA300 genomic DNA by PCR using the primers listed in Table 2, before being cloned into the cloning vector pDrive (Qiagen) in Escherichia coli TOP10. The sequence of inserts in recombinant plasmids was verified by Sanger sequencing (Source Biosciences) before being subcloned on EcoRI or BamHI/HindIII restriction fragments into the E. coli-Staphylococcus shuttle plasmid pLI50. The plasmids were transformed by electroporation into the restriction-deficient strain RN4220, and subsequently into strain HoR34. All plasmid-harboring strains were cultured in medium supplemented with 100 μg/ml ampicillin (E. coli) or 10 μg/ml chloramphenicol (S. aureus) for plasmid selection.
Growth competition experiments.
Overnight cultures of USA300 and HoR34 cultures were diluted to an A600 of 0.05 in fresh BHI medium and grown for 6 h. The cell density of both exponential-phase cultures was adjusted to an A600 of 0.1 in 500-ml flasks containing 50 ml BHI or BHI supplemented with 0.5 μg/ml oxacillin and incubated at 37°C with shaking. The number of CFU in samples collected at 0, 2, 4, 8, 24, and 48 h was determined by plating serial dilutions on BHI agar. Colonies formed by each strain were readily differentiated based on their tetracycline resistance and appearance, i.e., the HoR34 colonies were tetracycline sensitive and had a white-colored, small-colony phenotype, whereas USA300 colonies were regular sized, tetracycline resistant, and pigmented.
Statistical analysis.
Two-tailed, two-sample equal-variance Student's t tests were used to determine statistically significant differences in assays performed during this study. A P value of <0.05 was deemed significant.
Quality control of genome sequence data.
Read quality was assessed by screening the read length, nucleotide, and quality score distributions using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and the FASTX-Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/index.html). The DNA reads were trimmed based on quality scores. Potential adaptor sequence was removed using Trimmomatic v0.32 (28), which scanned reads using a four-base sliding window and trimmed reads where the average Phred base quality of the window was below 30. All ambiguous “N” bases and reads shorter than 35 bp were removed. The first 20 bases of the DNA reads were removed because they had a nucleotide content that deviated from the expected 25% rate for each base. The DNA reads were corrected using BayesHammer (29) to reduce sequencing errors that can reduce the alignment quality, increase false-positive single nucleotide polymorphism (SNP) rates, and reduce the number of valid SNPs (30). These steps retained 84% of the initial DNA reads among HoR isolates from the chemostat, yielding median quality values of >30 across the reads. Insert sizes were an average of 185. Read lengths after trimming and filtering averaged 185 bp, and the average coverage per sample on the chromosome, calculated using the Bedtools genomecov function (31) on mapped reads, ranged from 47 to 197.
Genome assembly.
The error-corrected paired and unpaired reads for each DNA sample were assembled using SPAdes v3.1.1 (5) with k-mers 21, 33, 55, 77, 99, and 127 and the “careful” parameter, which minimized the number of mismatches in the contigs (32). The resulting assemblies were compared to the reference USA300_FPR3757 (42) chromosome using QUAST v2.3 (33). The GC content of each assembly was 32.6%, and there were between 31 and 51 scaffolds per assembly, with N50 values of >200 kb. One or two short gaps (<500 bp) were found in each assembly that could not be fully closed using Gapfiller (34).
Single nucleotide polymorphism calling using assembly and read mapping.
The chromosome and three plasmids (GenBank accession no. NC_007790 to NC_007793) were indexed with k-mer of 13 and step size of two using SMALT v5.7 (http://www.sanger.ac.uk/science/tools/smalt-0). The error-corrected DNA reads were mapped to the genome with SMALT, which applied a Smith-Waterman sequence alignment algorithm. The SAM (sequence alignment/map) files were converted to BAM (binary alignment/map) files using SAMtools v0.1.18 (35). The BAM files were then coordinate sorted, the paired and unpaired files were merged, and PCR duplicate reads were removed. Candidate SNPs were detected where the base quality (BQ) was >25, the mapping quality (MQ) was >30, and the read depth was <100 using SAMtools Mpileup v0.1.18, Bcftools v0.1.17-dev, and the SAMtools v0.1.11 vcfutils.pl function. The read depth allele frequency (RDAF) of the nonreference allele and local coverage were estimated using SAMtools Pileup v0.1.11.
To call SNPs using an assembly-based approach, the scaffolds produced by SPAdes were aligned to the USA300 reference genome using nucmer in the MUMmer v3.23 package. This was followed by eliminating conflicting repeat copies using the “delta-filter” command and the “show-snps” command to call SNPs and indels. The union of SNPs called by nucmer and SNPs called by Bcftools was used as a candidate SNP set. These sites were queried across all samples using the SAMtools Pileup files to find false-negative SNPs uncalled by nucmer or Bcftools. The RDAF of the nonreference alleles was reported for each SNP using SAMtools Pileup output. Each candidate SNP was assessed using the following additional criteria: (i) SNP quality (SQ) of >30; (ii) read coverage of >5; (iii) forward-reverse read coverage ratio between 0.1 and 0.9; (iv) nonreference read allele frequency of >0.1; (v) 2+ forward reads; (vi) 2+ reverse reads. Results were converted to variant call format (VCF) and annotated. SNPs were homozygous if the RDAF was ≥0.85 and heterozygous if 0.1 < RDAF < 0.85. Insufficient read depth coverage was present to predict SNPs with a RDAF of <0.1.
Indel calling using split-read mapping.
Deletions and short insertions (indels) were called using the samtopindel script to convert the BAM files, and then with Pindel (36) to keep only the indels with at least 10 supporting reads. The RDAF of the indels smaller than the read length were calculated using the BAM files in Integrative Genomics Viewer (IGV) (number of reads with indel at locus/all reads at the locus). For indels greater than 1 bp in length, the sum of the number of reads with the indel was divided by the sum of the number of reads at each site in the indel. This approach may be limited by uneven coverage at a locus. If the indel was longer than the read length, then a lack of read coverage at the sites predicted to have the mutation was considered evidence of the deletion, and the RDAF was set at one.
Variant annotation.
The functional effects of SNPs and indels was estimated by annotation with SnpEff v4.0e (37) using the Staphylococcus_aureus_USA300_FPR3757_uid58555 database file from the SnpEff database. Results were manually checked using the reference genome annotation.
Copy number variation detection using read coverage.
Copy number variants (CNVs) were screened using the BAM files containing reads with MQs of >30 to reduce false-positive rates (12–14). Coverage was calculated for every base using genomecov in Bedtools with the “-d” flag (31) so that the median chromosomal coverage could be calculated for each sample. Genome-wide coverage levels were analyzed in 10-kb and 25-kb windows and plotted as 5-kb sliding windows with a 2.5-kb step using the Bedtools makewindows function (31). Coverage for each window was normalized by dividing it by the median coverage of the chromosome to produce a copy number estimate. Windows with a copy number of ≥2 were reported. The copy number of plasmids was determined by dividing the median read coverage of the plasmid by the median read coverage of the chromosome.
MinION long-read genome sequencing.
To evaluate the number of SCCmec copies and their location contiguous with or excised from the chromosome, genomic DNA from strain HoR34 was amplified to generate long reads using an Oxford Nanopore Technologies (ONT) MinION sequencing device. MinION sequencing library construction was carried out according to the manufacturer's instructions and as previously described (38). The library was sequenced on an R7.3 MinION flow cell using the two-dimensional (2D) sequencing protocol. The run produced 26,859 FAST5 files, which were processed using poRe (39), yielding 17,254 2D reads. These reads were used with the MiSeq data in a hybrid assembly using SPAdes (32) and SSPACE-LongRead (40) to produce a single contig.
ACKNOWLEDGMENTS
This study was funded by grants from the Irish Health Research Board (HRA-POR-2012-51 and HRA-POR-2015-1158) (to J.P.O.).
We thank Cyril Carroll and Claire Fingleton for assistance with the chemostat experiment and analysis of strain HoR34.
REFERENCES
- 1.Bal AM, Coombs GW, Holden MT, Lindsay JA, Nimmo GR, Tattevin P, Skov RL. 2016. Genomic insights into the emergence and spread of international clones of healthcare-, community- and livestock-associated methicillin-resistant Staphylococcus aureus: blurring of the traditional definitions. J Glob Antimicrob Resist 6:95–101. doi: 10.1016/j.jgar.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. 2012. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 8:e1002626. doi: 10.1371/journal.ppat.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson DI, Levin BR. 1999. The biological cost of antibiotic resistance. Curr Opin Microbiol 2:489–493. doi: 10.1016/S1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 4.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJ. 2006. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312:1944–1946. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 5.Sieradzki K, Tomasz A. 1997. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J Antimicrob Chemother 39(Suppl A):47–51. doi: 10.1093/jac/39.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- 6.Dengler V, McCallum N, Kiefer P, Christen P, Patrignani A, Vorholt JA, Berger-Bachi B, Senn MM. 2013. Mutation in the c-di-AMP cyclase dacA affects fitness and resistance of methicillin resistant Staphylococcus aureus. PLoS One 8:e73512. doi: 10.1371/journal.pone.0073512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dordel J, Kim C, Chung M, Pardos de la Gandara M, Holden MT, Parkhill J, de Lencastre H, Bentley SD, Tomasz A. 2014. Novel determinants of antibiotic resistance: identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. mBio 5:e01000. doi: 10.1128/mBio.01000-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domanski TL, de Jonge BL, Bayles KW. 1997. Transcription analysis of the Staphylococcus aureus gene encoding penicillin-binding protein 4. J Bacteriol 179:2651–2657. doi: 10.1128/jb.179.8.2651-2657.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulhbacher J, Brouillette E, Allard M, Fortier LC, Malouin F, Lafontaine DA. 2010. Novel riboswitch ligand analogs as selective inhibitors of guanine-related metabolic pathways. PLoS Pathog 6:e1000865. doi: 10.1371/journal.ppat.1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek KT, Bowman L, Millership C, Dupont Sogaard M, Kaever V, Siljamaki P, Savijoki K, Varmanen P, Nyman TA, Grundling A, Frees D. 2016. The cell wall polymer lipoteichoic acid becomes nonessential in Staphylococcus aureus cells lacking the ClpX chaperone. mBio 7:e01228-16. doi: 10.1128/mBio.01228-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shahmirzadi SV, Nguyen MT, Gotz F. 2016. Evaluation of Staphylococcus aureus lipoproteins: role in nutritional acquisition and pathogenicity. Front Microbiol 7:1404. doi: 10.3389/fmicb.2016.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proctor RA, Kriegeskorte A, Kahl BC, Becker K, Loffler B, Peters G. 2014. Staphylococcus aureus small colony variants (SCVs): a road map for the metabolic pathways involved in persistent infections. Front Cell Infect Microbiol 4:99. doi: 10.3389/fcimb.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek KT, Grundling A, Mogensen RG, Thogersen L, Petersen A, Paulander W, Frees D. 2014. Beta-lactam resistance in methicillin-resistant Staphylococcus aureus USA300 is increased by inactivation of the ClpXP protease. Antimicrob Agents Chemother 58:4593–4603. doi: 10.1128/AAC.02802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao W, Monk IR, Tobias NJ, Gladman SL, Seemann T, Stinear TP, Howden BP. 2015. Large tandem chromosome expansions facilitate niche adaptation during persistent infection with drug-resistant Staphylococcus aureus. Microb Genom 1:e000026. doi: 10.1099/mgen.0.000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilbronner S, Monk IR, Brozyna JR, Heinrichs DE, Skaar EP, Peschel A, Foster TJ. 2016. Competing for iron: duplication and amplification of the isd locus in Staphylococcus lugdunensis HKU09-01 provides a competitive advantage to overcome nutritional limitation. PLoS Genet 12:e1006246. doi: 10.1371/journal.pgen.1006246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, Jones A, Palazzolo-Ballance AM, Perdreau-Remington F, Sensabaugh GF, DeLeo FR, Chambers HF. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis 197:1523–1530. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 18.Higgins PG, Rosato AE, Seifert H, Archer GL, Wisplinghoff H. 2009. Differential expression of ccrA in methicillin-resistant Staphylococcus aureus strains carrying staphylococcal cassette chromosome mec type II and IVa elements. Antimicrob Agents Chemother 53:4556–4558. doi: 10.1128/AAC.00395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mir-Sanchis I, Roman CA, Misiura A, Pigli YZ, Boyle-Vavra S, Rice PA. 2016. Staphylococcal SCCmec elements encode an active MCM-like helicase and thus may be replicative. Nat Struct Mol Biol 23:891–898. doi: 10.1038/nsmb.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovett ST, Drapkin PT, Sutera VA Jr, Gluckman-Peskind TJ. 1993. A sister-strand exchange mechanism for recA-independent deletion of repeated DNA sequences in Escherichia coli. Genetics 135:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandegren L, Andersson DI. 2009. Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nat Rev Microbiol 7:578–588. doi: 10.1038/nrmicro2174. [DOI] [PubMed] [Google Scholar]
- 22.Noto MJ, Fox PM, Archer GL. 2008. Spontaneous deletion of the methicillin resistance determinant, mecA, partially compensates for the fitness cost associated with high-level vancomycin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 52:1221–1229. doi: 10.1128/AAC.01164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong SY, Nelson J, Paterson DL, Fowler VG Jr, Howden BP, Cheng AC, Chatfield M, Lipman J, Van Hal S, O'Sullivan M, Robinson JO, Yahav D, Lye D, Davis JS, CAMERA2 Study Group, Australasian Society for Infectious Diseases Clinical Research Network. 2016. CAMERA2 - combination antibiotic therapy for methicillin-resistant Staphylococcus aureus infection: study protocol for a randomised controlled trial. Trials 17:170. doi: 10.1186/s13063-016-1295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis JS, Sud A, O'Sullivan MV, Robinson JO, Ferguson PE, Foo H, van Hal SJ, Ralph AP, Howden BP, Binks PM, Kirby A, Tong SY, Combination Antibiotics for MEthicillin Resistant Staphylococcus aureus (CAMERA) Study Group, Australasian Society for Infectious Diseases Clinical Research Network. 2016. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 62:173–180. doi: 10.1093/cid/civ808. [DOI] [PubMed] [Google Scholar]
- 25.Fleming GTA, Patching JW. 2008. The fermenter in research and development. Wiley, Chichester, England. [Google Scholar]
- 26.Conlon KM, Humphreys H, O'Gara JP. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J Bacteriol 184:4400–4408. doi: 10.1128/JB.184.16.4400-4408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy H, Waters EM, Bose JL, Foster S, Bayles KW, O'Neill E, Fey PD, O'Gara JP. 2016. The major autolysin is redundant for Staphylococcus aureus USA300 LAC JE2 virulence in a murine device-related infection model. FEMS Microbiol Lett 363:fnw087. doi: 10.1093/femsle/fnw087. [DOI] [PubMed] [Google Scholar]
- 28.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolenko SI, Korobeynikov AI, Alekseyev MA. 2013. BayesHammer: Bayesian clustering for error correction in single-cell sequencing. BMC Genomics 14(Suppl 1):S7. doi: 10.1186/1471-2164-14-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley DR, Schatz MC, Salzberg SL. 2010. Quake: quality-aware detection and correction of sequencing errors. Genome Biol 11:R116. doi: 10.1186/gb-2010-11-11-r116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boetzer M, Pirovano W. 2012. Toward almost closed genomes with GapFiller. Genome Biol 13:R56. doi: 10.1186/gb-2012-13-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. 2009. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 25:2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Risse J, Thomson M, Patrick S, Blakely G, Koutsovoulos G, Blaxter M, Watson M. 2015. A single chromosome assembly of Bacteroides fragilis strain BE1 from Illumina and MinION nanopore sequencing data. Gigascience 4:60. doi: 10.1186/s13742-015-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson M, Thomson M, Risse J, Talbot R, Santoyo-Lopez J, Gharbi K, Blaxter M. 2015. poRe: an R package for the visualization and analysis of nanopore sequencing data. Bioinformatics 31:114–115. doi: 10.1093/bioinformatics/btu590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boetzer M, Pirovano W. 2014. SSPACE-LongRead: scaffolding bacterial draft genomes using long read sequence information. BMC Bioinformatics 15:211. doi: 10.1186/1471-2105-15-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367:731–739. [DOI] [PubMed] [Google Scholar]