ABSTRACT
This retrospective cohort study included 53 patients admitted to the intensive care unit (ICU), with an average age of 69 years, without neurologic disorder before initiation of a continuous piperacillin infusion at the standard dose and who underwent piperacillin serum concentration monitoring. Among them, 23 developed a neurologic disorder for which the piperacillin causality was chronologically and semiologically suggestive. A concentration threshold of 157.2 mg/liter independently predicted neurotoxicity with 96.7% specificity and 52.2% sensitivity and may constitute a limitation when targeting less susceptible pathogens.
KEYWORDS: β-lactams, intensive care unit, neurotoxicity, sepsis, therapeutic drug monitoring, β-lactams
TEXT
Piperacillin is a β-lactam antibiotic, allowing bactericidal activity both against Gram-negative and Gram-positive bacteria. In combination with tazobactam as a β-lactamase inhibitor, it is among the most used broad-spectrum antibiotics in intensive care units (ICU) (1). Due to the variability of the pharmacokinetics (PK) in critically ill patients, therapeutic drug monitoring (TDM) of piperacillin is recommended in the ICU to adjust the dose according to the serum concentration (2). The pharmacokinetic-pharmacodynamic (PK/PD) relationship of β-lactams has been extensively studied to improve efficacy optimization, and the dose is adjusted according to the time the free (or unbound) drug concentration remains above the MIC of the infecting pathogen (fT > MIC) (2). Moreover, administration of piperacillin by continuous infusion leads to higher median concentrations than standard bolus dosing and thus allows a higher probability of attainment of the 100% fT > 4× MIC minimum PK/PD target, which would be required in critically ill patients (3–6). Conversely, the upper value above which serum concentrations are not required is usually set at 100% fT = 10× MIC for dose reduction (2), but the toxic concentration threshold, especially when using continuous infusion, is unknown. Piperacillin like other β-lactams can be neurotoxic if excessively accumulated (7). The aim of our study was thus to determine the serum concentration of piperacillin administered by continuous infusion that would predict neurotoxicity in critically ill patients and thus improve the development of a rational strategy for dose adjustment based on routine TDM.
This retrospective cohort study was conducted at the medical ICU of the University Hospital of Amiens, Amiens, France. The patients included consisted of adults who received continuous intravenous (i.v.) infusion of piperacillin-tazobactam at the standard dose adjusted to the glomerular filtration rate (GFR) estimated by the modification of diet in renal disease (MDRD) formula (12 or 16 g/24 h for an estimated GFR [eGFR] of ≥40 ml/liter/min/1.73 m2, 12 g/24 h for an eGFR of ≥20 ml/min/1.73 m2, and 8 g/24 h for an eGFR of <20 ml/min/1.73 m2) and who underwent monitoring of the piperacillin serum concentration over a 22-month period. The total serum concentration of piperacillin was measured after at least 48 h of treatment by a validated method combining high-performance liquid chromatography with photodiode array detection. For each patient, at least two investigators retrospectively reviewed the neurological evaluations daily recorded by the intensivists using the medical software of the ICU (Centricity Critical Care-Anandic Medical Systems AG, Switzerland). The basic neurological evaluation consisted of examination of the patient's mental status, cranial nerves, motor and sensory functions, coordination, and reflexes. For sedated patients, daily sedation interruption or alleviation allows assessment of neurologic function and detection of potential neurologic morbidity, as this has been proposed to shorten the length of stay in ICU (8). Patients who were showing any neurological disorder (ND) before the initiation of piperacillin and patients who did not undergo serum piperacillin measurement within the 24 h before or after the onset of an ND during piperacillin treatment were excluded. Patients who were pharmacologically sedated at the initiation of piperacillin or who had been sedated during piperacillin treatment were included if they were not showing any ND before the sedation induction. For included patients, the causality of piperacillin for an ND was considered suggestive when the 3 following criteria were met: (i) the ND presented as a symptom/sign consistent with piperacillin neurotoxicity (confusion, depressed level of consciousness, hallucinations, myoclonia, seizures according to the Common Terminology Criteria for Adverse Events of the National Institutes of Health, and awakening delayed by more than 24 h after stopping sedation), (ii) the ND occurred at least 48 h after the initiation of piperacillin, and (iii) the ND resolved or improved within 2 days after piperacillin discontinuation or dose reduction without starting renal replacement therapy. For each patient included, demographic data, medical history, and the simplified acute physiology score (SAPS II) at admission were collected. On the day of the piperacillin serum concentration measurement, we also examined the dose of piperacillin per 24 h the patient was receiving and whether the patient was febrile (body temperature more than 38°C), was septic according to standard criteria (9), and was also receiving or had also received up to 24 h previously renal replacement therapy, mechanical ventilation, vasopressive drugs, or potentially neurotoxic comedication (other β-lactams, fluoroquinolones, drugs of the central nervous system, drugs with atropine-like effects, and curare-like drugs). We also extracted biological data that are of interest for neurological evaluation. When a patient underwent several serum piperacillin measurements and did not show an ND during the treatment, the day of the highest measured concentration was selected for statistical analysis, so that each patient was included only once. The characteristics of the patients who developed an ND for which piperacillin causality was suggestive (piperacillin neurotoxicity group) were compared to those of the patients who did not develop an ND or who developed an ND for which piperacillin causality was not suggestive (the rest of the cohort). Categorical variables were analyzed using a chi-square or Fisher's exact test, and continuous variables were analyzed using Student's t test or a Mann-Whitney U test for parametric or nonparametric variables, respectively. A receiver operating characteristic (ROC) analysis was performed to determine the serum concentration value of piperacillin that predicts piperacillin neurotoxicity. A multivariate analysis of usual risk factors for neurotoxicity in the ICU was performed using a backward stepwise method for multivariable logistic regression models. All variables with a P value of <0.1 in the univariate analysis were included in the final regression model. All tests were two tailed, and a P value of <0.05 was considered to be statistically significant. Statistical analyses were performed using IBM SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA).
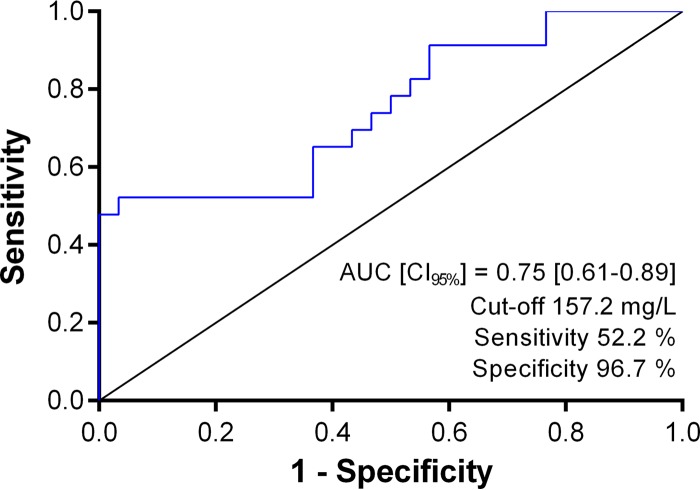
During the study period, 214 patients were treated with piperacillin-tazobactam. Among them, 129 (60.3%) underwent at least one piperacillin serum measurement, and 53 (24.8%) have been included for analysis according to previously described criteria. The clinical and biological data collected for included patients are detailed in Table 1. Reported NDs for which the piperacillin causality was considered suggestive were depressed level of consciousness in 11 patients (47.8%), delayed awakening after stopping sedation in 10 patients (43.5%), confusion in 5 patients (21.7%), myoclonia in 3 patients (13%), seizures in 1 patient (4.3%), and hallucinations in 1 patient (4.3%). Patients in the piperacillin neurotoxicity group were more septic (65.2 versus 26.7%; P = 0.0006), on vasopressive drugs (82.6 versus 46.7%; P = 0.01), and showed a lower eGFR (18 versus 50 ml/min/1.73 m2; P = 0.0142) than patients in the rest of cohort. The median piperacillin dose normalized to eGFR was higher in the piperacillin neurotoxicity group than in the rest of the cohort (48 versus 22 g/24 h per 100 ml/min/1.73 m2; P = 0.0111), while it was not different at the piperacillin initiation time (36.4 versus 28.6 g/24 h per 100 ml/min/1.73 m2; P = 0.5634). Indeed, the median dose and the median eGFR at the start of the treatment were not statistically different in patients who subsequently developed a suggestive piperacillin neurotoxicity compared to those who did not (dose of 12 versus 12 g/24 h, P = 0.4779, and eGFR of 33 versus 44 ml/min/1.73 m2, P = 0.3792, respectively). Excluding patients who were on renal replacement therapy, we observed that the median percentage of decrease of eGFR between piperacillin initiation and piperacillin concentration measurements (on average 3 days) was significantly higher in patients with sepsis (−35.6% versus −6.1%, respectively; n = 39, P = 0.0269). Then we found that the piperacillin serum concentration was significantly higher in the piperacillin neurotoxicity group than in the rest of the cohort (159.9 versus 91.3 mg/ml; P = 0.0016). It could thus be hypothesized that the dose has not been always adjusted adequately to the sepsis-associated GFR changes and that the first piperacillin serum measurement was generally too late to anticipate the rapid piperacillin accumulation. Based on the concentration data from the overall cohort, ROC analysis found a predictive serum concentration threshold for the occurrence of piperacillin neurotoxicity of 157.2 mg/liter with 96.7% specificity and 52.2% sensitivity (area under the concentration-time curve [AUC], 0.75; 95% confidence interval [CI], 0.61 to 0.89) (Fig. 1). The high specificity of this threshold suggests that piperacillin neurotoxicity would be almost systematic at a higher concentration. This was corroborated by multivariable analysis showing that a piperacillin concentration equal or higher than 157.2 mg/liter was an independent risk factor of piperacillin neurotoxicity with an odds ratio of 14.86 (95% CI, 1.27 to 173.23; P = 0.0313) (Table 2). Conversely, none of the other factors, including sepsis, were found as independent risk factors. Although sepsis is a well-known cause of encephalopathy in ICUs (10), our results suggest that sepsis may constitute an indirect cause of NDs, promoting drug neurotoxicity at least by inducing GFR decrease and then drug accumulation. However, the 157.2-mg/liter concentration threshold also exhibits moderate sensitivity, indicating that piperacillin neurotoxicity would still occur at a lower concentration. The neurotoxic piperacillin concentration threshold is thus likely to be lower in patients that have accumulated several neurological risk factors, especially causes of blood-brain barrier permeability increase.
TABLE 1.
Comparison between patients who developed neurotoxicity for which piperacillin causality was suggestive and patients who did not show piperacillin neurotoxicitya
| Parameterb | Result forc: |
P valued | |
|---|---|---|---|
| Piperacillin neurotoxicity group (n = 23) | Rest of cohort (n = 30) | ||
| Demographic data and medical history at admission | |||
| Women, % | 39.1 | 23.3 | 0.2423 |
| Age, median yr | 65 (61.5–71.5) | 71.5 (64.3–79.5) | 0.0502 |
| Wt, median kg | 87 (68–99) | 87.5 (78.3–99) | 0.1778 |
| BMI, median kg/m2 | 29.6 (23.3–32.8) | 28.4 (25–32.7) | 0.6416 |
| History of brain injury (stroke or trauma), % | 13.0 | 0 | 0.0760 |
| History of epilepsy, % | 8.7 | 3.3 | 0.5730 |
| History of alcoholism, % | 17.4 | 13.3 | 0.7150 |
| History of drug abuse, % | 0 | 0 | |
| History of cardiovascular disease, % | 82.6 | 76.7 | 0.7379 |
| History of cancer, % | 43.4 | 60 | 0.2756 |
| SAPS II, median | 58 (46–76) | 54 (42–73) | 0.4454 |
| Clinical data from day of piperacillin serum measurement | |||
| Fever, % | 30.4 | 20 | 0.5220 |
| Sepsis, % | 65.2 | 26.7 | 0.0006* |
| Mechanical ventilation, % | 60.9 | 53.3 | 0.7800 |
| Renal replacement therapy, % | 26.1 | 26.7 | 1 |
| Vasopressive comedications, % | 82.6 | 46.7 | 0.0100* |
| Potentially neurotoxic comedications, % | 73.9 | 80 | 0.7430 |
| Hepatic impairment, % | 30.4 | 13.3 | 0.1770 |
| Biological data from day of piperacillin serum measurement | |||
| Creatininemia, median μM | 261 (170–349.5) | 122 (54–242) | 0.0169* |
| eGFR, median ml/min/1.73 m2 | 18 (15–37) | 50 (12–83) | 0.0142* |
| Proteinemia, median g/liter | 55 (51–60) | 55 (42–64) | 0.7060 |
| Natremia, median mM | 139 (137–144) | 138 (130–141) | 0.3726 |
| Calcemia, median mM | 1.9 (1.8–2) | 2 (1.8–2.2) | 0.1368 |
| Lactatemia, median mM | 1.6 (1.1–2.4) | 1.5 (1.1–1.9) | 0.3528 |
| CRP, median mg/liter | 149.6 (103–281.2) | 144.3 (89.9–190.1) | 0.1537 |
| PCT, median μg/liter | 25 (1.9–35.6) | 2.48 (2.5–9.45) | 0.0072* |
| pH, median | 7.39 (7.35–7.47) | 7.44 (7.39–7.47) | 0.3173 |
| Piperacillin treatment at initiation time | |||
| Dose, median g/24 h | 12 (12–12) | 12 (12–12) | 0.4866 |
| Dose normalized to eGFR, median g/24 h/100 ml/min/1.73 m2 | 36.4 (16–58.6) | 28.6 (12.8–52.5) | 0.5634 |
| Piperacillin treatment on day of piperacillin serum measurement | |||
| Dose, median g/24 h | 12 (8–12) | 12 (9–12) | 0.9207 |
| Dose normalized to eGFR, median g/24 h/100 ml/min/1.73 m2 | 48 (35.3–69.7) | 22 (14.3–54) | 0.0111* |
| Serum concn, median mg/liter | 156.9 (95.4–236) | 91.3 (68.6–126.8) | 0.0016* |
| Time from treatment initiation, median days | 3 (2–5) | 3 (2–5) | 0.7819 |
| fC/MIC, median | 9.72 (5.35–15.06) | 5.4 (3.6–7.9) | 0.0174* |
Patients who developed neurotoxicity for which the piperacillin causality was suggestive are represented by the piperacillin neurotoxicity group, and those who did not show piperacillin neurotoxicity are indicated as the rest of the cohort.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; fC/MIC, free concentration of piperacillin over the MIC of the infective pathogen; PCT, procalcitonin; SAPS II, simplified acute physiology score.
Interquartile ranges are shown in parentheses.
*, significant at P <0.05.
FIG 1.
Receiver operating characteristic (ROC) curve of piperacillin serum concentration for predicting neurotoxicity.
TABLE 2.
Logistic regression analysis of risk factors for piperacillin neurotoxicity in critically ill patients
| Risk factor of neurotoxicity | Odds ratio (95% CI) | P value |
|---|---|---|
| Univariate analysis | ||
| Women | 2.11 (0.64–6.94) | 0.2183 |
| Age of >68 yr | 0.49 (0.16–1.48) | 0.2082 |
| History of brain injury (stroke or trauma) | NDa | |
| History of epilepsy | 2.76 (0.23–32.5) | 0.4193 |
| History of alcoholism | 1.37 (0.3–6.18) | 0.6833 |
| Fever of >38°C | 1.75 (0.5–6.17) | 0.3843 |
| Sepsis | 5.16 (1.59–16.77) | 0.0064 |
| Invasive mechanical ventilation | 1.36 (0.45–4.1) | 0.5837 |
| Renal replacement therapy | 0.97 (0.28–3.33) | 0.9622 |
| Vasopressive comedication | 5.43 (1.49–19.82) | 0.0105 |
| Potentially neurotoxic comedication | 0.70 (0.19–2.57) | 0.6006 |
| Hepatic impairment | 2.84 (0.72–11.27) | 0.1369 |
| eGFR of <30 ml/min/1.73 m2 | 2.81 (0.91–8.68) | 0.0721 |
| Natremia of <135 mM | 1.37 (0.30–6.17) | 0.6833 |
| Calcemia of <2 mM | 2.61 (0.83–8.18) | 0.0993 |
| Lactatemia of >5 mM | ND | |
| pH of <7.35 | 3.18 (0.7–14.42) | 0.1343 |
| Piperacillin concn of ≥157.2 mg/liter | 26.58 (3.08–229.32) | 0.0028 |
| Multivariate analysis | ||
| eGFR of <30 ml/min/1.73 m2 | 1.37 (0.31–5.97) | 0.6769 |
| Vasopressive comedications | 1.78 (0.37–8.54) | 0.4705 |
| Sepsis | 2.36 (0.52–10.66) | 0.2656 |
| Calcemia of <2 mM | 3.55 (0.78–16.18) | 0.1013 |
| Piperacillin concn of ≥157.2 mg/liter | 14.86 (1.27–173.23) | 0.0313 |
ND, not determinable.
Nevertheless, the neurotoxic threshold of the piperacillin serum concentration shouldn't be interpreted without considering PK/PD targets for dose adaptation. The clinical benefits of the continuous infusion strategy are supported by the results of recent meta-analyses, especially for patients with a high sickness severity and infected by less-susceptible pathogens, such as nonfermenting Gram-negative bacilli (11–13). In our cohort, the infective organism was not identified for 19 of the 53 patients (35.8%). It was an Enterobacteriaceae family member in 17 patients (32.1%), Pseudomonas aeruginosa in 10 patients (18.9%), Staphylococcus aureus in 3 patients (5.7%), and another organism in 4 patients (7.5%). Considering 30% serum protein binding and EUCAST's epidemiological MIC cutoff values for identified organisms or the MIC of 16 mg/liter for nonidentified organisms, the median free piperacillin serum concentration over MIC ratio was significantly higher in the piperacillin neurotoxicity group than in the rest of the cohort (9.7 versus 5.4, respectively, P = 0.0174). Importantly, the 100% fT = 10× MIC published threshold for dose reduction would allow neurotoxic piperacillin concentrations in cases of MICs from 12 mg/liter. A 16-mg/ml susceptibility breakpoint for piperacillin-tazobactam against Pseudomonas aeruginosa may thus incite clinicians to maintain a potentially neurotoxic dosage of piperacillin since the therapeutic window for 100% fT = 4× MIC to fT = 10× MIC would set the total serum concentration target between 83.2 and 208 mg/liter.
The primary limitations of this study are the retrospective design and the small cohort. Larger prospective and multicenter clinical studies including standardization of the neurological evaluation of ICU patients are needed to define more accurately the neurotoxic concentration threshold of piperacillin and risk factors. Moreover, the value we found here applies only to continuous infusion, and thresholds should be specifically defined for intermittent and extended infusions. Another important limitation is the use of MDRD in daily estimation of GFR for dose adjustment and for describing renal dysfunction. Creatinine-based GFR estimates rely on stable serum creatinine concentrations which cannot be present in the ICU. Nevertheless, MDRD may still be relevant in the ICU to predict clearance changes and PK/PD target attainment, as described for ceftazidime (14, 15).
Finally, this study is the first interested in the concentration-neurotoxicity relationship of piperacillin administered combined with tazobactam through continuous infusion in critically ill patients. A piperacillin serum concentration of 157.2 mg/liter would be predictive of neurotoxicity and constitute a concrete and useful criterion for the decision to reduce the piperacillin dose or, in case of a low-susceptibility pathogen, to switch to another antibiotic according to microbiological data and close TDM.
ACKNOWLEDGMENT
We declare we have no conflicts of interest regarding the present work.
REFERENCES
- 1.Thomas Z, Bandali F, Sankaranarayanan J, Reardon T, Olsen KM, Critical Care Pharmacotherapy Trials Network. 2015. A multicenter evaluation of prolonged empiric antibiotic therapy in adult ICUs in the United States. Crit Care Med 43:2527–2534. doi: 10.1097/CCM.0000000000001294. [DOI] [PubMed] [Google Scholar]
- 2.Wong G, Brinkman A, Benefield RJ, Carlier M, De Waele JJ, El Helali N, Frey O, Harbarth S, Huttner A, McWhinney B, Misset B, Pea F, Preisenberger J, Roberts MS, Robertson TA, Roehr A, Sime FB, Taccone FS, Ungerer JPJ, Lipman J, Roberts JA. 2014. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother 69:1416–1423. doi: 10.1093/jac/dkt523. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Piperacillin penetration into tissue of critically ill patients with sepsis—bolus versus continuous administration? Crit Care Med 37:926–933. doi: 10.1097/CCM.0b013e3181968e44. [DOI] [PubMed] [Google Scholar]
- 4.Rafati MR, Rouini MR, Mojtahedzadeh M, Najafi A, Tavakoli H, Gholami K, Fazeli MR. 2006. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents 28:122–127. doi: 10.1016/j.ijantimicag.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Duszynska W, Taccone FS, Switala M, Hurkacz M, Kowalska-Krochmal B, Kübler A. 2012. Continuous infusion of piperacillin/tazobactam in ventilator-associated pneumonia: a pilot study on efficacy and costs. Int J Antimicrob Agents 39:153–158. doi: 10.1016/j.ijantimicag.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Dulhunty JM, Roberts JA, Davis JS, Webb SAR, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Lipman J. 2013. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis 56:236–244. doi: 10.1093/cid/cis856. [DOI] [PubMed] [Google Scholar]
- 7.Chow KM, Hui AC, Szeto CC. 2005. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis 24:649–653. doi: 10.1007/s10096-005-0021-y. [DOI] [PubMed] [Google Scholar]
- 8.Barr J, Kishman CP, Jaeschke R. 2013. The methodological approach used to develop the 2013 Pain, Agitation, and Delirium Clinical Practice Guidelines for adult ICU patients. Crit Care Med 41:S1–S15. doi: 10.1097/CCM.0b013e3182a167d7. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent J-L, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS. 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 10.Gofton TE, Young GB. 2012. Sepsis-associated encephalopathy. Nat Rev Neurol 8:557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 11.Teo J, Liew Y, Lee W, Kwa AL-H. 2014. Prolonged infusion versus intermittent boluses of β-lactam antibiotics for treatment of acute infections: a meta-analysis. Int J Antimicrob Agents 43:403–411. doi: 10.1016/j.ijantimicag.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JA, Abdul-Aziz M-H, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, Bellomo R, Lipman J. 2016. Continuous versus intermittent β-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 194:681–691. doi: 10.1164/rccm.201601-0024OC. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Zhang C, Zhou Q, Wang Y, Chen L. 2015. Clinical outcomes with alternative dosing strategies for piperacillin/tazobactam: a systematic review and meta-analysis. PLoS One 10:e0116769. doi: 10.1371/journal.pone.0116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georges B, Conil J-M, Ruiz S, Seguin T, Cougot P, Fourcade O, Houin G, Saivin S. 2012. Ceftazidime dosage regimen in intensive care unit patients: from a population pharmacokinetic approach to clinical practice via Monte Carlo simulations. Br J Clin Pharmacol 73:588–596. doi: 10.1111/j.1365-2125.2011.04117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georges B, Conil J-M, Seguin T, Ruiz S, Minville V, Cougot P, Decun J-F, Gonzalez H, Houin G, Fourcade O, Saivin S. 2009. Population pharmacokinetics of ceftazidime in intensive care unit patients: influence of glomerular filtration rate, mechanical ventilation, and reason for admission. Antimicrob Agents Chemother 53:4483–4489. doi: 10.1128/AAC.00430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]