ABSTRACT
Cefiderocol (formerly S-649266) is an investigational siderophore cephalosporin. Iron-depleted cation-adjusted Mueller-Hinton broth (ID-CAMHB) was prepared according to the Clinical and Laboratory Standards Institute (CLSI) protocol and used to perform broth microdilution testing of cefiderocol against a 2014-2015 collection of clinical isolates of Gram-negative bacilli from North America (n = 4,239) and Europe (n = 4,966). The concentrations of cefiderocol inhibiting 90% of isolates tested (MIC90s) were 0.5 μg/ml (North America; n = 3,007) and 1 μg/ml (Europe; n = 3,080) for all isolates of Enterobacteriaceae; 1 μg/ml (North America; n = 30) and 4 μg/ml (Europe; n = 139) for meropenem-nonsusceptible (MIC ≥ 2 μg/ml) isolates of Enterobacteriaceae; 0.5 μg/ml for both North American (n = 765) and European (n = 765) isolates of Pseudomonas aeruginosa; 0.5 μg/ml (North America; n = 151) and 1 μg/ml (Europe; n = 202) for meropenem-nonsusceptible (MIC ≥ 4 μg/ml) isolates of P. aeruginosa; 1 μg/ml for both North American (n = 309) and European (n = 839) isolates of all Acinetobacter baumannii strains as well as for both North American (n = 173) and European (n = 595) isolates of meropenem-nonsusceptible A. baumannii; and 0.5μg/ml (North America; n = 152) and 0.25 μg/ml (Europe; n = 276) for isolates of Stenotrophomonas maltophilia. MICs of cefiderocol were ≤4 μg/ml for 99.9% (6,078/6,087) of all Enterobacteriaceae, 97.0% (164/169) of meropenem-nonsusceptible Enterobacteriaceae, 99.9% (1,529/1,530) of all P. aeruginosa isolates, 100% (353/353) of meropenem-nonsusceptible P. aeruginosa isolates, 97.6% (1,120/1,148) of all A. baumannii isolates, 96.9% (744/768) of meropenem-nonsusceptible A. baumannii isolates, 100% of isolates of S. maltophilia (428/428) and 93.8% of isolates of Burkholderia cepecia (11/12). We conclude that cefiderocol demonstrated potent in vitro activity against a recent collection of clinical isolates of commonly encountered Gram-negative bacilli, including carbapenem-nonsusceptible isolates.
KEYWORDS: cefiderocol, siderophore, carbapenem-resistant, Chelex 100 resin, Gram-negative bacteria
INTRODUCTION
Carbapenems provide effective therapy for patients infected with extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae and are considered agents of choice for many Gram-negative infections. Carbapenem-resistant Enterobacteriaceae have emerged or are in the process of emerging in many countries worldwide (1, 2). Carbapenem resistance is also significant among nonfermenting pathogens such as Pseudomonas aeruginosa and Acinetobacter baumannii (3, 4). Observed increases in carbapenem resistance among Enterobacteriaceae and nonfermenters are concerning, as there are few antimicrobial agents available that are safe and effective in the treatment of these infections and the development and distribution of new, more potent agents have not kept pace with increasing and diversifying resistance, particularly for commonly encountered pathogenic Gram-negative bacilli (5, 6). Multidrug-resistant phenotypes, including resistance to cephalosporins, aminoglycosides, and fluoroquinolones, are characteristic of most carbapenem-resistant isolates and present clinicians with difficult decisions to optimize therapy for patients (7). Carbapenem resistance in Gram-negative bacilli may arise by acquisition of class A (e.g., Klebsiella pneumoniae carbapenemase [KPC]), class B (e.g., NDM, IMP, VIM), or class D (e.g., OXA-48) β-lactamases (1, 2, 7–9), by overproduction of chromosomal AmpC, or by acquisition of an ESBL in combination with a porin (e.g., OprD) deficiency or the presence of efflux pumps (9–12).
Cefiderocol, formerly known as S-649226, is a novel siderophore cephalosporin for injection discovered by and currently in clinical development with Shionogi & Co., Ltd., to treat infections caused by carbapenem-resistant Gram-negative bacteria. Cefiderocol has a unique mechanism of cell entry. The cephalosporin moiety of cefiderocol binds primarily to bacterial penicillin binding protein 3 (PBP 3), similar to other cephalosporins, while the catechol moiety at the 3-position side chain of the cephalosporin contributes to forming a chelated complex with ferric iron that facilitates cefiderocol's crossing of the outer membrane of Gram-negative bacilli using the receptor-mediated bacterial iron transport system (13, 14). Bacterial iron transport systems accelerate and increase the influx of cefiderocol to the periplasmic space of Gram-negative bacteria, where its cephalosporin moiety can inhibit cell wall synthesis, thereby enhancing its antimicrobial activity relative to carbapenems, β-lactam/β-lactamase inhibitor combinations, and advanced-generation cephalosporins (14, 15). Cefiderocol has been reported to be active against carbapenem-resistant Gram-negative bacilli harboring various carbapenemases and to be more stable than other β-lactam agents such as ceftazidime, cefepime, and meropenem against class A, B, and D carbapenemases such as KPC, VIM, IMP, NDM, and OXA (15–17). Cefiderocol is also active against ESBL-producing Escherichia coli and Klebsiella pneumoniae (15) as well as against meropenem-resistant P. aeruginosa and A. baumannii (18).
Accurate in vitro susceptibility testing of cefiderocol by broth microdilution requires the use of iron-depleted conditions because such conditions induce the production of ferric iron transporters, which are strongly regulated by proximal iron concentrations. Iron-depleted conditions mimic the conditions faced by bacteria infecting human tissues and fluids (15, 17, 19). In January 2016, the Clinical and Laboratory Standards Institute (CLSI) Subcommittee on Antimicrobial Susceptibility Testing approved broth microdilution and disk diffusion methods and quality control MIC ranges for cefiderocol (20). Broth microdilution testing of cefiderocol requires iron-depleted cation-adjusted Mueller-Hinton broth (ID-CAMHB) (20). MICs determined using ID-CAMHB have been shown to be reproducible and to correlate well with in vivo efficacy in animal models (21–23). MIC assays for cefiderocol performed in CAMHB with iron concentrations of >0.03 μg/ml show highly variable results and do not correlate with in vivo efficacy (22).
The current study, SIDERO-WT-2014, tested a 2014-2015 collection of 9,205 clinical isolates of Gram-negative bacilli from patients in North America and Europe against cefiderocol and comparators using CLSI broth microdilution methodology. This study generated the first in vitro surveillance testing data for cefiderocol using the recently approved CLSI broth microdilution MIC determination method (20).
RESULTS
The in vitro activities of cefiderocol and comparators are summarized in Table 1 for the 4,239 isolates collected from North American medical center laboratories and in Table 2 for the 4,966 isolates from European medical center laboratories. The concentrations of antimicrobial agent inhibiting 90% of isolates tested (MIC90s) for cefiderocol against Enterobacteriaceae were 0.5 μg/ml (North America, n = 3,007 isolates) and 1 μg/ml (Europe, n = 3,080 isolates). The MIC range for cefiderocol was ≤0.002 to 8 μg/ml for both sets of isolates, and 99.9% (6,078/6,087) of all Enterobacteriaceae had cefiderocol MICs of ≤4 μg/ml. One isolate of Serratia marcescens from North America had a MIC to cefiderocol of 8 μg/ml, while six isolates of K. pneumoniae, one isolate of Enterobacter aerogenes, and one isolate of S. marcescens from Europe also had a MIC to cefiderocol of 8 μg/ml. Five of the nine isolates with cefiderocol MICs of 8 μg/ml were nonsusceptible to meropenem. Against meropenem-nonsusceptible (MIC ≥ 2 μg/ml) isolates from North America (Fig. 1) and Europe (Fig. 2), MIC90 values were 1 μg/ml (n = 30) and 4 μg/ml (n = 139); 97.0% (164/169) of all meropenem-nonsusceptible Enterobacteriaceae had MICs to cefiderocol of ≤4 μg/ml. In comparison, testing of recently approved antimicrobial agents ceftazidime-avibactam and ceftolozane-tazobactam against the same geographic sets of isolates of meropenem-nonsusceptible Enterobacteriaceae demonstrated MIC90 values of 4 and >64 μg/ml, respectively, for isolates from North America (Table 1) and >64 and >64 μg/ml, respectively, for isolates from Europe (Table 2). Against meropenem-susceptible (MIC ≤1 μg/ml) isolates from North America and Europe, MIC90 values for cefiderocol were 0.5 μg/ml (n = 2,977) and 1 μg/ml (n = 2,941); 99.9% (5,914/5,918) of all meropenem-susceptible Enterobacteriaceae had MICs to cefiderocol of ≤4 μg/ml. Figure 3 depicts the cumulative percentages of all meropenem-nonsusceptible isolates of Enterobacteriaceae that were susceptible to increasing concentrations of cefiderocol and its comparators.
TABLE 1.
In vitro activity of cefiderocol and comparators against Gram-negative bacilli (n = 4,239) isolated by 50 medical center laboratories in North America from 2014 to 2015
| Family/genus/species (no. of isolates) | Antimicrobial agent | MIC (μg/ml)a |
MIC interpretationb |
||||
|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | % susceptible | % intermediate | % resistant | ||
| Enterobacteriaceae (3,007) | Cefiderocol | ≤0.002–8 | 0.06 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.5 | 93.7 | 2.2 | 4.2 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.5 | 99.9 | 0 | 0.1 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 1 | 94.3 | 1.8 | 4.0 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | >8 | 86.2 | 1.4 | 12.3 | |
| Colistin | ≤0.25 to >8 | 0.5 | >8 | 82.4 | 0 | 17.6 | |
| Meropenem | ≤0.06 to >64 | ≤0.06 | ≤0.06 | 99.0 | 0.1 | 0.9 | |
| Meropenem-nonsusceptible Enterobacteriaceae (30) | Cefiderocol | 0.008–2 | 0.12 | 1 | |||
| Cefepime | ≤0.06 to >64 | 16 | >64 | 10.0 | 23.3 | 66.7 | |
| Ceftazidime-avibactam | 0.12 to >64 | 1 | 4 | 96.7 | 0 | 3.3 | |
| Ceftolozane-tazobactam | 1 to >64 | 64 | >64 | 13.3 | 6.7 | 80.0 | |
| Ciprofloxacin | ≤0.12 to >8 | 8 | >8 | 20.0 | 16.7 | 63.3 | |
| Colistin | ≤0.25 to >8 | 0.5 | >8 | 66.7 | 0 | 33.3 | |
| Meropenem | 2 to >64 | 8 | 64 | 0 | 13.3 | 86.7 | |
| Klebsiella spp.c (1,010) | Cefiderocol | ≤0.002–4 | 0.06 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.5 | 93.9 | 2.0 | 4.2 | |
| Ceftazidime-avibactam | ≤0.06–8 | 0.12 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 1 | 96.8 | 0.6 | 2.6 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 0.5 | 92.9 | 0.8 | 6.3 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 99.4 | 0 | 0.6 | |
| Meropenem | ≤0.06 to >64 | ≤0.06 | ≤0.06 | 98.4 | 0.1 | 1.5 | |
| Klebsiella pneumoniae (765) | Cefiderocol | ≤0.002–4 | 0.03 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.5 | 92.7 | 2.2 | 5.1 | |
| Ceftazidime-avibactam | ≤0.06–8 | 0.12 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 1 | 96.3 | 0.8 | 2.9 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 91.5 | 1.1 | 7.5 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 99.4 | 0 | 0.7 | |
| Meropenem | ≤0.06 to >64 | ≤0.06 | ≤0.06 | 98.0 | 0.1 | 1.8 | |
| Klebsiella oxytoca (245) | Cefiderocol | ≤0.002–2 | 0.06 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.12 | 97.6 | 1.2 | 1.2 | |
| Ceftazidime-avibactam | ≤0.06–2 | 0.12 | 0.25 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06–64 | 0.25 | 0.5 | 98.4 | 0 | 1.6 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | ≤0.12 | 97.1 | 0 | 2.9 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 99.6 | 0 | 0.4 | |
| Meropenem | ≤0.06–8 | ≤0.06 | ≤0.06 | 99.6 | 0 | 0.4 | |
| Escherichia coli (740) | Cefiderocol | ≤0.002–2 | 0.06 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 8 | 88.8 | 1.8 | 9.5 | |
| Ceftazidime-avibactam | ≤0.06–2 | 0.12 | 0.25 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 0.5 | 97.4 | 1.0 | 1.6 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | >8 | 66.0 | 0.1 | 33.9 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 99.7 | 0 | 0.3 | |
| Meropenem | ≤0.06–4 | ≤0.06 | ≤0.06 | 99.9 | 0 | 0.1 | |
| Serratia spp.d (503) | Cefiderocol | ≤0.002–8 | 0.06 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.25 | 98.0 | 1.2 | 0.8 | |
| Ceftazidime-avibactam | ≤0.06–2 | 0.12 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | 0.12 to >64 | 0.5 | 1 | 97.2 | 1.4 | 1.4 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 90.3 | 4.8 | 5.0 | |
| Colistin | 0.5 to >8 | >8 | >8 | 5.0 | 0 | 95.0 | |
| Meropenem | ≤0.06–64 | ≤0.06 | 0.12 | 98.2 | 0.4 | 1.4 | |
| Serratia marcescens (472) | Cefiderocol | 0.004–8 | 0.06 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.25 | 97.9 | 1.3 | 0.9 | |
| Ceftazidime-avibactam | ≤0.06–2 | 0.12 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | 0.12 to >64 | 0.5 | 1 | 97.0 | 1.5 | 1.5 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 2 | 89.6 | 5.1 | 5.3 | |
| Colistin | 0.5 to >8 | >8 | >8 | 5.1 | 0 | 94.9 | |
| Meropenem | ≤0.06–64 | ≤0.06 | 0.12 | 98.1 | 0.4 | 1.5 | |
| Enterobacter spp.e (494) | Cefiderocol | 0.004–4 | 0.25 | 1 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 1 | 93.9 | 4.5 | 1.6 | |
| Ceftazidime-avibactam | ≤0.06–4 | 0.25 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06–64 | 0.25 | 8 | 81.8 | 6.3 | 11.9 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 0.25 | 94.9 | 1.8 | 3.2 | |
| Colistin | ≤0.25 to >8 | 0.5 | 2 | 91.3 | 0 | 8.7 | |
| Meropenem | ≤0.06–32 | ≤0.06 | 0.12 | 99.6 | 0 | 0.4 | |
| Enterobacter aerogenes (238) | Cefiderocol | 0.004–4 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06–16 | ≤0.06 | 0.5 | 97.1 | 2.5 | 0.4 | |
| Ceftazidime-avibactam | ≤0.06–4 | 0.25 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06–16 | 0.25 | 4 | 81.9 | 10.1 | 8.0 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 0.25 | 97.5 | 0.4 | 2.1 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 98.3 | 0 | 1.7 | |
| Meropenem | ≤0.06–8 | ≤0.06 | 0.12 | 99.6 | 0 | 0.4 | |
| Enterobacter cloacae (213) | Cefiderocol | 0.008–4 | 0.25 | 1 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 4 | 89.2 | 7.5 | 3.3 | |
| Ceftazidime-avibactam | ≤0.06–4 | 0.25 | 1 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06–64 | 0.5 | 8 | 80.3 | 3.3 | 16.4 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 92.5 | 3.3 | 4.2 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 94.8 | 0 | 5.2 | |
| Meropenem | ≤0.06–32 | ≤0.06 | 0.12 | 99.5 | 0 | 0.5 | |
| Enterobacter asburiae (30) | Cefiderocol | ≤0.06–0.5 | 0.25 | 1 | |||
| Cefepime | ≤0.06–2 | ≤0.06 | 0.25 | 100 | 0 | 0 | |
| Ceftazidime-avibactam | 0.12–16 | 0.25 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.12 to >8 | 0.25 | 8 | 86.7 | 0 | 13.3 | |
| Ciprofloxacin | ≤0.25 to >8 | ≤0.12 | 0.5 | 93.3 | 0 | 6.7 | |
| Colistin | ≤0.06–0.25 | >8 | >8 | 13.3 | 0 | 86.7 | |
| Meropenem | ≤0.06–0.5 | ≤0.06 | 0.12 | 100 | 0 | 0 | |
| Citrobacter spp.f (260) | Cefiderocol | ≤0.002–2 | 0.12 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 0.25 | 97.7 | 1.5 | 0.8 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.25 | 99.2 | 0 | 0.8 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 1 | 93.1 | 0.8 | 6.2 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 0.5 | 93.9 | 0.4 | 5.8 | |
| Colistin | ≤0.25–2 | 0.5 | 1 | 100 | 0 | 0 | |
| Meropenem | ≤0.06–8 | ≤0.06 | ≤0.06 | 99.2 | 0.4 | 0.4 | |
| Citrobacter freundii (143) | Cefiderocol | ≤0.002–1 | 0.06 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 1 | 96.5 | 2.1 | 1.4 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.5 | 99.3 | 0 | 0.7 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 8 | 88.1 | 1.4 | 10.5 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 91.6 | 0.7 | 7.7 | |
| Colistin | ≤0.25–2 | 0.5 | 1 | 100 | 0 | 0 | |
| Meropenem | ≤0.06–8 | ≤0.06 | ≤0.06 | 98.6 | 0.7 | 0.7 | |
| Citrobacter koseri (99) | Cefiderocol | 0.06–2 | 0.25 | 0.5 | |||
| Cefepime | ≤0.06–0.25 | ≤0.06 | ≤0.06 | 100 | 0 | 0 | |
| Ceftazidime-avibactam | ≤0.06–0.5 | 0.12 | 0.12 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | ≤0.06–1 | 0.25 | 0.5 | 100 | 0 | 0 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | ≤0.12 | 96.0 | 0 | 4.0 | |
| Colistin | ≤0.25–1 | ≤0.25 | 1 | 100 | 0 | 0 | |
| Meropenem | ≤0.06–0.12 | ≤0.06 | ≤0.06 | 100 | 0 | 0 | |
| Pseudomonas aeruginosa (765) | Cefiderocol | ≤0.002–8 | 0.06 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | 4 | 16 | 85.5 | 8.1 | 6.4 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 2 | 8 | 98.0 | 0 | 2.0 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.5 | 2 | 97.7 | 1.2 | 1.2 | |
| Ciprofloxacin | ≤0.12 to >8 | 0.25 | 8 | 77.9 | 7.5 | 14.6 | |
| Colistin | ≤0.25–4 | 1 | 2 | 99.5 | 0.5 | 0 | |
| Meropenem | ≤0.06 to >64 | 0.25 | 8 | 80.3 | 6.0 | 13.7 | |
| Meropenem-nonsusceptible Pseudomonas aeruginosa (151) | Cefiderocol | ≤0.002–4 | 0.06 | 0.5 | |||
| Cefepime | 1 to >64 | 8 | 32 | 53.6 | 21.9 | 24.5 | |
| Ceftazidime-avibactam | 0.5 to >64 | 4 | 8 | 90.7 | 0 | 9.3 | |
| Ceftolozane-tazobactam | 0.25 to >64 | 1 | 4 | 90.1 | 4.6 | 5.3 | |
| Ciprofloxacin | ≤0.12 to >8 | 2 | >8 | 43.7 | 12.6 | 43.7 | |
| Colistin | ≤0.25–4 | 1 | 1 | 99.3 | 0.7 | 0 | |
| Meropenem | 4 to >64 | 8 | 16 | 0 | 30.5 | 69.5 | |
| Acinetobacter baumannii (309) | Cefiderocol | ≤0.002–8 | 0.12 | 1 | |||
| Cefepime | 0.25 to >64 | 16 | 64 | 49.2 | 20.1 | 30.7 | |
| Ceftazidime-avibactam | 1 to >64 | 16 | >64 | ||||
| Ceftolozane-tazobactam | ≤0.06 to >64 | 8 | >64 | ||||
| Ciprofloxacin | ≤0.12 to >8 | >8 | >8 | 34.3 | 0.3 | 65.4 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 94.8 | 0 | 5.2 | |
| Meropenem | ≤0.06 to >64 | 8 | >64 | 44.0 | 1.6 | 54.4 | |
| Meropenem-nonsusceptible Acinetobacter baumannii (173) | Cefiderocol | ≤0.002–8 | 0.25 | 1 | |||
| Cefepime | 4 to >64 | 32 | >64 | 16.8 | 31.8 | 51.5 | |
| Ceftazidime-avibactam | 4 to >64 | 32 | >64 | ||||
| Ceftolozane-tazobactam | 0.5 to >64 | 16 | >64 | ||||
| Ciprofloxacin | 0.25 to >8 | >8 | >8 | 1.7 | 0 | 98.3 | |
| Colistin | ≤0.25 to >8 | 0.5 | 2 | 91.3 | 0 | 8.7 | |
| Meropenem | 4 to >64 | 64 | >64 | 0 | 2.9 | 97.1 | |
| Stenotrophomonas maltophilia (152) | Cefiderocol | ≤0.002–4 | 0.06 | 0.5 | |||
| Cefepime | 1 to >64 | 32 | >64 | ||||
| Ceftazidime-avibactam | 0.5 to >64 | 8 | 64 | ||||
| Ceftolozane-tazobactam | 0.12 to >64 | 8 | >64 | ||||
| Ciprofloxacin | 0.25 to >8 | 2 | >8 | ||||
| Colistin | ≤0.25 to >8 | 2 | >8 | ||||
| Meropenem | 1 to >64 | >64 | >64 | ||||
| Burkholderia cepacia (6) | Cefiderocol | 0.015–16 | |||||
| Cefepime | 16 to >64 | ||||||
| Ceftazidime-avibactam | 4 | ||||||
| Ceftolozane-tazobactam | 2–16 | ||||||
| Ciprofloxacin | 0.5–8 | ||||||
| Colistin | >8 | ||||||
| Meropenem | 2–8 | 83.3 | 16.7 | 0 | |||
MIC50 and MIC90 calculated only for genus or species where >30 isolates were tested. Species of Enterobacteriaceae with <30 isolates were grouped with the overall genus data.
Blank spaces mean that there are no CLSI, EUCAST, or FDA MIC breakpoints available for this agent.
The 1,010 isolates of Klebsiella spp. were composed of 765 Klebsiella pneumoniae and 245 Klebsiella oxytoca isolates.
The 503 isolates of Serratia spp. were composed of 472 Serratia marcescens, 22 Serratia liquefaciens, 4 Serratia ureilytica, 3 Serratia odorifera, 1 Serratia grimesii, and 1 Serratia rubidaea isolates.
The 494 isolates of Enterobacter spp. were composed of 238 Enterobacter aerogenes, 213 Enterobacter cloacae, 30 Enterobacter asburiae, 10 Enterobacter kobei, 2 Enterobacter ludwigii, and 1 Enterobacter amnigenus isolates.
The 260 isolates of Citrobacter spp. were composed of 143 Citrobacter freundii, 99 Citrobacter koseri, 10 Citrobacter braakii, 5 Citrobacter amalonaticus, 2 Citrobacter farmeri, and 1 Citrobacter sedlakii isolates.
TABLE 2.
In vitro activity of cefiderocol and comparators against Gram-negative bacilli (n = 4,966) isolated by 49 medical center laboratories in Europe from 2014 to 2015
| Family/genus/species (no. of isolates) | Antimicrobial agent | MIC (μg/ml)a |
MIC interpretationb |
||||
|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | % susceptible | % intermediate | % resistant | ||
| Enterobacteriaceae (3,080) | Cefiderocol | ≤0.002–8 | 0.12 | 1 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | >64 | 81.6 | 3.1 | 15.4 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.5 | 98.6 | 0 | 1.4 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 8 | 87.1 | 2.4 | 10.5 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | >8 | 80.5 | 1.8 | 17.8 | |
| Colistin | ≤0.25 to >8 | 0.5 | >8 | 82.0 | 0 | 18.0 | |
| Meropenem | ≤0.06 to >64 | ≤0.06 | 0.12 | 95.0 | 0.5 | 4.1 | |
| Meropenem-nonsusceptible Enterobacteriaceae (139) | Cefiderocol | 0.008–8 | 1 | 4 | |||
| Cefepime | 0.25 to >64 | >64 | >64 | 6.5 | 1.4 | 92.1 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 1 | >64 | 71.9 | 0 | 28.1 | |
| Ceftolozane-tazobactam | 0.5 to >64 | >64 | >64 | 5.0 | 2.2 | 92.8 | |
| Ciprofloxacin | ≤0.12 to >8 | >8 | >8 | 10.8 | 1.4 | 87.8 | |
| Colistin | ≤0.25 to >8 | 1 | >8 | 72.7 | 0 | 27.3 | |
| Meropenem | 2 to >64 | 16 | >64 | 0 | 10.1 | 89.9 | |
| Klebsiella spp.c (1,021) | Cefiderocol | ≤0.002–8 | 0.12 | 2 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | >64 | 70.3 | 3.3 | 26.4 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 1 | 97.8 | 0 | 2.3 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 64 | 83.0 | 1.3 | 15.8 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | >8 | 72.3 | 2.6 | 25.1 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 95.0 | 0 | 5.0 | |
| Meropenem | ≤0.06 to >64 | ≤0.06 | 2 | 89.9 | 1.0 | 9.1 | |
| Klebsiella pneumoniae (761) | Cefiderocol | ≤0.002–8 | 0.12 | 2 | |||
| Cefepime | ≤0.06 to >64 | 0.12 | >64 | 63.1 | 3.0 | 33.9 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.25 | 1 | 97.1 | 0 | 2.9 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.5 | 64 | 78.2 | 1.3 | 20.5 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | >8 | 64.4 | 2.8 | 32.9 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 93.3 | 0 | 6.7 | |
| Meropenem | ≤0.06 to >64 | ≤0.06 | 8 | 86.7 | 1.3 | 12.0 | |
| Klebsiella oxytoca (260) | Cefiderocol | ≤0.002–2 | 0.03 | 0.25 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 1 | 91.5 | 4.2 | 4.2 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.25 | 99.6 | 0 | 0.4 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 0.5 | 96.9 | 1.2 | 1.9 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | ≤0.12 | 95.4 | 2.3 | 2.3 | |
| Colistin | ≤0.25–2 | 0.5 | 1 | 100 | 0 | 0 | |
| Meropenem | ≤0.06–8 | ≤0.06 | ≤0.06 | 99.2 | 0 | 0.8 | |
| Escherichia coli (789) | Cefiderocol | ≤0.002–4 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 64 | 81.6 | 3.6 | 14.8 | |
| Ceftazidime-avibactam | ≤0.06–32 | 0.12 | 0.25 | 99.8 | 0 | 0.3 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.25 | 0.5 | 96.5 | 0.4 | 3.2 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | >8 | 72.9 | 0.5 | 26.6 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 99.5 | 0 | 0.5 | |
| Meropenem | ≤0.06–4 | ≤0.06 | ≤0.06 | 99.6 | 0 | 0.4 | |
| Serratia spp.d (493) | Cefiderocol | ≤0.002–8 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | 0.12 | 0.5 | 94.1 | 1.0 | 4.9 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.5 | 99.2 | 0 | 0.8 | |
| Ceftolozane-tazobactam | 0.12 to >64 | 0.5 | 1 | 95.3 | 1.8 | 2.8 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 93.7 | 2.2 | 4.1 | |
| Colistin | 0.5 to >8 | >8 | >8 | 5.1 | 0 | 94.9 | |
| Meropenem | ≤0.06–64 | ≤0.06 | 0.12 | 99.0 | 0 | 1.0 | |
| Serratia marcescens (455) | Cefiderocol | ≤0.002–8 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | 0.12 | 0.5 | 93.6 | 1.1 | 5.3 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.5 | 99.1 | 0 | 0.9 | |
| Ceftolozane-tazobactam | 0.25 to >64 | 0.5 | 1 | 95.2 | 2.0 | 2.9 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 93.4 | 2.4 | 4.2 | |
| Colistin | 0.5 to >8 | >8 | >8 | 5.5 | 0 | 94.5 | |
| Meropenem | ≤0.06–64 | ≤0.06 | 0.12 | 98.9 | 0 | 1.1 | |
| Serratia liquefaciens (33) | Cefiderocol | 0.015–0.25 | 0.06 | 0.12 | |||
| Cefepime | ≤0.06–0.25 | ≤0.06 | 0.12 | 100 | 0 | 0 | |
| Ceftazidime-avibactam | 0.12–1 | 0.25 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | 0.25–1 | 0.5 | 1 | 100 | 0 | 0 | |
| Ciprofloxacin | ≤0.12–0.5 | ≤0.12 | ≤0.12 | 100 | 0 | 0 | |
| Colistin | >8 | >8 | >8 | 0 | 0 | 100 | |
| Meropenem | ≤0.06–0.25 | ≤0.06 | 0.12 | 100 | 0 | 0 | |
| Enterobacter spp.e (530) | Cefiderocol | 0.008–8 | 0.25 | 1 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 16 | 85.7 | 4.2 | 10.2 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.25 | 0.5 | 97.9 | 0 | 2.1 | |
| Ceftolozane-tazobactam | ≤0.06–64 | 0.5 | 8 | 75.7 | 7.6 | 16.8 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 90.0 | 2.1 | 7.9 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 94.3 | 0 | 5.7 | |
| Meropenem | ≤0.06–64 | ≤0.06 | 0.12 | 96.2 | 0.6 | 3.2 | |
| Enterobacter cloacae (301) | Cefiderocol | 0.008–4 | 0.25 | 1 | |||
| Cefepime | ≤0.06 to >64 | 0.12 | 32 | 78.4 | 6.6 | 15.0 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.25 | 1 | 96.4 | 0 | 3.7 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.5 | 16 | 74.1 | 3.0 | 22.9 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 8 | 85.1 | 2.7 | 12.3 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 95.7 | 0 | 4.3 | |
| Meropenem | ≤0.06–64 | ≤0.06 | 0.25 | 93.7 | 1.0 | 5.3 | |
| Enterobacter aerogenes (204) | Cefiderocol | 0.015–8 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 1 | 94.6 | 1.0 | 4.4 | |
| Ceftazidime-avibactam | ≤0.06–8 | 0.25 | 0.5 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | 0.12–32 | 0.5 | 4 | 77.0 | 14.2 | 8.8 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 0.25 | 96.1 | 1.5 | 2.5 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 98.5 | 0 | 1.5 | |
| Meropenem | ≤0.06–64 | ≤0.06 | 0.12 | 99.5 | 0 | 0.5 | |
| Citrobacter spp.f (247) | Cefiderocol | 0.004–4 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 1 | 94.3 | 2.0 | 3.6 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.5 | 98.4 | 0 | 1.6 | |
| Ceftolozane-tazobactam | 0.12 to >64 | 0.25 | 8 | 82.6 | 3.2 | 14.2 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 1 | 91.5 | 0.4 | 8.1 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 99.6 | 0 | 0.4 | |
| Meropenem | ≤0.06–8 | ≤0.06 | ≤0.06 | 96.8 | 0.4 | 2.8 | |
| Citrobacter freundii (160) | Cefiderocol | 0.004–2 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | ≤0.06 | 2 | 92.5 | 2.5 | 5.0 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 0.12 | 0.5 | 98.1 | 0 | 1.9 | |
| Ceftolozane-tazobactam | 0.12 to >64 | 0.25 | 16 | 75.6 | 3.8 | 20.6 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | 4 | 88.1 | 0 | 11.9 | |
| Colistin | ≤0.25–2 | 0.5 | 1 | 100 | 0 | 0 | |
| Meropenem | ≤0.06–8 | ≤0.06 | 0.12 | 95.6 | 0.6 | 3.8 | |
| Citrobacter koseri (73) | Cefiderocol | 0.008–4 | 0.25 | 0.5 | |||
| Cefepime | ≤0.06–8 | ≤0.06 | ≤0.06 | 98.6 | 1.4 | 0 | |
| Ceftazidime-avibactam | ≤0.06–1 | 0.12 | 0.25 | 100 | 0 | 0 | |
| Ceftolozane-tazobactam | 0.12–4 | 0.25 | 0.5 | 98.6 | 1.4 | 0 | |
| Ciprofloxacin | ≤0.12 to >8 | ≤0.12 | ≤0.12 | 98.6 | 0 | 1.4 | |
| Colistin | ≤0.25 to >8 | 0.5 | 1 | 98.6 | 0 | 1.4 | |
| Meropenem | ≤0.06–0.12 | ≤0.06 | ≤0.06 | 100 | 0 | 0 | |
| Pseudomonas aeruginosa (765) | Cefiderocol | ≤0.002–4 | 0.12 | 0.5 | |||
| Cefepime | ≤0.06 to >64 | 4 | 32 | 82.1 | 7.5 | 10.5 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 2 | 8 | 91.6 | 0 | 8.4 | |
| Ceftolozane-tazobactam | ≤0.06 to >64 | 0.5 | 4 | 90.9 | 2.1 | 7.1 | |
| Ciprofloxacin | ≤0.12 to >8 | 0.25 | >8 | 74.0 | 4.1 | 22.0 | |
| Colistin | ≤0.25 to >8 | 1 | 2 | 98.7 | 0.5 | 0.8 | |
| Meropenem | ≤0.06 to >64 | 0.5 | 16 | 73.6 | 5.2 | 21.2 | |
| Meropenem-nonsusceptible Pseudomonas aeruginosa (202) | Cefiderocol | 0.008–4 | 0.25 | 1 | |||
| Cefepime | 1 to >64 | 16 | >64 | 47.5 | 18.3 | 34.2 | |
| Ceftazidime-avibactam | 1 to >64 | 8 | 64 | 68.3 | 0 | 31.7 | |
| Ceftolozane-tazobactam | 0.5 to >64 | 1 | >64 | 67.3 | 5.9 | 26.7 | |
| Ciprofloxacin | ≤0.12 to >8 | 8 | >8 | 34.7 | 5.0 | 60.4 | |
| Colistin | ≤0.25–4 | 1 | 1 | 99.0 | 1.0 | 0 | |
| Meropenem | 4 to >64 | 8 | 16 | 0 | 19.8 | 80.2 | |
| Acinetobacter baumannii (839) | Cefiderocol | 0.004–64 | 0.12 | 1 | |||
| Cefepime | ≤0.06 to >64 | 64 | >64 | 26.5 | 12.5 | 61.0 | |
| Ceftazidime-avibactam | ≤0.06 to >64 | 16 | >64 | ||||
| Ceftolozane-tazobactam | ≤0.06 to >64 | 8 | 64 | ||||
| Ciprofloxacin | ≤0.12 to >8 | >8 | >8 | 21.9 | 0.1 | 78.0 | |
| Colistin | ≤0.25 to >8 | 1 | >8 | 87.5 | 0 | 12.5 | |
| Meropenem | ≤0.06 to >64 | 32 | >64 | 29.1 | 0.6 | 70.3 | |
| Meropenem-nonsusceptible Acinetobacter baumannii (595) | Cefiderocol | 0.004–64 | 0.12 | 1 | |||
| Cefepime | 4 to >64 | 64 | >64 | 2.4 | 13.6 | 84.0 | |
| Ceftazidime-avibactam | 1 to >64 | 32 | >64 | ||||
| Ceftolozane-tazobactam | 1 to >64 | 16 | >64 | ||||
| Ciprofloxacin | ≤0.12 to >8 | >8 | >8 | 0.2 | 0 | 99.8 | |
| Colistin | ≤0.25 to >8 | 1 | >8 | 82.7 | 0 | 17.3 | |
| Meropenem | 4 to >64 | 64 | >64 | 0 | 0.8 | 99.2 | |
| Stenotrophomonas maltophilia (276) | Cefiderocol | 0.004–2 | 0.06 | 0.25 | |||
| Cefepime | 0.5 to >64 | 32 | >64 | ||||
| Ceftazidime-avibactam | 0.5 to >64 | 16 | 64 | ||||
| Ceftolozane-tazobactam | 0.12 to >64 | 8 | >64 | ||||
| Ciprofloxacin | ≤0.12 to >8 | 2 | 8 | ||||
| Colistin | ≤0.25 to >8 | 1 | >8 | ||||
| Meropenem | 0.12 to >64 | >64 | >64 | ||||
| Burkholderia cepacia (6) | Cefiderocol | 0.004–1 | |||||
| Cefepime | 16 to >64 | ||||||
| Ceftazidime-avibactam | 2–32 | ||||||
| Ceftolozane-tazobactam | 1–64 | ||||||
| Ciprofloxacin | 0.5 to >8 | ||||||
| Colistin | ≤0.25 to >8 | ||||||
| Meropenem | 2–16 | 66.7 | 0 | 33.3 | |||
MIC50 and MIC90 calculated only for genus or species where >30 isolates were tested. Species of Enterobacteriaceae with <30 isolates were grouped with the overall genus data.
Blank spaces mean that there are no CLSI, EUCAST, or FDA MIC breakpoints available for this agent.
The 1,021 isolates of Klebsiella spp. were composed of 761 Klebsiella pneumoniae and 260 Klebsiella oxytoca isolates.
The 493 isolates of Serratia spp. were composed of 455 Serratia marcescens, 33 Serratia liquefaciens, 3 Serratia ureilytica, 1 Serratia odorifera, and 1 Serratia rubidaea isolates.
The 530 isolates of Enterobacter spp. were composed of 301 Enterobacter cloacae, 204 Enterobacter aerogenes, 19 Enterobacter asburiae, 4 Enterobacter kobei, and 2 Enterobacter ludwigii isolates.
The 247 isolates of Citrobacter spp. were composed of 160 Citrobacter freundii, 73 Citrobacter koseri, 9 Citrobacter braakii, 3 Citrobacter amalonaticus, and 2 Citrobacter farmeri isolates.
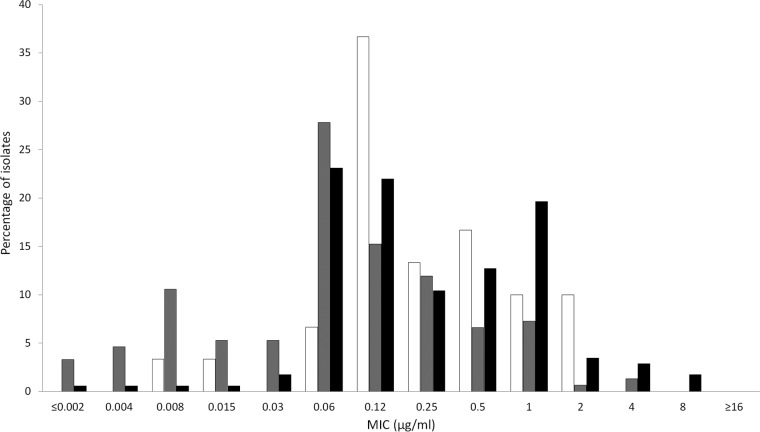
FIG 1.
Cefiderocol MIC distributions for meropenem-nonsusceptible Enterobacteriaceae (white bars; n = 30), P. aeruginosa (gray bars; n = 151), and A. baumannii (black bars; n = 173) isolates collected by North American medical center laboratories.
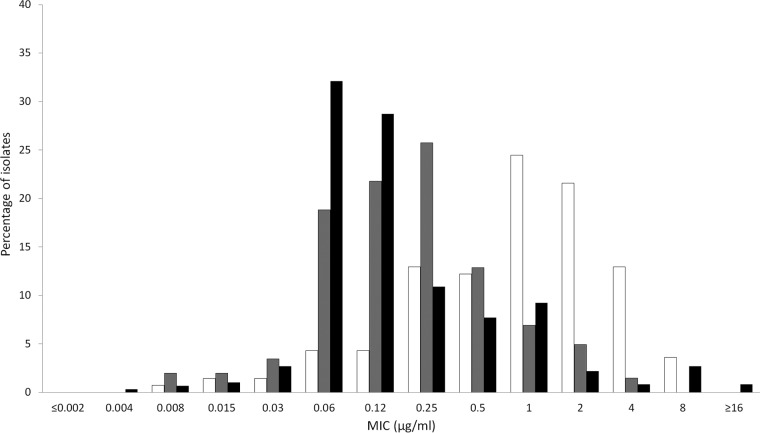
FIG 2.
Cefiderocol MIC distributions for meropenem-nonsusceptible Enterobacteriaceae (white bars; n = 139), P. aeruginosa (gray bars; n = 202), and A. baumannii (black bars; n = 595) isolates collected by European medical center laboratories.
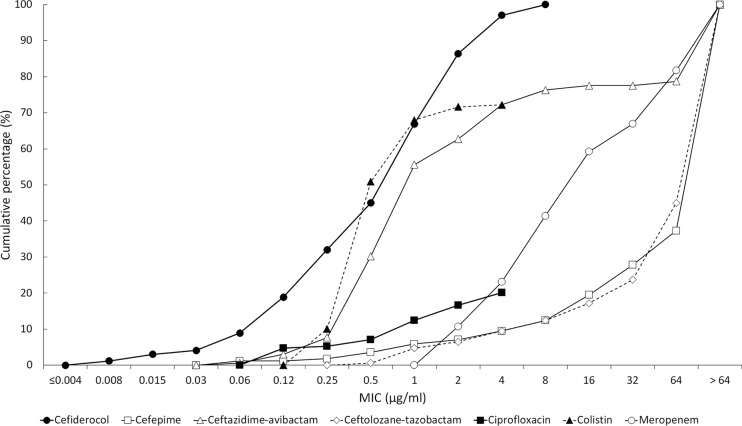
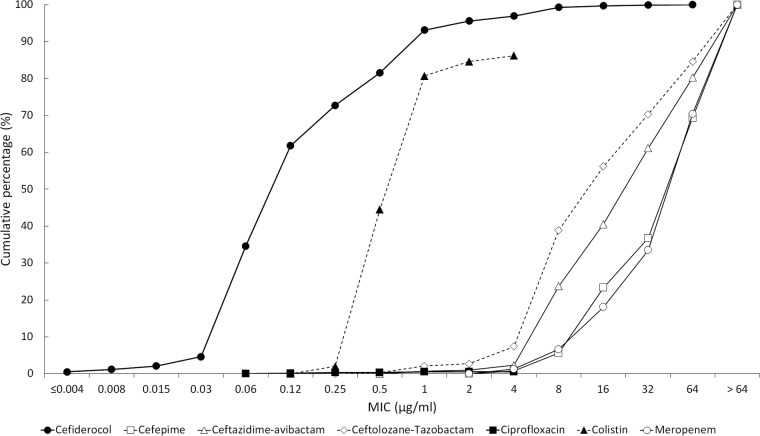
FIG 3.
Cumulative MIC susceptibility curves for cefiderocol and comparators against 169 meropenem nonsusceptible Enterobacteriaceae from North American and European medical center laboratories. For colistin and ciprofloxacin, the endpoint of their respective cumulative MIC susceptibility curves represents the highest concentration tested. The remaining tested isolates had MICs higher than their endpoint value.
The MIC90 values for cefiderocol against P. aeruginosa were 0.5 μg/ml (North America, n = 765 isolates) and 0.5 μg/ml (Europe, n = 765 isolates); 99.9% (1,529/1,530) of all P. aeruginosa isolates had MICs of ≤4 μg/ml. The one isolate with a MIC to cefiderocol of 8 μg/ml was from North America. Against meropenem-nonsusceptible (MIC ≥ 4 μg/ml) isolates from North America (Fig. 1) and Europe (Fig. 2), MIC90 values for cefiderocol were 0.5 μg/ml (n = 151) and 1 μg/ml (n = 202); all 353 isolates of P. aeruginosa that were meropenem nonsusceptible had MICs to cefiderocol of ≤4 μg/ml. In comparison, the MIC90 values for ceftazidime-avibactam and ceftolozane-tazobactam against isolates of meropenem-nonsusceptible P. aeruginosa from North America (Table 1) and Europe (Table 2) were 8 and 4 μg/ml and 64 and >64 μg/ml, respectively. Against meropenem-susceptible (MIC ≤ 2 μg/ml) isolates from North America and Europe, MIC90 values for cefiderocol were 0.5 μg/ml (n = 614) and 0.5 μg/ml (n = 563); 99.9% (1,176/1,177) of all meropenem-susceptible isolates of P. aeruginosa had MICs to cefiderocol of ≤4 μg/ml. Figure 4 depicts the cumulative percentages of all meropenem-nonsusceptible isolates of P. aeruginosa that were susceptible to increasing concentrations of cefiderocol and its comparators.
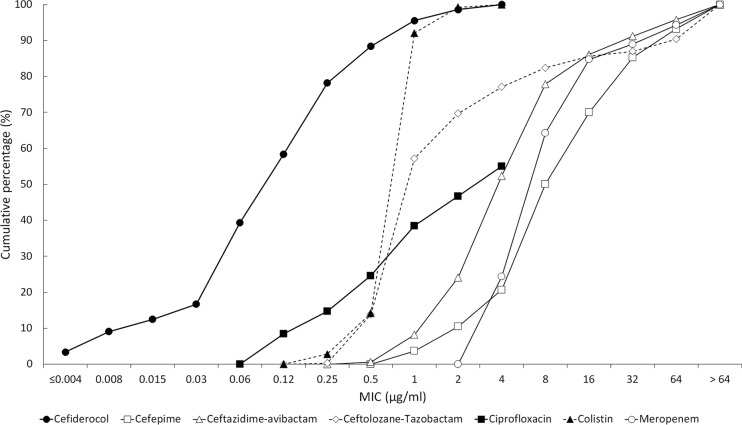
FIG 4.
Cumulative MIC susceptibility curves for cefiderocol and comparators against 353 meropenem-nonsusceptible P. aeruginosa isolates from North American and European medical center laboratories. For colistin and ciprofloxacin, the endpoint of their respective cumulative MIC susceptibility curves represents the highest concentration tested. The remaining tested isolates had MICs higher than their endpoint value.
The MIC90 values for cefiderocol against A. baumannii were 1 μg/ml for both North American (n = 309) and European (n = 839) isolates; 97.6% (1,120/1,148) of all A. baumannii had MICs to cefiderocol of ≤4 μg/ml. Of the 28 isolates with cefiderocol MIC values of >4 μg/ml, 25 were from European medical laboratories (18 isolates from two sites in Russia; 6 isolates from one site in Turkey; and 1 isolate from Sweden) and 3 isolates were from two sites in the United States. Twenty-four of these 28 isolates (85.7%) were also nonsusceptible to meropenem. Against meropenem-nonsusceptible isolates of A. baumannii from North America (Fig. 1; n = 173) and Europe (Fig. 2; n = 595), MIC90 values were 1 μg/ml for both data sets; 96.9% (744/768) of meropenem-nonsusceptible A. baumannii isolates had MICs to cefiderocol of ≤4 μg/ml. Against meropenem-susceptible isolates from North America and Europe, MIC90 values for cefiderocol were 0.25 μg/ml (n = 136) and 0.25 μg/ml (n = 244); 99.0% (376/380) of meropenem-nonsusceptible A. baumannii isolates had MICs to cefiderocol of ≤4 μg/ml. Of the other agents tested against isolates of A. baumannii, colistin was the only one demonstrating significant in vitro activity. Figure 5 depicts the cumulative percentages of all meropenem-nonsusceptible isolates of A. baumannii that were susceptible to increasing concentrations of cefiderocol and its comparators.
FIG 5.
Cumulative MIC susceptibility curves for cefiderocol and comparators against 768 meropenem-nonsusceptible A. baumannii isolates from European and North American medical center laboratories. For colistin and ciprofloxacin, the endpoint of their respective cumulative MIC susceptibility curves represents the highest concentration tested. The remaining tested isolates had MICs higher than their endpoint value.
The MIC90 values for cefiderocol against Stenotrophomonas maltophilia were 0.5 μg/ml (North America, n = 152 isolates) and 0.25 μg/ml (Europe, n = 276 isolates); all isolates of S. maltophilia had MICs to cefiderocol of ≤4 μg/ml, while the MIC90s for cefepime, ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem were ≥64 μg/ml and for colistin and ciprofloxacin were ≥8 μg/ml. There are no published CLSI breakpoints for S. maltophilia for any of the other antimicrobial agents tested in this study.
The MIC range for cefiderocol for 12 isolates of Burkholderia cepacia was 0.008 to 16 μg/ml. Three isolates were meropenem nonsusceptible (MIC ≥ 8 μg/ml). Too few isolates were collected to generate MIC50 and MIC90 values. One isolate from the United States had a cefiderocol MIC of 16 μg/ml; all other isolates (93.8%, 11/12) had cefiderocol MICs of ≤1 μg/ml.
If the entire data set is considered (n = 9,205 isolates) and species intrinsically resistant to colistin (B. cepacia, Serratia spp.) and species for which colistin MIC breakpoints are not available (S. maltophilia) are excluded, colistin nonsusceptibility was observed for 273 isolates (121 A. baumannii, 73 Enterobacter species, 57 Klebsiella species, 14 P. aeruginosa, 6 E. coli, and 2 Citrobacter koseri isolates). The cefiderocol MIC range and MIC90 for colistin-nonsusceptible isolates were ≤0.002 to 8 μg/ml and 2 μg/ml, respectively; 99.6% (272/273) isolates had cefiderocol MICs of ≤4 μg/ml.
DISCUSSION
Carbapenem-resistant Enterobacteriaceae, P. aeruginosa, and A. baumannii are frequently multidrug resistant. Currently, there are very few antimicrobial agents available to clinicians to treat patients infected with carbapenem-resistant Gram-negative bacilli, and the few agents that are accessible to treat systemic infections are associated with considerable toxicities, increasing resistance, and in the case of colistin, intrinsic resistance to several species of Enterobacteriaceae, including Proteus spp., Providencia spp., Morganella morganii, and Serratia spp. In addition, new β-lactam/β-lactamase inhibitor combinations, ceftazidime-avibactam and ceftolozane-tazobactam, have recently been approved in several countries, but neither of these agents is active against isolates producing class B metallo-β-lactamases (1, 2). In the current study, meropenem-nonsusceptible isolates of Enterobacteriaceae and P. aeruginosa challenged the in vitro activities of ceftazidime-avibactam and ceftolozane-tazobactam, particularly isolates from European medical center laboratories (Table 2).
The current study demonstrated that the in vitro activity of the novel siderophore cephalosporin, cefiderocol, was superior to that of comparators against recent clinical isolates of meropenem-nonsusceptible Enterobacteriaceae, P. aeruginosa, and A. baumannii from North America and Europe (Fig. 3 to 5), including isolates that were resistant to colistin and the β-lactam/β-lactamase inhibitor combinations ceftazidime-avibactam and ceftolozane-tazobactam. Cefiderocol also demonstrated potent activity against S. maltophilia, while all six comparators were inactive. Cefiderocol exhibited MIC90s against P. aeruginosa and S. maltophilia that were 4 to >64 times lower than those of comparator agents. Against A. baumannii, cefiderocol (MIC90, 1 μg/ml) was up to 64 times more potent than the comparator agents tested, with the exception of colistin, which also had an MIC90 of 1 μg/ml.
Cefiderocol at a concentration of ≤4 μg/ml inhibited 99.9% (6,078/6,087) of all isolates of Enterobacteriaceae, 99.9% (1,529/1,530) of all isolates of P. aeruginosa, 97.6% (1,120/1,148) of all isolates of A. baumannii, and 100% (428/428) of all isolates of S. maltophilia. The highest MIC for cefiderocol for Enterobacteriaceae (seven isolates) and P. aeruginosa (one isolate) was 8 μg/ml. Against A. baumannii, 28 isolates (2.3%) had MICs of >4 μg/ml. One isolate of Burkholderia cepacia, of the 12 tested, had a cefiderocol MIC of 16 μg/ml (from the United States); all other isolates had cefiderocol MICs of ≤1 μg/ml.
Previous studies have tested the in vitro activity of cefiderocol against genetically characterized ESBL- and carbapenemase-producing isolates of Gram-negative bacilli as well as against isolates of Gram-negative bacilli resistant to carbapenems by mechanisms other than carbapenemases (15–17, 24). Isolates of E. coli, K. pneumoniae, S. marcescens, Citrobacter freundii, and Enterobacter cloacae harboring ESBLs (e.g., CTX-type, SHV-type, TEM-type), KPC-type carbapenemases, and OXA-type carbapenemases as well as K. pneumoniae, S. marcescens, C. freundii, and E. cloacae harboring VIM-type and IMP-type carbapenemases were all inhibited by cefiderocol at a MIC of ≤4 μg/ml (15). Most NDM-1-producing isolates of E. coli (73.7%, 14/19), K. pneumoniae (100%, 24/24), and S. marcescens, C. freundii, and E. cloacae (100%, 6/6) also tested with MICs to cefiderocol of ≤4 μg/ml (15). Ito et al. reported that among 33 isolates of P. aeruginosa, MICs to cefiderocol were ≤2 μg/ml for isolates harboring GIM-1, IMP-type, or SPM-1 carbapenemases and that 14 of 16 (87.5%) VIM-positive isolates had a MIC to cefiderocol of ≤4 μg/ml (17). Against 29 isolates of A. baumannii shown to be β-lactamase-producing, MICs to cefiderocol were ≤4 μg/ml for isolates harboring IMP-1, OXA-51, and OXA-58; some isolates that were positive for OXA-23 or OXA-24 were less susceptible to cefiderocol than were other OXA-type-positive isolates (17).
The mechanism(s) responsible for elevated MICs for cefiderocol in the limited number of isolates that have been observed are currently unknown. If one were to hypothesize a mechanism of resistance to cefiderocol, it would likely involve either a reduction in production of one or more components of the iron transport system or one or more mutations in the binding site for the iron transport system on the outer membrane of Gram-negative bacteria, as this has been reported previously for other siderophore β-lactams (25, 26). The adaption-based resistance to other siderophore-conjugated antibacterial agents, such as MB-1, attributed to competition with native siderophores in P. aeruginosa (26), has not been observed for cefiderocol tested against isolates of P. aeruginosa, A. baumannii, or Enterobacteriaceae (21). The mechanism(s) of resistance to cefiderocol that appeared in the small subset of isolates with higher MICs to cefiderocol (≥8 μg/ml) in the current study requires future study.
Cefiderocol has been tested in several animal models and has shown in vivo efficacy against ESBL-producing, KPC-producing, and multidrug-resistant isolates of Gram-negative bacilli (21, 23, 27). In a neutropenic murine thigh infection model, cefiderocol treatment consistently generated CFU reductions of >1 log and sustained antibacterial effects against all isolates tested (23). In the same study, the effectiveness of cefiderocol was demonstrated to correlate well with the pharmacodynamics parameter % fT>MIC (the percentage of a 24-hour period in which the unbound drug concentration exceeded the MIC) (23). Cefiderocol has also demonstrated efficacy against multidrug-resistant and carbapenem-resistant isolates of P. aeruginosa, A. baumannii, and Enterobacteriaceae in a murine lung infection model (21) and against ESBL-producing or KPC-producing isolates of Enterobacteriaceae in various animal infection models (27). In pharmacokinetic modeling of human drug exposures using a dose of 2 g every 8 h infused over 3 h, cefiderocol achieves a 100% probability to target attainment for organisms with an MIC of 4 μg/ml (28). Importantly, in a rat lung infection model that reproduced human pharmacokinetic and pharmacodynamics parameters, cefiderocol demonstrated bactericidal activity against carbapenem-resistant (KPC- and NDM-1-positive) isolates of K. pneumoniae, with cefiderocol MICs as high as 4 μg/ml (29). A clinical trial evaluating the efficacy and safety of cefiderocol versus imipenem or cilastatin in complicated urinary tract infections is currently ongoing (30) and will be followed by a clinical trial comparing cefiderocol to the best available therapies for the treatment of serious infections caused by carbapenem-resistant Gram-negative pathogens (31).
The current study tested cefiderocol, a promising, novel siderophore cephalosporin, using the CLSI-approved broth microdilution method with ID-CAMHB prepared by preincubation with Chelex 100 resin (20), and found it to demonstrate potent in vitro activity against Enterobacteriaceae, P. aeruginosa, A. baumannii, S. maltophila, and B. cepacia, including carbapenem-resistant isolates. A cefiderocol MIC of ≤4 μg/ml was observed for 99.6% (9,166/9,205) of all isolates of Gram-negative bacilli tested in the current study. Previous studies that performed molecular characterization of isolates for β-lactamase genes have reported that cefiderocol is stable against a broad range of β-lactamases, including ESBL-, AmpC-, and carbapenemase-producing (class A, B, and D enzymes) isolates, including metallo-β-lactamases (e.g., NDM-1, VIM, IMP), as well as against isolates resistant to carbapenems by alternate mechanisms (15–17, 24). Cefiderocol represents a potentially significant advance in the treatment options available to clinicians to care for patients infected with antimicrobial-resistant Gram-negative bacilli. Further in vitro and clinical work with cefiderocol is warranted.
MATERIALS AND METHODS
Bacterial isolates.
From 1 November 2014 to 31 October 2015, 99 medical center laboratories from 13 countries (Canada, 9 medical center laboratories; United States, 41; Czech Republic, 3; France, 5; Germany, 6; Greece, 4; Hungary, 4; Italy, 5; Russia, 5; Spain, 5; Sweden, 2; Turkey, 5; United Kingdom, 5) were each requested to collect 100 clinical isolates with a specific species distribution (15 for E. coli, 15 for K. pneumoniae, 5 for Klebsiella spp. other than K. pneumoniae, 10 for Enterobacter spp., 10 for Serratia spp., 5 for Citrobacter spp., 15 for P. aeruginosa, 15 for A. baumannii, 5 for Burkholderia cepacia, and 5 for Stenotrophomonas maltophilia) from patients with documented intra-abdominal, urinary tract, skin and soft tissue, lower respiratory tract, or bloodstream infections. Only one isolate per patient infection episode was accepted. All isolates were shipped to International Health Management Associates, Inc. (IHMA, Schaumburg, IL, USA), where their identities were confirmed using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Billerica, MA, USA). In total, 9,205 isolates of Gram-negative bacilli were collected by the 50 medical center laboratories in North America (n = 4,239) and the 49 medical center laboratories in Europe (n = 4,966).
Antimicrobial susceptibility testing.
All aspects of antimicrobial susceptibility testing, including broth microdilution panel production, inoculation, incubation, and interpretation, adhered to current CLSI methods (32, 33) and were conducted by IHMA. Broth microdilution panels included the following antimicrobial agents: cefiderocol (doubling dilution range tested, 0.002 to 256 μg/ml), cefepime (0.06 to 64 μg/ml), ceftazidime-avibactam (0.06 and 4 μg/ml to 64 and 4 μg/ml), ceftolozane-tazobactam (0.06 and 8 μg/ml to 64 and 8 μg/ml), ciprofloxacin (0.12 to 8 μg/ml), colistin (0.25 to 8 μg/ml), and meropenem (0.06 to 64 μg/ml). Cefiderocol and ceftolozane were obtained from Shionogi & Co., Ltd. (Osaka, Japan). Avibactam was obtained from Biochempartner (Wuhan, China). Other antimicrobial agents were obtained from the U.S. Pharmacopeia (Rockville, MD). All antimicrobial agents were dissolved and diluted according to CLSI guidelines (20, 32). Cefiderocol was dissolved and diluted in sterile normal saline (20). CAMHB was prepared according to the manufacturer's (BBL, Becton-Dickinson, Sparks, MD) instructions and was used as recommended by the CLSI for broth microdilution panel preparation (32, 33). Cefiderocol was tested using ID-CAMHB prepared by adding 100 g of Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA) to 1 liter of autoclaved CAMHB and stirred for 2 h at room temperature (23°C) to remove cations in the medium. The iron-depleted broth was then filtered using a 0.2-μm filter, its pH was adjusted to 7.3 using 0.1 M hydrochloric acid, and then the broth was supplemented with calcium (CaCl2), magnesium (MgCl2), and zinc (ZnSO4) to final concentrations of 22.5 mg/liter (range, 20 to 25 mg/liter), 11.25 mg/liter (range, 10 to 12.5 mg/liter), and 10 μM (0.56 mg/liter; range 0.5 to 1.0 mg/liter), respectively, and again passed through a 0.2-μm filter. The method of preparation of ID-CAMHB described above was approved by the CLSI Subcommittee on Antimicrobial Susceptibility Testing in January 2016 (20, 34) and has supplanted previous medium preparation methods, including those using 20 μM human apotransferrin and Chelex-treated Iso-Sensitest broth due to MIC reproducibility issues or to the very limited number of manufacturers able to provide media (15–17, 24). The final concentration of iron in ID-CAMHB prepared using the above-described method is ≤0.03 mg/liter (20).
The broth microdilution panels included growth control wells for both CAMHB and ID-CAMHB. The panels were incubated at 35°C for 20 h in ambient air before MIC endpoints were read. ID-CAMHB did not significantly affect the growth of any quality control or test organism. Reading the MIC of cefiderocol was contingent on the presence of strong growth in the ID-CAMHB growth control (i.e., a button of approximately 2 mm or greater). The cefiderocol MIC was read as the first panel well in which isolate growth was significantly reduced (i.e., a button of <1 mm or light/faint turbidity) relative to the growth observed in the ID-CAMHB growth control well. The method described above for reading MIC endpoints for cefiderocol was approved by the CLSI Subcommittee on Antimicrobial Susceptibility Testing in January 2016 (20, 34).
CLSI interpretive criteria, when available (32), and FDA interpretive criteria for ceftazidime-avibactam (35) were used to interpret MICs. For colistin, CLSI interpretive criteria were used to interpret colistin MICs for P. aeruginosa and A. baumannii. Colistin lacks CLSI or FDA breakpoints for Enterobacteriaceae; therefore, European Committee on Antimicrobial Susceptibility Testing (EUCAST) MIC breakpoints for Enterobacteriaceae were applied to Enterobacteriaceae tested against colistin (susceptible, ≤2 μg/ml; resistant, ≥4 μg/ml) (36). Quality control testing was performed each day of testing using E. coli ATCC 25922, P. aeruginosa ATCC 27853, and K. pneumoniae ATCC 700603. All quality control results were within specified CLSI ranges (32), including the CLSI-approved but not-yet-published ranges for cefiderocol (E. coli ATCC 25922, 0.06 to 0.5 μg/ml; P. aeruginosa ATCC 27853, 0.06 to 0.5 μg/ml) (20).
ACKNOWLEDGMENTS
We extend our thanks to all participants from the 99 medical centers in North America and Europe that provided bacterial isolates for this study.
The study was funded by Shionogi & Co., Ltd., Osaka, Japan, and funding included compensation fees for services in relation to preparing the manuscript.
M.A.H. and D.F.S. are employees of International Health Management Associates, Inc. M.T. and Y.Y. are employees of Shionogi & Co. R.E. is a consultant to Shionogi and was compensated for supporting this research. J.A.K. is an employee of the University of Manitoba and Diagnostic Services Manitoba. The IHMA authors and J.A.K. do not have personal financial interests in the sponsor of this paper (Shionogi & Co., Ltd.).
Footnotes
[This article was published on 24 August 2017 with a standard copyright line (“© 2017 American Society for Microbiology. All Rights Reserved.”). The authors elected to pay for open access for the article after publication, necessitating replacement of the original copyright line with the one above, and this change was made on 8 September 2017.]
REFERENCES
- 1.Kazmierczak KM, Biedenbach DJ, Hackel M, Rabine S, de Jonge BLM, Bouchillon SK, Sahm DF, Bradford PA. 2016. Global dissemination of blaKPC into bacterial species beyond Klebsiella pneumoniae and in vitro susceptibility to ceftazidime-avibactam and aztreonam-avibactam. Antimicrob Agents Chemother 60:4490–4500. doi: 10.1128/AAC.00107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jonge BLM, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. 2016. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014). Antimicrob Agents Chemother 60:3163–3169. doi: 10.1128/AAC.03042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zilberberg MD, Kollef MH, Shorr AF. 2016. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: a survey study. J Hosp Med 11:21–26. doi: 10.1002/jhm.2477. [DOI] [PubMed] [Google Scholar]
- 4.Labarca JA, Costa Sallas MJ, Seas C, Guzmàn-Blanco M. 2016. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit Rev Microbiol 42:276–292. doi: 10.3109/1040841X.2014.940494. [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Talbot GH, Bradley JS, Edwards JE Jr, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2014. Antimicrobial resistance global report on surveillance. World Health Organization, Geneva, Switzerland: http://www.who.int/drugresistance/documents/surveillancereport/en/. [Google Scholar]
- 7.Woodford N, Turton JF, Livermore DM. 2011. Multiresistant gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 8.Biedenbach D, Bouchillon S, Hackel M, Hoban D, Kazmierczak K, Hawser S, Badal R. 2015. Dissemination of NDM metallo-β-lactamase genes among clinical isolates of Enterobacteriaceae collected during the SMART global surveillance study from 2008 to 2012. Antimicrob Agents Chemother 59:826–830. doi: 10.1128/AAC.03938-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh TR. 2010. Emerging carbapenemases: a global perspective. Int J Antimicrob Agents 36(Suppl 3):S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 10.Bonomo RA, Szabo D. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis 43(Suppl 2):S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 11.Poirel L, Nordmann P. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 12.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito A, Nishikawa Masumoto TS, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito A, Nishikawa T, Oota M, Kanazawa S, Fukuhara N, Yamaguchi T, Nakamura R, Tsuji M, Yamano Y. 2015. S-649266, a novel siderophore cephalosporin: binding affinity to PBP and bactericidal activity, abstr ECCMID-1871 Abstr 25th Eur Congr Clin Microbiol Infect Dis. [Google Scholar]
- 15.Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 60:729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito-Horiyama T, Ishii Y, Ito A, Sato T, Nakamura R, Fukuhara N, Tsuji M, Yamano Y, Yamaguchi K, Tatedo K. 2016. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 60:4384–4386. doi: 10.1128/AAC.03098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito A, Kohira N, Bouchillon SK, West J, Rittenhouse S, Sader HS, Rhomberg PR, Jones RN, Yoshizawa H, Nakamura R, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against nonfermenting Gram-negative bacteria. J Antimicrob Chemother 71:670–677. doi: 10.1093/jac/dkv402. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji M, Ito A, Nakamura R, Yamano Y, Shimada J. 2014. S-649266, a novel siderophore cephalosporin: in vitro activity against gram-negative bacteria. Open Forum Infect Dis 1(Suppl 1):S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otto BR, Verweij-van Vught AMJJ, MacLaren DM. 1992. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol 18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2016. Antimicrobial susceptibility testing meeting minutes and presentations, January 2016. CLSI, Wayne, PA: http://www.clsi.org/standards/micro/microbiology-files. [Google Scholar]
- 21.Tsuji M, Horiyama T, Toba S, Nakamura R, Yamano Y. 2015. S-649266, a novel siderophore cephalosporin: in vivo efficacy in murine infection model caused by multidrug-resistant gram-negative bacteria, abstr ECCMID-0253 Abstr 25th Eur Congr Clin Microbiol Infect Dis. [Google Scholar]
- 22.Tsuji M, Nakamura R, Hackel M, Sahm DF, Sato T, Echols R, Yamano Y. 2015. Use of iron-depleted cation-adjusted Mueller Hinton broth (ID-CAMHB) for microdilution testing of S-649266, a novel siderophore cephalosporin, abstr ECCMID-0808 Abstr 25th Eur Congr Clin Microbiol Infect Dis. [Google Scholar]
- 23.Ghazi I, Tsuji M, Nicolau DP. 2016. Activity of S-649266 siderophore cephalosporin and comparators against Pseudomonas aeruginosa in murine thigh infection model, abstr Microbe-M-516 ASM Microbe 2016. [Google Scholar]
- 24.Ito A, Kohira N, Nakamura R, Tsuji M, Kreiswirth B, Yamano Y. 2015. S-649266, a novel siderophore cephalosporin: in vitro activity against gram-negative bacteria including carbapenem resistant strains, abstr ECCMID-0252 Abstr 25th Eur Congr Clin Microbiol Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatsumi Y, Maejima T, Mitsuhashi S. 1995. Mechanism of tonB-dependent transport of KP-736, a 1,5-dihydroxy-4-pyridone-substituted cephalosporin, into Escherichia coli K-12 cells. Antimicrob Agents Chemother 39:613–619. doi: 10.1128/AAC.39.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomaras AP, Crandon JL, McPherson CJ, Banevicius MA, Finegan SM, Irvine RL, Brown MF, O'Donnell JP, Nicolau DP. 2013. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:4197–4207. doi: 10.1128/AAC.00629-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura R, Toba S, Tsuji M, Yamano Y, Shimada J. 2014. S-649266, a novel siderophore cephalosporin: IV. In vivo efficacy in various murine infection models, abstr F-1558 Abstr 54th Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 28.Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. 2016. Pharmacokinetic/pharmacodynamics modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother doi: 10.1128/AAC.01381-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ClinicalTrials.gov. A study of efficacy/safety of intravenous S-649266 versus imipenem/cilastatin in complicated urinary tract infections. ClinicalTrials registration no. NTC02321800. https://clinicaltrials.gov/ct2/results?term=S-649266&Search. Accessed 28 July 2016.
- 30.ClinicalTrials.gov. Study of S-649266 or best available therapy for the treatment of severe infections caused by carbapenem-resistant gram-negative pathogens. ClinicalTrials registration no. NTC02321800. https://clinicaltrials.gov/ct2/show/NCT02714595?term=S-649266&rank=2. Accessed 28 July 2016.
- 31.Tsuji M, Horyama T, Nakamura R, Echols R, Singley C, Rittenhouse S, Yamano Y. 2014. S-649266, a novel siderophore cephalosporin: efficacy against Klebsiella pneumoniae producing NDM-1 or KPC in rat lung infection model with recreated humanized exposure profile of 2 g dose with 1 or 3 h infusion, abstr 248 IDWeek 2014. [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing. Twenty-sixth informational supplement M100-S26. CLSI, Wayne, PA. [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 10th ed Approved standard M07-A10. CLSI, Wayne, PA. [Google Scholar]
- 34.Tsuji M, Hackel M, Echols R, Sahm D, Yamano Y. 2016. MIC reproducibility of iron depleted cation adjusted-Mueller Hinton broth (ID-CAMHB) for microdilution testing of S-649266, a novel siderophore cephalosporin, abstr Microbe-M-470. ASM Microbe 2016. [Google Scholar]
- 35.Allergan plc. 2015. Avycaz package insert. Allergan plc, Dublin, Ireland. [Google Scholar]
- 36.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0 (valid from 2016-01-01). http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf Accessed 28 July 2016.