ABSTRACT
The use of rapid diagnostic tests (RDTs) enhances antimicrobial stewardship program (ASP) interventions in optimization of antimicrobial therapy. This quasi-experimental cohort study evaluated the combined impact of an ASP/RDT bundle on the appropriateness of empirical antimicrobial therapy (EAT) and time to de-escalation of broad-spectrum antimicrobial agents (BSAA) in Gram-negative bloodstream infections (GNBSI). The ASP/RDT bundle consisted of system-wide GNBSI treatment guidelines, prospective stewardship monitoring, and sequential introduction of two RDTs, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) and the FilmArray blood culture identification (BCID) panel. The preintervention period was January 2010 through December 2013, and the postintervention period followed from January 2014 through June 2015. The postintervention period was conducted in two phases; phase 1 followed the introduction of MALDI-TOF MS, and phase 2 followed the introduction of the FilmArray BCID panel. The interventions resulted in significantly improved appropriateness of EAT (95% versus 91%; P = 0.02). Significant reductions in median time to de-escalation from combination antimicrobial therapy (2.8 versus 1.5 days), antipseudomonal beta-lactams (4.0 versus 2.5 days), and carbapenems (4.0 versus 2.5 days) were observed in the postintervention compared to the preintervention period (P < 0.001 for all). The reduction in median time to de-escalation from combination therapy (1.0 versus 2.0 days; P = 0.03) and antipseudomonal beta-lactams (2.2 versus 2.7 days; P = 0.04) was further augmented during phase 2 compared to phase 1 of the postintervention period. Implementation of an antimicrobial stewardship program and RDT intervention bundle in a multihospital health care system is associated with improved appropriateness of EAT for GNBSI and decreased utilization of BSAA through early de-escalation.
KEYWORDS: antimicrobial stewardship, bloodstream infection, rapid diagnostics
INTRODUCTION
The benefit of timely appropriate antimicrobial therapy on mortality in bloodstream infection (BSI) and sepsis is well described (1–4). The use of rapid diagnostic tests (RDTs) enhances early identification of select causative organisms and may help avoid immediate delays in appropriate therapy (5, 6). A large systematic review and meta-analysis of the impact of RDTs on BSI outcomes suggested that RDTs could improve the timeliness of appropriate therapy and could have positive clinical benefits, including reduced mortality, when combined with direct provider communication (7).
Antimicrobial stewardship programs (ASPs) strive to optimize antimicrobial use while minimizing its potential consequences, e.g., antibiotic resistance, adverse events, hospital-acquired infections, and added cost. Antimicrobial optimization is often accomplished by streamlining therapy to reduce the unnecessary use of broad-spectrum antimicrobial agents (BSAA). Several studies have demonstrated positive impacts on appropriate antimicrobial therapy and improved patient outcomes when implementing RDTs into an ASP model as a bundle (8–12). Others have shown the use of RDTs without active ASP intervention may not provide the expected patient benefits (11, 13–15). Current studies have not fully evaluated the synergistic impact of a comprehensive stewardship intervention and RDT bundle specifically in a multihospital health care system. The benefit of sequential implementation of multiple RDTs on antibiotic therapy in Gram-negative BSI (GNBSI) is also unknown.
The current study was designed to evaluate the combined impact of an ASP bundle and multiple RDTs on the appropriateness of empirical antimicrobial therapy (EAT) and timeliness to de-escalation of BSAA in GNBSI. The bundle consisted of (i) implementation of system-wide evidence-based GNBSI antimicrobial management guidelines (see Supplement File 1 in the supplemental material), (ii) prospective antimicrobial stewardship review and feedback on all positive blood cultures for Gram-negative bacilli, and (iii) sequential introduction of two RDTs, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) and the FilmArray blood culture identification (BCID) panel.
RESULTS
Demographics and clinical characteristics.
A total of 1,163 unique patients with GNBSI were included in the study, 830 in the preintervention group and 333 in the postintervention group. Demographic and clinical characteristics were comparable between the two groups (Table 1). Overall, the most common source of GNBSI was the urinary tract (51.2%), and 40.6% of GNBSIs were community acquired. Escherichia coli was the most common bloodstream isolate, followed by Klebsiella spp., Proteus mirabilis, and Pseudomonas aeruginosa (Fig. 1).
TABLE 1.
Demographics and clinical characteristics of patients with a Gram-negative bloodstream infection
| Variablea | Preintervention group (n = 830) | Postintervention group (n = 333) |
|---|---|---|
| Age (median [IQR]) (yrs) | 65 (54–78) | 64 (54–76) |
| Female (n [%]) | 448 (54) | 167 (50) |
| Race (n [%]) | ||
| White | 400 (48) | 141 (42) |
| Black | 401 (48) | 179 (54) |
| Other | 29 (3) | 13 (4) |
| Comorbid conditions (n [%]) | ||
| Diabetes mellitus | 316 (38) | 133 (40) |
| Cancer | 152 (18) | 49 (15) |
| Immunocompromised | 106 (13) | 36 (11) |
| End-stage renal disease | 77 (9) | 41 (12) |
| Liver cirrhosis | 30 (4) | 25 (8) |
| Catheters (n [%]) | ||
| Central venous catheter | 181 (22) | 58 (17) |
| Foley catheter | 102 (12) | 35 (11) |
| Severity of illness | ||
| Intensive care unit | 205 (25) | 111 (33) |
| Pitt bacteremia score ≥4 | 190 (23) | 65 (20) |
| Site of acquisition (n [%]) | ||
| Community acquired | 344 (41) | 129 (39) |
| Health care associated | 316 (38) | 108 (32) |
| Hospital acquired | 170 (20) | 96 (29) |
| Source of BSI (n [%]) | ||
| Urine | 444 (53) | 152 (46) |
| GI/biliary tract | 106 (13) | 38 (12) |
| Central venous catheter | 59 (7) | 33 (10) |
| Respiratory system | 49 (6) | 15 (5) |
| Skin and skin structure | 34 (4) | 16 (5) |
| Other | 15 (2) | 4 (1) |
| Unknown | 123 (15) | 75 (23) |
IQR, interquartile range; GI, gastrointestinal.
FIG 1.
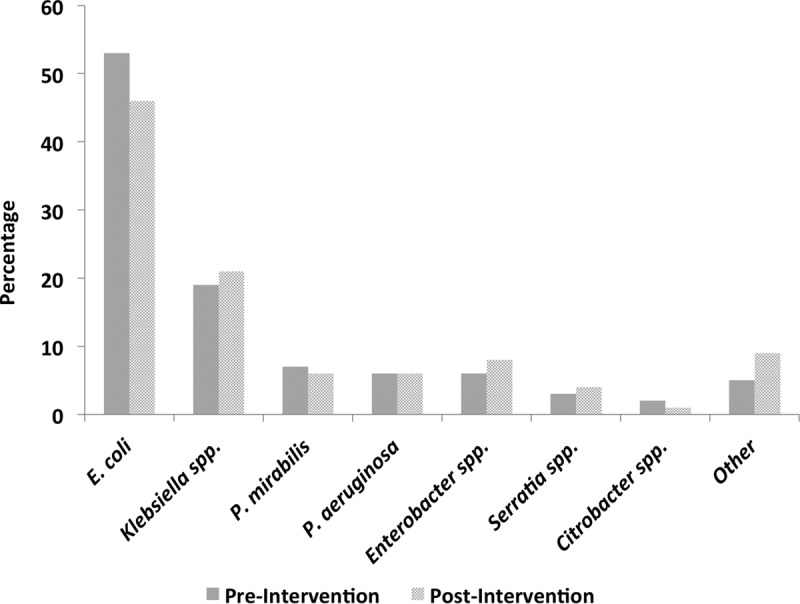
Microbiology of Gram-negative bloodstream infections.
Preintervention versus postintervention periods.
Clinical outcomes in the preintervention versus postintervention periods are summarized in Table 2. Empirical combination therapy was used significantly less often in the postintervention group than in the preintervention group (27% versus 12%; P < 0.001). Empirical anti-pseudomonal agents were also used less frequently than in the preintervention group, with an absolute decrease of 6% in the postintervention period (82% versus 76%; P = 0.01). The interventions were associated with significantly improved appropriateness of EAT both overall (95% versus 91%; P = 0.02) and in patients with BSI due to P. aeruginosa/chromosomally mediated AmpC-producing Enterobacteriaceae (CAE) (97% versus 87%; P = 0.02). The appropriateness of EAT also improved from 89% to 97% in critically ill patients with aPitt bacteremia score of ≥4, although this finding did not reach statistical significance (P = 0.06).
TABLE 2.
Clinical outcomes in preintervention and postintervention periods
| Outcomea | Preintervention, n = 830 | Postintervention, n = 333 | P value |
|---|---|---|---|
| Empirical antimicrobial therapy within 48 h (n [%]) | |||
| Combination therapy | 225 (27) | 49 (15) | <0.001 |
| Antipseudomonal agents | 684 (82) | 252 (76) | 0.01 |
| Carbapenems | 89 (11) | 32 (10) | 0.57 |
| Appropriate empirical therapy within 48 h (n [%]) | |||
| Overall | 758 (91) | 317 (95) | 0.02 |
| Pitt bacteremia score of ≥4 | 170/190 (89) | 63/65 (97) | 0.06 |
| BSI due to P. aeruginosa/CAE | 134/154 (87) | 66/68 (97) | 0.02 |
| Time to de-escalation (median [IQR]) (days)b | |||
| Combination therapy | 2.8 (2.5–3.0) | 1.5 (1.0–2.0) | <0.001 |
| Antipseudomonal beta-lactams | 4.0 (3.6–4.2) | 2.5 (2.3–2.7) | <0.001 |
| Carbapenems | 4.0 (3.7–4.9) | 2.5 (2.0–3.0) | <0.001 |
CAE, AmpC-producing Enterobacteriaceae; IQR, interquartile range.
Number of subjects eligible for de-escalation in pre- and postintervention periods was 225 and 49 for combination therapy, 388 and 156 for antipseudomonal beta-lactams, and 181 and 52 for carbapenems, respectively.
The antimicrobial stewardship and RDT intervention bundle resulted in significant reductions in time to de-escalation from combination antimicrobial therapy (2.8 versus 1.5 days), antipseudomonal beta-lactams (APBLs) (4.0 versus 2.5 days), and carbapenems (4.0 versus 2.5 days) compared to those of the preintervention group (P < 0.001 for all).
Phase 1 versus phase 2 of intervention.
The median time to Gram staining and final susceptibility reports were comparable between phase 1 and phase 2 of the intervention period. However, the time to organism identification was significantly reduced from 1.7 to 0.8 days (P < 0.001) when BCID was introduced in phase 2 compared to phase 1 (MALDI-TOF MS only). Antimicrobial utilization and the appropriateness of EAT were comparable between phase 1 and phase 2 within the postintervention group (Table 3). Time to de-escalation from combination therapy (2 days in phase 1 versus 1 day in phase 2; P = 0.03), APBLs (2.7 days versus 2.2 days; P = 0.04) and carbapenems (2.8 days versus 2.1 days; P = 0.32) was further reduced in phase 2 compared to phase 1 of the postintervention period (Table 3). It was noted that de-escalation from APBLs in the postintervention period started as early as 1 day following collection of index blood culture. Ultimately, 61% and 78% of patients in phase 1 and phase 2 of the intervention period, respectively, were de-escalated off APBL within 3 days per the respective Kaplan-Meier curves (Fig. 2). De-escalation was often prior to availability of final in vitro antimicrobial susceptibility testing results (Table 3). Among patients who were de-escalated from APBLs or carbapenems, none underwent subsequent escalation of antimicrobial therapy based on in vitro antimicrobial susceptibility testing results.
TABLE 3.
Clinical outcomes in phases 1 and 2 of postintervention perioda
| Outcomeb | Phase 1 (n = 182) | Phase 2 (n = 151) | P value |
|---|---|---|---|
| Microbiology results (median [IQR]) (days) | |||
| Gram stain | 0.7 (0.7–0.7) | 0.7 (0.6–0.7) | 0.28 |
| Organism identification | 1.7 (1.7–1.9) | 0.8 (0.7–0.8) | <0.001 |
| Final antimicrobial susceptibility report | 3.3 (2.9–3.8) | 3.0 (2.7–3.9) | 0.93 |
| Empirical antimicrobial therapy within 48 h (n [%]) | |||
| Combination therapy | 26 (14) | 23 (15) | 0.81 |
| Antipseudomonal agents | 133 (73) | 119 (79) | 0.22 |
| Carbapenems | 22 (12) | 10 (7) | 0.10 |
| Appropriate empirical therapy within 48 h (n [%]) | |||
| Overall | 173 (95) | 144 (95) | 0.90 |
| Pitt bacteremia score of ≥4 | 27/28 (96) | 36/37 (97) | 0.99 |
| BSI due to P. aeruginosa/CAE | 34/36 (94) | 32/32 (100) | 0.49 |
| Time to de-escalation (median [IQR]) (days)c | |||
| Combination therapy | 2.0 (1.0–2.8) | 1.0 (1.0–2.0) | 0.03 |
| Antipseudomonal beta-lactams | 2.7 (2.5–3.0) | 2.2 (2.0–2.5) | 0.04 |
| Carbapenems | 2.8 (2.0–3.3) | 2.1 (1.0–2.9) | 0.32 |
Phase 1, MALDI-TOF MS; phase 2, MALDI-TOF MS plus FilmArray BCID.
IQR, interquartile range; CAE, AmpC-producing Enterobacteriaceae.
Numbers of subjects eligible for de-escalation in phases 1 and 2 of the postintervention period were 26 and 23 for combination therapy, 87 and 69 for antipseudomonal beta-lactams, and 35 and 17 for carbapenems, respectively.
FIG 2.

Time to de-escalation off antipseudomonal beta-lactams in patients with bloodstream infection due to Enterobacteriaceae. Log-rank P value is <0.001. Number of subjects with bloodstream infections due to E. coli, Klebsiella spp., P. mirabilis, and Salmonella spp. who were empirically started on anti-pseudomonal beta-lactams: preintervention, 388; phase 1, 87; phase 2, 69. Dotted black line, preintervention period; solid black line, phase 1 intervention; solid gray line, phase 2 intervention.
DISCUSSION
This study demonstrates the first multisystem stewardship bundle combining 4 independent strategies, guidelines, prospective stewardship monitoring and feedback, and 2 independent RDTs (MALDI-TOF MS and FilmArray BCID) to improve antimicrobial utilization, the appropriateness of EAT, and the timeliness of de-escalation. Both passive and active guideline-recommended stewardship program elements were uniquely combined to improve antimicrobial therapy in serious, invasive Gram-negative infections (16, 17). Additionally, use of sound local evidence from well-designed studies allowed for development and implementation of local stewardship guidelines (4). Appropriate therapy within 48 h of BSI was improved in the postintervention period, with 95% of patients having an appropriate agent, dose, and route for the identified pathogen compared to 91% in the preintervention period. However, the magnitude of EAT improvement was notably observed in critically ill patients with Pitt bacteremia scores ≥4 (97% post- versus 89% preinterventions). This was likely due to relatively more conservative local guidelines and more aggressive antimicrobial stewardship and support team (ASST) recommendations due to lifesaving opportunities with appropriate therapy (4, 18, 19). Despite the extensive use of antipseudomonal and combination antimicrobial therapy in the preintervention period, only 87% of patients with BSI due to P. aeruginosa or CAE received appropriate EAT. This improved to 97% in in the postintervention period. Local institutional guidelines and the addition of RDTs, particularly BCID, improved the identification of patients with BSI due to these organisms. This improvement was achieved while using significantly less empirical antipseudomonal agents and nearly 50% fewer combination regimens in the post- versus preintervention periods. The institutional guidelines discouraged the nonstratified use of APBLs. Combination therapy was also discouraged, especially among patients with BSI due to Enterobacteriaceae based on local evidence (C. Troficanto, J. Kohn, J. A. Justo, P. B. Bookstaver, H. Albrecht, and M. N. Al-Hasan, presented at ASM Microbe Annual Meeting, Boston, MA, June 2016).
In the present study, de-escalation from combination therapy and APBLs, including carbapenems, was performed approximately 1.5 days sooner following implementation of the stewardship and RDT intervention bundle. The implementation of the FilmArray BCID in phase 2 of the intervention period resulted in faster identification of bloodstream isolates by nearly 1 day compared to phase 1 (MALDI-TOF MS). This contributed to an even shorter time to de-escalation from APBLs and carbapenems in phase 2 compared with that of phase 1 of the intervention. Of note, two-thirds of de-escalations from APBLs and carbapenems occurred prior to the availability of final antimicrobial susceptibility testing results (Fig. 2). Identification of E. coli, Klebsiella species, or P. mirabilis provided enough information for the ASST to recommend stopping APBLs and carbapenems in the absence of risk factors for extended-spectrum β-lactamase (ESBL)-producing bacteria (20, 21). Initial de-escalation to ceftriaxone based on organism identification from FilmArray BCID was recommended prior to the availability of antimicrobial susceptibility testing results in patients with low predicted probabilities of BSI due to ESBL-producing Enterobacteriaceae (20; C. Troficanto, J. Kohn, J. A. Justo, P. B. Bookstaver, H. Albrecht, and M. N. Al-Hasan, presented at ASM Microbe Annual Meeting, Boston, MA, June 2016). A second intervention to a narrower spectrum antimicrobial agent than ceftriaxone was recommended upon reporting of antimicrobial susceptibility testing results. It was also common practice that ASST interventions would include de-escalation from empirical Gram-positive therapy (e.g., vancomycin). Vancomycin use decreased from 87.3 days of therapy/1,000 patient days prior to the intervention compared to 79.9 days of therapy/1,000 patient days following the intervention, representing a 9% decline in overall vancomycin utilization in the health care system. Although the majority of antimicrobial interventions involved de-escalation from BSAA, it was not unusual for the ASST to recommend escalation of EAT to ertapenem in patients with Enterobacteriaceae bloodstream isolates with high predicted probabilites of ESBL production or combination antimicrobial therapy in critically ill patients with P. aeruginosa or Acinetobacter baumanii BSI who had received recent APBLs (20). However, this did not necessarily reflect increased rates of escalation in EAT following interventions. In the preintervention period, 140 (17%) patients underwent escalation in EAT compared to 30 (16%) and 18 (12%) in phase 1 and phase 2 of the postintervention period, respectively.
Prior data suggest that the introduction of technology alone without ASP intervention does not provide the same benefit – which is typically needed to justify the costs of implementing a novel RDT (6, 9, 15). This was supported by our findings that nearly 40% of patients remained on APBLs for longer than 5 days in the preintervention period even in the absence of BSI due to P. aeruginosa (Fig. 2). Continual provider-level education combined with effective communication strategies by the ASST were essential for compliance with recommendations. Managing provider perceptions of and potential biases against complying with institutional guidelines or stewardship recommendations is a key element to a successful ASP (22, 23).
While the impact of RDTs on early appropriate therapy has been documented, the impact on early streamlined definitive therapy achieved in this study has many potential additional clinical benefits. Early de-escalation from APBLs, including piperacillin-tazobactam and carbapenems, may avoid the additive risk of nephrotoxicity and the risk of colonization with carbapenem-resistant Enterobacteriaceae (CRE), respectively (24–26). Use of these agents, especially in a combination antibiotic regimen, significantly disrupts the gut microbiome and increases the risk of Clostridium difficile infection (27, 28). Future studies should examine the long-term cumulative impact of these interventions on patient morbidity and mortality and antimicrobial resistance rates once this practice of early de-escalation of BSAA is extended to other infectious syndromes.
The main strength in this study is the inclusion of a large number of patients with GNBSI from 2 different hospitals with full implementation and consistent electronic medical records (EMRs). This study also presents the first data evaluating the impact of multiple RDTs implemented over time (with ASP support). Although this concept has been described in patients with BSI due to Gram-positive cocci, early de-escalation from BSAA based on organism identification prior to in vitro antimicrobial susceptibility testing results is a relatively new concept in antimicrobial management of patients with GNBSI. However, this practice should be undertaken only with the complete understanding of local antimicrobial resistance mechanisms, including ESBL production, preferably with derivation of local clinical prediction tools or validation of currently existing ones (20, 21). While FilmArray BCID is broad in reporting, certain Gram-negative bacteria (e.g., Stenotrophomonas maltophilia) and resistance mechanisms (e.g., CTX, OXA, or NDM) are not included. Early de-escalation from BSAA may not be appropriate in the presence of specific host risk factors for antimicrobial resistance.
The study has limitations. The quasi-experimental design lacks random assignment and thus is subject to some biases related to unknown confounders. Additionally, the preintervention data were assessed by retrospective chart review, leading to some limitations of data accessibility. However, the same definitions were used in the preintervention and postintervention periods for sources of infection and other clinical variables. Second, since multiple interventions were introduced simultaneously, it is difficult to assess the benefit of each individual component of the intervention bundle. For example, the definition of appropriate EAT was based on antibiotic therapy in the first 48 h from index blood culture collection. It remains difficult to determine from the current design whether improvements in EAT were mostly driven by institutional management guidelines, RDTs, direct ASST interventions, or health care provider education performed by ASST at the time of implementation of institutional guidelines and RDTs. It is safe to conclude, however, that this bundle of interventions worked well together. It is conceivable that faster identification of BSIs after the addition of BCID in phase 2 compared to MALDI-TOF MS alone in phase 1 has assisted in faster de-escalation of BSAA. However, it remains possible that this further improvement is due to continued active stewardship and provider education throughout the postintervention phase, with primary health care providers becoming more accustomed to using the guidelines and interpreting the RDT results. Lastly, both hospitals included in this study are within the same health care system and geographical location. Previous work demonstrated that 4.6% of E. coli, Klebsiella species, and P. mirabilis bloodstream isolates produced ESBLs, and only one CRE bloodstream isolate was detected at the two hospitals during the study period (21). Multicenter studies from hospitals in different health care settings and geographic locations may provide more diverse patient populations, microbiology, and antimicrobial resistance patterns.
In conclusion, implementation of an antimicrobial stewardship and RDT intervention bundle in a multihospital health care system is associated with improved appropriateness of EAT for GNBSI while also resulting in a significant decrease in the utilization of BSAA. This emphasizes the benefits of systematic use of antimicrobial agents in the postintervention period compared to individual decisions made by primary health care providers in the preintervention period. The combination of passive guidance via evidence-based institutional guidelines, active monitoring and feedback of prescribing patterns by an ASST, and real-time data from novel RDTs contributed to improvements in local antimicrobial management of GNBSIs. Faster identification of BSIs through the addition of BCID to existing robust interventions was associated with further reduction in the duration of therapy with BSAA.
MATERIALS AND METHODS
Study design and definitions.
The study was a quasi-experimental cohort design conducted at Palmetto Health Richland, a community teaching hospital, and Palmetto Health Baptist, a community hospital, in Columbia, South Carolina. It was approved by the Palmetto Health Institutional Review Board, and a waiver of informed consent was granted. Eligible patients were adults (>18 years of age) with a first episode of positive blood culture for a Gram-negative bacillus between 1 January 2010 and 30 June 2015. Only monomicrobial BSIs were included. The sources of BSIs were defined according to the Centers for Disease Control and Prevention criteria (CDC) (29). The site of acquisition (community-acquired, health care-associated, or hospital-acquired) was defined according to the criteria described by Friedman and colleagues (30). Blood cultures from each hospital were processed centrally using standard microbiology techniques according to the CLSI. In vitro antimicrobial susceptibilities were determined using the Vitek-2 system and were utilized to define appropriateness of therapy. The appropriateness of EAT within 48 h of collection of the index blood culture was defined previously (4). In brief, therapy was considered appropriate if the isolate was susceptible to the agent(s) according to CLSI criteria and if the agent was administered at the minimum acceptable dose via the intravenous route. The preintervention period was 1 January 2010 through 31 December 2013, and the postintervention period followed from 1 January 2014 through 30 June 2015.
Intervention bundle.
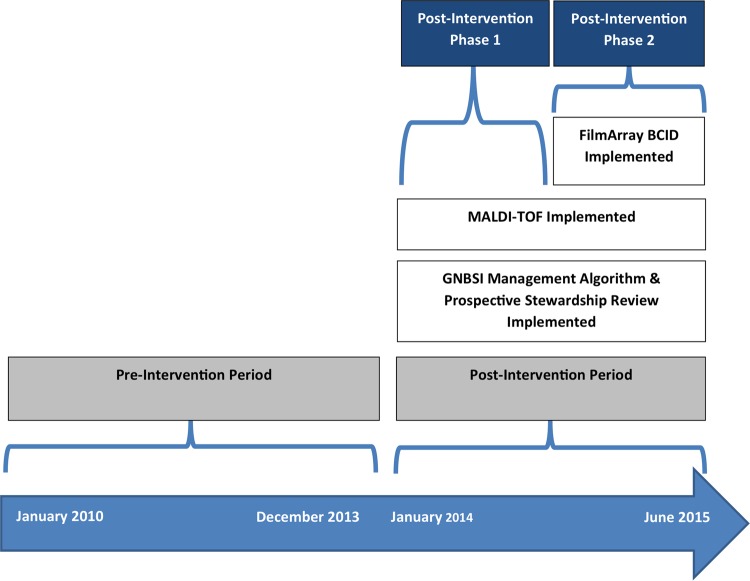
The postintervention period consisted of two phases, phase 1, from 1 January 2014 through 30 September 2014, and phase 2, from 1 October 2014 through 30 June 2015 (Fig. 3). The comprehensive stewardship and rapid diagnostic bundle was composed of three key interventions in phase 1: (i) implementation of evidence-based institutional management guidelines for GNBSIs based on site of acquisition, bloodstream antibiogram, and acute severity of illness (see Supplement File 1 in the supplemental material); (31) (ii) prospective stewardship monitoring triggered by positive blood culture notifications through the clinical decision support system, TheraDoc, allowing real-time recommendations to optimize EAT and streamline to definitive therapy; and (iii) introduction of MALDI-TOF MS on all positive blood cultures. Phase 2 included the introduction of FilmArray BCID on all positive blood cultures, in addition to the previously established interventions. MALDI-TOF MS and BCID results also triggered real-time notification through TheraDoc to members of the stewardship team for interpretation and intervention. The institutional antimicrobial stewardship and support team, defined locally as ASST, was composed of three infectious diseases pharmacists, a critical care pharmacist, an infectious diseases physician, and intermittent pharmacy and physician trainees. One stewardship pharmacist at each hospital routinely evaluated antibiotic therapy for appropriateness on weekdays from 7:30 a.m. to 4:30 p.m. and facilitated patient-specific interventions. Although ASST coverage was typically not available at nights or on weekends, the microbiology lab routinely performed and reported results of MALDI-TOF MS and BCID throughout.
FIG 3.
Implementation timeline of Gram-negative bloodstream infection stewardship bundle.
Outcomes.
The primary outcome of this study was the difference in proportion of patients who received appropriate EAT in the preintervention and postintervention phases. Subgroup analyses were performed to evaluate the appropriateness of EAT by acute severity of illness using the Pitt bacteremia score and by organism (e.g., P. aeruginosa or CAE) (32). CAE included Enterobacter, Serratia, Citrobacter, Morganella, Providencia, Hafnia, and indole-positive Proteus species (33). Secondary outcomes included time to de-escalation from combination antibiotic therapy, APBLs, or carbapenems in the preintervention and postintervention phases. Times of initiation and discontinuation of antimicrobial agents were recorded from the medication administration records in the EMRs.
Other secondary outcomes included examination of median times to organism identification, appropriateness of EAT, and times to antibiotic de-escalation in phase 1 (MALDI-TOF MS) and phase 2 (MALDI-TOF MS plus BCID) of the intervention period.
Statistical analysis.
Descriptive statistics were used to summarize basic patient demographics, prevalence of comorbid conditions, site and source of infection, and frequency of identified organisms.
Chi-square or Fisher's exact test was used, as appropriate, to compare the appropriateness of EAT between the preintervention and postintervention periods, between phase 1 and phase 2 of the intervention period overall, and within subgroups of patients with BSI due to P. aeruginosa/CAE and critically ill patients with a Pitt bacteremia score of ≥4.
Kaplan Meier analysis was used to determine times to de-escalation from combination, APBL, or carbapenem therapy. This allowed censoring of patients who expired while still receiving combination, APBL, or carbapenem therapy in the respective analysis. Only patients who were eligible for de-escalation of antimicrobial therapy were included in the respective analysis. De-escalation of combination therapy included all patients who were initially started on combination antimicrobial regimens. De-escalation of APBLs included patients with BSI due Enterobacteriaceae (Escherichia coli, Klebsiella spp., Proteus mirabilis, and Salmonella spp.) who received APBLs. De-escalation of carbapenem analysis included patients with BSI due to non-ESBL-producing bacteria who received carbapenem therapy. Log-rank P value was reported to determine statistical significance in time to de-escalation of antimicrobial therapy in the preintervention and postintervention periods and in phase 1 versus phase 2 of interventions.
The Wilcoxon-rank sum test was used to compare median time to Gram stain report, organism identification, and final in vitro antimicrobial susceptibility testing results between phase 1 and phase 2 of the intervention period.
All outcomes were considered significant if the P value was <0.05, unless otherwise indicated. JMP Pro version 12. 1 (SAS Institute, Inc., Cary, NC, USA) was used for all statistical analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the members of the Palmetto Health Antimicrobial Stewardship and Support team and the Palmetto Health Microbiology Laboratory in Columbia, SC, USA, for their help in facilitating the conduct of this study.
The preliminary results of this study were presented in part at the IDWeek Annual Meeting in October 2015 in San Diego, CA, USA (abstract 52168).
E.B.N. and M.N.A. have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
The study received funding from the Grant in Aid Program at Palmetto Health Richland in Columbia, SC, USA. The funding source had no role in study design. We report no other sources of funding.
P.B.B. received research funding from Allergan PLC and is a content development and honorarium recipient from Rockpointe Inc. and FreeCe.com. M.N.A. is a content development and honorarium recipient from Rockpointe Inc. E.B.N., T.J.S., J.A.J., J.K., K.L.H., C.T., and H.A.A. have no conflicts and nothing to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00189-17.
REFERENCES
- 1.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, Peters C, Ahsan M, Chateau D, Cooperative Antimicrobial Therapy of Septic Shock Database Research Group. 2009. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136:1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 3.McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP Jr, Miller RR, Furuno JP. 2007. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin Infect Dis 45:329–337. doi: 10.1086/519283. [DOI] [PubMed] [Google Scholar]
- 4.Cain SE, Kohn J, Bookstaver PB, Albrecht H, Al-Hasan MN. 2015. Stratification of the impact of inappropriate empirical antimicrobial therapy for Gram-negative bloodstream infections by predicted prognosis. Antimicrob Agents Chemother 59:245–250. doi: 10.1128/AAC.03935-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goff DA, Jankowski C, Tenover FC. 2012. Using rapid diagnostic tests to optimize antimicrobial selection in antimicrobial stewardship programs. Pharmacotherapy 32:677–687. doi: 10.1002/j.1875-9114.2012.01137.x. [DOI] [PubMed] [Google Scholar]
- 6.Bauer KA, Perez KK, Forrest GN, Goff DA. 2014. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis 59(Suppl 3):S134–S145. doi: 10.1093/cid/ciu547. [DOI] [PubMed] [Google Scholar]
- 7.Buehler SS, Madison B, Snyder SR, Derzon JH, Cornish NE, Saubolle MA, Weissfeld AS, Weinstein MP, Liebow EB, Wolk DM. 2016. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev 29:59–103. doi: 10.1128/CMR.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martiny D, Debaugnies F, Gateff D, Gerard M, Aoun M, Martin C, Konopnicki D, Loizidou A, Georgala A, Hainaut M, Chantrenne M, Dediste A, Vandenberg O, Van Praet S. 2013. Impact of rapid microbial identification directly from positive blood cultures using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry on patient management. Clin Microbiol Infect 19:E568-81. doi: 10.1111/1469-0691.12282. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee R, Teng CB, Cunningham SA, Ihde SM, Steckelberg JM, Moriarty JP, Shah ND, Mandrekar JN, Patel R. 2015. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 61:1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Peterson LE, Musser JM. 2014. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 69:216–225. doi: 10.1016/j.jinf.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Vardakas KZ, Anifantaki FI, Trigkidis KK, Falagas ME. 2015. Rapid molecular diagnostic tests in patients with bacteremia: evaluation of their impact on decision making and clinical outcomes. Eur J Clin Microbiol Infect Dis 34:2149–2160. doi: 10.1007/s10096-015-2466-y. [DOI] [PubMed] [Google Scholar]
- 12.Messacar K, Hurst AL, Child J, Campbell K, Palmer C, Hamilton S, Dowell E, Robinson CC, Parker SK, Dominguez SR. 2016. Clinical impact and provider acceptability of real-time antimicrobial stewardship decision support for rapid diagnostics in children with positive blood culture results. J Pediatric Infect Dis Soc pii:piw047. doi: 10.1093/jpids/piw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtzman C, Whitney D, Barlam T, Miller NS. 2011. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol 49:1581–1582. doi: 10.1128/JCM.02461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terp S, Krishnadasan A, Bowen W, Joo J, Furoy D, Chan J, Moran G, Talan D. 2014. Introduction of rapid methicillin-resistant Staphylococcus aureus polymerase chain reaction testing and antibiotic selection among hospitalized patients with purulent skin infections. Clin Infect Dis 58:e129–132. doi: 10.1093/cid/ciu039. [DOI] [PubMed] [Google Scholar]
- 15.Cosgrove SE, Li DX, Tamma PD, Avdic E, Hadhazy E, Wakefield T, Gherna M, Carroll KC. 2016. Use of PNA FISH for blood cultures growing Gram-positive cocci in chains without a concomitant antibiotic stewardship intervention does not improve time to appropriate antibiotic therapy. Diagn Microbiol Infect Dis 86:86–92. doi: 10.1016/j.diagmicrobio.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM, Infectious Diseases Society of America, Society for Healthcare Epidemiology of America. 2007. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 17.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO, Hamilton CW, Jenkins TC, Lipsett PA, Malani PN, May LS, Moran GJ, Neuhauser MM, Newland JG, Ohl CA, Samore MH, Seo SK, Trivedi KK. 2016. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 62:e51–77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. 2010. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retamar P, Portillo MM, Lopez-Prieto MD, Rodriguez-Lopez F, de Cueto M, Garcia MV, Gomez MJ, Del Arco A, Munoz A, Sanchez-Porto A, Torres-Tortosa M, Martin-Aspas A, Arroyo A, Garcia-Figueras C, Acosta F, Corzo JE, Leon-Ruiz L, Escobar-Lara T, Rodriguez-Bano J, SAEI/SAMPAC Bacteremia Group. 2012. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother 56:472–478. doi: 10.1128/AAC.00462-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumbarello M, Trecarichi EM, Bassetti M, De Rosa FG, Spanu T, Di Meco E, Losito AR, Parisini A, Pagani N, Cauda R. 2011. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother 55:3485–3490. doi: 10.1128/AAC.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Augustine MR, Testerman TL, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2017. Clinical risk score for prediction of extended-spectrum beta-lactamase producing Enterobacteriaceae in bloodstream isolates. Infect Control Hosp Epidemiol 38:266–272. doi: 10.1017/ice.2016.292. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein EJ, Goff DA, Reeve W, Naumovski S, Epson E, Zenilman J, Kaye KS, File TM Jr. 2016. Approaches to modifying the behavior of clinicians who are noncompliant with antimicrobial stewardship program guidelines. Clin Infect Dis 63:532–538. doi: 10.1093/cid/ciw247. [DOI] [PubMed] [Google Scholar]
- 23.Grigoryan L, Naik AD, Horwitz D, Cadena J, Patterson JE, Zoorob R, Trautner BW. 2016. Survey finds improvement in cognitive biases that drive overtreatment of asymptomatic bacteriuria after a successful antimicrobial stewardship intervention. Am J Infect Control 44:1544–1548. doi: 10.1016/j.ajic.2016.04.238. [DOI] [PubMed] [Google Scholar]
- 24.Burgess LD, Drew RH. 2014. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 34:670–676. doi: 10.1002/phar.1442. [DOI] [PubMed] [Google Scholar]
- 25.Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R. 2009. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 30:666–671. doi: 10.1086/598244. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 27.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. 2012. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 67:742–748. doi: 10.1093/jac/dkr508. [DOI] [PubMed] [Google Scholar]
- 28.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. 2011. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 53:42–48. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 29.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 31.Nimmich E, Bookstaver PB, Kohn J, Justo JA, Hammer KL, Albrecht H, Al-Hasan MN. 2017. Development of institutional guidelines for management of Gram-negative bloodstream infections: incorporating local evidence. Hosp Pharm, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann Intern Med 140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 33.Hammer KL, Stoessel A, Justo JA, Bookstaver PB, Kohn J, Derrick CB, Albrecht H, Al-Hasan MN. 2016. Association between chronic hemodialysis and bloodstream infections caused by chromosomally mediated AmpC-producing Enterobacteriaceae. Am J Infect Control 44:1611–1616. doi: 10.1016/j.ajic.2016.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.