ABSTRACT
Enteropathogenic Escherichia coli (EPEC) is a leading cause of severe infantile diarrhea in developing countries. Previous research has focused on the diversity of the EPEC virulence plasmid, whereas less is known regarding the genetic content and distribution of antibiotic resistance plasmids carried by EPEC. A previous study demonstrated that in addition to the virulence plasmid, reference EPEC strain B171 harbors a second, larger plasmid that confers antibiotic resistance. To further understand the genetic diversity and dissemination of antibiotic resistance plasmids among EPEC strains, we describe the complete sequence of an antibiotic resistance plasmid from EPEC strain B171. The resistance plasmid, pB171_90, has a completed sequence length of 90,229 bp, a GC content of 54.55%, and carries protein-encoding genes involved in conjugative transfer, resistance to tetracycline (tetA), sulfonamides (sulI), and mercury, as well as several virulence-associated genes, including the transcriptional regulator hha and the putative calcium sequestration inhibitor (csi). In silico detection of the pB171_90 genes among 4,798 publicly available E. coli genome assemblies indicates that the unique genes of pB171_90 (csi and traI) are primarily restricted to genomes identified as EPEC or enterotoxigenic E. coli. However, conserved regions of the pB171_90 plasmid containing genes involved in replication, stability, and antibiotic resistance were identified among diverse E. coli pathotypes. Interestingly, pB171_90 also exhibited significant similarity with a sequenced plasmid from Shigella dysenteriae type I. Our findings demonstrate the mosaic nature of EPEC antibiotic resistance plasmids and highlight the need for additional sequence-based characterization of antibiotic resistance plasmids harbored by pathogenic E. coli.
KEYWORDS: EPEC, Escherichia coli, mosaic, plasmid
INTRODUCTION
There has been an alarming increase in the frequency of antibiotic resistance in bacterial pathogens. Multidrug-resistant Enterobacteriaceae are of particular concern and include some groups of disease-causing Escherichia coli (1–3). E. coli strains have been identified that are resistant to all routinely prescribed antibiotics, including colistin, which is a last resort treatment option for carbapenem-resistant Enterobacteriaceae (4–7). The most widely reported antibiotic-resistant E. coli strains are those belonging to multilocus sequence type (MLST) 131 (ST131), which are usually identified as extraintestinal pathogenic E. coli (ExPEC) and often exhibit resistance to fluoroquinolones, as well as extended-spectrum cephalosporins (1, 8). E. coli can also serve as a reservoir of antibiotic resistance genes, including mph(A), which confers resistance to macrolides such as azithromycin (9). E. coli strains from healthy volunteers in different geographic locations were also identified with resistance to antibiotics, including ampicillin, trimethoprim, and ciprofloxacin (10), and E. coli strains carrying tetracycline resistance genes were isolated from infants that had not received tetracycline (11). E. coli resistance genes and elements have been demonstrated to exist in fecal E. coli reservoirs and spread internationally for decades without detection (12).
Previous studies have also demonstrated that other pathotypes of disease-causing E. coli, such as the enteropathogenic E. coli (EPEC), can exhibit resistance to one or more antibiotics (13–17). One study demonstrated that EPEC strains from Brazil exhibited resistance to tetracycline, sulfonamides, ampicillin, chloramphenicol, and streptomycin (18). EPEC is one of six diarrheagenic pathotypes (EPEC, enterohemorrhagic E. coli [EHEC], enterotoxigenic E. coli [ETEC], enteroaggregative E. coli [EAEC], enteroinvasive E. coli [EIEC], and diffusely adherent E. coli [DAEC]) of disease-causing E. coli that cause human gastrointestinal illness (19–21). EPEC was also recently characterized in the Global Enteric Multisite Study as a leading cause of lethal diarrheal illness among infants in developing countries (22). Similar to the other E. coli pathotypes, EPEC can be molecularly classified by the presence of canonical virulence factors that are associated with mechanisms of pathogenesis or by phenotypes that are unique to each of the E. coli pathotypes (19–21). EPEC and EHEC are collectively called the attaching and effacing E. coli (AEEC) due to the chromosomally encoded locus of enterocyte effacement (LEE) pathogenicity island, which contains the intimin protein, involved in adherence, and a type III secretion system that delivers effector proteins into host cells (19, 20, 21, 23, 24). In addition to the LEE region, EHEC contains the Shiga toxin, which is carried by a phage and is absent from EPEC (20, 25). Meanwhile, EPEC strains are subclassified as typical EPEC (tEPEC) strains that contain the plasmid-encoded bundle-forming pilus (BFP) or atypical EPEC (aEPEC) strains that contain the LEE region but do not have the Shiga toxin genes or BFP genes (21).
E. coli plasmids and phage carry many of the virulence genes that contribute to differences in pathogenesis, and these virulence genes are used to distinguish among the different E. coli pathotypes (19, 20, 26). Many of the virulence plasmids and virulence-associated phage of E. coli have been sequenced and described in detail for the E. coli pathotypes, including EHEC, EPEC, ETEC, and EAEC (26–33). The EPEC adherence factor (EAF) virulence plasmid, which carries genes encoding the BFP of tEPEC, has been sequenced and described for several reference strains, including EPEC strains E2348/69 (29, 34) and B171 (28). In a previous study, we sequenced and compared the EAF plasmids from genomically diverse EPEC strains, demonstrating considerable differences in the gene content and stability of this canonical tEPEC virulence plasmid (35).
In contrast to our knowledge of the diversity of EPEC virulence plasmids, much less is known about the sequence diversity and distribution of EPEC antibiotic resistance plasmids. A large multidrug resistance plasmid from EPEC strain MB80 was previously characterized using subtractive hybridization (36). The traI and traC genes of the conjugative transfer system were absent from the virulence plasmid of EPEC reference strain E2348/69; however, they were detected in other EPEC strains, including the EPEC reference strain B171 (30, 36, 37). While portions of the MB80 plasmid were sequenced (36) and the draft genome sequence of EPEC strain B171 has been generated (30), the complete sequence of the resistance plasmid from these EPEC strains has not been previously described. Thus, to increase our understanding of EPEC antibiotic resistance plasmids, we sequenced the large, ∼90-kb antibiotic and heavy metal resistance plasmid, pB171_90, from the EPEC reference strain B171, which was previously determined to confer resistance to tetracycline, streptomycin, chloramphenicol, and sulfonamides (30, 36, 37). We describe the distribution of the pB171_90 genes among a publicly available collection of 4,798 E. coli genome assemblies in GenBank, which includes E. coli of diverse pathotypes as well as commensal E. coli that lack canonical pathotype-specific virulence genes. We also investigated whether the pB171_90 plasmid genes may have been disseminated to other species of enteric bacteria. Thus, the current study provides a comprehensive description of the genetic content of an antibiotic resistance plasmid from a tEPEC reference strain as well as the distribution of the plasmid genes among a diverse collection of E. coli and other enteric pathogens.
RESULTS
Sequence characterization of the large antibiotic resistance plasmid, pB171_90, from EPEC strain B171.
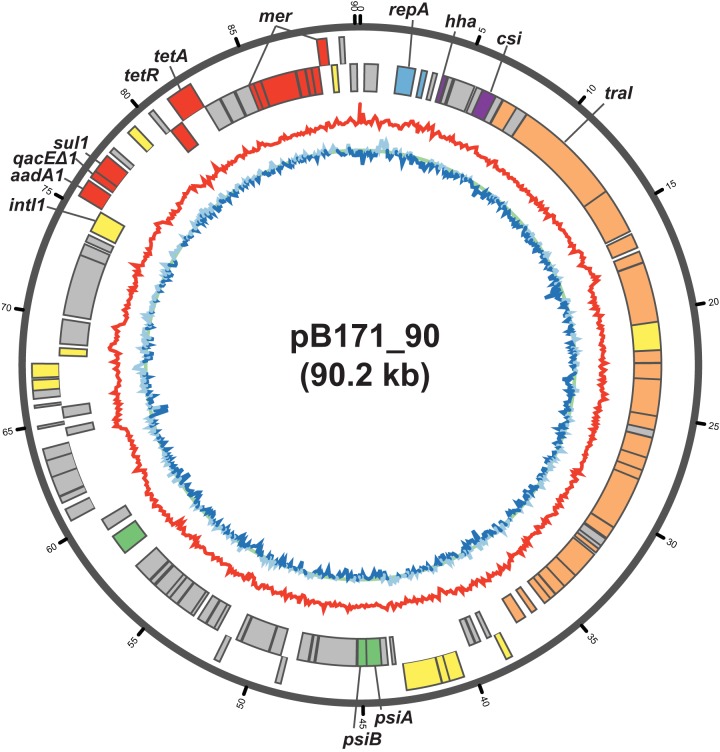
In the current study, we describe the complete sequence of the large antibiotic resistance plasmid from EPEC strain B171 to gain additional insight into its genetic content and potential role in antibiotic resistance and/or pathogenesis. We have designated this plasmid pB171_90 (GenBank accession number CP021212), as it is from EPEC strain B171 and has a sequence length of 90,229 bp. Plasmid pB171_90 has a GC content of 54.55% and contains 108 predicted protein-coding genes (Fig. 1). The predicted functions of the pB171_90 protein-coding genes include conjugative transfer, plasmid stability, and antibiotic resistance (Table 1; Fig. 1). Among the pB171_90 antibiotic resistance genes are a spectinomycin-streptomycin resistance gene (aadA1, pB171_90_88) (GenBank accession number NG_052027.1) (38), tetA (pB171_90_95) encoding a tetracycline efflux pump, and tetR (pB171_90_94) encoding the tetA repressor (39–41), a multidrug efflux pump (qacEΔ1, pB171_90_89) (GenBank accession number L06822.4) (42), and a dihydropteroate synthase gene, which can confer resistance to sulfonamides (sul1, pB171_90_90) (GenBank accession number NC_006671.1) (43) (Table 1). The genes for spectinomycin-streptomycin resistance (aadA1), sulfonamide resistance (sul1), and the qacEΔ1 efflux pump form a class 1 integron that includes a class 1 integrase gene, intI1 (GenBank accession number NC_007100.1), and is located within a region (coordinates 74,154 to 77,947) that has 100% nucleotide identity to a previously described Tn21 transposon from E. coli (GenBank accession number X12870.1) (44). Also carried on pB171_90 is a mer operon (pB171_90_99 to pB171_90_105), which contains all of the mer genes conferring narrow-spectrum mercury resistance, but the plasmid is lacking merB and merG, which are involved in organomercury detoxification and broad-spectrum mercury resistance (45).
FIG 1.
Circular plot of pB171_90. The outermost track of the plot contains the protein-coding genes on the positive strand, while the adjacent track contains the protein-coding genes on the negative strand. The predicted protein functions are indicated by colors as follows: replication (blue), virulence (purple), antibiotic resistance (red), conjugative transfer (orange), plasmid stability (green), transposition (yellow), and unknown (gray). The red line is the GC content along a 100-bp sliding window. The blue line is the GC skew along a 100-bp sliding window, with light blue indicating a positive skew and dark blue indicating a negative skew. The circular plot was generated using Circos 0.69-3 (80).
TABLE 1.
Annotation of the EPEC strain B171 resistance plasmid, pB171_90
| Gene ID or CDSb | Gene | Predicted protein function | Coding strand | Coordinates (bp) |
No. of E. coli genomes (%)a | |
|---|---|---|---|---|---|---|
| Start | Stop | |||||
| pB171_90_1 | CAAX protease self-immunity family protein | − | 850 | 197 | 956 (19.92) | |
| pB171_90_2 | repA | IncFII family plasmid replication initiator RepA | − | 2,649 | 1,792 | 3,214 (66.99) |
| pB171_90_3 | repB | Replication regulatory RepB family protein | − | 3,214 | 2,954 | 435 (9.07) |
| pB171_90_4 | Hok/Gef family protein | − | 3,704 | 3,498 | 1,359 (28.32) | |
| pB171_90_5 | hha | Hemolysin expression-modulating protein Hha | − | 4,202 | 3,993 | 2,209 (46.04) |
| pB171_90_6 | Conserved hypothetical protein | − | 4,494 | 4,258 | 33 (0.69) | |
| pB171_90_7 | Conserved hypothetical protein | − | 5,594 | 4,581 | 57 (1.19) | |
| pB171_90_8 | Putative gp46 | − | 6,009 | 5,701 | 58 (1.21) | |
| pB171_90_9 | csi | Putative Csi protein | − | 6,716 | 6,006 | 31 (0.65) |
| pB171_90_10 | Conserved hypothetical protein | − | 7,066 | 6,713 | 31 (0.65) | |
| pB171_90_11 | traX | Type F conjugative transfer system pilin acetylase TraX | − | 7,839 | 7,108 | 147 (3.06) |
| pB171_90_12 | Conserved hypothetical protein | − | 8,402 | 7,836 | 151 (3.15) | |
| pB171_90_13 | traI | Conjugative transfer relaxase protein TraI | − | 13,717 | 8,420 | 134 (2.79) |
| pB171_90_14 | traD | Type IV conjugative transfer system coupling protein TraD | − | 15,948 | 13,717 | 135 (2.81) |
| pB171_90_15 | traT | Enterobacterial TraT complement resistance family protein | − | 16,802 | 16,092 | 3,017 (62.88) |
| pB171_90_16 | traS | Putative protein TraS | − | 17,549 | 17,004 | 71 (1.48) |
| pB171_90_17 | traG | TraG-like, N-terminal region family protein | − | 20,474 | 17,571 | 134 (2.79) |
| pB171_90_18 | Conjugative relaxosome accessory transposon family protein | − | 21,837 | 20,476 | 135 (2.81) | |
| pB171_90_19 | trbB | Type F conjugative transfer system pilin assembly thiol-disulfide isomerase TrbB | − | 22,445 | 21,843 | 135 (2.81) |
| pB171_90_20 | traF | Type F conjugative transfer system pilin assembly protein TraF | − | 23,248 | 22,469 | 134 (2.79) |
| pB171_90_21 | traN | Type F conjugative transfer system mating-pair stabilization protein TraN | − | 25,059 | 23,245 | 133 (2.77) |
| pB171_90_22 | trbC | Type F conjugative transfer system pilin assembly protein TrbC | − | 25,693 | 25,013 | 132 (2.75) |
| pB171_90_23 | Conserved hypothetical protein | − | 26,058 | 25,690 | 113 (2.36) | |
| pB171_90_24 | traU | TraU family protein | − | 27,055 | 26,069 | 2,118 (44.14) |
| pB171_90_25 | traW | Type F conjugative transfer system protein TraW | − | 27,687 | 27,052 | 138 (2.88) |
| pB171_90_26 | trbI | Type F conjugative transfer system protein TrbI | − | 28,151 | 27,687 | 132 (2.75) |
| pB171_90_27 | traC | Type IV secretion system protein TraC | − | 30,739 | 28,148 | 134 (2.79) |
| pB171_90_28 | traV | Type IV conjugative transfer system protein TraV | − | 31,349 | 30,750 | 137 (2.86) |
| pB171_90_29 | Conserved hypothetical protein | − | 31,819 | 31,409 | 99 (2.06) | |
| pB171_90_30 | Conserved hypothetical protein | − | 31,988 | 31,803 | 102 (2.13) | |
| pB171_90_31 | traR | Prokaryotic DksA/TraR C4-type zinc finger family protein | − | 32,216 | 31,992 | 140 (2.92) |
| pB171_90_32 | trbI | Bacterial conjugation TrbI-like family protein | − | 33,692 | 32,328 | 136 (2.83) |
| pB171_90_33 | traK | Type F conjugative transfer system secretin TraK | − | 34,419 | 33,679 | 138 (2.88) |
| pB171_90_34 | traE | Type IV conjugative transfer system protein TraE | − | 34,972 | 34,409 | 167 (3.48) |
| pB171_90_35 | traL | Type IV conjugative transfer system protein TraL | − | 35,297 | 34,992 | 178 (3.71) |
| pB171_90_36 | traA | Type IV conjugative transfer system pilin TraA | − | 35,658 | 35,299 | 193 (4.02) |
| pB171_90_37 | traJ | Putative TraJ protein | − | 36,419 | 36,138 | 97 (2.02) |
| pB171_90_38 | traM | Relaxosome TraM domain protein | − | 37,283 | 36,783 | 71 (1.48) |
| pB171_90_39 | Integrase core domain protein | + | 38,211 | 38,504 | 29 (0.60) | |
| pB171_90_40 | Putative membrane protein | − | 38,880 | 38,665 | 257 (5.36) | |
| pB171_90_41 | Conserved hypothetical protein | − | 39,463 | 39,221 | 363 (7.57) | |
| pB171_90_42 | Conserved hypothetical protein | − | 39,786 | 39,505 | 2,115 (44.08) | |
| pB171_90_43 | Transposase family protein | + | 40,493 | 41,143 | 497 (10.36) | |
| pB171_90_44 | Putative transposase | + | 41,143 | 41,490 | 4,145 (86.39) | |
| pB171_90_45 | Transposase C of IS166 homeodomain protein | + | 41,510 | 43,081 | 1,225 (25.53) | |
| pB171_90_46 | Hok/Gef family protein | − | 43,577 | 43,416 | 2,991 (62.34) | |
| pB171_90_47 | Conserved hypothetical protein | − | 44,099 | 43,785 | 559 (11.65) | |
| pB171_90_48 | psiA | PsiA family protein | − | 44,815 | 44,096 | 2,803 (58.42) |
| pB171_90_49 | psiB | Protein PsiB | − | 45,246 | 44,812 | 3,155 (65.76) |
| pB171_90_50 | ParB-like nuclease domain protein | − | 47,268 | 45,301 | 2,661 (55.46) | |
| pB171_90_51 | Conserved hypothetical protein | − | 47,563 | 47,330 | 3,119 (65.01) | |
| pB171_90_52 | ssbF | Plasmid-derived single-stranded DNA-binding protein | − | 48,147 | 47,620 | 3,080 (64.19) |
| pB171_90_53 | Putative YddA protein | + | 48,630 | 48,875 | 1,310 (27.30) | |
| pB171_90_54 | Methyltransferase small domain protein | − | 49,524 | 48,961 | 2,546 (53.06) | |
| pB171_90_55 | Conserved hypothetical protein | − | 50,930 | 49,569 | 2,554 (53.23) | |
| pB171_90_56 | Putative conserved predicted protein | − | 51,212 | 50,982 | 2,622 (54.65) | |
| pB171_90_57 | Conserved hypothetical protein | + | 51,475 | 51,732 | 2,354 (49.06) | |
| pB171_90_58 | Putative YchA | − | 52,404 | 52,213 | 3,245 (67.63) | |
| pB171_90_59 | Conserved hypothetical protein | − | 52,823 | 52,401 | 3,210 (66.90) | |
| pB171_90_60 | Antirestriction family protein | − | 53,295 | 52,870 | 3,241 (67.55) | |
| pB171_90_61 | Putative YciA | − | 53,705 | 53,544 | 2,014 (41.98) | |
| pB171_90_62 | Putative YfdA | − | 54,478 | 53,708 | 2,911 (60.67) | |
| pB171_90_63 | Conserved hypothetical protein | − | 54,957 | 54,523 | 3,120 (65.03) | |
| pB171_90_64 | Conserved hypothetical protein | − | 55,192 | 54,971 | 3,039 (63.34) | |
| pB171_90_65 | DNA methylase family protein | − | 55,876 | 55,193 | 3,149 (65.63) | |
| pB171_90_66 | Putative YfbA | − | 56,203 | 55,952 | 1,906 (39.72) | |
| pB171_90_67 | Conserved hypothetical protein | − | 57,187 | 56,261 | 2,675 (55.75) | |
| pB171_90_68 | parM | Plasmid segregation protein ParM | − | 58,977 | 57,997 | 385 (8.02) |
| pB171_90_69 | Conserved hypothetical protein | − | 59,932 | 59,366 | 347 (7.23) | |
| pB171_90_70 | Bacterial regulatory, TetR family protein | + | 60,580 | 61,137 | 144 (3) | |
| pB171_90_71 | Conserved hypothetical protein | + | 61,446 | 61,736 | 142 (2.96) | |
| pB171_90_72 | Conserved hypothetical protein | + | 61,768 | 62,694 | 143 (2.98) | |
| pB171_90_73 | Conserved hypothetical protein | + | 62,663 | 63,421 | 141 (2.94) | |
| pB171_90_74 | DoxX family protein | + | 63,423 | 63,950 | 141 (2.94) | |
| pB171_90_75 | Conserved hypothetical protein | − | 64,645 | 64,283 | 123 (2.56) | |
| pB171_90_76 | Hypothetical protein | + | 64,852 | 64,971 | 395 (8.23) | |
| pB171_90_77 | def | Peptide deformylase | − | 65,655 | 65,146 | 4,764 (99.29) |
| pB171_90_78 | Hypothetical protein | + | 65,785 | 65,895 | 144 (3) | |
| pB171_90_79 | Conserved hypothetical protein | + | 66,183 | 66,548 | 1,079 (22.49) | |
| pB171_90_80 | Transposase zinc-binding domain protein | + | 66,548 | 67,015 | 1,440 (30.01) | |
| pB171_90_81 | Transposase family protein | + | 67,109 | 67,735 | 906 (18.88) | |
| pB171_90_82 | Transposase family protein | − | 68,490 | 68,125 | 3,488 (72.70) | |
| pB171_90_83 | Conserved hypothetical protein | − | 69,867 | 68,692 | 71 (1.48) | |
| pB171_90_84 | Conserved hypothetical protein | − | 73,048 | 70,082 | 797 (16.61) | |
| pB171_90_85 | Resolvase, N-terminal domain protein | − | 73,611 | 73,051 | 865 (18.03) | |
| pB171_90_86 | Conserved hypothetical protein | − | 74,087 | 73,737 | 794 (16.55) | |
| pB171_90_87 | intI1 | Integron integrase family protein | − | 75,303 | 74,290 | 1,093 (22.78) |
| pB171_90_88 | aadA1 | Streptomycin 3''-adenylyltransferase | + | 75,452 | 76,243 | 569 (11.86) |
| pB171_90_89 | qacEΔ1 | Small multidrug resistance family protein | + | 76,407 | 76,754 | 957 (19.95) |
| pB171_90_90 | sul1 | Dihydropteroate synthase | + | 76,748 | 77,587 | 942 (19.63) |
| pB171_90_91 | Putative Achromobacter xylosoxidans subsp. denitrificans plasmid pAX22, partial DNA for integron In70 | + | 77,784 | 78,041 | 345 (7.19) | |
| pB171_90_92 | Transposase DDE domain protein | + | 78,944 | 79,366 | 57 (1.19) | |
| pB171_90_93 | Putative relaxase/helicase | + | 80,191 | 80,433 | 897 (18.70) | |
| pB171_90_94 | tetR | Tetracycline repressor protein class B from transposon Tn10 | − | 81,115 | 80,465 | 956 (19.92) |
| pB171_90_95 | tetA | Tetracycline resistance protein, class C | + | 81,221 | 82,420 | 1,008 (21.01) |
| pB171_90_96 | Multidrug resistance efflux transporter family protein | − | 83,234 | 82,452 | 841 (17.53) | |
| pB171_90_97 | Helix-turn-helix domain protein | − | 84,004 | 83,297 | 809 (16.86) | |
| pB171_90_98 | EAL domain protein | − | 84,786 | 84,079 | 702 (14.63) | |
| pB171_90_99 | merE | MerE family protein | − | 85,019 | 84,783 | 715 (14.90) |
| pB171_90_100 | merD | Mercuric resistance transcriptional repressor protein MerD | − | 85,378 | 85,016 | 694 (14.46) |
| pB171_90_101 | merA | Mercuric reductase | − | 87,090 | 85,396 | 683 (14.24) |
| pB171_90_102 | merC | MerC mercury resistance family protein | − | 87,564 | 87,142 | 685 (14.28) |
| pB171_90_103 | merP | Mercuric transport protein periplasmic component | − | 87,875 | 87,600 | 720 (15.01) |
| pB171_90_104 | merT | MerT mercuric transport family protein | − | 88,239 | 87,889 | 733 (15.28) |
| pB171_90_105 | merR | Hg(II)-responsive transcriptional regulator | + | 88,311 | 88,745 | 757 (15.78) |
| pB171_90_106 | Putative transposase | − | 89,118 | 88,852 | 3,807 (79.35) | |
| pB171_90_107 | Hypothetical protein | + | 89,301 | 89,516 | 3,793 (79.05) | |
| pB171_90_108 | pemK | mRNA interferase PemK | − | 90,074 | 89,742 | 1,073 (22.36) |
The number and percentage of publicly available E. coli genomes that contained each gene with a BSR of ≥0.7. The total number of E. coli genome assemblies that were available in GenBank as of November 2016 was 4,798.
CDS, protein-coding genes.
The resistance plasmid, pB171_90, also carries two genes that were present on the EPEC resistance plasmid and not on the EPEC virulence plasmid and were previously suggested to have a role in pathogenesis (36). One potential pathogenicity gene is csi, a putative calcium sequestration inhibitor (36), whose function in virulence is poorly understood. The second virulence-associated gene of pB171_90, hha, encodes a transcriptional regulator of known virulence genes, including a hemolysin (46), genes involved in adherence and biofilm formation (47, 48), and genes involved in type III secretion (49). The hha gene has been identified on the chromosome and a virulence plasmid of E. coli O157:H7 Shiga toxin-producing E. coli (STEC) strains (50).
While sequencing the resistance plasmid of E. coli strain B171 using the Pacific Biosciences (PacBio) platform, we also took the opportunity to resequence the B171 EAF plasmid (here referred to as the virulence plasmid). The original sequence of the pB171 virulence plasmid was previously assembled from Sanger-sequenced plasmid subclones produced by different groups nearly 2 decades ago (28). To distinguish the PacBio-sequenced version of the plasmid from the original version, which is designated pB171 (GenBank accession number AB024946.1) (28), we have named the newly PacBio-sequenced version of the B171 virulence plasmid pB171_69 (GenBank accession number CP021211). The PacBio-sequenced virulence plasmid, pB171_69, has a GC content of 46.02% and a sequence length of 68,814 bp. The pB171_69 plasmid sequence is nearly identical to the original virulence plasmid, pB171, which has a GC content of 46% and a sequence length of 68,817 bp (28). Comparison of the two virulence plasmid sequences demonstrated that there were 21 single nucleotide polymorphisms (SNPs) and seven insertions or deletions in pB171_69 compared to the original version of the plasmid (see Table S2 in the supplemental material). These differences were identified in only 7 of the 80 previously described protein-coding genes (Table S2) (28). Overall, this comparative analysis demonstrates that the B171 virulence plasmids that were sequenced nearly 2 decades apart using different sequencing technologies are remarkably similar. Further investigation is necessary to determine whether these sequence differences are caused by the sequencing technology or whether they represent genetic changes that have been acquired by different laboratory strains of B171 over time and may influence any phenotypes of B171.
Distribution of the pB171_90 genes among diverse E. coli genomes.
To determine the distribution of the pB171_90 genes among diverse E. coli genomes, we used large-scale BLAST score ratio (LS-BSR) analysis to conduct an in silico screen of 4,798 E. coli genome assemblies available in GenBank as of November 2016 for the presence of pB171_90 genes. LS-BSR analysis demonstrated that some of the genes, such as pB171_90_77 encoding a peptide deformylase, were present in nearly all of the E. coli genomes (4,764 of 4,798 genomes, 99%) (Table 1). Meanwhile, genes that were previously described as being unique to the B171 strain and a few other related EPEC strains had a narrow distribution among the 4,798 E. coli genomes analyzed. The csi gene encoding a putative calcium sequestration inhibitor (GenBank accession number Y08258) (36) was, prior to this study, identified as unique in the B171 strain, and the traI gene encoding a conjugative transfer relaxase protein had only been reported from EPEC resistance plasmids (36). In this study, a much broader and sequence-based analysis identified the csi gene with significant similarity (BSR ≥ 0.7) in 31 of the 4,798 E. coli genomes (0.65%), including B171 (Table 2). Unlike the very narrow distribution of the csi gene, the traI gene was detected in more but still relatively few E. coli genomes (134 genomes total; 2.79% of the E. coli genomes) that did not contain csi (Table 2). Also, both csi and traI were significantly more prevalent among the AEEC genomes, such as the EPEC, compared to the non-AEEC genomes (chi-squared P value < 0.05) (Table 2). All of the E. coli genomes that contained csi also contained the traI gene, except for the genome of EPEC strain 302014, which contained csi but not traI (see Table S1 in the supplemental material). Interestingly, while several of the pB171_90 genes were significantly more associated with AEEC genomes (IncFII repA, hha, csi, and traI genes), the antibiotic resistance genes (tetA, sul1, and mer genes) were more frequently associated with the non-AEEC genomes (Table 2). These results highlight the mosaic nature of these plasmids, with the resistance genes having a different distribution from other genes, such as csi and hha, that are unique to pB171_90 and related plasmids.
TABLE 2.
In silico detection of select pB171_90 genes in AEEC genomes versus other E. coli
| Gene | No. of genomes (%)a |
P value (non-AEEC vs AEEC)e | ||
|---|---|---|---|---|
| All E. colib | Non-AEECc | AEECd | ||
| repA | 3,214 (66.99) | 2,225 (65.89) | 989 (69.6) | 0.01379 |
| hha | 2,209 (46.04) | 1,306 (38.67) | 903 (63.55) | <2.2e−16 |
| csi | 31 (0.65) | 9 (0.27) | 22 (1.55) | 1.16e−06 |
| traI | 134 (2.79) | 83 (2.46) | 51 (3.59) | 0.03796 |
| tetR | 956 (19.92) | 780 (23.1) | 176 (12.39) | <2.2e−16 |
| tetA | 1,008 (21.01) | 806 (23.87) | 202 (14.22) | 9.04e−14 |
| sulI | 942 (19.63) | 802 (23.75) | 140 (9.85) | <2.2e−16 |
| mer (≥4 genes)f | 719 (14.99) | 594 (17.59) | 125 (8.8) | 9.44e−15 |
The total number of genomes that contained each of the pB171_90 genes listed. The number in parentheses is the percentage of genomes with each gene.
The total number of E. coli genomes analyzed was 4,798. These were all of the E. coli assemblies available in GenBank as of November 2016.
The total number of non-AEEC genomes analyzed was 3,377.
The total number of AEEC genomes analyzed was 1,421. The AEEC genomes were identified by in silico analysis as those that had the LEE gene escV.
The numbers of non-AEEC versus AEEC genomes that contained each gene were compared using the chi-square test.
The number of genomes that contained ≥4 of the 7 total genes in the pB171_90 mer operon.
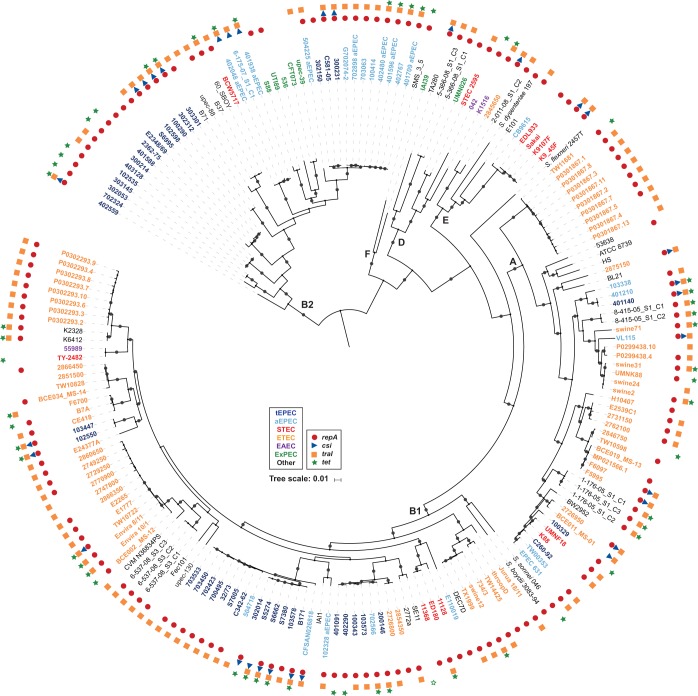
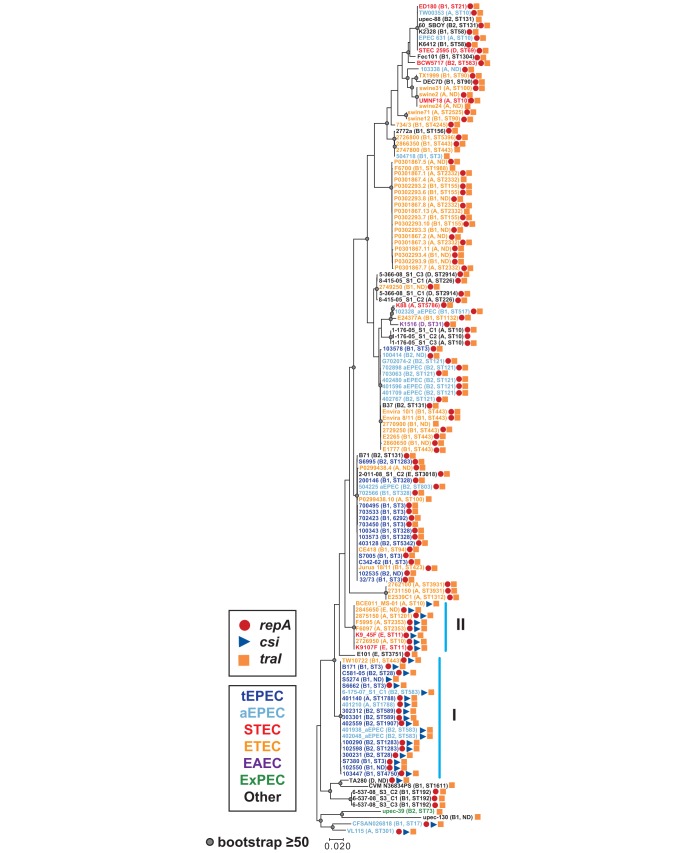
We further investigated the genome-wide diversity of the E. coli strains that contain the unique genes of pB171_90 (csi and traI) by constructing a whole-genome phylogeny. Phylogenomic analysis of the csi- and/or traI-containing genomes with a collection of diverse E. coli and Shigella reference genomes demonstrated that the csi- and/or traI-containing E. coli genomes occurred in five of the six E. coli phylogroups (A, B1, B2, D, and E) (Fig. 2). Overall, the genomes analyzed in the phylogenomic analysis grouped together into clades by pathotype (Fig. 2). The csi gene, which was among the pB171_90 genes that had the narrowest distribution, was identified in genomes belonging to four phylogroups, but these genomes were concentrated in multiple clades or doublets of ETEC or EPEC genomes (Fig. 2). EPEC strain B171 was present in a subclade of the EPEC2 lineage (51) that also includes other csi-containing EPEC genomes (Fig. 2). Interestingly, among the STEC genomes that contained the unique pB171_90 genes csi and traI were two strains (K9107F and K9_45F) that were phylogenomically related to genomes of O157:H7 strains and were determined by molecular serotyping to have the O157:H7 serotype (Fig. 2; Table S1). The findings from the phylogenomic analysis demonstrate that csi and traI have been acquired by genomically diverse E. coli and are not restricted to EPEC strains that are closely related to strain B171.
FIG 2.
Phylogenomic analysis of E. coli genomes that carry genes with similarity to those of pB171_90. A SNP-based phylogeny was generated using ISG as previously described (35, 70). There were 149,150 conserved SNP sites identified among all of the E. coli genomes analyzed relative to the reference E. coli strain IAI39 (GenBank accession number NC_011750.1) that were used to construct a maximum-likelihood phylogeny with 100 bootstrap values using RAxML v.7.2.8 (71). The phylogeny was midpoint rooted and labeled using iTOL v.3 (72). Bootstrap values of ≥80 are designated by a gray circle. The scale bar indicates the approximate distance of 0.01 nucleotide substitutions per site. The E. coli phylogroups B1, A, B2, D, E, and F are denoted by bold letters. Symbols designate the presence of pB171_90 genes in each genome with tetA and tetR both represented by green stars (both genes, solid green star; one gene, open green star). Colors of the genome labels indicate the presumptive pathotype that was determined by in silico detection of canonical virulence genes.
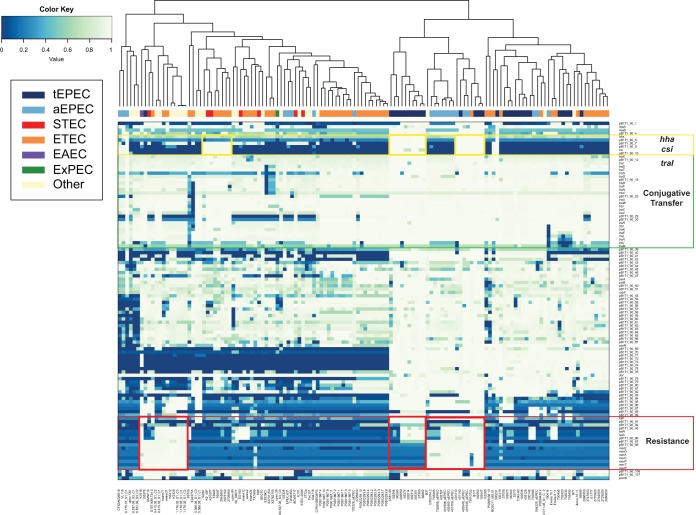
In silico detection of the 108 protein-coding genes of pB171_90 in the csi- and/or traI-containing genomes demonstrated that nearly all of the pB171_90 genes were present with significant similarity (BSR ≥ 0.7) in EPEC genomes 102550 and 103447 (Fig. 3). In addition, the genome of ETEC strain TW10722 contained nearly all of the pB171_90 genes but was lacking several genes from the mer operon (Fig. 3). The frequencies of genes in the different plasmid regions (putative virulence genes hha and csi, conjugative transfer genes, and resistance genes) were demonstrated in the clustered heatmap (Fig. 3). Since all but one of the genomes analyzed contain the traI gene, it was anticipated that these genomes would also contain most of the other genes involved in conjugative transfer (Fig. 3, green rectangle). The csi-containing genomes grouped together into several clusters (Fig. 3, yellow boxes); however, only two of the clusters also contained some or all of the resistance genes (Fig. 3, red boxes). The resistance genes were present mostly in genomes of three different clusters in the heatmap, of which two of the clusters also contained the csi and hha genes while the third cluster that did not (Fig. 3, red boxes). These results demonstrate that the unique genes of the pB171_90 plasmid (csi and hha) do not travel exclusively with the antibiotic resistance genes of the pB171_90 plasmid, highlighting the mosaic nature of pB171_90 and related plasmids.
FIG 3.
In silico detection of pB171_90 genes in diverse E. coli genomes. The presence or absence of all predicted protein-coding genes of pB171_90 in the 134 E. coli genomes that contained the pB171_90 genes csi and/or traI is indicated by the BSR values in the heatmap (see Table S1 in the supplemental material). The clustered heatmap was generated using the heatmap2 function of gplots v.3.0.1 in R v.3.3.2. The genomes were clustered using the complete linkage method with Euclidean distance estimation. The pB171_90 genes are represented by the rows, while each column represents a different E. coli genome. The predicted pathotype of each E. coli genome is indicated by the color of the rectangle at the top of the heatmap (see legend for detail).
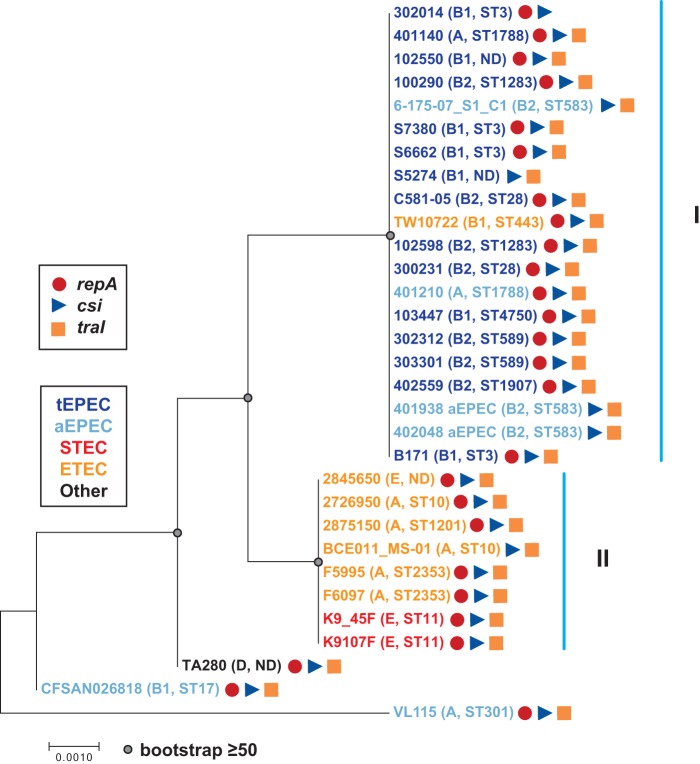
Phylogenetic analysis of the csi nucleotide sequences demonstrated that csi sequences from all but three of the genomes (aEPEC strains VL115 and CFSAN026818 and E. coli strain TA280) were present in two clades that we have designated clades I and II (Fig. 4). Clade I contained all of the csi sequences from tEPEC genomes, four of the csi sequences from aEPEC genomes, and one csi sequence from an ETEC genome (Fig. 4). The genomes of clade I belonged to phylogroups A, B1, and B2 and had at least 10 different MLSTs, while clade II contained csi sequences from six out of seven (85.7%) of the ETEC genomes and both of the STEC genomes (Fig. 4). Clade I genomes belonging to phylogroups A and E had four different MLSTs (Fig. 4). Thus, the csi genes exhibit the greatest similarity among genomes belonging to the same E. coli pathotype rather than from genomes of the same E. coli phylogroup.
FIG 4.
Phylogenetic analysis of csi. The csi nucleotide sequences of pB171_90 and other E. coli genomes were aligned using ClustalW. The alignment was used to construct a maximum-likelihood phylogeny with the Kimura 2-parameter model and 1,000 bootstraps using MEGA7 (79). The scale bar represents the approximate distance of 0.001 nucleotide substitutions per site. Bootstrap values of ≥50 are indicated by a gray circle. The predicted E. coli pathotypes are indicated by the color of their genome label (see legend), and the phylogroup and MLST of each genome are denoted in parentheses.
Phylogenetic analysis of the traI gene sequences demonstrated that there was greater pathotype specificity than phylogroup specificity (Fig. 5). There were clades of traI sequences from ETEC genomes of different phylogroups (A or B1) and other clades that contained nearly all EPEC genomes also from multiple phylogroups (A, B1, and B2). The genomes that formed clades I and II in the csi phylogeny formed similar clades in the traI phylogeny (Fig. 5). The consistent sequence similarity of the csi and traI genes occurring in the same genomes, along with the close proximity of csi (pB171_90_9) and traI (pB171_90_13) in the pB171_90 plasmid (Table 1), suggests that the csi and traI genes travel together.
FIG 5.
Phylogenetic analysis of traI. The traI nucleotide sequences of pB171_90 and other E. coli strains were aligned using ClustalW. The alignment was used to construct a maximum-likelihood phylogeny with the Kimura 2-parameter model and 1,000 bootstraps using MEGA7 (79). The scale bar represents the approximate distance of 0.02 nucleotide substitutions per site. Bootstrap values of ≥50 are indicated by a gray circle. The predicted E. coli pathotypes are indicated by the color of their genome label (see legend), and the phylogroup and MLST of each genome is denoted in parentheses. The blue vertical lines indicate the two clades identified in the csi phylogeny (Fig. 4).
The replication protein-coding gene of pB171_90, repA (pB171_90_2), was also significantly more associated with the AEEC genomes (69%) than with the non-AEEC genomes (65%), with a chi-squared P value of 0.013 (Table 2). The pB171_90 repA gene is most similar to the IncFIIA repA gene that has been identified in previously sequenced E. coli virulence plasmids, including plasmids from AEEC genomes (26, 35). Based on the IncF replicon typing scheme of Villa et al., the pB171 virulence plasmid belongs to the F13:A−:B4 IncF replicon type (52), while the resistance plasmid pB171_90 belongs to the F79:A−:B− replicon type as determined by plasmid multilocus sequence typing (pMLST) analysis (53). Alignment of the repA nucleotide sequences from plasmids pB171_90 and pB171_69 indicated that they have 95% nucleotide identity (816/858 identical bases). Thus, both plasmids have related, but distinct, plasmid replicon types. In a previous study investigating the diversity of AEEC plasmids, we also identified multiple IncFII replicon types in the same genome (35). In contrast to the formation of clades I and II by the csi-containing genomes in both the csi and traI phylogenies, phylogenetic analysis of repA sequences demonstrated that the similarity of the repA gene sequences differs among some of the csi-containing genomes. The repA sequences from genomes in clade I of the csi and traI phylogenies formed a similar clade, while the genomes from clade II did not form a clade and were instead distributed throughout the phylogeny (see Fig. S1 in the supplemental material). Interestingly, the genomes of clade I were primarily tEPEC and aEPEC genomes with one ETEC genome, while the genomes of clade II were ETEC and STEC (Fig. 4 and 5; Fig. S1). This suggests that the csi and traI genes were likely acquired as part of the same plasmid in the EPEC strains, whereas the csi and traI genes of the STEC and ETEC genomes are associated with plasmids that contain a different IncFII replicon type.
Another gene of interest on pB171_90 is the virulence gene transcriptional regulator, hha, which was identified in 46% of the E. coli genomes and was significantly more associated with AEEC genomes over non-AEEC genomes (chi-squared P value < 2.2e−16) (Tables 1 and 2). Similar to the repA gene, phylogenetic analysis of the hha genes identified in the csi and/or traI-containing genomes demonstrated that the clade I genomes from the csi phylogeny also grouped together in the hha phylogeny, while the clade II genomes were distributed throughout the phylogeny (see Fig. S2 in the supplemental material). Interestingly, the hha genes identified in the csi and/or traI-containing genomes were more similar to the hha genes from plasmid pB171_90 and the E. coli O157:H7 plasmid pO157 from strain EDL933 (Fig. S2). These findings suggest that the hha genes identified in the clade I genomes may be located on plasmids containing the repA, csi, and traI genes, demonstrating that plasmids with similarity to pB171_90 exist among genomically diverse EPEC strains (Fig. S2).
Identification of pB171_90 genes in the genomes of other human enteric pathogens.
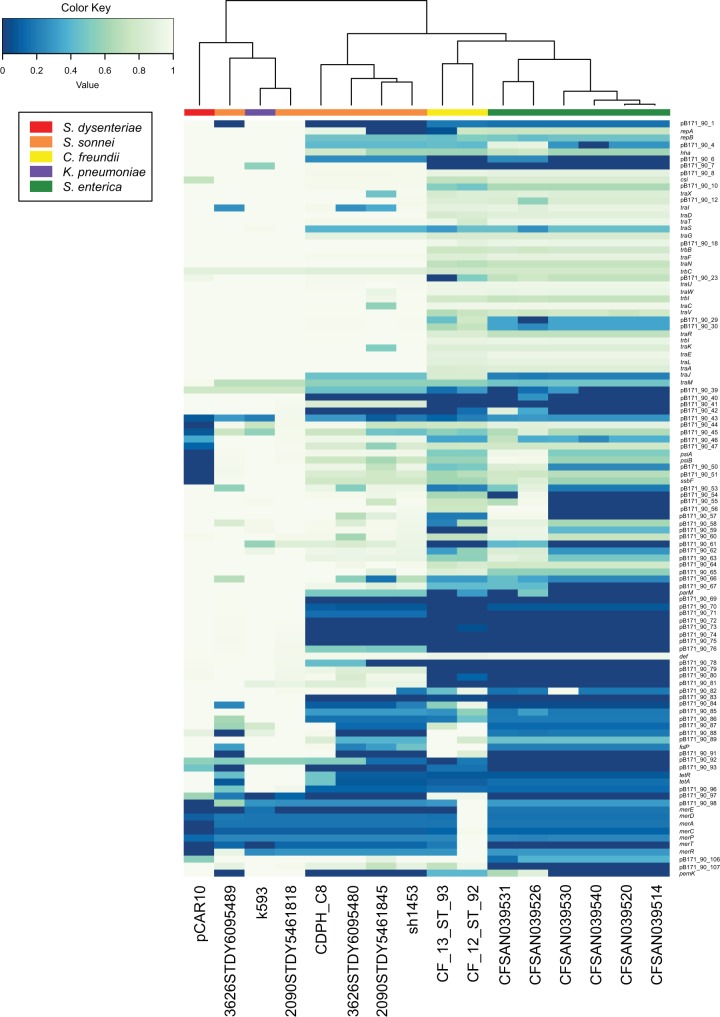
We further investigated whether unique genes of pB171_90, such as csi, were present in other species of bacteria by using BLASTP to compare the predicted amino acid sequence of the Csi peptide to the nonredundant (nr) protein sequence database of GenBank. This query identified 16 genome or plasmid sequences of enteric pathogens other than E. coli that contained a region encoding a protein with similarity to Csi (Fig. 6; Table S1). Among these non-E. coli csi-containing genomes were a Shigella dysenteriae type I plasmid, pCAR10 (GenBank accession number KT754161.1) (54), and whole-genome sequences of six Shigella sonnei, two Citrobacter freundii, one Klebsiella pneumoniae, and six Salmonella enterica strains (Fig. 6).
FIG 6.
In silico detection of pB171_90 genes in other human enteric pathogens. The predicted protein-coding genes of pB171_90 were identified in additional human enteric pathogens that contained the pB171_90 gene csi and are listed in Table S1 in the supplemental material. The clustered heatmap was generated using the heatmap2 function of gplots v.3.0.1 in R v.3.3.2. The genomes were clustered using the complete linkage method with Euclidean distance estimation. The pB171_90 genes are represented by the rows, while each column represents a different genome. The species are designated by the color of the rectangle at the top of the heatmap (see legend).
Phylogenetic analysis of the csi genes from the E. coli genomes compared with the csi genes that were identified in other human enteric pathogens demonstrated that the csi genes formed three different phylogenetic groups (see Fig. S3 in the supplemental material). Group 1 contained all of the csi genes from the E. coli genomes as well as those from the Shigella genomes (S. dysenteriae and S. sonnei) and the single sequence from a Klebsiella genome (Fig. S3). Group 2 contained only the csi sequences from Salmonella genomes, while group 3 was made up of the csi sequences from the Citrobacter genomes (Fig. S3). The csi phylogenetic analysis demonstrates that there is species specificity among the csi genes.
In silico detection of the 108 protein-coding genes of pB171_90 in each of the csi-containing enteric species genomes demonstrated that >50% of the plasmid genes were present with significant similarity (BSR ≥ 0.7) in the S. dysenteriae type I plasmid pCAR10 and also in the K. pneumoniae genome and two of the S. sonnei genomes (Fig. 6). The Tn21 transposon of pB171_90 that contains the aadA1, sul1, and qacEΔ1, was also detected with 100% nucleotide identity in sequenced plasmids and genomes from other species, including several plasmids from S. dysenteriae (p69-3818, GenBank accession number KT754167.1; p92-9000, GenBank accession number KT754166.1; and p80-547, GenBank accession number KT754160.1).
Comparison of the predicted protein-coding genes of the S. dysenteriae type I plasmid, pCAR10 (GenBank accession number KT754161.1) (54), with those of pB171_90, highlighted the significant similarity of these two antibiotic resistance carrying plasmids both in terms of gene content and order and also their levels of nucleotide similarity (Fig. 6; see also Fig. S4 in the supplemental material). Interestingly, the replication protein-coding gene, the csi gene, and the conjugative transfer genes were highly conserved (BSR ≥ 0.7) between the two plasmids (Fig. S4). However, some genes, such as those involved in plasmid stability and the antibiotic resistance genes, differed between pB171_90 and pCAR10 (Fig. S4). Of the antibiotic resistance genes identified on pB171_90, pCAR10 also carries the tetracycline resistance gene tetA, the tetA repressor tetR, and the sulfonamide resistance gene sul1 (Table 1; Fig. S4). However, pCAR10 contains additional antibiotic resistance genes that are missing from pB171_90, including a predicted blaTEM β-lactamase gene and a dihydrofolate reductase gene, dfrA15 (Table 1; Fig. S4). The pCAR10 plasmid also contains a small transporter designated emrE, which confers resistance to ethidium bromide, and has 100% nucleotide identity to the small multidrug resistance protein-coding gene qacEΔ1 (pB171_90_89) of pB171_90 (Table 1; Fig. S4). The similarity of pB171_90 genes to genes identified in other species indicates that EPEC resistance plasmids may be transferred between EPEC and other human pathogens, including S. dysenteriae type I.
DISCUSSION
An increased prevalence of antibiotic resistance among certain groups of disease-causing E. coli, and the identification of E. coli as a reservoir of antibiotic resistance for routinely prescribed antibiotics (1, 9, 10, 55), highlights the importance of investigating the diversity of plasmids involved in disseminating resistance genes among E. coli. The pB171_90 plasmid belongs to the IncFII plasmid family, which counts among its members many of the virulence plasmids of diverse pathotypes of E. coli, including the virulence plasmids of EPEC (26). IncFII plasmids have also been described that carry antibiotic resistance genes among E. coli and other members of the family Enterobacteriaceae (26, 52) and have been reported to carry extended-spectrum β-lactamases (ESBLs), such as blaCTX-M-15 (8, 56, 57). To provide insight into the diversity of resistance plasmids among EPEC strains, we provide the complete sequence and description of the antibiotic and heavy metal resistance plasmid, pB171_90, from the E. coli reference strain B171.
By investigating the presence of the pB171_90 genes among a large collection of E. coli genomes, we determined that genes such as csi and traI that were previously identified as unique to the B171 resistance plasmid (36) can be found in genomically diverse E. coli belonging to phylogroups A, B1, B2, D, and E (Fig. 2) as well as other enteric pathogens. Although the majority of the genomes that exhibited similarity to the genes of pB171_90 were identified as EPEC or ETEC, the pB171_90 genes were also identified in genomes of other diverse pathotypes (EAEC, STEC, ExPEC/uropathogenic E. coli [UPEC]) as well as E. coli genomes that didn't contain any of the pathotype-specific genes and may not be associated with illness (see Table S1 in the supplemental material). Interestingly, pB171_90 also contained a copy of the transcriptional regulator hha, which has been previously identified on the chromosome and plasmid of E. coli O157:H7 (46, 50). The hha gene was initially described as a regulator of hemolysin production but has since been described as a regulator of the LEE pathogenicity region and biofilm formation (47–49). Further studies are necessary to determine the role of hha in regulating virulence genes of EPEC strain B171. The pB171_90 plasmid also had significant similarity with genes of two O157:H7 E. coli genomes as well as the pCAR10 plasmid from a S. dysenteriae type I strain (54). This finding suggests that an exchange of resistance plasmids has occurred between EPEC and the phylogenomically related EHEC O157:H7 and S. dysenteriae, which cooccur together in E. coli phylogroup E.
Another notable feature of the pB171_90 resistance plasmid is the presence of a Tn21 transposon containing a spectinomycin-streptomycin resistance gene, a sulfonamide resistance gene, a small efflux pump, and a class 1 integrase gene, intI1. In a study investigating the prevalence of class 1 integrons among E. coli and Klebsiella isolated from hospitalized patients, the class 1 integrons were detected in 49% of the E. coli strains and were significantly associated with resistance to several classes of antibiotics (58). Comparison of pB171_90 with the sequenced EPEC and EHEC resistance plasmids pO111-CRL115 (GenBank accession number KC340959) and pO26-CRL125 (GenBank accession number KC340960) demonstrated that the tetracycline resistance and mercury resistance regions are conserved between these plasmids. Although plasmids pO111-CRL115 and pO26-CRL125 also contain a class 1 integron (59), the antibiotic resistance genes in this cassette differed from those of pB171_90, emphasizing the modular structure of E. coli resistance plasmids.
In summary, by characterizing the resistance plasmid of the EPEC reference strain B171, we provide further insight into the sequence diversity and host range of antibiotic and heavy metal resistance plasmids of E. coli. There were only a few publicly available E. coli genomes that contained nearly all of the resistance plasmid genes; however, genes from certain plasmid regions, including the antibiotic and heavy metal resistance genes, were present in diverse E. coli and other members of the family Enterobacteriaceae. Our study highlights the need for additional complete sequencing of resistance plasmids from EPEC and other pathotypes of E. coli to provide further insight into the dissemination and evolution of the E. coli resistance plasmids.
MATERIALS AND METHODS
Plasmid sequencing.
Plasmid DNA of EPEC strain B171 (37) was isolated from 1 liter of culture grown in L broth using alkaline lysis extraction method followed by ethidium bromide-CsCl density gradient centrifugation as previously described (60). The plasmid band was isolated and rebanded (190,000 × g) overnight on a second identical gradient. Plasmid was siphoned using a 3-ml syringe and an 18-gauge needle. Ethidium bromide was removed using isopropanol-CsCl-saturated 10 mM Tris, 5 mM EDTA, and 10 mM NaCl (pH 8) and then dialyzed against three changes of 10 mM Tris and 1 mM EDTA (pH 8). Plasmid DNA was ethanol precipitated using 0.125 M NaCl (final) and dissolved in water prior to sequencing.
The purified plasmid DNA was sequenced using the Pacific Biosciences RS II platform with the P6C4 chemistry in a single flow cell. Pacific Biosciences sequencing was completed by generating a sequencing library from 5 to 20 kb using standard methods at the Institute for Genome Sciences Genomics Resource Center (http://www.igs.umaryland.edu/resources/grc/). A total of 115,336 reads were generated with an average read length of 7,036 bp and a maximum read length of 38,154 bp. PacBio raw data were corrected and assembled using HGAP (SMRTAnalysis 2.3.0) (61), Canu assembler v.1.2 (62), and Celera assembler v.8.2 (63) run with default parameters. The assemblies were assessed for inconsistencies and misassembly using Nucmer whole-genome alignments (64) and Circleator plots (GC skew) (65). A BLAST search against NCBI's complete nucleotide database was used to confirm that all of the contigs were plasmids (65). The Canu assembler generated the most complete assembly for each of the plasmids in this sample. Circular contigs were identified and Minimus2 was used for circularization (66). The final circularized assembly was polished using Quiver (SMRTAnalysis 2.3.0) (61).
In silico multilocus sequence typing and serotyping.
The seven loci (adk, gyrB, fumC, icd, mdh, purA, and recA) of the multilocus sequence typing (MLST) scheme developed by Wirth et al. (67) were identified in each of the genomes listed in Table S1 in the supplemental material. The allele sequences were used to query BIGSdb (68) to obtain the allele numbers and sequence type of each of the E. coli genomes analyzed.
The molecular serotype of each genome was determined using Serotype Finder v.1.1 (https://cge.cbs.dtu.dk/services/SerotypeFinder/) with the default settings of an 85% identity threshold and 60% minimum alignment length (69).
Phylogenomic analysis.
The 134 E. coli genomes that contained genes with similarity to csi and/or traI of the B171 resistance plasmid were compared with 55 previously sequenced E. coli and Shigella genomes using the SNP-based in silico genotyper (ISG) (70) as previously described (35) (Table S1). There were 149,150 conserved SNP sites identified among all of the E. coli genomes analyzed relative to the E. coli reference strain IAI39 (GenBank accession number NC_011750.1) that were used to generate a maximum-likelihood phylogeny with 100 bootstrap values using RAxML v.7.2.8 (71). The phylogeny was constructed using the general time reversible (GTR) model of nucleotide substitution with the GAMMA model of rate heterogeneity and 100 bootstrap replicates. The phylogeny was then midpoint rooted and labeled using the interactive tree of life software (iTOL v.3) (72).
In silico detection of pB171_90 genes.
The genes of pB171_90 were predicted and annotated using an in-house annotation pipeline (73). These genes were then detected in a collection of 4,798 E. coli genome assemblies available in GenBank as of November 2016, using large-scale BLAST score ratio (LS-BSR) analysis as previously described (74, 75). The pB171_90 protein-coding genes were compared to each genome listed in Table S1 using TBLASTN (76) with composition-based adjustment turned off. The TBLASTN scores were used to generate a BSR indicating the detection of each gene cluster in each of the genomes (Table S1). The BSR was determined by dividing the score of a gene compared to a genome by the score of the gene compared to its own sequence. The genes that were identified with a BSR of ≥0.7 were considered present in the genomes analyzed. The numbers of genes that were present in AEEC compared to non-AEEC genomes was examined for statistical significance using the chi-squared test with Yates' continuity correction performed using R v.3.3.2. The clustered heatmap was generated using the heatmap2 function of gplots v.3.0.1 in R v.3.3.2. The BSR values of the genomes were clustered in the heatmap using the complete linkage method with Euclidean distance estimation.
Pathotype-specific virulence genes were identified in each of the genomes by in silico analysis using LS-BSR as described above. The following canonical virulence genes, which have been previously used for the molecular-based classification of clinical E. coli strains to each of the pathotypes, were detected in each of the following E. coli genomes analyzed: tEPEC (LEE genes eae and escV and BFP genes bfpA and bfpB), aEPEC (LEE genes eae and escV only; no BFP or Shiga toxin genes), ETEC (eltA and eltB encoding the heat-labile toxin LT and/or est encoding the heat-stable toxin ST), EAEC (the transcriptional regulator aggR, aatA encoding an outermembrane protein, or pic and/or pet encoding autotransporters), STEC (Shiga toxin genes stxA and stxB), EIEC (ipaB, mxi-spa, and/or ospB, which are all involved in type III secretion), and ExPEC/UPEC (papG encoding a fimbrial adhesion precursor) (19–21, 77, 78).
Gene phylogenies.
Individual gene phylogenies were constructed for the repA, hha, csi, and traI sequences that were detected by in silico analysis of each of the E. coli genomes analyzed (Table S1). A csi phylogeny was also constructed with the csi sequences from E. coli and other species of human enteric pathogens listed in Table S1. The nucleotide sequences of each gene were aligned using ClustalW, and maximum-likelihood phylogenies were constructed using the Kimura 2-parameter model and 1,000 bootstraps with MEGA7 (79).
Accession number(s).
The assemblies of pB171_90 and pB171_68 are deposited in GenBank under the accession numbers CP021212 and CP021211, respectively.
Supplementary Material
ACKNOWLEDGMENT
This project was funded in part by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under grant U19 AI110820.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00995-17.
REFERENCES
- 1.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 51:286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 2.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 4.Mediavilla JR, Patrawalla A, Chen L, Chavda KD, Mathema B, Vinnard C, Dever LL, Kreiswirth BN. 2016. Colistin- and carbapenem-resistant Escherichia coli harboring mcr-1 and blaNDM-5, causing a complicated urinary tract infection in a patient from the United States. mBio 7:e01191-16. doi: 10.1128/mBio.01191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz S, Johnson AP. 2016. Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother 71:2066–2070. doi: 10.1093/jac/dkw274. [DOI] [PubMed] [Google Scholar]
- 6.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, Hinkle M, Whitman T, Lesho E, Schaecher KE. 2016. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother 60:4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Qu F, Shan B, Huang B, Jia W, Chen C, Li A, Miao M, Zhang X, Bao C, Xu Y, Chavda KD, Tang YW, Kreiswirth BN, Du H, Chen L. 2016. Detection of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae from different hospitals in China. Antimicrob Agents Chemother 60:5033–5035. doi: 10.1128/AAC.00440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoesser N, Sheppard AE, Pankhurst L, De Maio N, Moore CE, Sebra R, Turner P, Anson LW, Kasarskis A, Batty EM, Kos V, Wilson DJ, Phetsouvanh R, Wyllie D, Sokurenko E, Manges AR, Johnson TJ, Price LB, Peto TE, Johnson JR, Didelot X, Walker AS, Crook DW, Modernizing Medical Microbiology Informatics Group. 2016. Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. mBio 7:e02162. doi: 10.1128/mBio.02162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phuc Nguyen MC, Woerther PL, Bouvet M, Andremont A, Leclercq R, Canu A. 2009. Escherichia coli as reservoir for macrolide resistance genes. Emerg Infect Dis 15:1648–1650. doi: 10.3201/eid1510.090696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erb A, Sturmer T, Marre R, Brenner H. 2007. Prevalence of antibiotic resistance in Escherichia coli: overview of geographical, temporal, and methodological variations. Eur J Clin Microbiol Infect Dis 26:83–90. doi: 10.1007/s10096-006-0248-2. [DOI] [PubMed] [Google Scholar]
- 11.Karami N, Nowrouzian F, Adlerberth I, Wold AE. 2006. Tetracycline resistance in Escherichia coli and persistence in the infantile colonic microbiota. Antimicrob Agents Chemother 50:156–161. doi: 10.1128/AAC.50.1.156-161.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labar AS, Millman JS, Ruebush E, Opintan JA, Bishar RA, Aboderin AO, Newman MJ, Lamikanra A, Okeke IN. 2012. Regional dissemination of a trimethoprim-resistance gene cassette via a successful transposable element. PLoS One 7:e38142. doi: 10.1371/journal.pone.0038142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nataro JP, Maher KO, Mackie P, Kaper JB. 1987. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun 55:2370–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross RJ, Ward LR, Threlfall EJ, King H, Rowe B. 1982. Drug resistance among infantile enteropathogenic Escherichia coli strains isolated in the United Kingdom. Br Med J (Clin Res Ed) 285:472–473. doi: 10.1136/bmj.285.6340.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra B, Junker E, Schroeter A, Helmuth R, Guth BE, Beutin L. 2006. Phenotypic and genotypic characterization of antimicrobial resistance in Escherichia coli O111 isolates. J Antimicrob Chemother 57:1210–1214. doi: 10.1093/jac/dkl127. [DOI] [PubMed] [Google Scholar]
- 16.Moyenuddin M, Wachsmuth IK, Moseley SL, Bopp CA, Blake PA. 1989. Serotype, antimicrobial resistance, and adherence properties of Escherichia coli strains associated with outbreaks of diarrheal illness in children in the United States. J Clin Microbiol 27:2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vignoli R, Varela G, Mota MI, Cordeiro NF, Power P, Ingold E, Gadea P, Sirok A, Schelotto F, Ayala JA, Gutkind G. 2005. Enteropathogenic Escherichia coli strains carrying genes encoding the PER-2 and TEM-116 extended-spectrum beta-lactamases isolated from children with diarrhea in Uruguay. J Clin Microbiol 43:2940–2943. doi: 10.1128/JCM.43.6.2940-2943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaletsky IC, Souza TB, Aranda KR, Okeke IN. 2010. Genetic elements associated with antimicrobial resistance in enteropathogenic Escherichia coli (EPEC) from Brazil. BMC Microbiol 10:25. doi: 10.1186/1471-2180-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 21.Nisa S, Scanlon KM, Donnenberg MS. 2013. Enteropathogenic Escherichia coli, p 75–119. In Donnenberg MS. (ed), Escherichia coli pathotypes and principles of pathogenesis, 2nd ed Academic Press, San Diego, CA. [Google Scholar]
- 22.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 23.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A 92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel TK, Kaper JB. 1997. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol 23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 25.Tarr PI, Gordon CA, Chandler WL. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 26.Johnson TJ, Nolan LK. 2009. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol Mol Biol Rev 73:750–774. doi: 10.1128/MMBR.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Xiong Y, Lan R, Ye C, Wang H, Ren J, Jing H, Wang Y, Zhou Z, Cui Z, Zhao H, Chen Y, Jin D, Bai X, Zhao A, Wang Y, Zhang S, Sun H, Li J, Wang T, Wang L, Xu J. 2011. pO157_Sal, a novel conjugative plasmid detected in outbreak isolates of Escherichia coli O157:H7. J Clin Microbiol 49:1594–1597. doi: 10.1128/JCM.02530-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobe T, Hayashi T, Han CG, Schoolnik GK, Ohtsubo E, Sasakawa C. 1999. Complete DNA sequence and structural analysis of the enteropathogenic Escherichia coli adherence factor plasmid. Infect Immun 67:5455–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkley C, Burland V, Keller R, Rose DJ, Boutin AT, Klink SA, Blattner FR, Kaper JB. 2006. Nucleotide sequence analysis of the enteropathogenic Escherichia coli adherence factor plasmid pMAR7. Infect Immun 74:5408–5413. doi: 10.1128/IAI.01840-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasko DA, Rosovitz MJ, Myers GS, Mongodin EF, Fricke WF, Gajer P, Crabtree J, Sebaihia M, Thomson NR, Chaudhuri R, Henderson IR, Sperandio V, Ravel J. 2008. The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. J Bacteriol 190:6881–6893. doi: 10.1128/JB.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crossman LC, Chaudhuri RR, Beatson SA, Wells TJ, Desvaux M, Cunningham AF, Petty NK, Mahon V, Brinkley C, Hobman JL, Savarino SJ, Turner SM, Pallen MJ, Penn CW, Parkhill J, Turner AK, Johnson TJ, Thomson NR, Smith SG, Henderson IR. 2010. A commensal gone bad: complete genome sequence of the prototypical enterotoxigenic Escherichia coli strain H10407. J Bacteriol 192:5822–5831. doi: 10.1128/JB.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burland V, Shao Y, Perna NT, Plunkett G, Sofia HJ, Blattner FR. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res 26:4196–4204. doi: 10.1093/nar/26.18.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhuri RR, Sebaihia M, Hobman JL, Webber MA, Leyton DL, Goldberg MD, Cunningham AF, Scott-Tucker A, Ferguson PR, Thomas CM, Frankel G, Tang CM, Dudley EG, Roberts IS, Rasko DA, Pallen MJ, Parkhill J, Nataro JP, Thomson NR, Henderson IR. 2010. Complete genome sequence and comparative metabolic profiling of the prototypical enteroaggregative Escherichia coli strain 042. PLoS One 5:e8801. doi: 10.1371/journal.pone.0008801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iguchi A, Thomson NR, Ogura Y, Saunders D, Ooka T, Henderson IR, Harris D, Asadulghani M, Kurokawa K, Dean P, Kenny B, Quail MA, Thurston S, Dougan G, Hayashi T, Parkhill J, Frankel G. 2009. Complete genome sequence and comparative genome analysis of enteropathogenic Escherichia coli O127:H6 strain E2348/69. J Bacteriol 191:347–354. doi: 10.1128/JB.01238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazen TH, Kaper JB, Nataro JP, Rasko DA. 2015. Comparative genomics provides insight into the diversity of the attaching and effacing Escherichia coli virulence plasmids. Infect Immun 83:4103–4117. doi: 10.1128/IAI.00769-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nwaneshiudu AI, Mucci T, Pickard DJ, Okeke IN. 2007. A second large plasmid encodes conjugative transfer and antimicrobial resistance in O119:H2 and some typical O111 enteropathogenic Escherichia coli strains. J Bacteriol 189:6074–6079. doi: 10.1128/JB.00349-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley LW, Junio LN, Libaek LB, Schoolnik GK. 1987. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun 55:2052–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liebert CA, Hall RM, Summers AO. 1999. Transposon Tn21, flagship of the floating genome. Microbiol Mol Biol Rev 63:507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith LD, Bertrand KP. 1988. Mutations in the Tn10 tet repressor that interfere with induction. Location of the tetracycline-binding domain. J Mol Biol 203:949–959. [DOI] [PubMed] [Google Scholar]
- 40.Aldema ML, McMurry LM, Walmsley AR, Levy SB. 1996. Purification of the Tn10-specified tetracycline efflux antiporter TetA in a native state as a polyhistidine fusion protein. Mol Microbiol 19:187–195. doi: 10.1046/j.1365-2958.1996.359886.x. [DOI] [PubMed] [Google Scholar]
- 41.Roberts MC. 2005. Update on acquired tetracycline resistance genes. FEMS Microbiol Lett 245:195–203. doi: 10.1016/j.femsle.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Paulsen IT, Littlejohn TG, Radstrom P, Sundstrom L, Skold O, Swedberg G, Skurray RA. 1993. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother 37:761–768. doi: 10.1128/AAC.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vedantam G, Guay GG, Austria NE, Doktor SZ, Nichols BP. 1998. Characterization of mutations contributing to sulfathiazole resistance in Escherichia coli. Antimicrob Agents Chemother 42:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundstrom L, Radstrom P, Swedberg G, Skold O. 1988. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet 213:191–201. [DOI] [PubMed] [Google Scholar]
- 45.Boyd ES, Barkay T. 2012. The mercury resistance operon: from an origin in a geothermal environment to an efficient detoxification machine. Front Microbiol 3:349. doi: 10.3389/fmicb.2012.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieto JM, Carmona M, Bolland S, Jubete Y, de la Cruz F, Juarez A. 1991. The hha gene modulates haemolysin expression in Escherichia coli. Mol Microbiol 5:1285–1293. doi: 10.1111/j.1365-2958.1991.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 47.Sharma VK, Carlson SA, Casey TA. 2005. Hyperadherence of an hha mutant of Escherichia coli O157:H7 is correlated with enhanced expression of LEE-encoded adherence genes. FEMS Microbiol Lett 243:189–196. doi: 10.1016/j.femsle.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Sharma VK, Bearson BL. 2013. Hha controls Escherichia coli O157:H7 biofilm formation by differential regulation of global transcriptional regulators FlhDC and CsgD. Appl Environ Microbiol 79:2384–2396. doi: 10.1128/AEM.02998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma VK, Zuerner RL. 2004. Role of hha and ler in transcriptional regulation of the esp operon of enterohemorrhagic Escherichia coli O157:H7. J Bacteriol 186:7290–7301. doi: 10.1128/JB.186.21.7290-7301.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paytubi S, Dietrich M, Queiroz MH, Juarez A. 2013. Role of plasmid- and chromosomally encoded Hha proteins in modulation of gene expression in E. coli O157:H7. Plasmid 70:52–60. doi: 10.1016/j.plasmid.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Hazen TH, Sahl JW, Fraser CM, Donnenberg MS, Scheutz F, Rasko DA. 2013. Refining the pathovar paradigm via phylogenomics of the attaching and effacing Escherichia coli. Proc Natl Acad Sci U S A 110:12810–12815. doi: 10.1073/pnas.1306836110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villa L, Garcia-Fernandez A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother 65:2518–2529. doi: 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 53.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Njamkepo E, Fawal N, Tran-Dien A, Hawkey J, Strockbine N, Jenkins C, Talukder KA, Bercion R, Kuleshov K, Kolinska R, Russell JE, Kaftyreva L, Accou-Demartin M, Karas A, Vandenberg O, Mather AE, Mason CJ, Page AJ, Ramamurthy T, Bizet C, Gamian A, Carle I, Sow AG, Bouchier C, Wester AL, Lejay-Collin M, Fonkoua MC, Hello SL, Blaser MJ, Jernberg C, Ruckly C, Merens A, Page AL, Aslett M, Roggentin P, Fruth A, Denamur E, Venkatesan M, Bercovier H, Bodhidatta L, Chiou CS, Clermont D, Colonna B, Egorova S, Pazhani GP, Ezernitchi AV, Guigon G, Harris SR, Izumiya H, Korzeniowska-Kowal A, et al. . 2016. Global phylogeography and evolutionary history of Shigella dysenteriae type 1. Nat Microbiol 1:16027. doi: 10.1038/nmicrobiol.2016.27. [DOI] [PubMed] [Google Scholar]
- 55.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Pendyala S, Debroy C, Nowicki B, Rice J. 2010. Escherichia coli sequence type ST131 as an emerging fluoroquinolone-resistant uropathogen among renal transplant recipients. Antimicrob Agents Chemother 54:546–550. doi: 10.1128/AAC.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novais A, Viana D, Baquero F, Martinez-Botas J, Canton R, Coque TM. 2012. Contribution of IncFII and broad-host IncA/C and IncN plasmids to the local expansion and diversification of phylogroup B2 Escherichia coli ST131 clones carrying blaCTX-M-15 and qnrS1 genes. Antimicrob Agents Chemother 56:2763–2766. doi: 10.1128/AAC.06001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao AN, Barlow M, Clark LA, Boring JR III, Tenover FC, McGowan JE Jr. 2006. Class 1 integrons in resistant Escherichia coli and Klebsiella spp., US hospitals. Emerg Infect Dis 12:1011–1014. doi: 10.3201/eid1206.051596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venturini C, Hassan KA, Roy Chowdhury P, Paulsen IT, Walker MJ, Djordjevic SP. 2013. Sequences of two related multiple antibiotic resistance virulence plasmids sharing a unique IS26-related molecular signature isolated from different Escherichia coli pathotypes from different hosts. PLoS One 8:e78862. doi: 10.1371/journal.pone.0078862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambrook JF, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 61.Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 62.Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 15 March 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berlin K, Koren S, Chin CS, Drake JP, Landolin JM, Phillippy AM. 2015. Assembling large genomes with single-molecule sequencing and locality-sensitive hashing. Nat Biotechnol 33:623–630. doi: 10.1038/nbt.3238. [DOI] [PubMed] [Google Scholar]
- 64.Delcher AL, Salzberg SL, Phillippy AM. 2003. Using MUMmer to identify similar regions in large sequence sets. Curr Protoc Bioinformatics Chapter 10:Unit 10.13. [DOI] [PubMed] [Google Scholar]
- 65.Crabtree J, Agrawal S, Mahurkar A, Myers GS, Rasko DA, White O. 2014. Circleator: flexible circular visualization of genome-associated data with BioPerl and SVG. Bioinformatics 30:3125–3127. doi: 10.1093/bioinformatics/btu505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sommer DD, Delcher AL, Salzberg SL, Pop M. 2007. Minimus: a fast, lightweight genome assembler. BMC Bioinformatics 8:64. doi: 10.1186/1471-2105-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jolley KA, Maiden MC. 2010. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. 2015. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J Clin Microbiol 53:2410–2426. doi: 10.1128/JCM.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahl JW, Beckstrom-Sternberg SM, Babic-Sternberg JS, Gillece JD, Hepp CM, Auerbach RK, Tembe W, Wagner DM, Keim PS, Pearson T. 2015. The in silico genotyper (ISG): an open-source pipeline to rapidly identify and annotate nucleotide variants for comparative genomics applications. bioRxiv doi: 10.1101/015578. [DOI] [Google Scholar]
- 71.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 72.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galens K, Orvis J, Daugherty S, Creasy HH, Angiuoli S, White O, Wortman J, Mahurkar A, Giglio MG. 2011. The IGS standard operating procedure for automated prokaryotic annotation. Stand Genomic Sci 4:244–251. doi: 10.4056/sigs.1223234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahl JW, Caporaso JG, Rasko DA, Keim P. 2014. The large-scale blast score ratio (LS-BSR) pipeline: a method to rapidly compare genetic content between bacterial genomes. PeerJ 2:e332. doi: 10.7717/peerj.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hazen TH, Donnenberg MS, Panchalingam S, Antonio M, Hossain A, Mandomando I, Ochieng JB, Ramamurthy T, Tamboura B, Qureshi S, Quadri F, Zaidi A, Kotloff KL, Levine MM, Barry EM, Kaper JB, Rasko DA, Nataro JP. 2016. Genomic diversity of EPEC associated with clinical presentations of differing severity. Nature Microbiol 1:15014. doi: 10.1038/nmicrobiol.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gertz EM, Yu YK, Agarwala R, Schaffer AA, Altschul SF. 2006. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol 4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hazen TH, Leonard SR, Lampel KA, Lacher DW, Maurelli AT, Rasko DA. 2016. Investigating the relatedness of enteroinvasive Escherichia coli to other E. coli and Shigella isolates by using comparative genomics. Infect Immun 84:2362–2371. doi: 10.1128/IAI.00350-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nataro JP, Steiner T, Guerrant RL. 1998. Enteroaggregative Escherichia coli. Emerg Infect Dis 4:251–261. doi: 10.3201/eid0402.980212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.