ABSTRACT
The prolonged use of injectable agents in a regimen for the treatment of multidrug-resistant tuberculosis (MDR-TB) is recommended by the World Health Organization, despite its association with ototoxicity and nephrotoxicity. We undertook this study to look at the relative adverse effects of capreomycin and amikacin. We reviewed the case notes of 100 consecutive patients treated at four MDR-TB treatment centers in the United Kingdom. The median total duration of treatment with an injectable agent was 178 days (interquartile range [IQR], 109 to 192 days; n = 73) for those with MDR-TB, 179 days (IQR, 104 to 192 days; n = 12) for those with MDR-TB plus fluoroquinolone resistance, and 558 days (IQR, 324 to 735 days; n = 8) for those with extensively drug-resistant tuberculosis (XDR-TB). Injectable use was longer for those started with capreomycin (183 days; IQR, 123 to 197 days) than those started with amikacin (119 days; IQR, 83 to 177 days) (P = 0.002). Excluding patients with XDR-TB, 51 of 85 (60%) patients were treated with an injectable for over 6 months and 12 of 85 (14%) were treated with an injectable for over 8 months. Forty percent of all patients discontinued the injectable due to hearing loss. Fifty-five percent of patients experienced ototoxicity, which was 5 times (hazard ratio [HR], 5.2; 95% confidence interval [CI], 1.2 to 22.6; P = 0.03) more likely to occur in those started on amikacin than in those treated with capreomycin only. Amikacin was associated with less hypokalemia than capreomycin (odds ratio, 0.28; 95% CI, 0.11 to 0.72), with 5 of 37 (14%) patients stopping capreomycin due to recurrent electrolyte loss. There was no difference in the number of patients experiencing a rise in the creatinine level of >1.5 times the baseline level. Hearing loss is frequent in this cohort, though its incidence is significantly lower in those starting capreomycin, which should be given greater consideration as a first-line agent.
KEYWORDS: antibiotic resistance, antimicrobial safety, tuberculosis
INTRODUCTION
Treatment of multidrug-resistant tuberculosis (MDR-TB) is challenging and requires extensive treatment with multidrug combinations for up to 2 years. These treatments are associated with significant adverse effects (1). Current treatment for MDR-TB is largely dependent on World Health Organization (WHO) guidelines (2–4), which are based on data from cohort studies and meta-analyses and on expert opinion. These guidelines recommend that all patients be initially treated (during the intensive phase) with an injectable agent in the form of an aminoglycoside (kanamycin or amikacin) or polypeptide (capreomycin). The duration of the intensive phase recommended by WHO rose from a minimum of 6 months to 8 months in 2011 (3, 4), with even longer durations of treatment being recommended for cases with more extensive resistance. The recommendation was based on a large meta-analysis of patient outcomes and did not take into account the side effects or other costs of these drugs (5).
The injectable agents have significant side effects in the form of permanent and potentially progressive postcessation ototoxicity and usually reversible nephrotoxicity (6–9). The frequency of ototoxicity and nephrotoxicity experienced by patients varies between studies, and most focus on the side effects of the aminoglycosides rather than those of the polypeptide capreomycin. Limited evidence suggests that capreomycin may be less ototoxic than amikacin (10).
No randomized controlled trial of different injectable agents has been performed, but better data are needed to inform policy. We performed a detailed service evaluation cohort study within four specialist MDR-TB treatment centers in the United Kingdom to compare the outcomes with different injectable agents in a real-world setting.
RESULTS
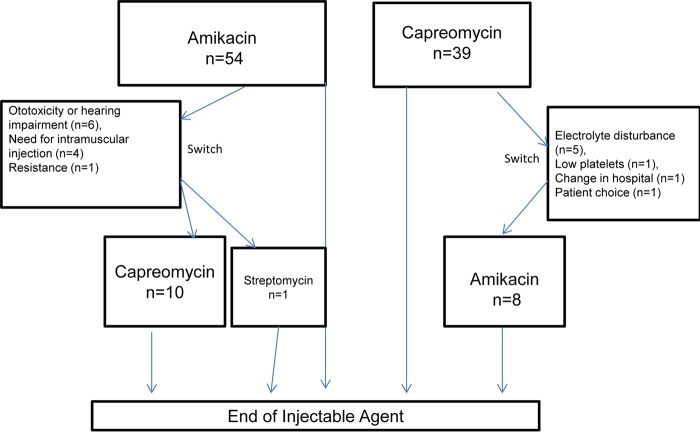
Fifty-four patients were started on amikacin, and 39 were started on capreomycin (total, n = 93). Nineteen patients switched injectable agent for the reasons stated in Fig. 1. The background characteristics and demographic of the patients and the characteristics of their cases of tuberculosis can be seen in Table 1.
FIG 1.
Flow diagram showing injectable agent use in cohort.
TABLE 1.
Background characteristics and demographics of patientsa
| Characteristic | Value |
|---|---|
| Median (IQR) age (mo) (n = 93) | 28 (24–38) |
| No. (%) of male patients (n = 93) | 64 (68) |
| No. (%) of HIV-infected patients (n = 93) | 5 (5) |
| No. (%) of patients with the following country of birth (n = 93): | |
| United Kingdom | 9 (10) |
| Other western and northern Europe | 1 (1) |
| Chinese subcontinent | 10 (10) |
| Indian subcontinent | 36 (38) |
| Africa | 15 (16) |
| Eastern Europe and Russia | 22 (24) |
| No. (%) of patients with the following type of TB (n = 93): | |
| MDR-TB | 73 (78) |
| MDR-TB + FLQr | 12 (13) |
| XDR-TB | 8 (9) |
| No. (%) of patients with the following location of TB (n = 93): | |
| Pulmonary | 41 (44) |
| Extrapulmonary only | 31 (33) |
| Both pulmonary and extrapulmonary | 21 (23) |
| No. (%) of patients receiving the following injectable agent (n = 93): | |
| Capreomycin | 31 (33) |
| Amikacin | 43 (46) |
| Amikacin and capreomycin (sequentially, either order) | 18 (19) |
| Amikacin followed by streptomycin | 1 (1) |
| Baseline (IQR) creatinine concn (μmol/liter) (n = 87) | 66 (58–75) |
| Median (IQR) creatinine clearance (n = 81) | 116.2 (75.7–179.1) |
| Median (IQR) initial dose of injectable agent (mg/kg) (n = 82) | 14.81 (14.06–16.13) |
| Median (IQR) no. of amikacin trough tests/wk (those on amikacin) (n = 58) | 1.01 (0.76–1.29) |
Data are for 93 patients. IQR, interquartile range; FLQr, fluoroquinolone resistance.
Total duration of treatment with an injectable agent.
The median total duration of treatment with an injectable agent was 178 days (interquartile range [IQR], 109 to 192 days; n = 73) for those with MDR-TB, 179 days (IQR, 104 to 192 days; n = 12) for those with MDR-TB plus fluoroquinolone (FLQ) resistance (MDR-TB plus FLQr), and 558 days (IQR, 324 to 735 days; n = 8) for those with extensively drug-resistant tuberculosis (XDR-TB).
Excluding those with XDR-TB, 51 of 60 (60%) patients were treated for 6 months or more and 12 of 85 (14%) patients were treated for 8 months or more. In the early cohort, the median duration of treatment was 165 days (IQR, 107 to 187 days; n = 42); of the patients in this cohort, 23 of 42 (55%) achieved the target treatment duration of 6 months and 3 of 42 (7%) were treated for 8 months or longer. In the latter cohort, the median duration of treatment was 183 days (IQR, 109 to 210 days; n = 43 days); of the patients in this cohort, 28 of 43 (65%) were treated for 6 months or more and 9 of 43 (21%) achieved the target treatment duration of 8 months or more. There was no statistically significant difference in the duration between the early and late cohorts (P = 0.19).
Seven of 8 (87%) patients with XDR-TB were treated for 6 months or more and 6 of 8 (75%) patients with XDR-TB were treated for 8 months or more.
The reasons for not achieving 6 months or more of treatment for all groups of patients were hearing loss (composite) for 14 of 35 (40%) patients, physician choice for 8 of 35 (23%) patients, drug resistance for 4 of 35 (11%) patients, compliance concerns for 3 of 35 (9%) patients, and other reasons for 6 of 35 (17%) patients.
The median duration of treatment with the first-line injectable agent was 160 days (IQR, 91 to 186 days) for all patients. The median total duration was 183 days (IQR, 123 to 197 days) for those started on capreomycin and 119 days (IQR, 83 to 177 days) for those started on amikacin (P = 0.002).
Ototoxicity.
The proportion of cases that met the criteria for ototoxicity assessment was 55 of 93 (59%) patients (Table 2), of whom 39 were started on amikacin and 16 were started on capreomycin. Clinical notes were available for all 55 patients. Ototoxicity occurred in 30 of the 55 (55%) patients at a median duration of 112.5 days (IQR, 91 to 177 days), and 18 of the 55 (60%) patients had bilateral changes. Deterioration at frequencies of 6 to 8 kHz was seen in only 19 of the 55 (63%) cases, deterioration at frequencies 4 to 8 kHz was seen in only 3 of the 55 (10%) cases, deterioration at frequencies of 2 to 8 kHz was seen in only 6 of the 55 (20%) cases, and deterioration across all frequencies tested (250 Hz to 8 kHz) was seen in 2 of the 55 (7%) cases. The median maximum change in hearing from that at the baseline at the worst frequency affected was 40 dB (IQR, 25 to 55 dB). At the time that ototoxicity was detected, 8 of the 55 (27%) patients reported new-onset hearing disturbance and tinnitus, 8 of the 55 (27%) patients reported tinnitus only, 3 of the 55 (10%) patients reported hearing disturbance only, and 11 of the 55 (37%) patients did not report any symptoms.
TABLE 2.
Ototoxicity and hearing loss (composite) definitionsa
| Term | Hearing loss | No hearing loss | Unable to classify |
|---|---|---|---|
| Ototoxicity | A significant deterioration (as determined by ASHA criteria) between an audiogram performed before or during therapy and one performed later during therapy or after completion of therapy in the presence of normal tympanogramsb | A normal audiogram in the last month or after completion of injectable therapyb; no significant deterioration (as determined by ASHA criteria) between an audiogram performed in the last month or after injectable therapy stopped and one performed within the first month of therapyb; no significant deterioration (ASHA criteria) between an audiogram performed after 365 days of injectable therapy and one performed within the first month of therapy | An abnormal audiogram without an earlier audiogram for comparisonb; a normal final audiogram before the last month of therapy (unless it was performed after 365 days on therapy) |
| Hearing loss (composite) | As for ototoxicity; a clinical report of new hearing impairment or tinnitus during or after therapy with an injectable agent in association with an abnormal audiogram; no prior audiogram required; a clinical report of new hearing impairment or tinnitus during or after therapy with an injectable agent in association with a significant deterioration (as determined by ASHA criteria) between an audiogram performed before or during therapy and one performed later during therapy or after completion of therapy above the 8-kHz range | No report of hearing impairment or tinnitus and the hearing loss does not fit the criteria for ototoxicity; a clinical report of new hearing impairment or tinnitus during or after therapy with an injectable agent in association with a normal audiogram or no deterioration in audiograms performed within a month of starting and at the time of or after the onset of symptoms | Unable to report symptoms (the patient was intubated or had extreme psychosis) or a full set of medical or nursing notes was missing |
| Worsening ototoxicity after stopping injectable agent | A significant deterioration (as determined by ASHA criteria) between an audiogram performed 30 days or more after the end of injectable therapy and one performed in the month before the end of therapy or on the stop date | No significant deterioration (as determined by ASHA criteria) between an audiogram performed 30 days or more after the end of injectable therapy and one performed in the month before the end of therapy or on the stop date | Any case not fitting either of the definitions |
PTA, pure-tone audiometry; normal audiogram, ability to hear all frequencies above 25 dB; abnormal audiogram, an abnormal audiogram according to the criteria of the American Speech-Language-Hearing Association (ASHA).
Based on definitions of hearing loss proposed by Seddon et al. (9).
Ototoxicity occurring during amikacin treatment.
Twenty-eight cases of ototoxicity occurred while the patients were receiving treatment with amikacin (n = 23) or after they had stopped treatment with amikacin (n = 5). The median total number of amikacin trough levels did not differ between those with ototoxicity (1.03; IQR, 0.77 to 1.28) and those without ototoxicity (1.21; IQR, 1 to 1.43) (P = 0.10). The proportions of patients with one or more amikacin trough levels above 2.5 were 12/28 (40%) for those treated with amikacin and who experienced ototoxicity and 5/14 (36%) for those treated with amikacin and who did not experience ototoxicity (P = 0.66).
Three cases who had initially been treated with capreomycin experienced ototoxicity while receiving treatment with amikacin. They had been switched to amikacin due to an electrolyte disturbance (n = 2) or resistance (n = 1). Two of the patients had normal audiograms (which were the same as their baseline audiograms) at the time of the switch (at 174 and 164 days of treatment, respectively), and the third had a normal audiogram at the start of capreomycin followed by an abnormal audiogram after 282 days of amikacin treatment, when newly reported tinnitus led to testing. Fifteen of 28 (54%) patients had audiograms sufficient to assess deterioration after the cessation of amikacin; 10 of 15 (67%) progressed, 1 of 28 (7%) improved, and the audiograms of 4 of 15 (27%) did not change.
Ototoxicity occurring during capreomycin treatment.
Two cases of ototoxicity occurred while patients were receiving capreomycin. Both were in patients with XDR-TB in whom stopping of the regimen would have reduced the number of active drugs below 4, and so despite early detection, treatment was continued with monitoring. Neither case experienced any permanent symptoms. Both cases had normal audiograms on the first assessment, and sensorineural hearing loss was identified on the second audiogram performed after the baseline, which was at day 33 of treatment (performed due to vague symptoms of muffled hearing, which went away) and day 112 of treatment (performed for screening with no symptoms), respectively. Changes were seen bilaterally in both cases at the 6-kHz and 8-kHz frequencies. There was a drop of 10 to 20 dB in case 1 and a drop of 30 to 55 dB in case 2. A further 3 and 4 audiograms were performed until days 434 (case 1) and 447 (case 2) of treatment, respectively, and no further deterioration was seen. Both patients continued treatment after this period of monitoring with no change in symptoms, but no further audiograms were performed.
Multivariable analysis using only the patients who fitted the ototoxicity criteria showed that ototoxicity was five times more likely for patients started on amikacin than for those treated with capreomycin only (hazard ratio, 5.2; 95% CI, 1.2 to 22.6; P = 0.03).
Hearing loss (composite).
Three of 93 patients did not have sufficient medical notes (n = 1) or could not express a loss of hearing (because of psychosis [n = 1] or intubation [n = 1]) to be included in this analysis. Thirty-four of the 90 (38%) patients meeting the criteria for inclusion experienced hearing loss (composite). The multivariable analysis showed that the likelihood of hearing loss (composite) was 14 times greater for patients started on amikacin than those treated with capreomycin only (hazard ratio, 13.9; 95% CI, 3.25 to 59; P < 0.001) (Table 3). Predicted survival analysis also showed that the probability of not developing hearing loss beyond 90 days was 0.99 (95% CI, 0.95 to 1.00) for those treated with capreomycin only, whereas it was 0.85 (95% CI, 0.73 to 0.92) for those started on amikacin. Furthermore, the probability of surviving without hearing loss beyond 180 days was 0.97 (95% CI, 0.86 to 0.99%) for those on capreomycin only, whereas it was 0.58 (95% CI, 0.41 to 0.72%) for those started on amikacin (Fig. 2).
TABLE 3.
Multivariable (adjusted) analysis investigating the predictors of hearing loss (composite)
| Variable | No. (%) of patients with: |
Univariate analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|---|
| Hearing loss | No hearing loss | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| All patients | 34 (38) | 56 (62) | ||||
| Therapeutic choice at start | ||||||
| Amikacin (n = 53) | 29 (55) | 24 (45) | 5.80 (2.23–15.04) | <0.001 | ||
| Capreomycin (n = 37) | 5a (14) | 32 (86) | ||||
| Treatment group comparison | ||||||
| Starting amikacin (n = 53) | 29 (55) | 24 (45) | 11.70 (2.78–49.20) | 0.001 | 13.85 (3.25–58.99) | <0.001 |
| Capreomycin only (n = 30) | 2 (7) | 28 (93) | ||||
| Capreomycin followed by amikacin (n = 7) | 3 (43) | 4 (57) | 6.29 (1.05–37.65) | 0.044 | 4.03 (0.66–24.63) | 0.13 |
| Capreomycin only (n = 30) | 2 (7) | 28 (93) | ||||
| Starting amikacin (n = 53) | 29 (55) | 24 (45) | 1.86 (0.56–6.13) | 0.307 | 3.44 (0.97–12.18) | 0.06 |
| Capreomycin followed by amikacin (n = 7) | 3 (43) | 4 (57) | ||||
| MDR-TB type comparison | ||||||
| MDR-TB + FLQr TB (n = 12) | 8 (67) | 4 (33) | 3.26 (1.44–7.36) | 0.005 | ||
| MDR-TB (n = 70) | 22 (31) | 48 (69) | ||||
| XDR-TB (n = 8) | 4 (50) | 4 (50) | 1.62 (0.55–4.73) | 0.378 | ||
| MDR-TB (n = 70) | 22 (31) | 48 (69) | ||||
| XDR-TB (n = 8) | 4 (50) | 4 (50) | 0.55 (0.17–1.83) | 0.331 | ||
| MDR-TB + FLQr TB (n = 12) | 8 (67) | 4 (33) | ||||
| FLQr TB (n = 20) | 12 (60) | 8 (40) | 2.43 (1.20–4.93) | 0.013 | 3.15 (1.45–6.88) | 0.004 |
| MDR-TB (n = 70) | 22 (31) | 48 (69) | ||||
| Median (IQR) dose of injectable at start (mg/kg) | 14.58 (13.82–15.51) | 14.94 (14.07–16.63) | 0.84 (0.71–1.00) | 0.047 | ||
| Median (IQR) baseline creatinine concn (μmol/liter, log scale) | 4.25 (4.13, 4.30) | 4.17 (4.04, 4.32) | 4.37 (1.12–17.11) | 0.034 | ||
| Median (IQR) creatinine clearance | 114.1 (99.5, 122.9) | 119. 3 (105.8–134.5) | 0.98 (0.97, 1.00) | 0.055 | 0.99 (0.97–1.00) | 0.11 |
| Median (IQR) age (yr) (1-yr effect) | 28.5 (25–39) | 27.5 (22.5–33.5) | 1.03 (0.99–1.07) | 0.127 | ||
Only two of these cases occurred while the patient was receiving capreomycin. The other three occurred while the patient was receiving amikacin, after the patients had been switched off capreomycin for other reasons. Two patients had normal pure-tone audiograms at the start of amikacin treatment.
FIG 2.
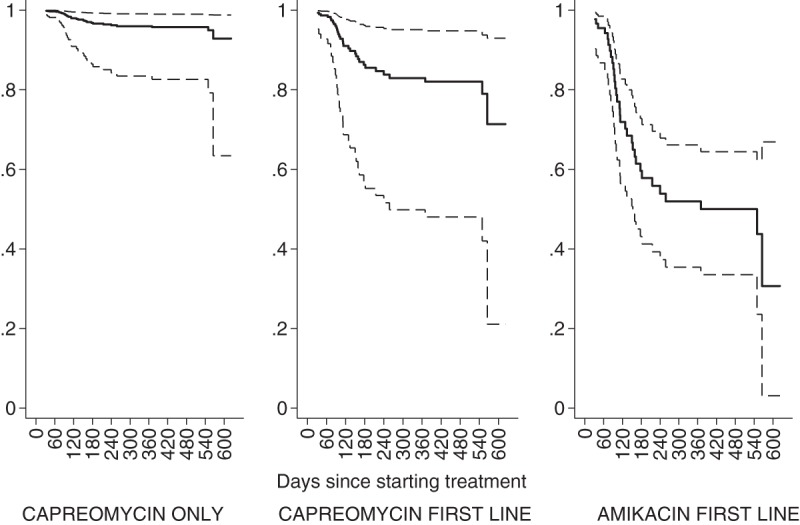
Predicted proportions (identified on the y axis) surviving without hearing loss by initial choice of injectable agent. The middle thick line represents the predicted proportion, and the outer thin lines represent 95% confidence intervals.
Nephrotoxicity.
Over the first 3 months, renal function monitoring was performed a median of 19 times (IQR, 14 to 25 times), and over months 4 to 6, renal function monitoring was performed a median of 9 times (IQR, 4 to 15 times).
Raised creatinine levels.
Eighty-five cases had a complete set of creatinine blood results. Twenty-five percent (21/85) of the patients had a rise in the creatinine blood level from the baseline level of 1.5 times or more; of these 85 patients, 3 (3.5%) had a rise from the baseline level of 3 times. The creatinine concentration returned to the baseline level (less than 1.5 times the normal level) in 19 of 21 cases: in 16 before the end of treatment with the injectable and in 3 before the end of MDR-TB treatment. Among the patients in whom the creatinine concentration did not return to the baseline level, one required hemodialysis after amikacin was stopped (he already had chronic kidney disease at the start of therapy for MDR-TB and a baseline creatinine level of 313 μmol/liter, which peaked at 846 μmol/liter), and the other died because of advanced HIV infection (CD4 cell count = 5). A multivariable model including the baseline creatinine concentration, the duration of treatment with the injectable agent, and the choice of injectable agent at the start of treatment showed that there was no significant difference in the odds of a raised creatinine concentration between the two injectable agents chosen at the start of treatment (P = 0.178) when adjusted for the total duration of treatment. However, some evidence suggests that an increasing duration of treatment may increase the odds of a raised creatinine concentration; i.e., an increase in the creatinine concentration at 30 days of treatment is associated with a 15% (95% CI, 25% to 32%) increase in the odds of raised creatinine levels (P = 0.04) (Table 4).
TABLE 4.
Multivariable model for creatinine concentration rise to over 1.5 times the baseline level and hypokalemia
| Variable | Multivariable model for creatinine concn rise of >1.5 times baseline |
Multivariable model for hypokalemia |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Baseline creatinine concn | 1.02 (0.99–1.05) | 0.145 | ||
| Amikacin vs capreomycin at start | 0.44 (0.14–1.45) | 0.178 | 0.28 (0.11–0.72) | 0.008 |
| Total duration (effect at 30 days) | 1.15 (1.02–1.32) | 0.040 | 1.00 (0.91–1.08) | 0.869 |
Electrolyte disturbance.
Eighty-six patients had a complete set of potassium concentration results; 37 of these started treatment for MDR-TB with capreomycin, and 49 started treatment with amikacin. Hypokalemia was found in 38 of 86 (44%) patients while they were being treated with an injectable agent: 23 of 38 (61%) were treated with capreomycin and 15 of 38 (39%) were treated with amikacin. Eighteen of 38 cases resolved alone, without potassium replacement. Seventeen required replacement with oral potassium (13 of these 17 patients were treated with capreomycin), 7 required replacement with intravenous potassium (all of these patients were treated with capreomycin), 4 had their dose reduced to 3 times per week (all of these patients were treated with capreomycin), and 3 required a switch in injectable agent (all of these patients were treated with capreomycin and then switched to amikacin). A multivariable model including the duration of treatment with an injectable agent and the initial injectable agent indicated that the odds of hypokalemia were approximately 4 times lower in those starting treatment with amikacin than for those starting treatment with capreomycin (odds ratio, 0.28; 95% CI, 0.11 to 0.72) (Table 4).
Regular testing for magnesium was performed for 15 of the patients receiving capreomycin and none of the patients receiving amikacin. Thirteen of the 15 patients were hypomagnesemic (11/13 had a reading below 0.5 mmol/liter), and 10 of these patients were treated with oral replacement, 9 were treated with intravenous replacement, and 4 required a switch to amikacin (3 of these also had reduced potassium levels and are inclusive of the 3 patients who required a switch in injectable agent because of hypokalemia mentioned above). One stopped treatment with the injectable earlier than planned due to hypomagnesemia.
A switch from capreomycin to amikacin or the early cessation of capreomycin treatment because of an electrolyte disturbance occurred in 5 of 37 patients at a median of 132 days (range, 53 to 207 days; n = 5). Of the four cases whose treatment was switched from capreomycin to amikacin, one subsequently suffered ototoxicity while receiving amikacin.
DISCUSSION
We present data showing that ototoxicity is very frequent and that in our cohort a third of patients do not reach the original 2008 WHO treatment guideline advising at least 6 months of treatment with an injectable agent. Even fewer reach the newer target of 8 months for the intensive phase. In a subcohort analysis, capreomycin was associated with less ototoxicity and/or hearing loss than amikacin, though its use was sometimes limited by electrolyte disturbances. Those starting capreomycin were also able to tolerate the injectable treatment for much longer.
The incidence of hearing loss during MDR-TB treatment is reported to be anywhere between 4.4% (1, 11) and 62% (12, 13), depending on the treatment duration, drug choice, dose (6), and type of monitoring. Studies with a clinical definition (in which patients report their symptoms) show lower levels than those with an audiogram-based definition (14), and the majority of studies have been performed in the presence of aminoglycosides, amikacin, or kanamycin (15 mg/kg of body weight/day). Kanamycin is closely related to amikacin and is more commonly used worldwide. The level of ototoxicity of 55% found in our study is similar to the findings of others using intense monitoring and aminoglycosides at 15 mg/kg (7, 12, 13, 15, 16). A retrospective cohort analysis suggests that the use of kanamycin is associated with less ototoxicity than the use of amikacin (16).
Few recent studies of capreomycin treatment of MDR-TB have investigated the hearing loss associated with capreomycin, possibly as its cost and the need for electrolyte monitoring put it out of reach for many low-income countries. However, although clearly defined methods for monitoring are not always described, there is a suggestion that the levels of hearing loss are lower for capreomycin, with the proportions of patients affected ranging from 0.7% to 25% (6, 17–20). Studies comparing amikacin to capreomycin have been limited to a small retrospective study conducted by our group that showed by univariate analysis that hearing loss is more often associated with amikacin use than capreomycin use (10) and a pharmacovigilance reporting study showing that spontaneous reports of deafness are disproportionately associated with amikacin, followed by kanamycin, than capreomycin (21). Our present study had larger numbers of patients than our earlier study and is not limited by reporting bias and other issues inherent in pharmacovigilance reporting. The main limitation of our study arises from the different audiogram policies at the various study sites. In the hearing loss (composite) analysis, there is the possibility that hearing loss caused by capreomycin was underestimated due to asymptomatic cases, in whom ototoxicity was less likely to be identified (ascertainment bias) than in the amikacin group, for whom audiograms were more routinely performed. However, to counter this possible bias, we performed the ototoxicity analysis by including in the denominators only those who had had an audiogram within a month of ending treatment with the injectable agent. Although the numbers of patients in that analysis was smaller than the total number of patients in the study, in this analysis, the possible bias works in the opposite direction because patients at the capreomycin treatment sites for whom audiograms were performed were more likely to be those with a perceived risk of ototoxicity. These issues probably account for the difference between the hazard ratio for the ototoxicity outcome (which was 5 times more likely with amikacin) and that for the composite hearing loss outcome (which was 15 times more likely with amikacin), and the real value may lie between the two numbers. We also consider that the character as well as the likelihood of occurrence of hearing loss can differ with capreomycin. The evidence for this suggestion is that the audiograms of the two patients who experienced ototoxicity during treatment with capreomycin did not display a progressive hearing loss, despite ongoing exposure (because of a lack of alternative drugs), which would be extremely unlikely for amikacin (8). However, further investigations on the type and degree of hearing loss caused by capreomycin in a randomized controlled trial are required. Reducing the proportions of patients experiencing hearing loss during treatment with amikacin may be possible with lower doses (7.5 mg/kg) and monitoring of the area under the concentration-time curve (22). However, the efficacy of this dose is unclear, and the use of this dose is not currently recommended. Other possibilities include the coadministration of N-acetylcysteine or other antioxidants (23), genetic testing for mutations in the mitochondrial gene encoding 12S rRNA (MT-RNR1), and avoiding aminoglycosides in patients with these mutations (24–26), though the prevalence of these mutations is low.
However, our findings support the initial use of capreomycin over amikacin as a means of reducing hearing loss. First-line capreomycin use has also been advocated when onward resistance patterns are considered; amikacin activity is often spared after the evolution of capreomycin resistance but not the other way round (27, 28). The disadvantage of capreomycin is the associated electrolyte disturbance, which led to the discontinuation or capreomycin or a switch to amikacin in 14% of patients treated with it in our study. Of note, however, electrolyte abnormalities were effectively managed in all patients with no long-term consequences. The association of capreomycin with electrolyte disturbance and renal impairment during treatment for tuberculosis (TB) is well reported (29–31). In settings in which regular rapid and reliable blood monitoring is not feasible, the nephrotoxicity of capreomycin may lead to death due to hypokalemia and renal failure (18, 29). Our data demonstrate that this is not the case in a well-resourced setting.
In summary, we provide evidence from a retrospective cohort study of high levels of ototoxicity and hearing loss in a cohort in the United Kingdom being treated for MDR-TB. Hearing loss was 14 times more likely with amikacin than capreomycin, while capreomycin was associated with electrolyte disturbance, leading to the cessation of treatment with the drug in 14% of those treated with it. Given the significance and the irreversibility of the hearing loss, in settings where blood monitoring is possible, we would favor starting with capreomycin rather than amikacin, until such time as short-course and injectable drug-free regimens incorporating the newer drugs have been shown to be effective (11).
MATERIALS AND METHODS
Setting.
Retrospective data were collected through clinical records and hospital database review at four tuberculosis (TB) treatment centers in the United Kingdom: St. Mary's Hospital, Imperial College NHS Trust, London (site 1); Heartlands Hospital, Birmingham (site 2); the Royal Free Hospital, London (site 3); and St. George's Hospital, London (site 4). These centers act as regional referral hubs for MDR-TB treatment. Data were also collected at referring hospitals if patients were treated under a shared care model. Standard definitions were used for MDR-TB, extensively drug resistant tuberculosis (XDR-TB), pulmonary TB (PTB), and extrapulmonary tuberculosis (EPTB) (32), and treatment was based on WHO guidelines (2, 3). At sites 1 and 2, amikacin was the preferred injectable agent, site 3 used a mix, and site 4 predominantly used capreomycin. All sites administered the drugs intravenously. All sites switched the injectable treatment used at the physician's discretion. All injectable agents were dosed initially at 15 mg/kg once a day, with trough drug levels for amikacin being determined at least weekly. A reduced frequency of dosing was used if side effects occurred. A duration of treatment of 6 months or more was defined as treatment for over 160 days, and a duration of treatment of 8 months or more was defined as treatment for over 220 days.
Study population and eligibility criteria.
The medical records of the first 100 consecutive patients over 14 years of age who had received a diagnosis of MDR-TB made in the United Kingdom and who initiated MDR-TB treatment at the four sites between 2008 and 2014 were reviewed. Seven patients were excluded due to a lack of injectable agent use (n = 2), streptomycin use at the start of treatment (n = 2), and over three initiations of treatment with medications for MDR-TB (n = 3). The cohort was split into two according to the date of treatment start (the 51st patient started treatment in spring 2011), which corresponded to the change in WHO advice regarding the duration of treatment with an injectable.
Renal function monitoring.
To be included in the analysis of renal function, patients required at least weekly blood results available for review. Renal impairment was defined as mild if the creatinine concentration was 1.5 times the baseline creatinine concentration and severe it was over 3 times the baseline creatinine concentration (33). Hypokalemia was defined as any drop in the potassium level to below 3.5 mmol/liter (29). Hypomagnesemia was defined as any magnesium concentration measurement below 0.7 mmol/liter (33).
Audiological monitoring.
All patients underwent pure-tone audiometry (PTA), performed to the standards of the British Society of Audiology (34), at the start of injectable therapy. All sites performed PTA if hearing loss, a change in symptoms, or any concern about hearing arose during treatment, and sites 1, 2, and 3 had, in addition, a policy of the monthly performance of PTA (limited by patient adherence to the protocol). Site 3 performed audiograms at frequencies above 9 to 20 kHz for a portion of the study period. Significant deterioration between audiograms was determined by use of the criteria of the American Speech-Language-Hearing Association (ASHA), which were as follows for the frequencies of between 250 Hz and 8 kHz tested: (i) a 20-dB decrease at any one test frequency, (ii) a 10-dB decrease at any two adjacent frequencies, and (iii) a loss of a response at any three adjacent frequencies at which responses were previously obtained (15). Two endpoints relating to hearing were chosen: an audiogram definition (ototoxicity) and a composite definition encompassing audiogram results and clinically reported hearing loss, referred to as hearing loss (composite) (Table 2). Patient-reported hearing impairment was defined as any report by the patient of a negative change in hearing while receiving injectable agents or after stopping treatment with the injectable, as documented by a nurse or doctor. Tinnitus was defined as any symptoms reported by the patient that were interpreted to be tinnitus by a doctor or nurse and documented in the records. The reasons for stopping injectable agents were collated from the medical notes according to what was written by the consultant in charge of treatment.
Statistics.
Patients were grouped according to the injectable agent that they were exposed to: (i) capreomycin only, (ii) amikacin to start (this group includes those treated only with amikacin and those treated with amikacin and switched to capreomycin or streptomycin because hearing loss was the main driver of this switch), and (iii) capreomycin and then a switch to amikacin (none of the patients was switched due to hearing loss). Hearing loss was analyzed within survival settings using Cox proportional hazard models, modeling the time since the treatment start time to the time of hearing loss. Raised creatinine concentrations and hypokalemia were investigated using logistic regression. Univariate analyses were initially undertaken and included all variables collected (age, gender, baseline creatinine concentration, baseline creatinine clearance [Cockroft-Gault equation], dose of drug, MDR-TB type, number of amikacin trough concentration determinations, treatment site, and amikacin and capreomycin group). Associations with resulting P values of less than 0.1 were further considered to form multivariable/adjusted models based on similar numbers of complete observations. Model selection was undertaken by choosing the most parsimonious model using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). The final models were further refined using multiple imputation methodologies, assuming the missing at random model to account for approximately 15% of the original data that were missing (35). Further details on statistical methodologies are given in Text S1 in the supplemental material. Stata software was employed for data analyses (Stata statistical software, release 14, 2015; StataCorp, College Station, TX).
Ethics.
The study was deemed by the NHS Ethics Board (NRES Committee London—City and East) to be a service evaluation. Consent for access to clinical records for review was given by the Confidentiality Advisory Group (GAG). The data were anonymized on-site for off-site analyses. Data sharing with public health was according to Caldicott principles.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Maria Mercer and Vera Pavlova (Respiratory Medicine, St. George's Hospital, London, United Kingdom), Marie O. Donoghue (Division of Medicine, Imperial College Hospital NHS Trust, London, United Kingdom), Angelita Solamalai (Respiratory Medicine, Royal Free Hospital, London, United Kingdom), Veronica White (Respiratory Medicine, Royal London NHS Trust, London, United Kingdom), and Lucy Baker (Respiratory Medicine, University Hospital Lewisham, Lewisham, United Kingdom).
This work was supported by St. George's Healthcare NHS Trust and The Jefferiss Charitable Trust (no grant number). G.S.C. is supported by BRC of Imperial College NHS Trust.
We have all completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare that A.A. has received research studentship funding from The Jefferiss Charitable Trust (no grant number) and P.B. is funded by Innovate UK (a UK government agency) in collaboration with QuantuMDx Ltd. No further information is declared.
A.A., T.S.H., M.D., G.S.C., O.M.K., A.L., and P.D.B. contributed to the design of the work. The acquisition of data was undertaken by A.A., M.D., O.M.K., M.L., and A.L. I.C.S. undertook statistical analysis with A.A. and T.S.H. All authors contributed to data interpretation and drafting of the manuscript and are responsible for the content.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02586-16.
REFERENCES
- 1.Wu S, Zhang Y, Sun F, Chen M, Zhou L, Wang N, Zhan S. 2013. Adverse events associated with the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Am J Ther 23:e521–e530. doi: 10.1097/01.mjt.0000433951.09030.5a. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.World Health Organization. 2011. Guidelines for the programmatic management of drug-resistant tuberculosis—2011 update. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 4.World Health Organization. 2016. The WHO treatment guidelines for drug-resistant tuberculosis (2016 update). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Becerra MC, Benedetti A, Burgos M, Centis R, Chan ED, Chiang CY, Cox H, D'Ambrosio L, DeRiemer K, Dung NH, Enarson D, Falzon D, Flanagan K, Flood J, Garcia-Garcia ML, Gandhi N, Granich RM, Hollm-Delgado MG, Holtz TH, Iseman MD, Jarlsberg LG, Keshavjee S, Kim HR, Koh WJ, Lancaster J, Lange C, de Lange WC, Leimane V, Leung CC, Li J, Menzies D, Migliori GB, Mishustin SP, Mitnick CD, Narita M, O'Riordan P, Pai M, Palmero D, Park SK, Pasvol G, Peña J, Pérez-Guzmán C, Quelapio MI, Ponce-de-Leon A. 2012. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med 9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duggal P, Sarkar M. 2007. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear Nose Throat Disord 7:5. doi: 10.1186/1472-6815-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melchionda V, Wyatt H, Capocci S, Garcia Medina R, Solamalai A, Katiri S, Hopkins S, Cropley I, Lipman M. 2013. Amikacin treatment for multidrug resistant tuberculosis: how much monitoring is required? Eur Respir J 42:1148–1150. doi: 10.1183/09031936.00184312. [DOI] [PubMed] [Google Scholar]
- 8.Seddon JA, Godfrey-Faussett P, Jacobs K, Ebrahim A, Hesseling AC, Schaaf HS. 2012. Hearing loss in patients on treatment for drug-resistant tuberculosis. Eur Respir J 40:1277–1286. doi: 10.1183/09031936.00044812. [DOI] [PubMed] [Google Scholar]
- 9.Seddon JA, Godfrey-Faussett P, Hesseling AC, Gie RP, Beyers N, Schaaf HS. 2012. Management of children exposed to multidrug-resistant Mycobacterium tuberculosis. Lancet Infect Dis 12:469–479. doi: 10.1016/S1473-3099(11)70366-8. [DOI] [PubMed] [Google Scholar]
- 10.Sturdy A, Goodman A, Jose RJ, Loyse A, O'Donoghue M, Kon OM, Dedicoat MJ, Harrison TS, John L, Lipman M, Cooke GS. 2011. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother 66:1815–1820. doi: 10.1093/jac/dkr221. [DOI] [PubMed] [Google Scholar]
- 11.Nunn AJ, Rusen I, Van Deun A, Torrea G, Phillips PP, Chiang C-Y, Squire SB, Madan J, Meredith SK. 2014. Evaluation of a standardized treatment regimen of anti-tuberculosis drugs for patients with multi-drug-resistant tuberculosis (STREAM): study protocol for a randomized controlled trial. Trials 15:353. doi: 10.1186/1745-6215-15-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modongo C, Sobota RS, Kesenogile B, Ncube R, Sirugo G, Williams SM, Zetola NM. 2014. Successful MDR-TB treatment regimens including amikacin are associated with high rates of hearing loss. BMC Infect Dis 14:542. doi: 10.1186/1471-2334-14-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris T, Bardien S, Schaaf HS, Petersen L, De Jong G, Fagan JJ. 2012. Aminoglycoside-induced hearing loss in HIV-positive and HIV-negative multidrug-resistant tuberculosis patients. S Afr Med J 102:363–366. doi: 10.7196/SAMJ.4964. [DOI] [PubMed] [Google Scholar]
- 14.Ramma LD, Ibekwe TS. 2012. Efficacy of utilising patient self-report of auditory complaints to monitor aminoglycoside ototoxicity. Int J Tuberc Lung Dis 16:283. doi: 10.5588/ijtld.11.0712. [DOI] [PubMed] [Google Scholar]
- 15.American Speech-Language-Hearing Association. 1994. Audiologic management of individuals receiving cochleotoxic drug therapy (guideline). American Speech-Language-Hearing Association, Rockville, MD. [Google Scholar]
- 16.Sagwa EL, Ruswa N, Mavhunga F, Rennie T, Leufkens HGM, Mantel-Teeuwisse AK. 2015. Comparing amikacin and kanamycin-induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort. BMC Pharmacol Toxicol 16:36. doi: 10.1186/s40360-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donomae I. 1966. Capreomycin in the treatment of pulmonary tuberculosis. Ann N Y Acad Sci 135:1011–1038. doi: 10.1111/j.1749-6632.1966.tb45543.x. [DOI] [PubMed] [Google Scholar]
- 18.Shean K, Streicher E, Pieterson E, Symons G, van Zyl Smit R, Theron G, Lehloenya R, Padanilam X, Wilcox P, Victor TC, van Helden P, Grobusch MP, Warren R, Badri M, Dheda K. 2013. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One 8:e63057. doi: 10.1371/journal.pone.0063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JD, Popplewell AG, Landwehr A, Greene ME. 1966. Toxicology studies in patients on prolonged therapy with capreomycin. Ann N Y Acad Sci 135:1047–1056. doi: 10.1111/j.1749-6632.1966.tb45545.x. [DOI] [PubMed] [Google Scholar]
- 20.Kass I. 1965. Chemotherapy regimens used in retreatment of pulmonary tuberculosis. Part II. Observations on the efficacy of combinations of ethambutol, capreomycin and companion drugs, including 4-4 diisoamyloxythiosemicarbanilide. Tubercle 46:166–177. [DOI] [PubMed] [Google Scholar]
- 21.Sagwa EL, Souverein PC, Ribeiro I, Leufkens HG, Mantel-Teeuwisse AK. 2016. Differences in VigiBase(R) reporting of aminoglycoside and capreomycin-suspected ototoxicity during tuberculosis treatment. Pharmacoepidemiol Drug Saf 26:1–8. doi: 10.1002/pds.4125. [DOI] [PubMed] [Google Scholar]
- 22.van Altena R, de Vries G, Haar C, de Lange W, Magis-Escurra C, van den Hof S, van Soolingen D, Boeree M, van der Werf T. 2015. Highly successful treatment outcome of multidrug-resistant tuberculosis in the Netherlands, 2000-2009. Int J Tuberc Lung Dis 19:406–412. doi: 10.5588/ijtld.14.0838. [DOI] [PubMed] [Google Scholar]
- 23.Kranzer K, Elamin WF, Cox H, Seddon JA, Ford N, Drobniewski F. 2015. A systematic review and meta-analysis of the efficacy and safety of N-acetylcysteine in preventing aminoglycoside-induced ototoxicity: implications for the treatment of multidrug-resistant TB. Thorax 70:1070–1077. doi: 10.1136/thoraxjnl-2015-207245. [DOI] [PubMed] [Google Scholar]
- 24.Hutchin T, Haworth I, Higashi K, Fischel-Ghodsian N, Stoneking M, Saha N, Arnos C, Cortopassi G. 1993. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res 21:4174–4179. doi: 10.1093/nar/21.18.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bindu LH, Reddy PP. 2008. Genetics of aminoglycoside-induced and prelingual non-syndromic mitochondrial hearing impairment: a review. Int J Audiol 47:702–707. doi: 10.1080/14992020802215862. [DOI] [PubMed] [Google Scholar]
- 26.Bardien S, Human H, Harris T, Hefke G, Veikondis R, Schaaf HS, van der Merwe L, Greinwald JH, Fagan J, de Jong G. 2009. A rapid method for detection of five known mutations associated with aminoglycoside-induced deafness. BMC Med Genet 10:2. doi: 10.1186/1471-2350-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukamura M. 1969. Cross-resistance relationships between capreomycin, kanamycin, and viomycin resistances in tubercle bacilli from patients. Am Rev Respir Dis 99:780–782. [DOI] [PubMed] [Google Scholar]
- 28.Caminero JA, Sotgiu G, Zumla A, Migliori GB. 2010. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 10:621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 29.Shin S, Furin J, Alcántara F, Hyson A, Joseph K, Sánchez E, Rich M. 2004. Hypokalemia among patients receiving treatment for multidrug-resistant tuberculosis. Chest 125:974–980. doi: 10.1378/chest.125.3.974. [DOI] [PubMed] [Google Scholar]
- 30.Holmes AM, Hesling CM, Wilson TM. 1970. Capreomycin-induced serum electrolyte abnormalities. Thorax 25:608–611. doi: 10.1136/thx.25.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aquinas M, Citron KM. 1972. Rifampicin, ethambutol and capreomycin in pulmonary tuberculosis, previously treated with both first and second line drugs: the results of 2 years chemotherapy. Tubercle 53:153–165. doi: 10.1016/0041-3879(72)90012-8. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 2014. 2013. Definitions and reporting framework for tuberculosis, revision (updated December (2014). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 33.U.S. Department of Health and Human Services. 2010. Common terminology criteria for adverse events, version 4.03. U.S. Department of Health and Human Services, Bethesda, MD: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Accessed 28 November 2016. [Google Scholar]
- 34.British Society of Audiology. 2011. Pure tone air and bone conduction threshold audiometry with and without masking and determination of uncomfortable loudness levels. British Society of Audiology, Reading, United Kingdom. [Google Scholar]
- 35.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. 2009. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.