ABSTRACT
Carbapenems are now being explored for treatment of multidrug-resistant tuberculosis (MDR-TB), especially in conjunction with clavulanate. Clinical use is constrained by the need for multiple parenteral doses per day and the lack of knowledge of the optimal dose for sterilizing effect. Our objective was to identify the ertapenem exposure associated with optimal sterilizing effect and then design a once-a-day dose for clinical use. We utilized the hollow-fiber system model of tuberculosis in a 28-day exposure-response study of 8 different ertapenem doses in combination with clavulanate. The systems were sampled at predetermined time points to verify the concentration-time profile and identify the total bacterial burden. Inhibitory sigmoid maximum-effect (Emax) modeling was used to identify the relationship between total bacterial burden and the drug exposure and to identify optimal exposures. Contrary to the literature, ertapenem-clavulanate combination demonstrated good microbial kill and sterilizing effect. In a dose fractionation hollow-fiber study, efficacy was linked to percentage of the 24-h dosing interval of ertapenem concentration persisting above MIC (%TMIC). We performed 10,000 MDR-TB patient computer-aided clinical trial simulations, based on Monte Carlo methods, to identify the doses and schedule that would achieve or exceed a %TMIC of ≥40%. We identified an intravenous dosage of 2 g once per day as achieving the target in 96% of patients. An ertapenem susceptibility breakpoint MIC of 2 mg/liter was identified for that dose. An ertapenem dosage of 2 g once daily is the most suitable to be tested in a phase II study of sterilizing effect in MDR-TB patients.
KEYWORDS: Mycobacterium tuberculosis, ertapenem, hollow-fiber infection model, MDR-TB, pharmacodynamics, pharmacokinetics
INTRODUCTION
The emergence of drug-resistant tuberculosis (TB), especially multidrug-resistant TB (MDR-TB), extensively drug-resistant TB (XDR-TB), and virtually incurable TB (termed totally drug-resistant TB by some), is a global emergency that threatens to undermine many gains of chemotherapy (1–4). As a result, there is currently a four-pronged effort to combat this problem: (i) identification of new small molecules to kill drug-resistant Mycobacterium tuberculosis, (ii) repurposing of antimicrobial drugs not currently used to treat TB into TB therapeutics, (iii) host-directed therapy, and (iv) use of pharmacokinetics/pharmacodynamics (PK/PD) science to optimize efficacy while suppressing emergence of acquired drug resistance (5–8). Carbapenems, extensively used to treat Gram-negative bacteria over the last 30 years, have also been shown to be effective against M. tuberculosis in vitro and in vivo when in the presence of a β-lactamase inhibitor (6, 9).
Several initiatives are ongoing to explore the added value of carbapenems given as part of a multidrug regimen for M/XDR-TB (10, 11). In murine TB, efficacy has been demonstrated for meropenem and imipenem with clavulanate; however, ertapenem was no better than nontreatment (9). In addition, ertapenem demonstrated high MICs, suggesting possible natural resistance. However, ertapenem degrades rapidly in in vitro growth media at incubation temperatures used to measure MICs with conventional methods (12). We have since developed an MIC assay that corrects for this degradation, which has demonstrated much lower MICs (12). The main advantage of ertapenem to patients could be its half-life of 4 h, which could allow a once-a-day schedule, as opposed to 0.6 to 0.7 h for meropenem and imipenem, which necessitates multiple and prolonged intravenous infusions per day (13). The multiple infusions per day with meropenem and imipenem make it rather difficult to administer long-duration therapy in M/XDR-TB. Recently, the first TB clinical data with ertapenem showed that it was well tolerated as part of a salvage regimen for MDR-TB patients (13, 14). Unfortunately, the efficacy of the drug could not be assessed, as it was used in a multidrug regimen; moreover, its sterilizing effect is unknown.
The hollow-fiber system model of TB (HFS-TB) has been used to examine the sterilizing effect of anti-TB agents, defined as the ability to kill either semidormant M. tuberculosis under acidic conditions or nonreplicating persisters under hypoxia (15–17). It was qualified by the European Medicines Agency and editorially endorsed by the U.S. Food and Drug Administration (http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2015/02/WC500181899.pdf). The HFS-TB in tandem with computer-aided clinical trial simulations was found to have a forecasting accuracy of >94% of observed optimal exposures and doses in TB patients in the clinic (18–20). This makes this model ideal to identify optimal doses for treatment of M/XDR-TB, which can directly be translated into clinical use. Our objective was to use these models to identify the optimal sterilizing-effect dose of ertapenem for treatment of MDR-TB.
RESULTS
Dose-effect HFS-TB study for sterilizing effect.
In the first HFS-TB, which was mainly a dose ranging study, we cultured M. tuberculosis H37Ra under acidic conditions to a semidormant state and then use it to inoculate HFS-TB units with circulating Middlebrook 7H9 acidified to a pH of 5.8, as described previously (15, 21). Different ertapenem exposures, based on human-equivalent doses of 0.25, 0.5, 1.0, 2.0, 3.0, 5.0, and 10.0 g, were administered into the central compartment of duplicate HFS-TB units via a computer-controlled syringe pump over 30 min, as in patients; drug concentrations achieved in each of the 16 HFS-TB were measured at 8 different time points over the first 24 h. Clavulanate was also dispensed via syringe pump to achieve a peak of 3 mg/liter at the end of 30 min of infusion. Pharmacokinetic modeling of the measured drug concentrations revealed that the lowest Akaike information criterion scores (22) were for a one-compartment model. The ertapenem total clearance (±standard deviation) was 4.11 ± 1.83 liters, and the volume was 22.55 ± 4.0 liters, which translates to a half-life of 3.80 h. The regression for observed concentrations versus pharmacokinetic model predicted concentrations had an r2 of 0.997 and the slope was 0.996 ± 0.006, which is close to unity. Thus, the one-compartment model described the data well, with no bias.
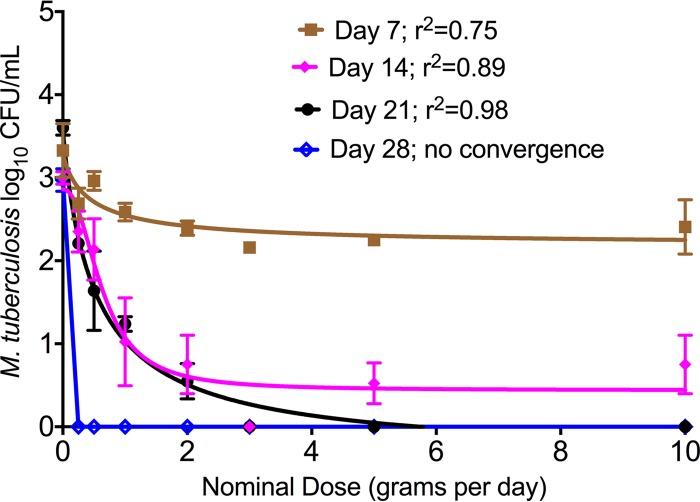
Figure 1 shows that ertapenem achieved a good sterilizing effect. The bacterial burden at the start of therapy was 4.0 log10 CFU/ml. The data are presented as inhibitory sigmoid maximum-effect (Emax) models between “nominal” human-equivalent dose and microbial burden. In Fig. 1, there was no model convergence on day 3, while on day 28, at the end of the experiment, all ertapenem-treated systems had bacterial burdens below limits of detection. All systems achieved percent time above MIC (%TMIC) of 100% of the dosing interval; the trough at 23.5 h was >4 mg/liter in all systems, and all achieved the same microbial kill on day 28.
FIG 1.
Ertapenem-clavulanate dose-effect sterilizing effect in the hollow-fiber model. Drug treatments are depicted as “nominal” human-equivalent doses. On day 3, inhibitory sigmoid Emax modeling demonstrated no model convergence, and there was very little kill; thus, regressions for day 3 were left out. However, by day 7 there was already good microbial kill, characterized by maximal kill (Emax) of 1.13 ± 0.34 log10 CFU/ml. By day 28, all ertapenem-treated HFS-TB units completely sterilized the bacteria.
Ertapenem dose fractionation study in the HFS-TB.
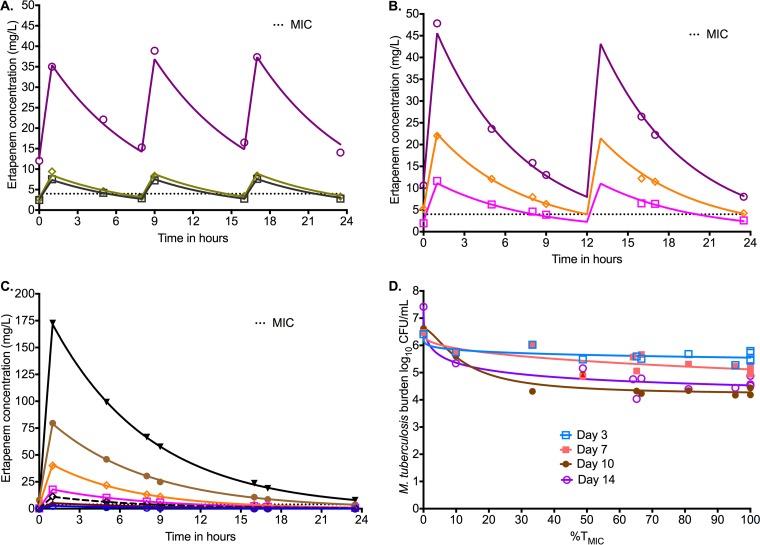
Next, we performed a new HFS-TB, this time using M. tuberculosis H37Rv and a dose fractionation design, for a treatment duration of 14 days. On measurement of ertapenem concentrations, similar to the first study, the concentrations were also best described using a one-compartment model; the observed versus predicted concentrations revealed a slope of 0.995 ± 0.002 (r2 > 0.999). The concentration-time profiles achieved with each dose are shown by dosing schedule in Fig. 2A to C, together with the ertapenem (plus clavulanate at 2.5 mg/liter) MIC of 4 mg/liter. Inhibitory sigmoid Emax model fitting by exposure, expressed as either maximum concentration (Cmax)/MIC, area under the concentration-time curve from 0 to 24 h (AUC0–24)/MIC, or %TMIC, revealed Akaike information criterion scores shown in Table 1. The lowest scores were for %TMIC, which means that this is the PK/PD index linked to microbial kill. Figure 2D shows the inhibitory sigmoid Emax MIC curves for each sampling day based on %TMIC. Based on day 10, which had the highest r2 (0.94), the relationship between %TMIC and bacterial burden was as follows: log10 CFU/ml = 5.68 − %TMIC2.56/[23.522.56 + %TMIC2.56]. From this relationship, we calculated the 80% effective concentration (EC80) as a %TMIC of 40.41% of the dosing interval. Indeed, this can be read off Fig. 2D as well, which shows that one gets the same exposure for optimal kill whichever sampling day is examined.
FIG 2.
Dose fractionation study to determine PK/PD index linked to ertapenem efficacy. The concentration time profiles are shown relative to the MIC. Symbols indicate measured concentrations and the lines modeled profile. (A) Concentration-time profiles of ertapenem identified in the HFS-TB with a dosing schedule of every 8 h. (B) Concentration-time profiles of ertapenem identified in the HFS-TB with a dosing schedule of every 12 h. (C) Concentration-time profiles of ertapenem identified in the HFS-TB with a dosing schedule of once a day. Given the concentration range, the scale obscures the time that concentrations persisted above MIC for some doses. For the blue open circles, the lowest concentration, the time above MIC was 0 h. For the dose shown by cayenne triangles the time was 3 h, for the black open diamonds it was 8.32 h, and for the open magenta squares it was 11.7 h. The rest can be read off the graph. (D) Inhibitory sigmoid Emax model for %TMIC versus bacterial burden. On day 7, the maximal kill (Emax) was 1.14 log10 CFU/ml, consistent with findings in the first HFS-TB dose-effect study. The study was carried out for only 14 days. Examination of the curves on each day shows that 80% of maximal kill occurs around a %TMIC of 40% on all sampling days except day 3, when it occurs with lower exposures.
TABLE 1.
Akaike information criterion scores for PK/PD index versus ertapenem sterilizing effect
| Parameter | Score on: |
|||
|---|---|---|---|---|
| Day 3 | Day 7 | Day 10 | Day 14 | |
| AUC0–24/MIC | −30.26 | −18.99 | −31.41 | −5.112 |
| Cmax/MIC | −30.63 | −17.19 | −31.55 | −2.144 |
| %TMIC | −60.39 | −62.96 | −49.39 | −45.06 |
Monte Carlo simulations to identify optimal ertapenem dose.
In TB patients, pharmacokinetic variability is one of the most important drivers of sterilizing effect (23–29). Therefore, in order to identify the optimal ertapenem dose for pulmonary TB, we performed Monte Carlo simulations of 10,000 patients with pulmonary TB, using the pharmacokinetic parameter estimates and between-patient variability indices shown in Table 2 based on previous studies (30–32). We also accounted for the ertapenem penetration into epithelial lining fluid (ELF) of 7.48% ± 8.17% (which mirrors the non-protein-bound concentration of 5 to 15%), and that in lung tissue of 23.6% ± 12.3% (33). We performed simulations to determine how much 1.0 g once a day, 1.0 g twice a day, 2.0 g once a day, 2.0 g twice a day, or 3.0 g once a day would achieve or exceed the target exposure, which is a %TMIC of 40.41%, associated with optimal sterilizing effect in ELF of patients. For internal validation, we compared the pharmacokinetic parameters in the 10,000 simulated patients to those of Burkhardt et al. (30) in Table 2, which shows that the simulations faithfully recapitulated the pharmacokinetic parameters and variability. As an extra external validation step, we compared the pharmacokinetic parameters in the simulations to those we actually observed in our MDR-TB patients in The Netherlands, as shown in Table 2 (13). Table 2 shows that the pharmacokinetic parameters and variance in our simulations were virtually identical to those we observed in patients. Therefore, the simulations were accurate in reproducing what is identified in the clinic.
TABLE 2.
Comparison of pharmacokinetic parameter and concentration estimates and ranges in 10,000 simulated patients to those actually observed in patients treated with 1 g
| Parameter | Value |
||
|---|---|---|---|
| Subroutine prior based on literature, mean ± SD | For 10,000 simulated TB patients, mean (range) | Observed in MDR-TB patients, mean (range) (13) | |
| Total clearance, liters/h | 2.63 ± 0.83 | 2.6 (0.02–6.00) | 2.1 (0.09–3.23) |
| Vol, liters | 10.6 ± 2.51 | 11 (1.2–19) | 7.3 (2.61–11.10) |
| Half-life, h | 2.8 (2.20–3.70) | 2.4 (2.05–3.53) | |
| AUC0–24, mg · h/liter | 448 (166–4,255) | 545 (309–1,130) | |
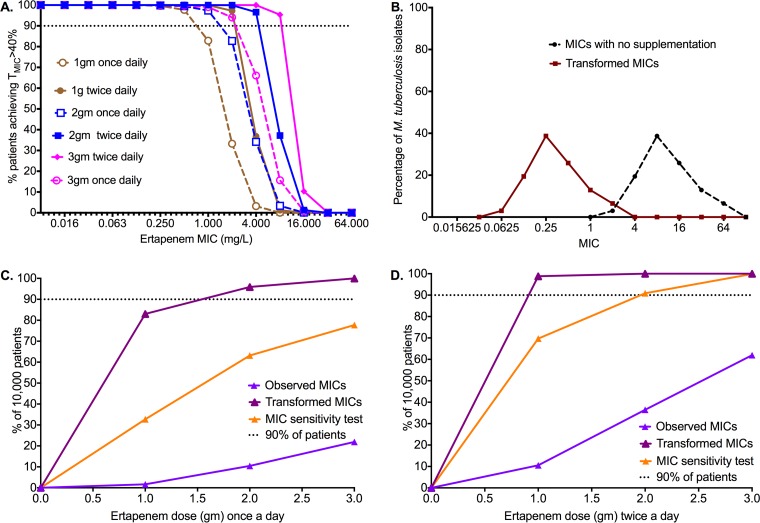
Figure 3A shows the target attainment probability (TAP) for each dose and dosing schedule as the MIC changes. On one extreme, the dosage of 1 g once a day had a TAP less than 90% once the MIC was 1 mg/liter, while the dosage of 3 g twice a day achieved a high TAP until 8 mg/liter and then fell precipitously at 16 mg/liter. For 2 g a day, the TAP fell at an MIC of 2 mg/liter. This means that the susceptibility breakpoint for ertapenem plus clavalunate will fall between MIC of 1 and 16 mg/liter and will depend on the final dose chosen.
FIG 3.
Target attainment probability and cumulative fraction of response for various ertapenem doses. (A) Target attainment probability for %TMIC of 40% as M. tuberculosis MIC changes. No dose or dosing schedule is effective once MICs are 16 mg/liter. (B) Ertapenem MIC distribution in isolates from The Netherlands, with and without transformation to account for ertapenem degradation. (C) Proportion of 10,000 patients who achieved or exceeded a %TMIC of 40% with once-a-day dosing. The proportion is highly sensitive to the MIC and fell on sensitivity analysis, a worst-case scenario. (D) Proportion of 10,000 patients who achieved or exceeded a %TMIC of 40% with twice-a-day dosing. The twice-a-day dosing schedule achieved the target in higher proportions of patients, even on sensitivity analysis. However, given the hardship of twice a day administration of therapy in TB, we chose the dosage of 2 g once a day as being most practical.
Since MIC variability is also an important determinant of therapy response in TB patients (25, 34–36), we also took into account the MIC distribution. Figure 3B shows the ertapenem MIC distribution from 33 MDR-TB patients isolates in The Netherlands, in the presence of clavulanate. Figure 3B shows that all isolates would have MICs between greater than 1 mg/liter and below 128 mg/liter. However, in the past we have shown that ertapenem degrades during the MIC testing, and if one accounts for the degradation, there is a 4-tube dilution decrease in MICs; if clavulanate is added and ertapenem is supplemented, there is a 7-tube dilution difference (12). Thus, we transformed the MICs for the 33 clinical isolates down by 4 tube dilutions as well, as shown in Fig. 3B. In that scenario, only 6.5% of isolates had an MIC greater than 1 mg/liter.
Summation of all TAPs to account for distribution of MICs gives the proportion of 10,000 TB patients who would achieve the target exposure of %TMIC of 40%, termed the cumulative fraction of response (CFR). Figure 3C shows the CFRs for the once-a-day dosing schedule for both observed MICs and transformed MICs. For the transformed MICs, the dosage of 2 g a day had a CFR of 96%. We also determined the MICs of ertapenem plus clavalunate for 4 clinical isolates incubated at 4°C versus 37°C to try and slow down drug degradation: MICs were lower at 4°C by 4, 2, 3, and 2 tube dilutions. Therefore, we performed sensitivity testing by examining CFR if MIC transformation was only 2 tube dilutions lower (worst-case scenario). Figure 3C shows that the dosage of 2 g once a day would not achieve the target in 90% of patients; nevertheless, it would achieve this in 63% of patients, which is still reasonable. Figure 3D shows the results of a twice-a-day dosing schedule; as would be expected from a %TMIC-driven drug, this dosing schedule performed better. The dosage of 1.0 g twice a day would achieve target exposure in 99% of patients and on sensitivity testing would still achieve this in 70% of patients. The dosage of 2 g twice a day would achieve >90% even on sensitivity testing.
DISCUSSION
This is the first study that showed the efficacy and sterilizing effect of ertapenem-clavulanate, unlike findings in the murine model, likely because the HFS-TB mimicked the half-life of 4 h encountered in patients, in contrast to 1.0 h in mice. We were able to recapitulate ertapenem's pharmacokinetics, and its half-life of 4 h, as encountered in TB patients, which likely explains the better efficacy in this model than that encountered in mice, in which the ertapenem half-life is 1 h. Moreover, dosages simulated in the model were in a range that would likely be tolerable in patients. This study showed the advantage of the hollow-fiber system, namely, a better recapitulation of human-like pharmacokinetics, and of microbial sterilizing-effect conditions. The Monte Carlo simulations then introduced the variability that would be encountered for pharmacokinetic parameters between patients and MICs between M. tuberculosis strains. Our two-step external validation approach in the simulations ensured that our simulations reflected clinical reality; sensitivity testing accounted for any uncertainty in MIC distribution. This allowed us to perform dose-effect studies that take into account the exposure-effect relationship as described for the hollow-fiber model, the essential aspects of drug behavior in patients, such as pharmacokinetic variability and the ratios for drug penetration to lungs, that are important in determining efficacy, and susceptibility of M. tuberculosis isolates encountered in hospitals. This approach, in many experiments based on the same M. tuberculosis isolate as we used in the current study (M. tuberculosis H37Rv), has been found to be >94% accurate in identifying clinical doses that are optimal in TB patients based on recent presentation for regulatory approval (19).
Ertapenem-clavulanate may play an important role in the intensive phase of TB treatment due to its sterilizing effect. In addition, intravenous administration may be more suitable for the intensive phase, in which M/XDR-TB patients are likely to be administered therapy in a TB clinic. As carbapenems are already part of the WHO list of TB drugs for M/XDR-TB, the next step is to explore the use of ertapenem-clavulanate in patients, using the dosage of 2 g once a day that we identified. Recently, it has been shown that meropenem-clavulanate has promising activity against MDR-TB in vitro (37, 38). Indeed, imipenem-clavulanate and meropenem-clavulanate were associated with a treatment success of >57% and culture conversion of >60% in a recent systemic analysis of five studies (39). However, since clavulanate is administered as oral amoxicillin-clavulanate, gastrointestinal side effects may become a problem if this formulation is administered for a prolonged duration multiple times a day with meropenem or imipenem, which would compromise absorption of other oral drugs. Unfortunately, the current suppliers of carbapenems are not interested in developing an infused combination of carbapenem and clavulanate. The main advantage of ertapenem is its long half-life, enabling once-daily dosing, which would also allow a once-a-day clavulanate dose, potentially reducing side effects. This may even facilitate dosing in an outpatient setting. Patients may present at the clinic once a day for their drug administration as part of directly observed treatment, or they could receive treatment as a once-a-day infusion at home when sputum culture negative in those countries where the drug is already part of home care for treatment of other chronic infections. Ertapenem has a labeled infusion time of only 30 min, which facilitates a relatively short stay at an outpatient clinic. Even more rapid infusion has been explored and showed similar drug exposure and tolerability (40). We show that a dosage of 2 g given once daily could contribute to an effective regimen. Ertapenem up to a dose of 3 g has been administered to healthy volunteers (41). Moreover, doses up to 2 g have been administered in 30 min without any additional complications (42). However, there is a need for a prospective phase II study exploring the safety and efficacy of 2 g of ertapenem with clavulanate once a day in MDR-TB patients.
On the other hand, the amount of time clavulanate has to be around to keep potentiating ertapenem is still unclear. Thus, the target concentration to aim with dosing is unclear. Clavulanate has a shorter half-life than does ertapenem. However, penetration into the bronchial mucosa is 118%, and its protein binding is minimal at 20%, and likely an effective concentration remains at the site of effect even when dosed once. Since clavulanate is renally eliminated, between-patient variability in systemic clearance, which is about 58%, is driven mainly by renal function: the lower the creatinine clearance, the less the drug is cleared (43). Separate dose-effect studies on the role of clavulanate will need to be conducted, after which simulations similar to the ones described here can be performed.
Finally, pharmacokinetics/pharmacodynamics-based susceptibility breakpoints in TB, mostly derived from hollow-fiber model monotherapy studies, have been shown to be highly accurate in delineating TB patients who fail or respond to combination therapy (25, 34, 35). The 2-mg/liter ertapenem susceptibility breakpoint we identified for the dosage of 2 g a day should thus be used by clinicians as decision-making tool to determine if a patient will respond to ertapenem therapy. This breakpoint will differ from the epidemiological cutoff value, which may be more useful for epidemiological tracking of acquired ertapenem resistance, as opposed to clinical decision-making.
There are some limitations to our study. First, we used two isolates M. tuberculosis for the sterilizing-effect experiments. Inclusion of a larger number of isolates could change the final target exposure associated with optimal efficacy. However, hollow-fiber studies in the past with these isolates were found to be predictive of the optimal exposure targets in patients for sterilizing effect (15, 24, 25, 44–46). A second limitation is that we used pharmacokinetic data from critically ill patients as prior data for our Monte Carlo simulations. The type of disease that a patient has can alter the pharmacokinetic parameters, so TB patients could have different pharmacokinetics. However, as shown in Table 2, the pharmacokinetic parameter estimates in simulated patients, and the AUC0–24 achieved with 1-g doses, were virtually identical to those we have identified in TB patients in The Netherlands as part of therapeutic drug monitoring. This validates that simulated patients had pharmacokinetic parameters and concentrations similar to those encountered in TB patients.
In conclusion, we have shown by simulation of human drug exposure of different dosages in an in vitro infection model of M. tuberculosis that ertapenem-clavunalate may be a valuable asset to TB treatment. Based on available pharmacokinetic data, we have identified that the dosage of ertapenem most suitable to be tested in a phase II study is 2 g once daily. An MIC of 2 mg/liter should be used to define resistance to this drug.
MATERIALS AND METHODS
We used M. tuberculosis H37Ra (ATCC 25177) and H37Rv (ATCC 27294) for our experiments, with growth and storage conditions described before (15). These isolates have been used in the HFS-TB before, with good forecasting accuracy. Ertapenem was purchased from Merck Sharp & Dohme. Clavulanate was purchased from Sigma-Aldrich. Drugs were dissolved in sterile water and syringe filtered for further use. Hollow-fiber cartridges were purchased from FiberCell (Frederick, MD).
Hollow-fiber system model of TB.
Construction of the HFS-TB to measure the sterilizing effect has been described in detail previously (15). The system recapitulates concentration-time profiles of drugs encountered in patients, taking into account the penetration into lungs. In the sterilizing-effect studies, semidormant M. tuberculosis growing in Middlebrook 7H9 broth acidified using acetic acid to a pH of 5.8 was used; this isolate grows at a rate 8- to 10-fold lower than that of log-phase M. tuberculosis (47). The HFS-TB in this model use acidified Middlebrook 7H9 broth without oleic acid, albumin, or catalase but with 20% dextrose. The peripheral compartment of each of 16 HFS-TB units with circulating acidified Middlebrook 7H9 broth was inoculated with M. tuberculosis. All HFS-TB units were incubated at 37°C under 5% CO2 for the entirety of the study. Different ertapenem exposures, based on human-equivalent doses of 0.25, 0.5, 1.0, 2.0, 3.0, 5.0, and 10.0 g, were administered into the central compartment via a computer-controlled syringe pump over 30 min, as in patients. The concentrations achieved with the doses were AUC0–24s of 0, 25, 50, 100, 200, 250, 500, and 1,000 mg · h/liter. There were two replicate hollow-fiber systems for each dose or AUC0–24. Clavulanate was also dispensed via syringe pump to achieve a peak of 3 mg/liter at the end of 30 min of infusion. Medium inflow and outflow were set to mimic the ertapenem half-life of 4 h encountered in patients; we took into account the degradation rate of the drug that we have identified in the past. We recapitulated pharmacokinetics as described in the package insert for INVANZ (ertapenem for injection) for intravenous or intramuscular administration.
The central compartments of each HFS-TB was sampled six times during the first 24 h, and ertapenem concentrations were measured using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method as described previously (48) in order to verify that human-like pharmacokinetics had been achieved. Ertapenem concentrations were modeled using a one-compartment pharmacokinetic model with first-order input and elimination, using ADAPT 5 software, as described previously (15, 24, 25, 44–46). These actual exposures achieved in the HFS-TB were subsequently used in the PK/PD analyses. In order to enumerate the M. tuberculosis burden as CFU per milliliter, the peripheral compartment of each HFS-TB unit was sampled on days 0, 3, 7, 14, 21, and 28. Samples were washed and processed as described previously (15) and spread on Middlebrook 7H10 agar supplemented with 10% oleic acid-dextrose-catalase. The cultures were incubated for 21 days at 37°C with 5% CO2 before the colonies were counted.
Identification of optimal ertapenem dose using computer-aided clinical trial simulations.
For the domain of input, we utilized the pharmacokinetic parameter estimates and between-patient variability indices identified by Burkhardt et al. (30). We performed simulations to determine how much 1.0 g once a day, 1.0 g twice a day, 2.0 g once a day, 2.0 g twice a day, 3.0 g once a day, or 3.0 g twice a day would achieve or exceed the target exposure, which is the %TMIC associated with optimal sterilizing effect in lung tissue of patients.
ACKNOWLEDGMENTS
This work was supported by National Institute of General Medical Sciences of the National Institutes of Health New Innovator Award DP2OD001886-0 and National Institute of Allergy and Infectious Diseases award R01AI079497 to Tawanda Gumbo.
T.G. is a consultant for Lumina Care solutions and founded Jacaranda Biomed, Inc. J.-W.C.A. reports personal fees from Pfizer, Astellas, MSD, and Gilead and grants from Pfizer, Astellas, and MSD, all outside the submitted work. All other authors have no conflict of interest.
REFERENCES
- 1.Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, Furin J, Nardell EA, London L, Lessem E, Theron G, van Helden P, Niemann S, Merker M, Dowdy D, Van Rie A, Siu GK, Pasipanodya JG, Rodrigues C, Clark TG, Sirgel FA, Esmail A, Lin HH, Atre SR, Schaaf HS, Chang KC, Lange C, Nahid P, Udwadia ZF, Horsburgh CR Jr, Churchyard GJ, Menzies D, Hesseling AC, Nuermberger E, McIlleron H, Fennelly KP, Goemaere E, Jaramillo E, Low M, Jara CM, Padayatchi N, Warren RM. 23 March 2017. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 2.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R. 2014. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2:321–338. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange C, Abubakar I, Alffenaar JW, Bothamley G, Caminero JA, Carvalho AC, Chang KC, Codecasa L, Correia A, Crudu V, Davies P, Dedicoat M, Drobniewski F, Duarte R, Ehlers C, Erkens C, Goletti D, Gunther G, Ibraim E, Kampmann B, Kuksa L, de Lange W, van Leth F, van Lunzen J, Matteelli A, Menzies D, Monedero I, Richter E, Rusch-Gerdes S, Sandgren A, Scardigli A, Skrahina A, Tortoli E, Volchenkov G, Wagner D, van der Werf MJ, Williams B, Yew W-W, Zellweger J-P, Cirillo DM. 2014. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J 44:23–63. doi: 10.1183/09031936.00188313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. 2012. Totally drug-resistant tuberculosis in India. Clin Infect Dis 54:579–581. doi: 10.1093/cid/cir889. [DOI] [PubMed] [Google Scholar]
- 5.Alsaad N, Wilffert B, van Altena R, de Lange WC, van der Werf TS, Kosterink JG, Alffenaar JW. 2014. Potential antimicrobial agents for the treatment of multidrug-resistant tuberculosis. Eur Respir J 43:884–897. doi: 10.1183/09031936.00113713. [DOI] [PubMed] [Google Scholar]
- 6.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolhuis MS, van der Laan T, Kosterink JG, van der Werf TS, van Soolingen D, Alffenaar JW. 2014. In vitro synergy between linezolid and clarithromycin against Mycobacterium tuberculosis. Eur Respir J 44:808–811. doi: 10.1183/09031936.00041314. [DOI] [PubMed] [Google Scholar]
- 8.Pasipanodya J, Gumbo T. 2011. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob Agents Chemother 55:24–34. doi: 10.1128/AAC.00749-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veziris N, Truffot C, Mainardi JL, Jarlier V. 2011. Activity of carbapenems combined with clavulanate against murine tuberculosis. Antimicrob Agents Chemother 55:2597–2600. doi: 10.1128/AAC.01824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiberi S, Sotgiu G, D'Ambrosio L, Centis R, Abdo Arbex M, Alarcon Arrascue E, Alffenaar JW, Caminero JA, Gaga M, Gualano G, Skrahina A, Solovic I, Sulis G, Tadolini M, Alarcon Guizado V, De Lorenzo S, Roby Arias AJ, Scardigli A, Akkerman OW, Aleksa A, Artsukevich J, Auchynka V, Bonini EH, Chong Marin FA, Collahuazo Lopez L, de Vries G, Dore S, Kunst H, Matteelli A, Moschos C, Palmieri F, Papavasileiou A, Payen MC, Piana A, Spanevello A, Vargas Vasquez D, Viggiani P, White V, Zumla A, Migliori GB. 2016. Comparison of effectiveness and safety of imipenem/clavulanate- versus meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur Respir J 47:1758–1766. doi: 10.1183/13993003.00214-2016. [DOI] [PubMed] [Google Scholar]
- 11.Tiberi S, Payen MC, Sotgiu G, D'Ambrosio L, Alarcon Guizado V, Alffenaar JW, Abdo Arbex M, Caminero JA, Centis R, De Lorenzo S, Gaga M, Gualano G, Roby Arias AJ, Scardigli A, Skrahina A, Solovic I, Sulis G, Tadolini M, Akkerman OW, Alarcon Arrascue E, Aleska A, Avchinko V, Bonini EH, Chong Marin FA, Collahuazo Lopez L, de Vries G, Dore S, Kunst H, Matteelli A, Moschos C, Palmieri F, Papavasileiou A, Spanevello A, Vargas Vasquez D, Viggiani P, White V, Zumla A, Migliori GB. 2016. Effectiveness and safety of meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur Respir J 47:1235–1243. doi: 10.1183/13993003.02146-2015. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava S, van Rijn SP, Wessels AM, Alffenaar JW, Gumbo T. 2016. Susceptibility testing of antibiotics that degrade faster than the doubling time of slow-growing mycobacteria: ertapenem sterilizing effect versus Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:3193–3195. doi: 10.1128/AAC.02924-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rijn SP, van Altena R, Akkerman OW, van Soolingen D, van der Laan T, de Lange WC, Kosterink JG, van der Werf TS, Alffenaar JW. 2016. Pharmacokinetics of ertapenem in patients with multidrug-resistant tuberculosis. Eur Respir J 47:1229–1234. doi: 10.1183/13993003.01654-2015. [DOI] [PubMed] [Google Scholar]
- 14.Tiberi S, D'Ambrosio L, De Lorenzo S, Viggiani P, Centis R, Sotgiu G, Alffenaar JW, Migliori GB. 2016. Ertapenem in the treatment of multidrug-resistant tuberculosis: first clinical experience. Eur Respir J 47:333–336. doi: 10.1183/13993003.01278-2015. [DOI] [PubMed] [Google Scholar]
- 15.Gumbo T, Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 53:3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heifets L, Lindholm-Levy P. 1992. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am Rev Respir Dis 145:1223–1225. [DOI] [PubMed] [Google Scholar]
- 17.Mitchison DA. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 18.Gumbo T, Pasipanodya JG, Nuermberger E, Romero K, Hanna D. 2015. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin Infect Dis 61(Suppl 1):S18–S24. doi: 10.1093/cid/civ426. [DOI] [PubMed] [Google Scholar]
- 19.Gumbo T, Pasipanodya JG, Romero K, Hanna D, Nuermberger E. 2015. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin Infect Dis 61(Suppl 1):S25–S31. doi: 10.1093/cid/civ427. [DOI] [PubMed] [Google Scholar]
- 20.Pasipanodya JG, Nuermberger E, Romero K, Hanna D, Gumbo T. 2015. Systematic analysis of hollow fiber model of tuberculosis experiments. Clin Infect Dis 61(Suppl 1):S10–S17. doi: 10.1093/cid/civ425. [DOI] [PubMed] [Google Scholar]
- 21.Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. 2015. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis 211(Suppl 3):S83–S95. doi: 10.1093/infdis/jiv183. [DOI] [PubMed] [Google Scholar]
- 22.Akaike H. 1974. A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723. [Google Scholar]
- 23.Swaminathan S, Pasipanodya JG, Ramachandran G, Hemanth Kumar AK, Srivastava S, Deshpande D, Nuermberger E, Gumbo T. 2016. Drug concentration thresholds predictive of therapy failure and death in children with tuberculosis: bread crumb trails in random forests. Clin Infect Dis 63:S63–S74. doi: 10.1093/cid/ciw471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chigutsa E, Pasipanodya JG, Visser ME, van Helden PD, Smith PJ, Sirgel FA, Gumbo T, McIlleron H. 2015. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 59:38–45. doi: 10.1128/AAC.03931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modongo C, Pasipanodya JG, Magazi BT, Srivastava S, Zetola NM, Williams SM, Sirugo G, Gumbo T. 25 July 2016. Artificial intelligence and amikacin exposures predictive of outcome in multidrug-resistant tuberculosis patients. Antimicrob Agents Chemother doi: 10.1128/AAC.00962-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasipanodya JG, Srivastava S, Gumbo T. 2012. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis 55:169–177. doi: 10.1093/cid/cis353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava S, Pasipanodya JG, Ramachandran G, Deshpande D, Shuford S, Crosswell HE, Cirrincione KN, Sherman CM, Swaminathan S, Gumbo T. 2016. A long-term co-perfused disseminated tuberculosis-3D liver hollow fiber model for both drug efficacy and hepatotoxicity in babies. EBioMedicine 6:126–138. doi: 10.1016/j.ebiom.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis 204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burkhardt O, Kumar V, Katterwe D, Majcher-Peszynska J, Drewelow B, Derendorf H, Welte T. 2007. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. J Antimicrob Chemother 59:277–284. doi: 10.1093/jac/dkl485. [DOI] [PubMed] [Google Scholar]
- 31.Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. 2015. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis 211 (Suppl 3):S96–S106. doi: 10.1093/infdis/jiu610. [DOI] [PubMed] [Google Scholar]
- 32.Romero K, Sinha V, Allerheiligen S, Danhof M, Pinheiro J, Kruhlak N, Wang Y, Wang SJ, Sauer JM, Marier JF, Corrigan B, Rogers J, Lambers Heerspink HJ, Gumbo T, Vis P, Watkins P, Morrison T, Gillespie W, Gordon MF, Stephenson D, Hanna D, Pfister M, Lalonde R, Colatsky T. 2014. Modeling and simulation for medical product development and evaluation: highlights from the FDA-C-Path-ISOP 2013 workshop. J Pharmacokinet Pharmacodyn 41:545–552. doi: 10.1007/s10928-014-9390-0. [DOI] [PubMed] [Google Scholar]
- 33.Burkhardt O, Majcher-Peszynska J, Borner K, Mundkowski R, Drewelow B, Derendorf H, Welte T. 2005. Penetration of ertapenem into different pulmonary compartments of patients undergoing lung surgery. J Clin Pharmacol 45:659–665. doi: 10.1177/0091270005276117. [DOI] [PubMed] [Google Scholar]
- 34.Gumbo T, Chigutsa E, Pasipanodya J, Visser M, van Helden PD, Sirgel FA, McIlleron H. 2014. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 69:2420–2425. doi: 10.1093/jac/dku136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gumbo T, Pasipanodya JG, Wash P, Burger A, McIlleron H. 2014. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob Agents Chemother 58:6111–6115. doi: 10.1128/AAC.03549-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng X, Zheng R, Hu Y, Werngren J, Davies FL, Mansjo M, Xu B, Hoffner S. 31 May 2016. Determination of minimum inhibitory concentration (MIC) breakpoints for second-line drugs associated with clinical outcomes in multidrug-resistant tuberculosis treatment in China. Antimicrob Agents Chemother doi: 10.1128/AAC.03008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies Forsman L, Giske CG, Bruchfeld J, Schon T, Jureen P, Angeby K. 2015. Meropenem-clavulanate has high in vitro activity against multidrug-resistant Mycobacterium tuberculosis. Int J Mycobacteriol 4(Suppl 1):S80–S81. doi: 10.1016/j.ijmyco.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalo X, Drobniewski F. 2013. Is there a place for beta-lactams in the treatment of multidrug-resistant/extensively drug-resistant tuberculosis? Synergy between meropenem and amoxicillin/clavulanate. J Antimicrob Chemother 68:366–369. doi: 10.1093/jac/dks395. [DOI] [PubMed] [Google Scholar]
- 39.Sotgiu G, D'Ambrosio L, Centis R, Tiberi S, Esposito S, Dore S, Spanevello A, Migliori GB. 2016. Carbapenems to treat multidrug and extensively drug-resistant tuberculosis: a systematic review. Int J Mol Sci 17:373. doi: 10.3390/ijms17030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiskirchen DE, Housman ST, Quintiliani R, Nicolau DP, Kuti JL. 2013. Comparative pharmacokinetics, pharmacodynamics, and tolerability of ertapenem 1 gram/day administered as a rapid 5-minute infusion versus the standard 30-minute infusion in healthy adult volunteers. Pharmacotherapy 33:266–274. doi: 10.1002/phar.1197. [DOI] [PubMed] [Google Scholar]
- 41.Majumdar AK, Musson DG, Birk KL, Kitchen CJ, Holland S, McCrea J, Mistry G, Hesney M, Xi L, Li SX, Haesen R, Blum RA, Lins RL, Greenberg H, Waldman S, Deutsch P, Rogers JD. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob Agents Chemother 46:3506–3511. doi: 10.1128/AAC.46.11.3506-3511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teppler H, Gesser RM, Friedland IR, Woods GL, Meibohm A, Herman G, Mistry G, Isaacs R. 2004. Safety and tolerability of ertapenem. J Antimicrob Chemother 53(Suppl 2):ii75–ii81. doi: 10.1093/jac/dkh209. [DOI] [PubMed] [Google Scholar]
- 43.Carlier M, Noe M, De Waele JJ, Stove V, Verstraete AG, Lipman J, Roberts JA. 2013. Population pharmacokinetics and dosing simulations of amoxicillin/clavulanic acid in critically ill patients. J Antimicrob Chemother 68:2600–2608. doi: 10.1093/jac/dkt240. [DOI] [PubMed] [Google Scholar]
- 44.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gumbo T, Louie A, Liu W, Brown D, Ambrose PG, Bhavnani SM, Drusano GL. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother 51:2329–2336. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. 2010. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J Infect Dis 201:1225–1231. doi: 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musuka S, Srivastava S, Siyambalapitiyage Dona CW, Meek C, Leff R, Pasipanodya J, Gumbo T. 2013. Thioridazine pharmacokinetic-pharmacodynamic parameters “wobble” during treatment of tuberculosis: a theoretical basis for shorter-duration curative monotherapy with congeners. Antimicrob Agents Chemother 57:5870–5877. doi: 10.1128/AAC.00829-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Rijn SP, Wessels AM, Greijdanus B, Touw DJ, Alffenaar JW. 2014. Quantification and validation of ertapenem using a liquid chromatography-tandem mass spectrometry method. Antimicrob Agents Chemother 58:3481–3484. doi: 10.1128/AAC.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]