ABSTRACT
Macrolide antibiotics are used as anti-inflammatory agents, e.g., for prevention of exacerbations in chronic obstructive pulmonary disease and cystic fibrosis. Several studies have shown improved outcomes after the addition of macrolides to β-lactam antibiotics for treatment of severe community-acquired pneumonia. However, a beneficial effect of macrolides in treating Gram-negative bacterial airway infections, e.g., those caused by Pseudomonas aeruginosa, remains to be shown. Macrolide antibiotics have significant side effects, in particular, motility-stimulating activity in the gastrointestinal tract and promotion of bacterial resistance. In this study, EM703, a modified macrolide lacking antibiotic and motility-stimulating activities but with retained anti-inflammatory properties, was used as an adjunct treatment for experimental P. aeruginosa lung infection, in combination with a conventional antibiotic. Airway infections in BALB/cJRj mice were induced by nasal instillation of P. aeruginosa; this was followed by treatment with the quinolone levofloxacin in the absence or presence of EM703. Survival, inflammatory responses, and cellular influx to the airways were monitored. Both pretreatment and simultaneous administration of EM703 dramatically improved survival in levofloxacin-treated mice with P. aeruginosa airway infections. In addition, EM703 reduced the levels of proinflammatory cytokines, increased the numbers of leukocytes in bronchoalveolar lavage fluid, and reduced the numbers of neutrophils present in lung tissue. In summary, the findings of this study show that the immunomodulatory properties of the modified macrolide EM703 can be important when treating Gram-negative pneumonia, as exemplified by P. aeruginosa infection in this study.
KEYWORDS: macrolide, EM703, host defense, anti-inflammatory, Pseudomonas aeruginosa
INTRODUCTION
Macrolide antibiotics (for example, erythromycin, azithromycin, and clarithromycin) have caught interest for use in the treatment of chronic airway inflammation (1). Reduction of pulmonary exacerbations has been demonstrated in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) (2, 3). Macrolides display broad biological responses, including bacteriostatic activity, stimulation of gastrointestinal motility, and anti-inflammatory properties (4–6). Each of these effects could be advantageous, depending on the clinical context; however, promotion of drug-resistant bacteria and diarrhea limit the clinical use of macrolides as anti-inflammatory agents.
In severe community-acquired pneumonia, which is often caused by the Gram-positive coccus Streptococcus pneumoniae, meta-analyses have shown that the addition of a macrolide antibiotic to a β-lactam has beneficial effects, reducing mortality rates (7–9). Pneumonia caused by Gram-negative bacteria, in particular, Pseudomonas aeruginosa, shows increased incidence in immunocompromised individuals (10–13). Recently, a retrospective study failed to show that macrolide treatment of P. aeruginosa airway infections during the first 48 h reduced mortality rates (14). In support of the idea that macrolides may have beneficial (possibly nonantibiotic) effects, a retrospective cohort study showed that macrolide use was associated with reduced mortality rates in patients with severe sepsis due to pneumonia and macrolide-resistant pathogens (15). Thus, it has not been clearly demonstrated that macrolides may have a role in improving the outcomes of P. aeruginosa airway infections.
Several explanations for the anti-inflammatory effect of macrolides have been proposed, including inhibition of proinflammatory cytokine production, reduced expression of adhesion molecules on neutrophils, and decreased production of mucus in the airways (16–18). In recent years, modified derivatives of erythromycin have been developed to circumvent unwanted side effects. The antimicrobial activity of erythromycin is highly dependent on the configuration of its lactone ring, and cleavage of the ring by an esterase attenuates the antibacterial activity (19). EM703 is a contracted 12-membered derivative of erythromycin with neither antibacterial nor motility-stimulating activity but with strong anti-inflammatory properties (6); this may also reduce the risk of promoting bacterial resistance. Previous studies have demonstrated potent anti-inflammatory effects of EM703 in a model of bleomycin-induced pulmonary fibrosis and oxidative stress in bronchial epithelial cells, caused by diesel exhaust particles (20, 21). This study set out to investigate the effect of EM703 as an adjunct anti-inflammatory treatment for experimental P. aeruginosa airway infections, in combination with a conventional antibiotic (i.e., levofloxacin).
RESULTS
EM703 improved survival rates in a model of levofloxacin-treated P. aeruginosa airway infection.
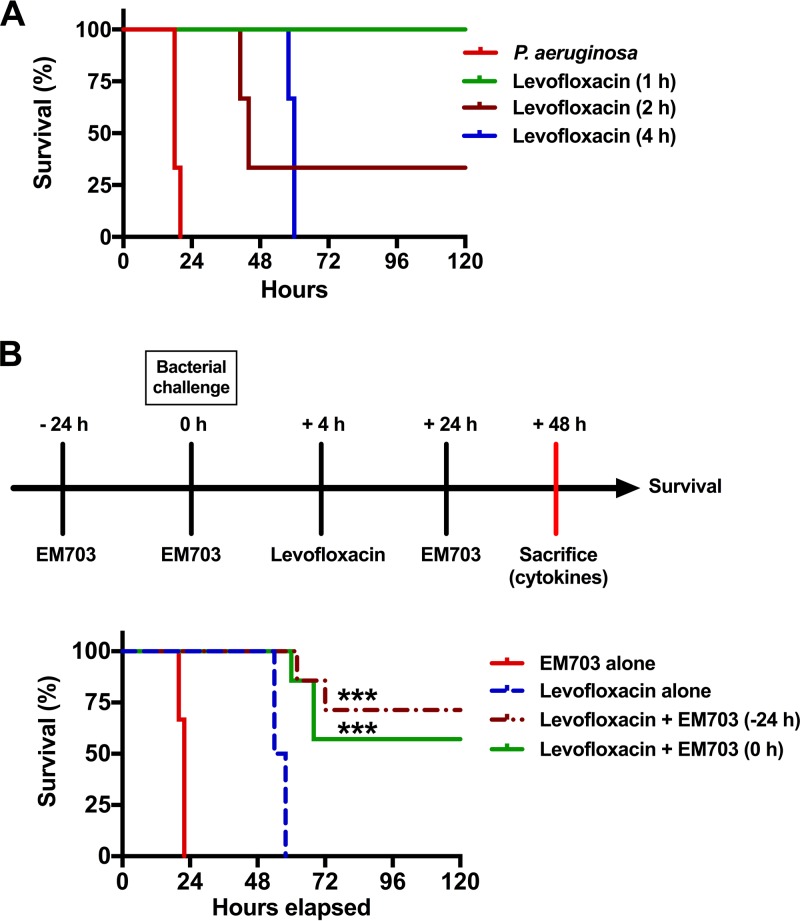
To investigate the possible beneficial anti-inflammatory effects of EM703 in combination with conventional quinolone treatment for P. aeruginosa airway infections, an animal model was developed. Mice were inoculated intranasally with P. aeruginosa (2 × 109 CFU/animal), followed by treatment with levofloxacin (100 mg/kg, subcutaneously) 1, 2, or 4 h after infection (three mice in each group, repeated in three separate experiments) (Fig. 1A). Based on the literature, a levofloxacin dose of 100 mg/kg was chosen to rapidly achieve complete bacterial killing and resulting high levels of release of bacterial components (22–25). The MIC of levofloxacin against P. aeruginosa (strain PAO1) is 0.25 μg/ml. Levofloxacin treatment 1 h postinfection completely cleared the bacterial load and rescued the mice, whereas treatment after 2 h resulted in a mortality rate of 30%. Mice that received treatment 4 h postinfection all died within 72 h (Fig. 1A). To confirm the eradication of bacteria, viable counts were performed with lung homogenates from mice treated with levofloxacin 4 h postinfection. The animals were sacrificed 48 h after the initiation of infection. No bacteria were detected, i.e., the number of CFU was below the limit of detection (<10 CFU/ml for samples diluted 1:10) (data not shown). This demonstrated that the time elapsed between the start of infection and treatment was important. In addition, the outcome was not related to bacterial eradication itself, suggesting involvement of a dysregulated host response.
FIG 1.
Survival times for P. aeruginosa airway infections with combined treatment with levofloxacin and EM703. (A) Survival graph. The survival of levofloxacin-treated mice infected with P. aeruginosa (2 × 109 bacterial CFU) was monitored for 7 days (only the course during the first 120 h is shown). Levofloxacin (100 mg/kg) was administered subcutaneously 1, 2, or 4 h after infection. The graph shows one representative experiment of three, with three mice in each group. (B) Schematic overview of the experimental design and time points (upper), using levofloxacin to treat P. aeruginosa-infected mice in the absence or presence of EM703, and survival graph (lower) showing the effect of EM703 treatment on the survival of infected mice treated with levofloxacin (seven mice in each group, in two separate experiments). Treatment with EM703 (either 24 h prior to or simultaneously with infection) in combination with levofloxacin (initiated 4 h after infection) improved survival. EM703 alone did not affect the survival of infected mice that did not receive levofloxacin. Statistical comparisons of survival curves were performed using the Mantel-Cox test, comparing the group treated with levofloxacin alone and the groups treated with a combination of levofloxacin and EM703. ***, P ≤ 0.001.
In an effort to improve the survival of levofloxacin-treated mice, the nonantibiotic (i.e., lacking antibacterial activity) and anti-inflammatory macrolide EM703 was administered orally (25 mg/kg). The dose of EM703 was chosen based on previous reports using this compound and related macrolides to achieve anti-inflammatory activity (20, 26, 27). To determine whether there was a time-dependent effect of EM703 treatment in relation to the initiation of infection, two different time points were chosen. Thus, mice were treated with EM703 either 24 h before infection (−24 h) or simultaneously with infection (0 h) and the drug was then administered once at 24 h (Fig. 1B, upper). Levofloxacin treatment was given 4 h after the instillation of bacteria. The addition of EM703, preceding or simultaneously with infection, in combination with levofloxacin improved the survival rate significantly (seven mice in each group, in two separate experiments; P = 0.009 at both −24 and 0 h with EM703) (Fig. 1B, lower). However, mice treated with levofloxacin alone or EM703 alone survived for less than 60 h and 24 h, respectively. The latter result was similar to findings observed for untreated mice (Fig. 1A).
Treatment with EM703 increased the numbers of immune cells in bronchoalveolar lavage fluid.
We next assessed whether the level of cellular immunity was affected by the addition of EM703. Following treatment of P. aeruginosa-infected mice with levofloxacin alone (at 4 h postinfection) in combination with EM703 (at −24 or 0 h) or vehicle, the mice were euthanized and bronchoalveolar lavage (BAL) fluid was collected (10 mice in each group, in two separate experiments). EM703 treatment combined with levofloxacin treatment caused a significant increase in the total number of cells (P = 0.0002 for EM703 added at −24 h and P = 0.0381 for EM703 added at 0 h), compared to treatment with levofloxacin alone (Table 1). Differential counts showed that the numbers of both neutrophils and macrophages were significantly elevated (P = 0.0002 and P < 0.0001, respectively) in mice pretreated with EM703 at 24 h before infection, with administration of levofloxacin 4 h later (Table 1).
TABLE 1.
Total and differential cell counts in bronchoalveolar fluid
| Treatment | Total cells |
Macrophages |
Neutrophils |
Eosinophils |
Lymphocytes |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells/ml (mean ± SEM) | Pa | Cells/ml (mean ± SEM) | P | Cells/ml (mean ± SEM) | P | Cells/ml (mean ± SEM) | P | Cells/ml (mean ± SEM) | P | |
| Levofloxacin alone | 5.0 × 105 ± 9.0 × 104 | 3.9 × 104 ± 8.2 × 103 | 4.5 × 105 ± 8.1 × 104 | 8.5 × 103 ± 3.6 × 103 | 1.3 × 104 ± 3.4 × 103 | |||||
| Levofloxacin plus EM703 at −24 h | 1.1 × 106 ± 1.1 × 105 | 0.0002 | 4.2 × 105 ± 7.9 × 104 | <0.0001 | 9.6 × 105 ± 8.8 × 104 | 0.0002 | 1.1 × 104 ± 4.3 × 103 | 0.9834 | 2.1 × 104 ± 5.8 × 103 | >0.9999 |
| Levofloxacin plus EM703 at 0 h | 9.0 × 105 ± 1.1 × 105 | 0.0381 | 1.6 × 105 ± 3.6 × 104 | 0.0263 | 7.4 × 105 ± 1.0 × 105 | 0.0544 | 1.2 × 104 ± 4.0 × 103 | >0.9999 | 1.4 × 104 ± 2.7 × 103 | 0.5298 |
P values were calculated using levofloxacin alone as a control.
Reduced lung damage and tissue infiltration of neutrophils were noted after the addition of EM703.
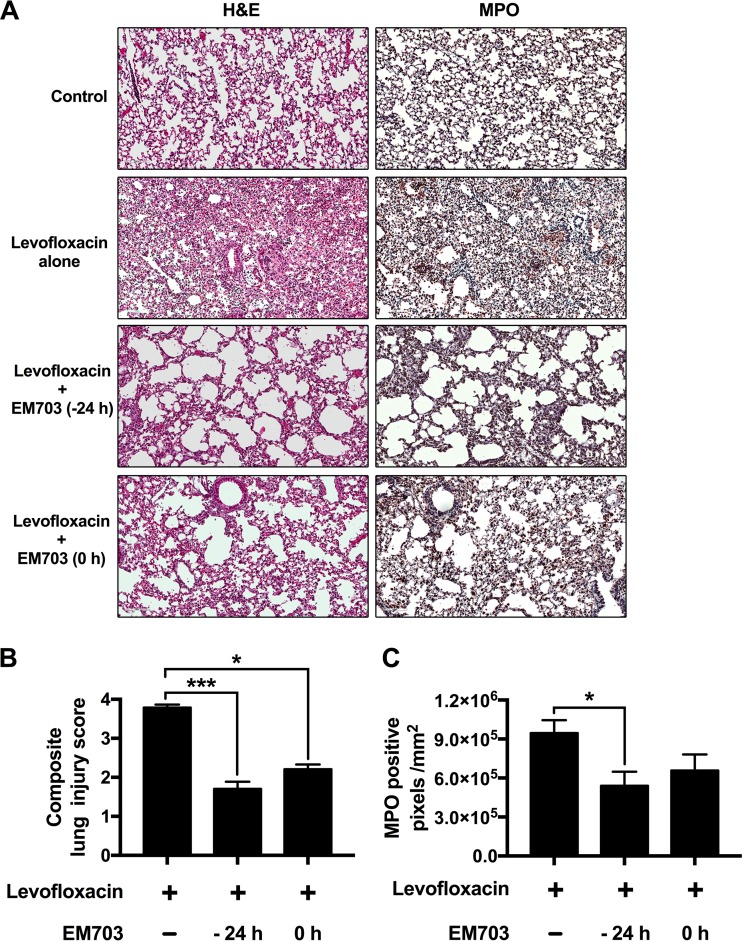
Lung morphology was investigated, comparing mice treated with vehicle alone, infected mice treated with levofloxacin alone, and mice treated with a combination of EM703 (24 h prior to or simultaneously with infection) and levofloxacin (eight mice in each group, in two separate experiments) (Fig. 2A). The addition of EM703 resulted in less dense cellularity in alveoli and in interstitial spaces and less alveolar septal thickening, as determined from hematoxylin and eosin (H&E) staining. Immunohistochemistry for detection of the specific granule protein myeloperoxidase (MPO) in neutrophils revealed an abundance of these cells in mice that were infected with P. aeruginosa and treated with levofloxacin alone (Fig. 2A). Mice pretreated with EM703 (−24 h) and mice treated simultaneously with EM703 (0 h) showed significantly smaller numbers of neutrophils, as detected by morphometric analysis (P = 0.0473) (Fig. 2A and C). An overall estimation of lung tissue damage was performed using a blinded lung injury scoring system in which neutrophils in the alveolar and interstitial spaces, the presence of hyaline membranes, proteinaceous debris filling the airspaces, and alveolar septal thickening were considered at higher magnification, confirming less damage after the addition of EM703 at −24 h (P = 0.0005) and 0 h (P = 0.0499) (Fig. 2B).
FIG 2.
Lung injury and numbers of neutrophils in lung tissue after EM703 pretreatment. (A) To investigate tissue damage and neutrophil infiltration, lung tissues were stained with H&E or processed for immunohistochemistry and stained to detect MPO. Dense infiltration of immune cells, alveolar thickening, and edema were seen in mice treated with levofloxacin alone, while the addition of EM703 (either 24 h before or at the time of infection) resulted in less pronounced changes. A high degree of neutrophil infiltration in mice treated with levofloxacin alone and less infiltration after the addition of EM703 were confirmed by immunohistological detection of MPO. (B) Blinded evaluation of tissue injury was performed using a lung injury scoring system and showed a significant reduction of lung injury after the addition of EM703 to levofloxacin treatment. (C) Quantification of MPO staining was performed with sections of lung tissue from 10 animals in each group. The results are expressed as the mean ± SEM (eight mice in each group). The Kruskal-Wallis nonparametric test, with Dunn's post hoc test for multiple comparisons, was used. *, P ≤ 0.05; ***, P ≤ 0.001.
Aspartate aminotransferase (AST), reflecting possible organ damage (e.g., liver, heart, and skeletal muscle), was measured in plasma (four mice in each group, in one experiment). Interestingly, the addition of EM703 reduced the increased AST levels seen with levofloxacin treatment alone, indicating a protective effect (P = 0.0121 for EM703 added at −24 h and P = 0.0997 for EM703 added at 0 h) (Fig. 3).
FIG 3.
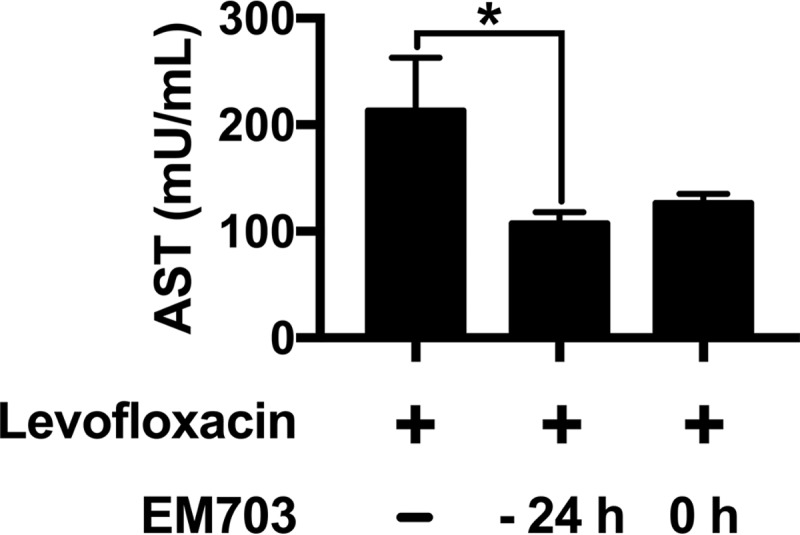
AST levels in plasma during treatment. The effects of different treatments on organ damage were estimated by measuring AST levels in plasma 48 h after bacterial inoculation in mice treated with levofloxacin alone or in combination with EM703. The results are expressed as the mean ± SEM (four mice in each group). The Kruskal-Wallis nonparametric test, with Dunn's post hoc test for multiple comparisons, was used. *, P ≤ 0.05.
EM703 reduced the levels of proinflammatory cytokines and chemokines during levofloxacin-treated P. aeruginosa airway infection.
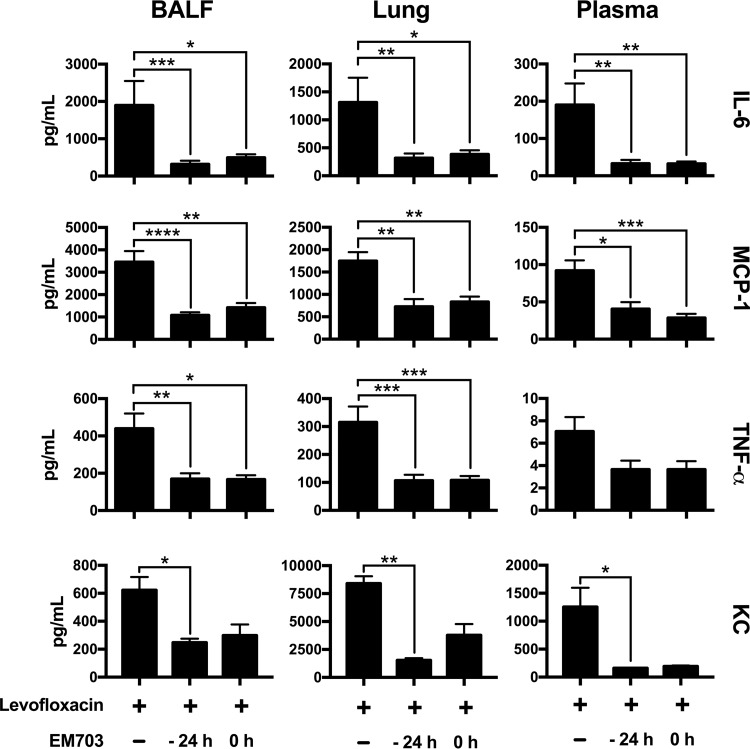
Mice were sacrificed 48 h after bacterial infection (10 mice in each group, in two separate experiments). The levels of key cytokines and chemokines involved in the regulation of inflammation were determined in BAL fluid, lung tissue homogenates, and plasma by using a colorimetric bead assay and an enzyme-linked immunosorbent assay (ELISA) (Fig. 4). Combining EM703 treatment with levofloxacin significantly reduced the levels of the proinflammatory cytokines interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) in BAL fluid and lung homogenates (treated either 24 h before or simultaneously with infection) (Fig. 4). Significant decreases were also observed in plasma but not in the case of TNF-α, IL-10, gamma interferon (IFN-γ), and IL-12-p70 (see Table S1 in the supplemental material). Generally, the effects of EM703 were seen in all compartments investigated, suggesting both systemic effects and effects within the lung.
FIG 4.
Cytokine and chemokine levels in BAL fluid, lung tissue, and plasma during infection. Cytokines and chemokines were measured in BAL fluid (BALF), lung tissue homogenate, and plasma 48 h after bacterial inoculation in mice treated with levofloxacin alone or in combination with EM703. To determine the levels of IL-6, MCP-1, and TNF-α, samples were obtained from 10 animals in each group, in two separate experiments (n = 4 and n = 6). In the case of KC, a separate ELISA was used to investigate samples from four mice in each group, in one experiment. The results are expressed as mean ± SEM. The Kruskal-Wallis nonparametric test, with Dunn's post hoc test for multiple comparisons, was used. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
EM703 has been reported to reduce chemokine production through inhibition of the transcription factor NF-κB. The levels of monocyte chemoattractant protein 1 (MCP-1) and the neutrophil-recruiting chemokine keratinocyte chemoattractant (KC) were significantly reduced when levofloxacin treatment was combined with EM703 (Fig. 4).
DISCUSSION
This study suggests that combining quinolone treatment with a nonantibiotic macrolide, as exemplified by EM703, may improve the outcomes in severe P. aeruginosa airway infections. The anti-inflammatory properties of EM703 are likely to be of major importance, as reflected by decreased levels of proinflammatory cytokines and reduced influx of neutrophils to the airways.
The exaggerated and dysregulated host responses to bacterial toxins are of critical importance during severe infections, and novel interventional strategies are a major focus of interest to improve prognoses (28). The importance of early antibiotic treatment during the course of infection and modulation of the inflammatory response are also illustrated in the model used in this study. In recent years, macrolides have caught increasing attention as anti-inflammatory agents, and several modes of action have been reported to explain these properties, including reduced production of proinflammatory cytokines, inhibited generation of reactive oxygen species (ROS), and increased neutrophil apoptosis (29–35). The development of novel erythromycin-derived macrolides such as EM703, targeting only inflammation and lacking unwanted side effects such as antibacterial activity and gastrointestinal motility-stimulating activity, make this therapeutic strategy more attractive. It was recently shown that EM703 reduces neutrophil generation of ROS, an effect mediated though inhibition of the NF-κB signaling pathway (6). In the present study, several proinflammatory cytokines were detected at reduced levels when levofloxacin treatment was combined with EM703. Adding EM703, either 24 h prior or simultaneously with levofloxacin treatment, resulted in reduced mortality rates and strong anti-inflammatory effects in both cases, indicating a rapid onset of the effects. The reduced levels of IL-6, a key regulator of acute-phase responses, reflected this. In a previous study investigating antibiotic treatment of P. aeruginosa airway infections in mice, high levels of IL-6 were associated with high mortality rates (36). Another significant effect of EM703 was reduction of levels of the proinflammatory chemokine MCP-1, which recruits immune cells and also causes weakness of the diaphragm in P. aeruginosa airway infections (37, 38).
TNF-α mediates many pathophysiological events during sepsis but also is a critical regulator of the innate immune response within the lung (28, 39, 40). Thus, an overwhelming initial TNF-α response may be deleterious, while a reduced later response can cause problems in clearing P. aeruginosa airway infections (41). Therefore, a balanced inflammatory response is of critical importance. The model used in this study reflects the initial phase and the bacteria are eradicated by the levofloxacin treatment, most likely causing extensive release of bacterial toxins. Therefore, reduced levels of TNF-α with the addition of EM703 are likely to be beneficial. The addition of EM703 to levofloxacin treatment also caused a nonsignificant increase of IL-10 levels in lung tissue. This is interesting, since this cytokine has been shown to improve survival and to reduce tissue injury during P. aeruginosa airway infections (42).
As mentioned, EM703 inhibits the activation of NF-κB, a key transcription factor in proinflammatory responses, including the promotion of increased CXC chemokine production, which is important for immune cell recruitment to sites of inflammation (21, 43). Reduced levels of the neutrophil-recruiting chemokine KC after the addition of EM703 were also reflected by lower levels of neutrophils in the lung tissue. However, the parallel increases of both neutrophil and macrophage numbers in BAL fluid when EM703 was added 24 h before levofloxacin treatment may seem paradoxical; this can be explained by the dynamic situation with regard to compartmentalization of neutrophils and macrophages at different stages of inflammation. During the resolution of inflammation, these cells may emigrate to the bronchial lumen as a mechanism of clearance, as described previously (44).
In clinical settings, the coverage of undetected atypical bacteria by macrolides, antibiotic synergy, and the immunomodulatory effects of macrolides have been suggested as explanations for their beneficial effects (17). However, the mouse model used in this study highlights the importance of immunomodulation, since there is no coinfection present, EM703 lacks antibacterial activity, and levofloxacin alone resulted in bacterial eradication.
Mice treated with EM703 at 24 h prior to infection had improved outcomes. This could translate to clinical settings, since many patients suffering from chronic airway inflammation (e.g., CF or COPD) are subject to long-term treatment with macrolides to prevent exacerbations. These patients are also at greater risk of acquiring infections with P. aeruginosa.
In contrast to a clinical setting, there was no chronic airway inflammation (e.g., CF or COPD) or other significant comorbidity (e.g., malignancy or immunosuppression) present in the animals in this study. This is a limitation that warrants further studies. In addition, clinical studies using nonantibiotic macrolides will be necessary to evaluate possible benefits in humans, whose immune responses may be affected differently.
EM703 has not yet been used clinically, and data from studies using classic macrolides in P. aeruginosa infections are limited. In one clinical study, macrolide therapy in the first 48 h after admission was not associated with decreased 30-day mortality rates, intensive care unit (ICU) admissions, need for mechanical ventilation, or lengths of hospital stays for patients with community-acquired P. aeruginosa pneumonia (14). The study was retrospective, and there was much heterogeneity regarding the antibiotics used against P. aeruginosa. In support of using macrolides when treating severe Gram-negative infections, a rabbit model of pyelonephritis caused by multidrug-resistant P. aeruginosa showed increased survival after coadministration of the macrolide clarithromycin and the aminoglycoside amikacin, which was probably attributable to the immunomodulatory properties of clarithromycin, as reflected by decreased levels of TNF-α (45).
Taken together, the results of this study show a possible beneficial role for novel nonantibiotic macrolides as an adjuvant treatment in severe P. aeruginosa airway infections. This strategy may result in improved outcomes, as reflected by reduced mortality rates.
MATERIALS AND METHODS
Animals.
Female BALB/cJRj mice (9 to 10 weeks of age, weighing 18 to 22 g; Janvier Labs, Le Genest-Saint-Isle, France) were maintained under specific-pathogen-free conditions and had free access to commercial chow and water. All mouse experiments were conducted according to institutional guidelines and were approved by the Malmö-Lund Animal Care Ethics Committee, Sweden (entry no. M187-15).
P. aeruginosa inoculation.
P. aeruginosa (strain Xen 41, derived from the parental pleural isolate P. aeruginosa PAO1; PerkinElmer, Waltham, MA) was grown aerobically in Todd-Hewitt (TH) broth at 37°C to the logarithmic growth phase (optical density at 620 nm [OD620] of ∼0.5), harvested, washed in phosphate-buffered saline (PBS), and diluted to 2 × 109 CFU/ml in the same buffer. Each animal was infected with 50 μl of the bacterial solution by intranasal instillation, whereas uninfected control animals received PBS alone. In order to maintain a consistent inoculum throughout the experiments, bacteria at a fixed OD620 value were used to infect the animals, and the CFU were confirmed by plating in every experiment.
Survival studies of levofloxacin-treated P. aeruginosa-infected mice.
The P. aeruginosa-infected animals were treated with levofloxacin (100 mg/kg; Sanofi-Aventis, Bromma, Sweden) by subcutaneous administration at different time points (1, 2, and 4 h) after the intranasal instillation of bacteria. Untreated animals were used as a control group. To standardize the timing of levofloxacin treatment, three separate experiments, with three or four animals in each group, were performed. For evaluation of animal survival, mice showing the defined and approved humane endpoint criteria (immobilization and shaking) were killed by an overdose of isoflurane (Abbott Laboratories, North Chicago, IL) and counted as nonsurvivors.
Macrolide treatment.
EM703 was a gift from Satoshi Omura (Kitasato Institute for Life Sciences and Graduate School of Infection Control Sciences, Kitasato University, Tokyo, Japan). EM703 has low aqueous solubility and therefore was formulated using nontoxic methylcellulose (0.5%) and polyethylene glycol (2.5%) (46–48). To ensure suspension homogeneity, the mixture was sonicated and orally administered to the mice (0.2 ml).
The animals treated with levofloxacin alone (4 h after infection) and control animals received oral administration of vehicle only (polyethylene glycol-methylcellulose; 0.2 ml) 24 h prior to infection or simultaneously with infection and also 24 h after the inoculation of bacteria. The groups treated with EM703 (25 mg/kg) received oral administration of the drug on a daily basis from 24 h prior to or the time of (0 h) inoculation of bacteria until 24 h postinfection (Fig. 1B, upper). The effect of combination treatment using levofloxacin and EM703 on the survival of mice was investigated using seven animals in each group (two experiments, n = 3 and n = 4). In subsequent experiments, to measure cell counts and inflammatory indices, 10 animals were used in each group (two separate experiments, n = 4 and n = 6).
Bronchoalveolar lavage and cell counts.
The mice were euthanized approximately 48 h after infection. BAL was performed with a total volume of 1 ml PBS containing 0.1 mM EDTA. When required, red blood cells were removed by resuspending the BAL fluid cells in 100 μl of lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA [pH 7.2]) for 2 min at room temperature, followed by washing in 1 ml PBS. The total number of cells was then counted and adjusted to cells per milliliter of BAL fluid. For differential counts, cytospin preparations of cells were stained with modified Wright-Giemsa stain (Sigma-Aldrich, St. Louis, MO), and at least 300 cells were counted in each BAL fluid sample.
Collection of lungs for immunohistochemistry and homogenization.
After sacrifice, the left lung lobes were perfused with Histofix (Histolab, Göteborg, Sweden) and submerged in buffered formaldehyde (4%). After dehydration and paraffin embedding, 3-μm sections were generated from the tissue blocks. After rehydration and antigen retrieval, sections were incubated with goat antibodies to murine MPO or preimmune goat IgG (R&D Systems, Abingdon, UK). Following rinsing, bound antibodies were detected using horseradish peroxidase-conjugated secondary rabbit anti-goat IgG antibodies (diluted 1:1,250) and were visualized using 3,3-diaminobenzidine as a chromogen. Some sections were also used for staining with H&E.
The right lung lobes were snap-frozen in liquid nitrogen and stored at −80°C until further analysis. The snap-frozen lungs were thawed and homogenized in tissue protein extraction reagent (T-PER) solution (Thermo Scientific, Göteborg, Sweden) containing protease inhibitor (Pefabloc SC; Sigma-Aldrich) at a final concentration of 1 mM. Lung homogenates were centrifuged at 9,000 × g for 10 min at 4°C, and the supernatants were collected.
Assessment of AST levels in plasma.
Following bacterial infection and treatment with levofloxacin in the absence or presence of EM703 at −24 and 0 h, animals were sacrificed 48 h postinfection and blood was collected, in EDTA-containing tubes, from four mice in each group. Plasma was isolated by centrifugation at 1,500 × g at 4°C for 10 min and was maintained at −80°C until it was used for further analysis. The levels of AST in plasma were assessed using an AST assay kit (Sigma-Aldrich), according to the manufacturer's instructions, in order to reflect the effects on organ damage following treatment.
Cytokine assay and ELISA.
Levels of the cytokines IL-6, IL-10, MCP-1, IFN-γ, TNF-α, and IL-12-p70 were measured in plasma, BAL fluid, and lung tissue homogenates from 10 mice from two separate experiments, 48 h after infection with P. aeruginosa. A cytometric bead assay (mouse inflammation kit; Becton Dickinson, Franklin Lakes, NJ) was used to measure cytokine levels, according to the manufacturer's instructions. KC levels in mice (four mice in each group, in one experiment) were determined using an ELISA kit (R&D Systems). The influence of the lung homogenate matrix was investigated using different spiked concentrations of the cytokines and chemokines. Similar recoveries were observed in lung homogenates and plasma. The assays were performed with single samples. According to the manufacturer, no cross-reactivity or background detection of proteins has been found using the bead-based assay. The detection limits were as follows: IL-6, 5 pg/ml; IL-10, 17.5 pg/ml; MCP-1, 52.7 pg/ml; IFN-γ, 2.5 pg/ml; TNF-α, 7.3 pg/ml; IL-12p70, 10.7 pg/ml. The intraassay coefficients of variability (CVs) were as follows: IL-6, 5%; IL-10, 6%; MCP-1, 5%; IFN-γ, 4%; TNF-α, 4%; IL-12p70, 8%; the interassay CVs were as follows: IL-6, 10%; IL-10, 10%; MCP-1, 9%; IFN-γ, 6%; TNF-α, 8%; IL-12p70, 6%. Concerning the KC ELISA, no cross-reactivity or interference with available related molecules (at 50 ng/ml) was observed, according to the manufacturer. The intraassay precision was 5%, and the interassay precision was 6%.
Statistical and sample size analyses.
When analyzing cytokines and cell counts, initial experiments were performed with four mice in each group, using IL-6 levels in BAL fluid as a measurement. The results showed a significant difference (P < 0.05) between mice treated with levofloxacin alone and the combination of levofloxacin and EM703. Therefore, it was decided to repeat the experiments with an additional six mice in each group, to increase the overall significance of the data. In total, 10 mice per group in two separate experiments were investigated for analyses of cytokines and cell counts.
Statistical comparisons of survival curves were performed using the Mantel-Cox test. Values for cell counts, AST levels, and cytokine levels are presented as the mean ± standard error of the mean (SEM). For statistical evaluation of more than two experimental groups, the Kruskal-Wallis nonparametric test, with Dunn's post hoc test for multiple comparisons, was used. Data graphics and statistical analysis were performed using GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA). P values of ≤0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Satoshi Omura (Kitasato University, Tokyo, Japan) for providing EM703. We also thank Sandra Jovic for excellent technical assistance and Alistair Kidd for linguistic editing of the manuscript.
The work was supported by grants from the Swedish Research Council, the Swedish Heart and Lung Foundation, the Swedish Government Funds for Clinical Research (ALF), the Swedish Foundation for Strategic Research, the Royal Physiographic Society in Lund, and the Alfred Österlund Foundation. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We declare we have no conflicts of interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02761-16.
REFERENCES
- 1.Spagnolo P, Fabbri LM, Bush A. 2013. Long-term macrolide treatment for chronic respiratory disease. Eur Respir J 42:239–251. doi: 10.1183/09031936.00136712. [DOI] [PubMed] [Google Scholar]
- 2.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR. 2011. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 4.Kibwage IO, Hoogmartens J, Roets E, Vanderhaeghe H, Verbist L, Dubost M, Pascal C, Petitjean P, Levol G. 1985. Antibacterial activities of erythromycins A, B, C, and D and some of their derivatives. Antimicrob Agents Chemother 28:630–633. doi: 10.1128/AAC.28.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber FH Jr, Richards RD, McCallum RW. 1993. Erythromycin: a motilin agonist and gastrointestinal prokinetic agent. Am J Gastroenterol 88:485–490. [PubMed] [Google Scholar]
- 6.Desaki M, Okazaki H, Sunazuka T, Omura S, Yamamoto K, Takizawa H. 2004. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-κB activation. Antimicrob Agents Chemother 48:1581–1585. doi: 10.1128/AAC.48.5.1581-1585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asadi L, Sligl WI, Eurich DT, Colmers IN, Tjosvold L, Marrie TJ, Majumdar SR. 2012. Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis 55:371–380. doi: 10.1093/cid/cis414. [DOI] [PubMed] [Google Scholar]
- 8.Nie W, Li B, Xiu Q. 2014. β-Lactam/macrolide dual therapy versus β-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother 69:1441–1446. doi: 10.1093/jac/dku033. [DOI] [PubMed] [Google Scholar]
- 9.Sligl WI, Asadi L, Eurich DT, Tjosvold L, Marrie TJ, Majumdar SR. 2014. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med 42:420–432. doi: 10.1097/CCM.0b013e3182a66b9b. [DOI] [PubMed] [Google Scholar]
- 10.Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, Torres A. 2002. Community-acquired pneumonia due to Gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med 162:1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 11.Murphy TF, Brauer AL, Eschberger K, Lobbins P, Grove L, Cai X, Sethi S. 2008. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177:853–860. doi: 10.1164/rccm.200709-1413OC. [DOI] [PubMed] [Google Scholar]
- 12.Sadikot RT, Blackwell TS, Christman JW, Prince AS. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Baum H, Welte T, Marre R, Suttorp N, Ewig S. 2010. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J 35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- 14.Laserna E, Sibila O, Fernandez JF, Maselli DJ, Mortensen EM, Anzueto A, Waterer G, Restrepo MI. 2014. Impact of macrolide therapy in patients hospitalized with Pseudomonas aeruginosa community-acquired pneumonia. Chest 145:1114–1120. doi: 10.1378/chest.13-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restrepo MI, Mortensen EM, Waterer GW, Wunderink RG, Coalson JJ, Anzueto A. 2009. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J 33:153–159. doi: 10.1183/09031936.00054108. [DOI] [PubMed] [Google Scholar]
- 16.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Iuvone T, D'Acquisto F, Di Rosa M. 2000. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther 292:156–163. [PubMed] [Google Scholar]
- 17.Lisboa T, Salluh JI, Friedman G. 2016. Macrolides and respiratory infection in critically ill patients: what is the next step? Minerva Anestesiol 82:221–229. [PubMed] [Google Scholar]
- 18.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, Tanaka M, Kasama T, Kobayashi K, Nakajima J, Ito K. 1997. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med 156:266–271. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 19.Barthelemy P, Autissier D, Gerbaud G, Courvalin P. 1984. Enzymic hydrolysis of erythromycin by a strain of Escherichia coli: a new mechanism of resistance. J Antibiot (Tokyo) 37:1692–1696. doi: 10.7164/antibiotics.37.1692. [DOI] [PubMed] [Google Scholar]
- 20.Li YJ, Azuma A, Usuki J, Abe S, Matsuda K, Sunazuka T, Shimizu T, Hirata Y, Inagaki H, Kawada T, Takahashi S, Kudoh S, Omura S. 2006. EM703 improves bleomycin-induced pulmonary fibrosis in mice by the inhibition of TGF-β signaling in lung fibroblasts. Respir Res 7:16. doi: 10.1186/1465-9921-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YJ, Shimizu T, Hirata Y, Inagaki H, Takizawa H, Azuma A, Kawada T, Sugawara I, Kudoh S, Sunazuka T, Omura S. 2013. EM, EM703 inhibit NF-κB activation induced by oxidative stress from diesel exhaust particle in human bronchial epithelial cells: importance in IL-8 transcription. Pulm Pharmacol Ther 26:318–324. doi: 10.1016/j.pupt.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Louie A, Liu W, VanGuilder M, Neely MN, Schumitzky A, Jelliffe R, Fikes S, Kurhanewicz S, Robbins N, Brown D, Baluya D, Drusano GL. 2015. Combination treatment with meropenem plus levofloxacin is synergistic against Pseudomonas aeruginosa infection in a murine model of pneumonia. J Infect Dis 211:1326–1333. doi: 10.1093/infdis/jiu603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith DC, Corcoran E, Lofland D, Lee A, Cho D, Lomovskaya O, Dudley MN. 2006. Pharmacodynamics of levofloxacin against Pseudomonas aeruginosa with reduced susceptibility due to different efflux pumps: do elevated MICs always predict reduced in vivo efficacy? Antimicrob Agents Chemother 50:1628–1632. doi: 10.1128/AAC.50.5.1628-1632.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie A, Fregeau C, Liu W, Kulawy R, Drusano GL. 2009. Pharmacodynamics of levofloxacin in a murine pneumonia model of Pseudomonas aeruginosa infection: determination of epithelial lining fluid targets. Antimicrob Agents Chemother 53:3325–3330. doi: 10.1128/AAC.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klesel N, Geweniger KH, Koletzki P, Isert D, Limbert M, Markus A, Riess G, Schramm H, Iyer P. 1995. Chemotherapeutic activity of levofloxacin (HR 355, DR-3355) against systemic and localized infections in laboratory animals. J Antimicrob Chemother 35:805–819. doi: 10.1093/jac/35.6.805. [DOI] [PubMed] [Google Scholar]
- 26.Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. 2010. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res 11:90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Wada H, Rossios C, Takagi D, Higaki M, Mikura S, Goto H, Barnes PJ, Ito K. 2013. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-κB inhibition. J Pharmacol Exp Ther 345:76–84. doi: 10.1124/jpet.112.200733. [DOI] [PubMed] [Google Scholar]
- 28.Angus DC, van der Poll T. 2013. Severe sepsis and septic shock. N Engl J Med 369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 29.Abdelghaffar H, Babin-Chevaye C, Labro MT. 2005. The macrolide roxithromycin impairs NADPH oxidase activation and alters translocation of its cytosolic components to the neutrophil membrane in vitro. Antimicrob Agents Chemother 49:2986–2989. doi: 10.1128/AAC.49.7.2986-2989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson R, Theron AJ, Feldman C. 1996. Membrane-stabilizing, anti-inflammatory interactions of macrolides with human neutrophils. Inflammation 20:693–705. doi: 10.1007/BF01488805. [DOI] [PubMed] [Google Scholar]
- 31.Hand WL, Hand DL, King-Thompson NL. 1990. Antibiotic inhibition of the respiratory burst response in human polymorphonuclear leukocytes. Antimicrob Agents Chemother 34:863–870. doi: 10.1128/AAC.34.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch CC, Esteban DJ, Chin AC, Olson ME, Read RR, Ceri H, Morck DW, Buret AG. 2000. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J Antimicrob Chemother 46:19–26. doi: 10.1093/jac/46.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Labro MT. 2004. Cellular and molecular effects of macrolides on leukocyte function. Curr Pharm Des 10:3067–3080. doi: 10.2174/1381612043383403. [DOI] [PubMed] [Google Scholar]
- 34.Schultz MJ, Speelman P, Zaat S, van Deventer SJ, van der Poll T. 1998. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob Agents Chemother 42:1605–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada K, Yanagihara K, Kaku N, Harada Y, Migiyama Y, Nagaoka K, Morinaga Y, Nakamura S, Imamura Y, Miyazaki T, Izumikawa K, Kakeya H, Hasegawa H, Mikamo H, Kohno S. 2013. Azithromycin attenuates lung inflammation in a mouse model of ventilator-associated pneumonia by multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 57:3883–3888. doi: 10.1128/AAC.00457-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coopersmith CM, Amiot DM II, Stromberg PE, Dunne WM, Davis CG, Osborne DF, Husain KD, Turnbull IR, Karl IE, Hotchkiss RS, Buchman TG. 2003. Antibiotics improve survival and alter the inflammatory profile in a murine model of sepsis from Pseudomonas aeruginosa pneumonia. Shock 19:408–414. doi: 10.1097/01.shk.0000054370.24363.ee. [DOI] [PubMed] [Google Scholar]
- 37.Divangahi M, Matecki S, Dudley RW, Tuck SA, Bao W, Radzioch D, Comtois AS, Petrof BJ. 2004. Preferential diaphragmatic weakness during sustained Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 169:679–686. doi: 10.1164/rccm.200307-949OC. [DOI] [PubMed] [Google Scholar]
- 38.Labbe K, Danialou G, Gvozdic D, Demoule A, Divangahi M, Boyd JH, Petrof BJ. 2010. Inhibition of monocyte chemoattractant protein-1 prevents diaphragmatic inflammation and maintains contractile function during endotoxemia. Crit Care 14:R187. doi: 10.1186/cc9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 40.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. 1996. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun 64:4733–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen GH, Reddy RC, Newstead MW, Tateda K, Kyasapura BL, Standiford TJ. 2000. Intrapulmonary TNF gene therapy reverses sepsis-induced suppression of lung antibacterial host defense. J Immunol 165:6496–6503. doi: 10.4049/jimmunol.165.11.6496. [DOI] [PubMed] [Google Scholar]
- 42.Sawa T, Corry DB, Gropper MA, Ohara M, Kurahashi K, Wiener-Kronish JP. 1997. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J Immunol 159:2858–2866. [PubMed] [Google Scholar]
- 43.Chen SM, Cheng DS, Williams BJ, Sherrill TP, Han W, Chont M, Saint-Jean L, Christman JW, Sadikot RT, Yull FE, Blackwell TS. 2008. The nuclear factor kappa-B pathway in airway epithelium regulates neutrophil recruitment and host defence following Pseudomonas aeruginosa infection. Clin Exp Immunol 153:420–428. doi: 10.1111/j.1365-2249.2008.03707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uller L, Persson CG, Erjefalt JS. 2006. Resolution of airway disease: removal of inflammatory cells through apoptosis, egression or both? Trends Pharmacol Sci 27:461–466. doi: 10.1016/j.tips.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Giamarellos-Bourboulis EJ, Adamis T, Laoutaris G, Sabracos L, Koussoulas V, Mouktaroudi M, Perrea D, Karayannacos PE, Giamarellou H. 2004. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 48:93–99. doi: 10.1128/AAC.48.1.93-99.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gad SC, Cassidy CD, Aubert N, Spainhour B, Robbe H. 2006. Nonclinical vehicle use in studies by multiple routes in multiple species. Int J Toxicol 25:499–521. doi: 10.1080/10915810600961531. [DOI] [PubMed] [Google Scholar]
- 47.Tojima I, Shimizu S, Ogawa T, Kouzaki H, Omura S, Sunazuka T, Shimizu T. 2015. Anti-inflammatory effects of a novel non-antibiotic macrolide, EM900, on mucus secretion of airway epithelium. Auris Nasus Larynx 42:332–336. doi: 10.1016/j.anl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Turner PV, Pekow C, Vasbinder MA, Brabb T. 2011. Administration of substances to laboratory animals: equipment considerations, vehicle selection, and solute preparation. J Am Assoc Lab Anim Sci 50:614–627. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.