ABSTRACT
Combining voriconazole and flucloxacillin is indicated in patient cohorts experiencing both invasive aspergillosis and Gram-positive infections (e.g., patients with chronic granulomatous disease or postinfluenza pulmonary aspergillosis). We report a highly relevant interaction between voriconazole and flucloxacillin, resulting in subtherapeutic plasma voriconazole concentrations in more than 50% of patients, that poses a severe threat if not managed properly.
KEYWORDS: aspergillosis, drug interactions, exposure, influenza, pharmacokinetics
TEXT
Voriconazole is a triazole antifungal that is used to treat a broad range of fungal infections (1). Its use is complicated by nonlinear pharmacokinetics and drug-drug interactions (2). Voriconazole may act as an inhibitor or as a substrate of the enzymes cytochrome P450 2C9 (CYP2C9), CYP2C19, and CYP3A4 and thus may alter its own pharmacokinetic profile and that of coadministered drugs (2). Many interactions have been well documented and can be managed through dose adjustments and close therapeutic drug monitoring of voriconazole, relevant coadministered drugs, or both (2).
However, more than once, drug-drug interactions have been discovered in daily practice in addition to those found during drug development. Here, we report a highly relevant interaction of voriconazole-flucloxacillin that resulted in suboptimal or even undetectable concentrations of voriconazole.
As concomitant voriconazole and flucloxacillin therapy is given to specific patient groups, such as those with chronic granulomatous disease or influenza-associated aspergillosis, this might be a threat to adequate antifungal treatment.
Patients were identified in five Dutch tertiary-care university hospitals from June 2014 to June 2016. Initially, three patients were identified on the basis of undetectable plasma voriconazole concentrations. This prompted us to search electronic patient records to retrospectively identify additional cases of the concomitant use of voriconazole and flucloxacillin. The data collected were gender, age, underlying disease, duration of voriconazole therapy, indication, voriconazole dose, plasma voriconazole and voriconazole N-oxide concentrations, duration of flucloxacillin therapy, indication, and flucloxacillin dose.
Twenty patients received voriconazole and flucloxacillin simultaneously. Patient characteristics, underlying disease, and indications for drug therapy are listed in Table 1. We observed subtherapeutic plasma voriconazole concentrations, defined as <1 mg/liter (local practice at the time of presentation), during flucloxacillin treatment in 11 of these 20 patients. The median voriconazole trough concentration in these 11 patients during flucloxacillin treatment was 0.20 (interquartile range [IQR], 0.1 to 0.8, n = 32) mg/liter, while the median voriconazole trough concentration in the other 9 patients was 1.45 (IQR, 1.0 to 4.9, n = 15) mg/liter. Of the 11 patients with subtherapeutic trough concentrations, multiple voriconazole dose increases only led to a significant rise in plasm a drug concentrations in 2 patients; both reached toxic concentrations (11.5 and 7.1 mg/liter) after receiving extremely high doses (48 mg/kg/day and 1,600 mg/day, respectively). This is shown for one patient in Fig. 1. The effect of flucloxacillin on plasma voriconazole concentrations was independent of the flucloxacillin dose administered.
TABLE 1.
Patient characteristics
| Gendera | Age (yr) | Underlying diseaseb | Voriconazole indication | Voriconazole administration route(s)c | Flucloxacillin indication | Flucloxacillin dose (mg/day) | Voriconazole started before flucloxacillin | Decrease in plasma voriconazole concn |
|---|---|---|---|---|---|---|---|---|
| M | 3 | ALL | Pulmonary aspergillosis | i.v., p.o. | Empirical therapy | 770d | Yes | No |
| F | 12 | ALL | Pulmonary and cerebral aspergillosis | i.v., p.o. | S. aureus infection | 12,000e | No | Yes |
| F | 17 | CF | Pulmonary aspergillosis | i.v. | Empirical therapy | 10,400f | No | Yes |
| M | 22 | CGD | Pulmonary and cerebral aspergillosis | p.o. | S. aureus infection | 12,000 | No | No |
| F | 23 | Hyper-IgE syndrome | Empirical therapy | p.o. | Empirical therapy | 6,000 | Yes | No |
| M | 24 | CGD | Empirical therapy | p.o. | Empirical therapy | 12,000 | No | Yes |
| M | 25 | CF, lung transplantation | Invasive aspergillosis | i.v. | Empirical therapy | 4,000 | Yes | Yes |
| M | 34 | CGD | Empirical therapy | p.o. | Empirical therapy | 12,000 | No | No |
| M | 38 | Hodgkin lymphoma | Probable pulmonary aspergillosis | i.v., p.o. | S. aureus infection | 6,000–8,000 | No | Yes |
| M | 42 | COPD, heart transplantation | Cryptococcus osteomyelitis | p.o. | S. aureus infection | 12,000 | Yes | Yes |
| M | 43 | CGD | Possible pulmonary aspergillosis | p.o. | Prophylaxis | 1,000 | No | No |
| F | 45 | CMV infection | Candidemia, ocular Candida glabrata infection | p.o. | Empirical therapy | 6,000 | Yes | No |
| F | 54 | Influenza | Pulmonary aspergillosis | p.o. | Empirical therapy | 4,000 | Yes | No |
| M | 55 | AML | Possible pulmonary aspergillosis | i.v., p.o. | S. aureus infection | 6,000 | No | Yes |
| F | 56 | Destroyed lung, COPD | Aspergilloma | p.o. | S. aureus infection | 6,000 | Yes | Yes |
| F | 65 | None | Pulmonary and cerebral aspergillosis | i.v. | S. aureus infection | 12,000 | Yes | Yes |
| M | 67 | AML | Probable pulmonary aspergillosis | i.v., p.o. | S. aureus infection | 6,000–12,000 | No | No |
| F | 68 | AML | Prophylaxis | p.o. | Unknown | 6,000 | Yes | No |
| M | 70 | Influenza | Possible pulmonary aspergillosis | i.v. | S. aureus infection | 12,000 | No | Yes |
| M | 71 | ITP, DMII | Possible pulmonary aspergillosis | p.o. | S. aureus infection | 6,000–12,000 | No | Yes |
M, male; F, female.
ALL, acute lymphatic leukemia; CGD, chronic granulomatous disease; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; CMV, cytomegalovirus; AML, acute myeloid leukemia; ITP, idiopathic thrombocytopenic purpura; DMII, diabetes mellitus type II.
i.v., intravenous; p.o., per os.
Equivalent to 50 mg/kg/day.
Equivalent to 226 mg/kg/day.
Equivalent to 208 mg/kg/day.
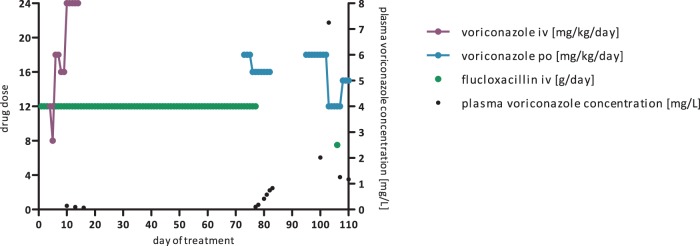
FIG 1.
Plasma voriconazole concentrations impacted by flucloxacillin, showing the day of flucloxacillin treatment on the x axis, flucloxacillin and voriconazole doses on the left y axis, and plasma voriconazole concentrations on the right y axis. A 12-year-old girl with relapsed acute lymphatic leukemia received flucloxacillin at 12 g/day (226 mg/kg/day) for Staphylococcus aureus sepsis and was started at 5 days after flucloxacillin initiation on voriconazole at 12 mg/kg/day for proven pulmonary and possible cerebral aspergillosis. Plasma voriconazole concentrations were routinely monitored and were extremely low on days 3, 6, 9, and 12 of voriconazole therapy (range, undetectable to 0.142 mg/liter) despite dose increases to 18 and 24 mg/kg/day. Voriconazole therapy was discontinued and switched to liposomal amphotericin B. Restart of voriconazole at 18 mg/kg/day on day 73 of flucloxacillin treatment did not lead to therapeutic voriconazole concentrations. Therefore, posaconazole was administered instead. Two weeks after discontinuation of flucloxacillin, posaconazole was switched back to voriconazole at 18 mg/kg/day, leading to adequate plasma voriconazole concentrations. A plasma voriconazole concentration increase to 7.3 mg/liter required a voriconazole dose reduction to 12 mg/kg/day. Rechallenge with a single dose of flucloxacillin again resulted in subtherapeutic plasma voriconazole concentrations.
Of nine patients who started voriconazole before flucloxacillin, four showed a significant decrease in voriconazole concentrations directly at the first measurement after initiation of flucloxacillin therapy (median of 2 [IQR, 0.25 to 5.25] days). Of 11 patients who started flucloxacillin before voriconazole or started both at the same time, 7 showed subtherapeutic voriconazole concentrations. After discontinuation of flucloxacillin, the voriconazole concentrations increased substantially within a week in four of six patients.
During voriconazole treatment, no drugs known to be CYP2C9, CYP2C19, or CYP3A4 inducers were used. The concentrations of other known CYP3A4 substrates (such as cyclosporine and tacrolimus) in plasma were not affected during flucloxacillin therapy.
All patients were Caucasian, apart from one Turkish patient. It would be very interesting to know the genotype, but unfortunately, this information is not available. The likelihood of encountering a biallelic *17 variation in Caucasians is low (3).
Metabolic ratios of plasma voriconazole to voriconazole N-oxide concentrations just prior to or after combination therapy and within a few days after the start of combination therapy were available for three of the patients who encountered the interaction. The median metabolic voriconazole N-oxide/voriconazole ratio without flucloxacillin was 1.5 (IQR, 0.5 to 3.8). The median metabolic voriconazole N-oxide/voriconazole ratio in the presence of flucloxacillin was 8.6 (IQR, 6.0 to 9.6). The statistical significance of the difference was not tested because of a lack of power.
In conclusion, we observed a strong effect of flucloxacillin on voriconazole concentrations in 11 of 20 patients who received both drugs concomitantly. In the other nine patients, no effect or a minor effect was found. To detect such a relevant drug interaction with very severe clinical consequences is striking, considering the long time since marketing introduction. From a mechanistic point of view, it is interesting that the interaction occurred in only half of the patients studied. This is the first report of a large cohort of patients who received the combination of flucloxacillin and voriconazole.
Voriconazole is metabolized in the liver via CYP450 enzymes (4) to voriconazole N-oxide (the most abundant circulating metabolite [4]), 4-hydroxyvoriconazole, and dihydroxyvoriconazole (5). These metabolites are further degraded via phase II reactions (5). Voriconazole N-oxide is formed via fluoropyrimidine N-oxidation reactions via CYP2C19 and CYP3A4, while 4-hydroxyvoriconazole is formed via methyl hydroxylation via CYP3A4 (4).
Although the CYP2C19 pathway has been considered the main pathway of voriconazole metabolism, hydroxylation via CYP3A4 has been found to contribute significantly to voriconazole metabolism, especially in poor CYP2C19 metabolizers (4, 6).
Pregnane X receptor (PXR) has been suggested as the most likely cause of the interaction between voriconazole and flucloxacillin (7), involving the upregulation specifically of CYP3A4. This hypothesis is supported by a recent study that envisioned a more dominant pathway involving CYP3A4 rather than CYP2C19 (8, 9). It is debatable whether an interaction with PXR (fully) explains the observed phenomenon for three reasons. First, the onset and cessation of the observed interaction in our patients do not comply with data regarding CYP3A4 induction in the literature. Flucloxacillin almost instantaneously reduced plasma voriconazole concentrations in our cohort, while induction of CYP3A4 generally takes about 1 to 2 weeks (10). The effect of induction is expected to gradually disappear approximately 2 weeks after discontinuation of the inducer (10), yet in most cases, we observed a quick, clinically relevant increase in plasma voriconazole concentrations after flucloxacillin discontinuation. Second, peak drug concentrations (data not shown) were very low to undetectable as well. In a setting of increased metabolism, one would expect peak drug concentrations to be within the normal range of single-dose administration. Third, the concentrations in plasma of other drugs metabolized by CYP3A4 remained unaffected.
The very low peak drug concentrations seen also argue against the possibility that CYP2C19 polymorphism caused the phenomenon observed, although lower peak drug concentrations have been described in ultrarapid metabolizers (11).
Several other mechanistic approaches may explain the effect of this interaction, such as a transporter-mediated shift in voriconazole distribution toward other cellular compartments or a shift in voriconazole metabolism pathways due to CYP3A4 competition, possibly leading to a subsequent increase in the role of flavin-containing mono-oxygenase 3 (12). The interaction requires a mechanistic investigation, including the prediction of which patients will be sensitive to this interaction.
Until more information is available, we advise physicians not to combine flucloxacillin and voriconazole or to closely monitor plasma voriconazole concentrations and quickly change therapy in cases of persistent subtherapeutic plasma voriconazole concentrations. It remains unknown whether other structurally related penicillins cause similar effects. It is unlikely that flucloxacillin will influence other azoles. Isavuconazole, however, is metabolized by CYP3A4 and might therefore not be the best alternative in the setting of this interaction until further elucidation of the mechanistic pathway.
In conclusion, the often prescribed combination of flucloxacillin and voriconazole leads to unmanageably low plasma voriconazole concentrations in half of the patients with primary and secondary immune deficiencies.
ACKNOWLEDGMENTS
No funding was obtained for this research. R.J.M.B. has served as a consultant to Astellas Pharma Inc., F2G, Gilead Sciences, Merck Sharpe and Dohme Corp. (MSD), and Pfizer Inc. and has received unrestricted and research grants from Astellas Pharma Inc., Gilead Sciences, MSD, and Pfizer Inc. All payments were invoiced by the Radboud university medical center and were outside the work described here. P.E.V. has received research grants from Astellas, Gilead Sciences, F2G, and MSD and honoraria for lectures from Gilead Sciences, Bio-Rad, and MSD. J.W.C.A. has received personal fees from Pfizer, Janssen, Astellas, MSD, and Gilead and grants from Pfizer and Astellas. All support received from MSD was outside the work described here.
REFERENCES
- 1.European Medicines Agency. 2017. Vfend. Annex I: summary of product characteristics. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000387/WC500049756.pdf Accessed 14 April 2017. [Google Scholar]
- 2.Brüggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. 2009. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 48:1441–1458. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 3.KNMP. 2016. Algemene achtergrondtekst Farmacogenetica—CYP2C19. KNMP, The Hague, The Netherlands: https://www.knmp.nl/downloads/g-standaard/farmacogenetica/Algemene-achtergrondtekst-Farmacogenetica-CYP2C19.pdf Accessed 10 July 2017. [Google Scholar]
- 4.Hyland R, Jones BC, Smith DA. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos 31:540–547. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 5.Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos 31:731–741. doi: 10.1124/dmd.31.6.731. [DOI] [PubMed] [Google Scholar]
- 6.Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK Jr, Thakker DR. 2010. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab Dispos 38:25–31. doi: 10.1124/dmd.109.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy B, Larcombe R, Chaptini C, Gordon DL. 2015. Interaction between voriconazole and flucloxacillin during treatment of disseminated Scedosporium apiospermum infection. J Antimicrob Chemother 70:2171–2173. doi: 10.1093/jac/dkv127. [DOI] [PubMed] [Google Scholar]
- 8.Geist MJ, Egerer G, Burhenne J, Riedel KD, Weiss J, Mikus G. 2013. Steady-state pharmacokinetics and metabolism of voriconazole in patients. J Antimicrob Chemother 68:2592–2599. doi: 10.1093/jac/dkt229. [DOI] [PubMed] [Google Scholar]
- 9.Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. 2007. Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol 73:2020–2026. doi: 10.1016/j.bcp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. 2003. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 42:819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 11.Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, Haefeli WE, Mikus G. 2009. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol 49:196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 12.Yanni SB, Annaert PP, Augustijns P, Bridges A, Gao Y, Benjamin DK Jr, Thakker DR. 2008. Role of flavin-containing monooxygenase in oxidative metabolism of voriconazole by human liver microsomes. Drug Metab Dispos 36:1119–1125. doi: 10.1124/dmd.107.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]