ABSTRACT
Thiram and disulfiram were evaluated as antibacterial agents against multidrug-resistant Staphylococcus aureus. Against a 30-member panel comprised of vancomycin-susceptible, vancomycin-intermediate, and vancomycin-resistant S. aureus strains, the MIC90 values of the respective test agents were 4 and 16 μg/ml. Additional analyses revealed that thiram and disulfiram are rapid-acting bacteriostatic agents with narrow, Gram-positive-bacterium spectrum activity. Synergy studies further determined that disulfiram increases the vancomycin susceptibility of three clinical vancomycin-resistant S. aureus strains in vitro, thus establishing a potential use in combination therapy.
KEYWORDS: thiram, disulfiram, vancomycin, Staphylococcus aureus, MRSA, VISA, VRSA, synergy, combination therapy
INTRODUCTION
Staphylococcus aureus is a common etiology of community- and health care-acquired infections (1). Antibiotic-resistant forms, including methicillin-resistant (MRSA), linezolid-resistant, vancomycin-intermediate (VISA), and vancomycin-resistant (VRSA) S. aureus variants are associated with invasive diseases in health care settings (2). Multidrug-resistant (MDR) strains possessing resistance factors for three or more antibiotic classes are further implicated in high rates of antimicrobial treatment failures and chronic infections. Accordingly, new mechanistic classes of antibiotics need to be developed to treat infections due to these resistant bacteria. One approach to expedite the development of new antibiotics is to repurpose preexisting drugs that have been approved for the treatment of other medical conditions.
Thiuram disulfides are a class of synthetic, organosulfur-based drugs whose members include thiram and disulfiram (Fig. 1). Thiram (THM), though not a candidate as a systemic antibiotic due to toxicity, was once used as an ectoparasitide for human scabies and remains in use as an agricultural fungicide in crop protection (3). Disulfiram (DSF) is an oral medication used as a deterrent to promote abstinence in chronic alcoholism treatment and is sold under the trade name Antabuse (4). In the body, DSF and its metabolites (e.g., diethyldithiocarbamate [DDTC]) inhibit aldehyde dehydrogenase (ALDH) presumably by thiol-disulfide exchange with cysteine residues in the active site (5). Irreversible inhibition of hepatic ALDH results in the bodily accumulation of toxic acetaldehyde produced during ethanol metabolism and an amplified “hangover” effect after alcohol is consumed (6).
FIG 1.

Chemical structures of thiram, disulfiram, and diethyldithiocarbamate.
Both THM and DSF are electrophiles that readily form disulfides with thiol-bearing substances. Bacteria possess a diverse range of intracellular cofactors (e.g., coenzyme A), metabolites (e.g., glutathione, mycothiol, and bacillithiol), and enzymes (e.g., thioredoxin) (7) containing thiophilic residues that THM and DSF can potentially modify by thiol-disulfide exchange to evoke antimicrobial effects. To this end, S. aureus is well known for its susceptibility toward natural thiol-reactive organosulfur compounds such as allicin (garlic) (8), and previous work (9) suggests that MDR strains, including VRSA, would be susceptible to THM and DSF. Here, the antibacterial activity of thiuram disulfides and their synergism with vancomycin (VAN) against VRSA is examined.
RESULTS
Antibacterial activity spectrum of thiram, disulfiram, and diethyldithiocarbamate.
Susceptibility testing by broth microdilution in 96-well plate format indicated that THM and DSF are narrow, Gram-positive spectrum antibacterial agents (Table 1). Clinically relevant pathogens VRE and MRSA were sensitive at an MIC range of 2 to 16 μg/ml. In comparison, the DSF metabolite DDTC exhibited weak antibacterial activity against the test panel. The Gram-negative species displayed low susceptibility to all three test agents with MICs of 32 to >64 μg/ml. To further establish the susceptibility range of S. aureus, THM, DSF, and DDTC were evaluated against strains possessing different antibiotic resistance determinants.
TABLE 1.
Antibacterial activities of thiram, disulfiram, and diethyldithiocarbamate compared to cefotaxime and vancomycina
| Speciesb | MIC (μg/ml) |
||||
|---|---|---|---|---|---|
| THM | DSF | DDTC | CTX | VAN | |
| Enterococcus faecium (VRE) | 8 | 16 | 32 | >64 | |
| Staphylococcus aureus (MSSA) | 2 | 8 | 32 | 1 | |
| Staphylococcus aureus (MRSA) | 2 | 8 | 16 | 1 | |
| Staphylococcus epidermidis | 1 | 1 | 32 | 2 | |
| Acinetobacter baumannii | 32 | 64 | 64 | >64 | |
| Klebsiella pneumoniae | 32 | 64 | 32 | 8 | |
| Pseudomonas aeruginosa | >64 | >64 | >64 | 16 | |
| Enterobacter cloacae | 32 | 64 | >64 | 16 | |
| Escherichia coli | 64 | >64 | >64 | >64 | |
THM, thiram; DSF, disulfiram; DDTC, diethyldithiocarbamate; CTX, cefotaxime; VAN, vancomycin.
The species examined included E. faecium ATCC 700221 (VanA type VRE), S. aureus ATCC 25293 (MSSA), S. aureus COL (MRSA), S. epidermidis ATCC 14990, A. baumannii ATCC 19606, E. cloacae ATCC 13047, E. coli BAA-2326 (EHEC O104:H4; ESBL producer), K. pneumoniae ATCC 700603 (K6; ESBL producer), and P. aeruginosa ATCC 15442.
Susceptibility of S. aureus to thiram, disulfiram, and diethyldithiocarbamate in comparison to other test agents.
Susceptibility testing against a diverse panel of characterized S. aureus isolates revealed moderate differences in sensitivities toward THM and DSF (Table 2). The MIC ranges were 2 to 8 μg/ml for THM and 8 to 32 μg/ml for DSF against oxacillin sodium (OXA)-, linezolid-, and VAN-resistant variants. As previously observed, DDTC displayed the weakest antibacterial activity of the three test agents, with an MIC range of 16 to 32 μg/ml. Additional tests revealed that THM and DSF display bacteriostatic properties. Each test agent demonstrated MBC:MIC ratios of ≥4, a metric used to define bacteriostatic agents (10). In contrast, VAN was confirmed to be bactericidal with an MBC:MIC ratio of ≤2. To further assess the susceptibility range of S. aureus to THM and DSF, the MIC50 and MIC90 values were determined for VAN-susceptible S. aureus (VSSA), VISA, and VRSA strains.
TABLE 2.
Comparison of disulfiram MICs and MBCs to other test agents against S. aureus
| Variant and straina | Test agentb | Concn (μg/ml) |
MBC:MIC | |
|---|---|---|---|---|
| MIC | MBC | |||
| MSSA NCTC 8325/RN1 | THM | 8 | 32 | 4:1 |
| DSF | 16 | >64 | >4:1 | |
| DDTC | 32 | 64 | 2:1 | |
| OXA | ≤0.5 | |||
| VAN | 1 | 2 | 2:1 | |
| CA-MRSA CA-347 | THM | 2 | 32 | 16:1 |
| DSF | 16 | >64 | >4:1 | |
| DDTC | 32 | 64 | 2:1 | |
| OXA | >64 | |||
| VAN | 1 | 1 | 1:1 | |
| HA-MRSA COL | THM | 2 | 16 | 8:1 |
| DSF | 8 | 64 | 8:1 | |
| DDTC | 16 | 64 | 4:1 | |
| OXA | >64 | |||
| VAN | 2 | 4 | 2:1 | |
| LRSA SA LinR #14 | THM | 4 | 32 | 8:1 |
| DSF | 8 | 64 | 8:1 | |
| DDTC | 16 | >64 | >4:1 | |
| OXA | >64 | |||
| VAN | 1 | 2 | 2:1 | |
| hVISA Mu3 ATCC 700698 | THM | 2 | 32 | 16:1 |
| DSF | 32 | >64 | >2:1 | |
| DDTC | 32 | >64 | >2:1 | |
| OXA | >64 | |||
| VAN | 2 | 4 | 2:1 | |
| VISA AR-215 | THM | 2 | 16 | 8:1 |
| DSF | 16 | >64 | >4:1 | |
| DDTC | 64 | |||
| OXA | >64 | |||
| VAN | 4 | 8 | 2:1 | |
| VRSA HIP11714 | THM | 2 | 16 | 8:1 |
| DSF | 16 | 64 | 4:1 | |
| DDTC | 32 | >64 | >2:1 | |
| OXA | >64 | |||
| VAN | >64 | |||
MSSA, methicillin-susceptible S. aureus; CA-MRSA, community-acquired methicillin-resistant S. aureus; HA-MRSA, hospital-acquired methicillin-resistant S. aureus; LRSA, linezolid-resistant S. aureus; hVISA, heterogeneous-resistant vancomycin-intermediate S. aureus; VISA, vancomycin-intermediate S. aureus; VRSA, vancomycin-resistant S. aureus.
THM, thiram; DSF, disulfiram; DDTC, sodium diethyldithiocarbamate trihydrate; OXA, oxacillin; VAN, vancomycin.
Comparison of MIC50 and MIC90 values for thiram, disulfiram, and diethyldithiocarbamate against VSSA, VISA, and VRSA.
Table 3 shows the MIC50 and MIC90 values for THM, DSF, and DDTC and the comparators OXA and VAN against VSSA, VISA, and VRSA. The panel, which included strains with resistance determinants for ampicillin (mecA and blaZ), vancomycin (vanA), trimethoprim (dfrG), aminoglycosides [aacA-aphD, aph(3′)-III, and aadD], tetracycline [tet(K) and tet(M)], and macrolides/streptogramins [erm(A), mph(C), and msr(A)], exhibited the highest susceptibility to THM, followed by DSF and DDTC. Cross-resistance was not observed with strains characterized for their resistance genes, which suggests an alternate mechanism of antibacterial action or cellular target. Notably, the THM and DSF MICs were equivalent between the VAN-susceptible and -resistant groups comprised of 10 clinical isolates each. In the final analysis, the MIC90 values for THM, DSF, and DDTC against the 30-strain panel were 4, 16, and 64 μg/ml, respectively.
TABLE 3.
Comparison of disulfiram MIC50 and MIC90 values to other test agents against S. aureus
| Variant (no. of strains)a | Test agentb | MIC (μg/ml) |
||
|---|---|---|---|---|
| MIC50 | MIC90 | Range | ||
| VSSA (10)c | THM | 2 | 4 | 2 to 4 |
| DSF | 8 | 16 | 8 to 32 | |
| DDTC | 32 | 64 | 16 to >64 | |
| OXA | 64 | >64 | ≤0.5 to >64 | |
| VAN | 1 | 1 | 1 to 2 | |
| VISA (10)d | THM | 2 | 4 | 2 to 8 |
| DSF | 16 | 16 | 4 to 32 | |
| DDTC | 32 | 64 | 16 to 64 | |
| OXA | >64 | >64 | ≤0.5 to >64 | |
| VAN | 4 | 8 | 4 to 8 | |
| VRSA (10)e | THM | 1 | 2 | 1 to 2 |
| DSF | 16 | 16 | 4 to 32 | |
| DDTC | 32 | 32 | 8 to 64 | |
| OXA | 256 | >256 | 128 to >256 | |
| VAN | 256 | >256 | 64 to >256 | |
VSSA, vancomycin-susceptible S. aureus (VAN MIC ≤ 2 μg/ml); VISA, vancomycin-intermediate S. aureus (VAN MIC = 4 to 8 μg/ml); VRSA, vancomycin-resistant S. aureus (VAN MIC ≥ 16 μg/ml).
THM, thiram; DSF, disulfiram; DDTC, diethyldithiocarbamate; OXA, oxacillin; VAN, vancomycin.
VSSA strains: MSSA (ATCC 25923, NCTC 8325); MRSA (C1999000193, CA-347, CM05, COL, JE2, N315, SA LinR #14, USA300-0114).
VISA strains: AR-215 to AR-224.
VRSA strains: 71080, AIS 080003, AIS 1000505, AIS 2006032, AIS 2006045, HIP11714, HIP11983, HIP13419, HIP14300, and HIP15178.
Effect of culture variables on the susceptibility of S. aureus.
The effect of different culture conditions on the susceptibility of S. aureus to THM, DSF, and DDTC was examined using the microdilution assay. Table 4 shows that an inoculum size greater than 106 CFU/ml and treatment times longer than 20 h increased the MICs of the test drugs. Medium variables that had an adverse effect on the MIC of THM included the surfactant Tween 80 and the blood components serum, albumin, and glutathione. In the case of DSF, serum and albumin did not appear to have an influence on antibacterial activity; however, decreased potency was detected when the culture media contained either glutathione or Tween 80. Physical and chemical variables that did not appear to obstruct THM and DSF activity were microaerobic and acidic environmental conditions. Nutritional supplements thymidine, vitamin K3 (menadione), and vitamin C also did not alter S. aureus susceptibility, although a 2-fold increase in the DSF MIC was observed when the antioxidant vitamin E (γ-tocopherol) was present.
TABLE 4.
Culture variable effects on S. aureus NCTC 8325 susceptibility to thiram, disulfiram, and diethyldithiocarbamate
| Variable(s) | MIC (μg/ml)a |
||||
|---|---|---|---|---|---|
| THM | DSF | DDTC | OXA | VAN | |
| 4.0 × 106 CFU/ml, 20 h | 8 | 16 | 64 | ≤0.5 | 1 |
| 4.0 × 106 CFU/ml, 24 h | 8 | 32 | 64 | ≤0.5 | 1 |
| 4.0 × 106 CFU/ml, 48 h | 32 | 64 | >64 | ≤0.5 | 2 |
| 3.3 × 108 CFU/ml, 24 h | 32 | 64 | >64 | ≤0.5 | 2 |
| 3.3 × 108 CFU/ml, 48 h | 64 | >64 | >64 | ≤0.5 | 4 |
| 5% CO2, 20 h | 8 | 16 | >64 | ≤0.5 | 1 |
| 5% CO2, 48 h | 16 | 64 | >64 | ≤0.5 | 1 |
| pH 5 | 2 | 4 | 32 | ≤0.5 | ≤0.5 |
| pH 6 | 8 | 16 | 64 | ≤0.5 | 1 |
| pH 7.4 | 8 | 16 | 64 | ≤0.5 | 1 |
| Serum, 5% (vol/vol) | 16 | 16 | >64 | ≤0.5 | 1 |
| Serum, 10% (vol/vol) | 16 | 16 | >64 | ≤0.5 | 1 |
| Albumin, 0.03% (vol/vol) | 16 | 16 | >64 | ≤0.5 | 1 |
| Glutathione, 100 μM | 16 | 32 | >64 | ≤0.5 | 1 |
| Thymidine, 100 μg/ml | 8 | 16 | 32 | ≤0.5 | 1 |
| Vitamin C, 20 μM | 8 | 16 | 64 | ≤0.5 | 1 |
| Vitamin E, 100 μM | 8 | 32 | 64 | ≤0.5 | 1 |
| Vitamin K3, 1 μg/ml | 8 | 16 | 32 | ≤0.5 | 1 |
| Tween 20, 0.1% (vol/vol) | 8 | 16 | >64 | ≤0.5 | 1 |
| Tween 80, 0.1% (vol/vol) | 16 | 32 | >64 | ≤0.5 | 1 |
| Oleic acid, 0.02% (vol/vol) | 8 | 16 | 64 | ≤0.5 | 2 |
Unless otherwise indicated, cultures were incubated at 37°C for 20 h in CAHMB. Susceptibility testing was performed by the broth microdilution method in 96-well plates using a 1:100 dilution of a 0.5 McFarland adjusted culture, and MIC values were recorded at 20 h.
Growth studies on thiram and disulfiram.
Treatment with 0.25× to 4× the MIC of THM and DSF inhibited the growth of S. aureus in a time- and dose-dependent manner (Fig. 2). In Fig. 2A, THM exhibited longer periods of sustained growth inhibition compared to DSF (Fig. 2B). Logarithmic growth was delayed for more than 18 h at 2× and 4× the MIC of THM. Conversely, growth initiated in less than 12 h at 2× and 4× the MIC of DSF with the same 108 CFU inoculum size. A potential reason for the disparity may be due to differences in the lethal dose threshold. To this end, Fig. 2A shows a slight decrease in CFU counts when S. aureus was treated with 4× the MIC of THM, which was not detected at 4× the MIC of DSF (Fig. 2B).
FIG 2.
Effects of thiram (A) and disulfiram (B) on the in vitro growth of S. aureus NCTC 8325.
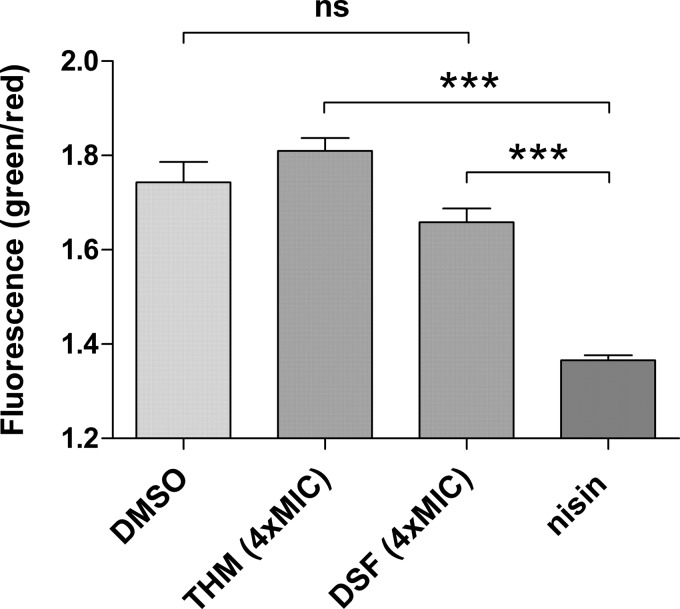
To further assess whether thiuram disulfides elicit killing effects on S. aureus, a Live/Dead BacLight bacterial viability assay was performed to detect dead and dying bacteria. Figure 3 shows negligible differences in cell viability between the vehicle (dimethyl sulfoxide [DMSO], 5%), THM, and DSF treatment groups in contrast to a significant decrease in viable bacteria treated with the pore-forming antibiotic nisin. These results further corroborated the initial bacteriostatic assessment for THM and DSF on the basis of their MBC:MIC ratios (Table 2).
FIG 3.
Effect of thiram, disulfiram, and nisin on cell viability. Shown are the ratios of green to red fluorescence (530/595 nm) for individual groups depicted as means ± the standard errors of the mean (n = 4).
Synergy studies with vancomycin against VRSA.
Synergy testing by the checkerboard microdilution assay in 96-well plate format (11) was used to evaluate the combined antibacterial effects of VAN with DSF on VanA-type VRSA. Table 5 shows that DSF lowers the MIC of VAN from >128 μg/ml to 4 to 16 μg/ml against three clinical VRSA isolates. The summation fractional inhibitory concentration indices (ΣFIC) calculated from the MIC values of VAN and DSF alone and in combination equaled ≤0.5, a metric used to define synergism (12).
TABLE 5.
Checkerboard assay results for disulfiram synergy with vancomycin in three VRSA strains
| Strain | MIC (μg/ml) |
FICa | ΣFICb | ||
|---|---|---|---|---|---|
| VAN | DSF | VAN/DSF | |||
| HIP14300 | >128 | 16 | 16/0.5 | 0.03 | <0.16 (S) |
| AIS 2006045 | >128 | 16 | 8/1 | 0.06 | <0.12 (S) |
| AIS 1000505 | >128 | 16 | 4/1 | 0.06 | <0.09 (S) |
That is, the lowest FIC measurement of DSF in combination with VAN.
That is, the lowest ΣFIC measurement. Results: synergy (S), ≤0.5; indifferent (I), 0.5 < to ≤4; antagonism (A), >4.
DISCUSSION
MDR S. aureus is associated with high rates of serious disease and treatment failures (2). As emerging threats in health care, few antibiotics are available to safely treat infections due to VISA and VRSA. Daptomycin and linezolid are among the limited number of anti-MRSA antibiotics approved in recent decades that are in use for these infections. Additional antibiotics remain a need, and repurposing effective drugs with established toxicology and pharmacokinetic data from clinical trials is a practical approach to fast-track such new therapies.
This research has shown that THM and DSF inhibit the in vitro growth of S. aureus strains with reduced VAN susceptibility at an MIC range of 1 to 8 μg/ml and 4 to 32 μg/ml, respectively (Table 3). Although THM exhibited greater overall efficacy against S. aureus, toxicity would prohibit its systemic use. Previous studies have revealed THM evokes micronucleus formation and increases preimplantation fetal deaths in mice (13). Conversely, DSF is an oral medication that is approved by the U.S. Food and Drug Administration (FDA) for administration for up to 500 mg daily (14). Pharmacokinetic studies in humans has shown that DSF has a half-life (t1/2) of 7.3 h and a mean plasma concentration of 1.3 nM, although significant intersubject variations are noted (6). Uniform tissue distribution of DSF and its metabolites (e.g., DDTC) are additional attributes established in animals studies (15). The toxicity of both DSF and DDTC have also been broadly investigated in cell and animal studies, which yielded no evidence for teratogenic, mutagenic, or carcinogenic effects (16).
The data in Fig. 2 further indicates that DSF inhibits the in vitro growth of S. aureus in a time- and dose-dependent manner. The immediate deceleration in growth on treatment is attributed to the rapid cleavage of DSF by thiophilic residues in proteins, metabolites, and cofactors, which is hypothesized to instigate an abrupt halt in metabolism. A similar mechanism of action is proposed for allicin and alike electrophilic disulfides (17). Upon cleavage of DSF, the mix disulfide product may further participate in a second thiol-disulfide exchange reaction with another endogenous thiol yielding a disulfide and DDTC. Based on the reaction profile of thiuram disulfides, thiol-disulfide exchange is expected to occur with cellular glutathione. Accordingly, the introduction of glutathione in the growth medium caused a 2-fold increase in the MICs of THM and DSF (Table 4). The antagonistic effect observed with glutathione, which is found in high abundance in Gram-negative bacteria but not in Gram-positive bacteria (7), is believed to account for the narrow activity spectrum of THM and DSF.
Another significant finding was the discovery that DSF potentiates VAN susceptibility in VRSA. The apparent synergy between the two agents suggests the potential use of DSF as an adjuvant for chronic and recurring S. aureus infections that do not completely respond to VAN monotherapy. Future experiments will include in vivo studies to evaluate DSF with VAN as a combination therapy for MDR S. aureus infections. Pharmacological and biochemical studies to elucidate the mechanism of growth inhibition and thiol-disulfide exchange sites will also be performed in conjunction with the work.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacteria were initially grown on Mueller-Hinton agar (MHA) from frozen stocks stored at −80°C in tryptic soy broth (TSB) containing 10% glycerol. Test cultures were obtained from uniform colonies and enumerated overnight in TSB at 37°C with constant rotation (250 rpm).
Preparation of antibiotics.
Thiram (THM), disulfiram (DSF), and sodium diethyldithiocarbamate trihydrate (DDTC) were purchased from Alfa Aesar (Haverhill, MA). Vancomycin hydrochloride (VAN), oxacillin sodium (OXA), and cefotaxime sodium were acquired from Chem-Impex International (Wood Dale, IL). Test drug stocks of THM and DSF were prepared in DMSO at 1 mg/ml. DDTC, VAN, and OXA were prepared in deionized water as 0.5-, 1-, or 10-mg/ml stocks. All drug stocks were used within 72 h of preparation and were not subject to freezing temperatures.
Susceptibility testing.
MICs were determined by the microdilution method in 96-well microplates in accordance to Clinical and Laboratory Standards Institute guidelines (18). Overnight cultures adjusted to a 0.5 McFarland standard and diluted to 1:100 in cation-adjusted Mueller-Hinton broth (CAMHB) were treated with serial dilutions of each antibiotic. Due to poor aqueous solubility, the highest test concentration of DSF that could be evaluated was 64 μg/ml. The plates were sealed with adhesive film and incubated at 37°C. After 20 h, the MICs were recorded as the lowest drug concentration that gave complete inhibition of visual growth. The lowest drug concentrations that inhibited 50 and 90% of the test strains were recorded as the MIC50 and MIC90 values, respectively. The minimal bactericidal concentrations (MBCs) were determined by spotting 5-μl aliquots from each well on MHA plates. After overnight incubation, the lowest concentration that inhibited complete visual growth was recorded as the MBC.
Growth studies.
An overnight innocuum of S. aureus NCTC 8325 was grown to an optical density at 600 nm (OD600) of 0.1 and treated with 0×, 0.5×, 1×, and 2× the MIC of the test drug in a 96-well plate. The plate was incubated at 37°C in a microplate reader (Multiskan GO; Thermo Scientific), and the OD600 was measured at 1-h intervals after 30 s of agitation. The results were plotted using GraphPad Prism software. Time-kill studies were performed using a 1:100 dilution of a 0.5 McFarland suspension of S. aureus NCTC 8325 treated with 0×, 1×, and 4× the MIC of antibiotic (19). At 0, 1, 2, 4, and 24 h, 10-μl samples were serially diluted in phosphate-buffered saline (PBS) and streaked onto MHA plates to enumerate the bacteria. The CFU were counted after 30 h of incubation, and the data were plotted using GraphPad Prism.
Viability studies.
A Live/Dead BacLight assay (Molecular Probes, Inc.) was performed on an overnight innocuum of S. aureus NCTC 8325. The cells were collected by centrifugation, washed twice with PBS, and resuspended to an OD600 of 0.7 in PBS supplemented with 5 mM MgSO4 and 20 mM glucose. After a 10-min adjustment period at 37°C, the cells were treated with either 4× the MIC of THM or DSF. DMSO (5%) and nisin (100 μg/ml), a pore-forming antibiotic, were used as controls. After 1 h of shaking incubation (200 rpm), 4× 100-μl samples were combined with 100 μl of a 2× staining reagent (SYTO 9/propidium iodide) in a black flat-bottom 96-well plate (Corning, Inc.). After 15 min, the green (λex, 485 nm; λem, 535 nm) and red (λex, 485 nm; λem, 595 nm) emissions were measured on a Filter Max F3 fluorescent plate reader (Molecular Devices, Inc.). Cell viability as a measure of membrane permeability was determined by calculating the green/red fluorescence ratio (λem, 535/595 nm), and the data were plotted using GraphPad Prism. One-way analysis of variance, followed by a Tukey's multiple-comparison test was used for statistical analysis.
Synergy studies.
Evaluation of synergism was performed by isobologram analysis using the classical checkerboard microdilution assay (11). A 1:100 dilution of a 0.5 McFarland suspension of VRSA was used to inoculate a 96-well plate containing 2-fold serial dilutions of DSF and VAN in 50 μl of CAMHB. The plates were sealed with adhesive film and incubated for 20 h at 37°C. The fractional inhibitory concentration (FIC) index was calculated by dividing the MIC of the VAN-DSF combination by the MIC of VAN or DSF alone. The summative ΣFIC value was calculated from the FIC values and interpreted according to standard metrics (synergy, ≤0.5; indifferent, 0.5 < to ≤ 4; antagonism, >4) (12).
ACKNOWLEDGMENTS
This research was supported by the Marshall University School of Pharmacy.
Bacterial strains were acquired from the American Type Culture Collection, the FDA-CDC Antimicrobial Resistance Isolate Bank, and the Network on Antimicrobial Resistance in Staphylococcus aureus for distribution by BEI Resources, National Institute of Allergy and Infectious Disease, National Institutes of Health.
REFERENCES
- 1.Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, Peters G. 2012. New insights into methicillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment, and resistance. Int J Antimicrob Agents 39:96–104. doi: 10.1016/j.ijantimicag.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Gould IM. 2013. Treatment of bacteremia: methicillin-resistant Staphylococcus aureus (MRSA) to vancomycin-resistant S. aureus (VRSA). Int J Antimicrob Agents 42:S17–S21. doi: 10.1016/j.ijantimicag.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Sharma VK, Aulakh JS, Malik AK. 2003. Thiram: degradation, applications, and analytical methods. J Environ Monit 5:717–723. doi: 10.1039/b304710e. [DOI] [PubMed] [Google Scholar]
- 4.Ellis PM, Dronsfield AT. 2013. Antabuse's diamond anniversary: still sparkling on? Drug Alcohol Rev 32:342–344. doi: 10.1111/dar.12018. [DOI] [PubMed] [Google Scholar]
- 5.Shen ML, Lipsky JJ, Naylor S. 2000. Role of disulfiram in the in vitro inhibition of rat liver mitochondrial aldehyde dehydrogenase. Biochem Pharmacol 60:947–953. doi: 10.1016/S0006-2952(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 6.Johansson B. 1992. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl 369:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 7.Loi VV, Rossius M, Antelmann H. 2015. Redox regulation by reversible protein S-thiolation in bacteria. Front Microbiol 6:187. doi: 10.3389/fmicb.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ankri S, Mirelman D. 1999. Antimicrobial properties of allicin from garlic. Microbes Infect 1:125–129. doi: 10.1016/S1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 9.Phillips M, Malloy G, Nedunchezian D, Lukrec A, Howard RG. 1991. Disulfiram inhibits the in vitro growth of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 35:785–787. doi: 10.1128/AAC.35.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pankey GA, Sabath LD. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis 38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 11.Moody JA. 1992. Synergism testing: broth microdilution checkboard and broth macrodilution methods, p 5.18.1–5.18.28. In Isenberg, HD (ed), Clinical procedures handbook. ASM Press, Washington, DC. [Google Scholar]
- 12.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal RC, Shukla Y, Mehrotra NK. 1997. Assessment of mutagenic potential of thiram. Food Chem Toxicol 35:523–525. [DOI] [PubMed] [Google Scholar]
- 14.Wright C, Moore RD. 1990. Disulfiram treatment of alcoholism. Am J Med 88:647–655. doi: 10.1016/0002-9343(90)90534-K. [DOI] [PubMed] [Google Scholar]
- 15.Faiman MD, Dodd DE, Hanzlik RE. 1978. Distribution of S35 disulfiram and metabolites in mice, and metabolism of S35 disulfiram in the dog. Res Commun Chem Pathol Pharmacol 21:543–567. [PubMed] [Google Scholar]
- 16.Gessner PK, Gessner T. 1992. Toxicology, p 335–345. In Disulfiram and its metabolite, diethyldithiocarbamate: pharmacology and status in the treatment of alcoholism, HIV infections, AIDS and heavy metal toxicity. Chapman and Hall, London, United Kingdom. [Google Scholar]
- 17.Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ. 2014. Allicin: chemistry and biological properties. Molecules 19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen JH, Turnidge JD. 2007. Antibacterial susceptibility tests: dilution and disk diffusion methods, p 1152–1172. In Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (ed), Manual of clinical microbiology. ASM Press, Washington, DC. [Google Scholar]
- 19.Verma P. 2007. Methods for determining bactericidal activity and antimicrobial interactions: synergy testing, time-kill curves, and population analysis, p 275–298. In Schwalbe R, Steele-Moore L, Goodwin AC (ed), Antimicrobial susceptibility testing protocols. CRC Press, Boca Raton, FL. [Google Scholar]