ABSTRACT
Prolonged hospitalization and antibiotic therapy are risk factors for the development of methicillin-resistant Staphylococcus aureus (MRSA) infections in thermal burn patients. We used a rat model to study the in vivo efficacy of daptomycin in the treatment of burn wound infections by S. aureus, and we evaluated the wound healing process through morphological and immunohistochemical analysis. A copper bar heated in boiling water was applied on a paraspinal site of each rat, resulting in two full-thickness burns. A small gauze was placed over each burn and inoculated with 5 × 107 CFU of S. aureus ATCC 43300. The study included two uninfected control groups with and without daptomycin treatment, an infected control group that did not receive any treatment, and two infected groups treated, respectively, with intraperitoneal daptomycin and teicoplanin. The main outcome measures were quantitative culture, histological evaluation of tissue repair, and immunohistochemical expression of wound healing markers: epidermal growth factor receptor (EGFR) and fibroblast growth factor 2 (FGF-2). The highest inhibition of infection was achieved in the group that received daptomycin, which reduced the bacterial load from 107 CFU/ml to about 103 CFU/g (P < 0.01). The groups treated with daptomycin showed better overall healing with epithelialization and significantly higher collagen scores than the other groups, and these findings were also confirmed by immunohistochemical data. In conclusion, our results support the hypothesis that daptomycin is an important modulator of wound repair by possibly reducing hypertrophic burn scar formation.
KEYWORDS: MRSA, burns, daptomycin, teicoplanin, wound healing
INTRODUCTION
Thermal burn patients acquire infections with staphylococci more frequently than other acutely ill surgical patients because of the loss of the skin barrier (1), decreased numbers of bactericidal polymorphonuclear cells (PMNs), and immune deficiency (2).
Additionally, prolonged hospitalization and antibiotic therapy are risk factors for the development of methicillin-resistant Staphylococcus aureus (MRSA) infection (3).
Colonization with MRSA increases the risk of bacteremia, septicemia, and serious clinical complications, including the loss of skin grafts in burn patients (4). For this reason, these infections are responsible for significant human mortality and morbidity, particularly in elderly people (5).
MRSA is able to secrete virulence factors, such as the extracellular adherence protein Eap, a member of the secretable expanded repertoire adhesive molecules that was previously demonstrated to impair wound healing by interfering with the proliferation and migration capacities of keratinocytes through the alteration of their morphology and adhesive properties (6). Moreover, it has been shown that the soluble products produced by both planktonic and biofilm MRSA cells have important effects on human fibroblast migration and viability by inducing apoptosis, and it is well-known that dermal fibroblasts play a pivotal role in the wound healing process (7).
Wound healing is a complex process which is regulated by an equally complex signaling network involving numerous growth factors, cytokines, and chemokines (8, 9).
Of particular interest are epidermal growth factor (EGF) and fibroblast growth factor (FGF) for their key roles in epithelial proliferation and migration (EGF receptor [EGFR], FGF-2) and granulation tissue formation, matrix deposition, and remodeling (FGF-2) (10). Within hours of injury, reepithelialization is initiated and the release of EGF and FGF stimulates epithelial cell migration and proliferation (10). EGF bind to the EGFR, a tyrosine kinase transmembrane protein (11) that plays an important role in reepithelialization by increasing keratinocyte proliferation and cell migration in acute wounds (12).
The FGF family is also involved in cutaneous wound healing, and in particular, FGF-2, or basic FGF (bFGF), plays a role in granulation tissue formation, reepithelialization, and tissue remodeling. FGF-2 regulates the synthesis and deposition of various extracellular matrix (ECM) components and increases keratinocyte motility during reepithelialization (13).
Full-thickness burns are those that damage all layers of the skin, and healing is a long process, especially in view of the major risk of infection. The current prevalence of antibiotic-resistant staphylococci has led to the study of promising alternatives to conventional medicines, such as daptomycin (14).
Daptomycin is a branched cyclic lipopeptide antibiotic of nonribosomal origin and the prototype of the acidic lipopeptide family (15). Daptomycin is an antimicrobial selectively active against aerobic, anaerobic, and facultative Gram-positive bacteria (14). It is also active against beta-hemolytic streptococci, MRSA, vancomycin-resistant enterococci (VRE), and vancomycin-resistant Staphylococcus aureus (VRSA) (16, 17). Daptomycin is recommended for the treatment of complicated skin, soft tissue, and bloodstream infections caused by S. aureus, including those associated with right-sided bacterial endocarditis (18).
Glycopeptides, in particular, teicoplanin, have come to play a significant role in therapy against Gram-positive bacterial infections. In particular, teicoplanin is the choice for the empirical therapy of these infections, primarily due to its activity against methicillin-resistant S. aureus, but its widespread use has caused a decrease in teicoplanin sensitivity in many countries (19).
The objective of the present study was to evaluate the in vivo efficacy of daptomycin compared to that of teicoplanin in the experimental treatment of burn wound infections by S. aureus in an animal model. In addition to direct antibacterial assays, we also studied the wound healing properties of these molecules through morphological evaluation and immunohistochemical analysis (EGFR, FGF-2).
RESULTS
Quantitative culture of excised tissues.
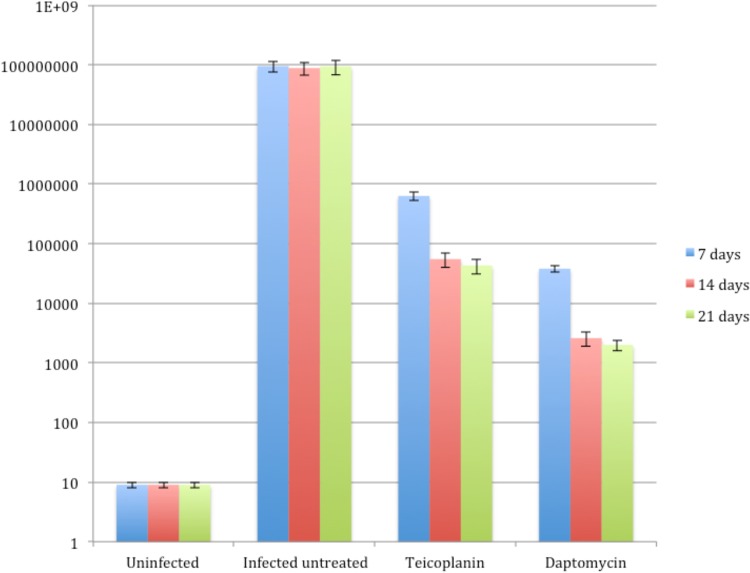
Daptomycin and teicoplanin exhibited MICs of 0.5 and 1 mg/liter, respectively. The mean bacterial numbers recovered from challenged but untreated controls (9.4 ×107 ± 2.6 ×107 CFU/g) were significantly higher than those recovered from all treatment groups. Specifically, the highest inhibition of the bacterial load was obtained in the group that received daptomycin (2.0 ×103 ± 0.4 × 103 CFU/g) (P < 0.01), while teicoplanin reduced the bacterial numbers to 4.3 ×104 ± 1.2 × 104 CFU/g (Fig. 1). No abnormal behavioral patterns, such as fatigue, stress, aggressiveness, weight loss, or change in movement, were observed among the rats at any time during the course of these experiments. Finally, no animal displayed signs of deeper infections, such as fever, abscesses, or inflammatory signs.
FIG 1.
Quantitative culture of excised tissues on 7, 14, and 21 days after burn injury. The numbers on the y axis are the number of CFU per gram.
Histological evaluation of wound healing.
Histological features of wound healing at 7, 14, and 21 days after burn injury are shown in Fig. 2.
FIG 2.
Representative images of hematoxylin- and eosin-stained histological sections of wound healing tissues from uninfected rats, infected rats treated with teicoplanin or daptomycin, and infected untreated rats on days 7, 14, and 21 after burn injury. (Insets) Lower-magnification images showing wound margins.
The wound healing scores are summarized in Table 1.
TABLE 1.
Wound healing scores in rats after burn injury
| Rat group | Wound healing scorea on the following day after burn injury: |
||
|---|---|---|---|
| 7 | 14 | 21 | |
| Uninfected | 1 | 2.5 | 4 |
| Infected without treatment | 0 | 1 | 2.5 |
| Infected treated with teicoplanin | 0.5 | 2 | 3 |
| Infected treated with daptomycin | 0.5 | 2 | 3.5 |
The wound healing scores refer to the morphological features described in Table 4.
At 7 days after burn injury, we observed in all biopsy specimens the detachment of the epidermis from the dermis, great damage in the dermis, and a strong presence of inflammatory cells, especially in the group of infected untreated rats, where a modest amount of granulation tissue with few cells and vessels was present. Untreated infected rats displayed the worst healing process compared to the uninfected and infected treated groups.
At 14 days after burn injury, the infected rats without treatment showed an overall impairment of the wound healing process: poor reepithelialization, an evident accumulation of granulation tissue with some cells and few fibers, a strong inflammatory response, and reduced collagen deposition.
The infected rats treated with teicoplanin and daptomycin showed incomplete reepithelialization; in particular, there was no evidence of features of differentiation, increased amounts of granulation tissue characterized by many cells and fibers, and a slight deposition of collagen and inflammatory infiltrate, although the global reepithelialization process was at a stage slightly less mature than that in the uninfected rats. At 21 days after burn injury, the untreated infected rats displayed incomplete reepithelialization, and the epithelium was still hypertrophic and poorly organized in well-differentiated layers. Abundant granulation tissue, few fibers, a modest deposition of collagen, and a severe inflammatory response were still present.
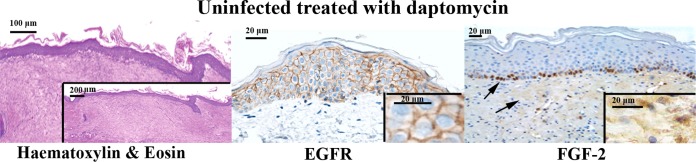
Biopsy specimens from uninfected rats treated or not treated with daptomycin (Fig. 3) and biopsy specimens from infected rats treated with teicoplanin and daptomycin showed robust epidermal coverage: we observed a reconstitution of the regular epidermal lining with evident keratinization, a substantial reduction of fibrinous exudation, and regular collagen deposition. In particular, rats treated with daptomycin displayed better epithelialization than rats treated with teicoplanin because they showed less hypertrophic epithelium. In addition, in this group we observed good collagen organization with many organized fibers, even if they were not always regular. We did not observe an evident inflammatory response.
FIG 3.
Histological sections of uninfected wounds treated with daptomycin stained with hematoxylin and eosin (for which the inset shows a lower-magnification image showing wound margins) and with EGFR and FGF-2 (immunoperoxidase reaction) (for which the insets show higher-magnification images).
Statistical analysis of histological wound healing parameters at day 21 postwounding.
At 21 days postwounding, untreated infected rats displayed the worst wound healing at both the epithelial and the dermal levels (P < 0.05) (Table 2). Reepithelialization in all treated groups progressed noticeably before day 21; however, in infected rats treated with teicoplanin and daptomycin, this was not observed at the same time: in rats treated with teicoplanin, the epithelium appeared to still be hypertrophic when it was compared with the epithelium of rats treated with daptomycin, and this difference was statistically significant (P < 0.05). Moreover, the collagen deposition appeared to be slightly less organized, even if this difference was not statistically significant. Only infected and uninfected rats treated with daptomycin displayed better collagen organization than untreated rats (P < 0.05) (Table 2).
TABLE 2.
Summary of wound healing parameters at day 21 postwounding in rat modela
| Rat group | Mean ± SD score |
||
|---|---|---|---|
| Epithelialization | Granulation tissue | Collagen organization | |
| Uninfected | 3.25 ± 0.40 | 2.80 ± 0.37 | 2.5 ± 0.60 |
| Uninfected treated with daptomycin | 3.30 ± 0.20 | 2.70 ± 0.30 | 2.6 ± 0.30 |
| Infected | 2.10 ± 0.10 | 1.58 ± 0.40 | 1.55 ± 0.40 |
| Infected treated with teicoplanin | 2.80 ± 0.20 | 2.35 ± 0.20 | 2.20 ± 0.50 |
| Infected treated with daptomycin | 3.15 ± 0.20 | 2.33 ± 0.50 | 2.40 ± 0.77 |
For the epithelialization score, P was <0.05 for the uninfected rats with and without daptomycin treatment and the infected rats with teicoplanin and daptomycin treatment versus infected rats and for the infected rats with daptomycin treatment versus infected rats with teicoplanin treatment. For the granulation tissue score, P was <0.05 for uninfected rats with and without daptomycin treatment and infected rats with teicoplanin and daptomycin treatment versus infected rats. For the collagen organization score, P was <0.05 for uninfected rats with and without daptomycin treatment and infected rats with daptomycin treatment versus infected rats. P values were determined by ANOVA and the Bonferroni test.
EGFR and FGF-2 staining.
The immunohistochemical data are shown in Table 3. Immunohistochemical staining of EGFR and FGF-2 was performed in the wounds on day 21 postburn (Fig. 3 and 4).
TABLE 3.
Immunohistochemical evaluation of EGFR and FGF-2 at day 21 postwounding in rat modela
| Rat group | % epithelial cells positive for EGFR | % dermal cells positive for FGF-2 |
|---|---|---|
| Uninfected | 50 ± 6.50 | 20 ± 3.60 |
| Uninfected treated with daptomycin | 51 ± 5.20 | 45 ± 4.30 |
| Infected | 20 ± 8.50 | 38 ± 6.70 |
| Infected treated with teicoplanin | 40 ± 6.20 | 70 ± 6.20 |
| Infected treated with daptomycin | 48 ± 3.60 | 85 ± 5.25 |
For EFGR, P was <0.05 for uninfected rats treated and not treated with daptomycin and infected rats treated with teicoplanin and daptomycin versus infected rats. For FGF-2, P was <0.05 for uninfected rats treated with daptomycin, infected rats treated with teicoplanin, and infected rats treated with daptomycin versus uninfected and infected rats and for infected rats treated with daptomycin versus infected rats treated with teicoplanin. P values were determined by ANOVA and the Bonferroni test.
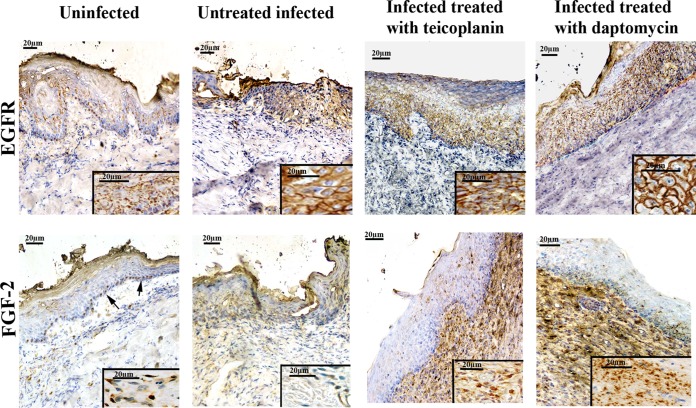
FIG 4.
Immunohistochemical expression of EGFR and FGF-2 in wound healing tissues from uninfected rats, infected rats treated with teicoplanin or daptomycin, and infected untreated rats on day 21 after burn injury (immunoperoxidase reaction). (Insets) Higher-magnification images.
In uninfected and treated infected wounds, EGFR was localized in the cell membrane in the epithelium (Fig. 3 and 4, insets). EGFR staining was found in both the basal layer and the suprabasal layers, and its expression was similar in these groups.
In infected untreated wounds, the amount of EGFR was significantly reduced compared to that in the other groups (P < 0.05); in addition, staining of EGFR was localized to the membrane, and it also showed a cytoplasmic presence (Fig. 3, insets).
In uninfected untreated wounds, FGF-2 staining in the dermis and epidermis was faint and sporadic and was more prominent in the basal cells (Fig. 4, arrows). Significant immunostaining was observed in the regenerated dermis of the uninfected treated wounds (Fig. 3, insets) and of the treated infected wounds (Fig. 4, insets). Treatment with daptomycin produced a significantly increased rate of positivity for FGF-2 in the dermis (P < 0.05), while the regenerated keratinocytes showed moderate and focal positivity for FGF-2 in the cytoplasm in the epithelium. In infected untreated wounds, the level of FGF-2 expression was decreased compared to that in the treated groups (P < 0.05).
DISCUSSION
MRSA acquisition and transmission are ongoing problems for burn patients, despite good adherence to infection control programs (20). The results of the in vivo study in our animal model showed that daptomycin demonstrated stronger antimicrobial activity than teicoplanin. The level of penetration of daptomycin into soft tissue is good, with reported concentrations of more than 70% of the plasma level being found within 2 h of administration and with these levels being maintained for 12 h (21).
In wound infections, the daily dosage of daptomycin is usually 4 mg/kg of body weight. However, in multicenter studies, high-dose daptomycin (a daily dose of more than 6 mg/kg) has been considered in patients with complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus (22).
The bactericidal activity of daptomycin is predominantly concentration dependent, and it has been demonstrated that suboptimal dosing may promote the selection of daptomycin-resistant bacteria, especially in Staphylococcus spp. (23). Moreover, the safety and tolerability of daptomycin and linear dose-proportional pharmacokinetics through the 6- to 12-mg/kg/day dosing range have been demonstrated in healthy volunteers (24). Given these considerations, we propose that daily doses of daptomycin of 7 mg/kg as well as a higher dose of teicoplanin be used for the treatment of MRSA infections, in order to increase the probability of achieving target concentrations within 72 h for teicoplanin and to enhance, for both antibiotics, clinical success rates. We also recommend these doses for the treatment of infections caused by pathogens that may be nonsusceptible to standard doses and to minimize the risk of resistance development in subjects who may need treatment for an extended duration, such as burn patients. Daptomycin has bactericidal activity caused by the calcium-dependent release of potassium and dissipation of the membrane potential. This mechanism ultimately leads to disruption of the cytoplasmic membrane and cell death. It has been observed that daptomycin preferentially inserts in the leading edges of the septal and forespore membranes, which also induces changes in the membrane structure (25).
Skin wound healing is a complex multifactorial process involving inflammation, cell proliferation, cell migration, reepithelialization, angiogenesis, extracellular matrix deposition, and remodeling (8, 9). We found that with both antibiotic treatments the appearance of the wound bed progressed beyond a little or no epithelial coverage with immature connective tissue and was instead characterized by robust epidermal coverage and an enhanced maturation of the dermis. The treatment with daptomycin proved to enhance wound healing during the third week, and at 21 days, the level of healing was comparable to that in the uninfected group and better than that in the teicoplanin group, in which the epithelium and collagen fiber deposition appeared to be slightly less organized.
Although the mechanisms of wound healing have been only partially elucidated, it is well-known that the proliferation of skin fibroblasts and keratinocytes is regulated in a complex manner by growth factors (GF), including basic fibroblast growth factor (bFGF, or FGF-2) and epidermal growth factor (EGF). The receptor tyrosine kinase epidermal growth factor receptor (EGFR) has also been implicated in this process. A transient elevation in the levels of EGFR and its ligands occurs in the skin following injury, and it is believed to contribute to the migratory and proliferative potential of keratinocytes adjacent to wound margins (26). The increased level of EGFR expression accelerates wound reepithelialization (27), enhancing angiogenesis, accelerating epithelial proliferation and migration, and stimulating the inflammatory reaction and wound contraction (28). Thus, expression of EGFR in the epithelium is a good indicator of the healing potential. In our study, uninfected and treated infected wounds showed similar good expression of EGFR, which was specifically localized in the cell membrane in the epithelium. On the contrary, in infected untreated wounds, the level of EGFR expression was significantly reduced compared to that in the other groups and was also localized in the cytoplasm. Our immunohistochemical expression profile has a biological correlation to our histological evaluation and suggests that teicoplanin and daptomycin were able to induce EGFR expression and, consequently, improve wound healing. In particular, our histological analyses demonstrated that the teicoplanin-treated group had a hyperkeratotic epidermis. Conversely, daptomycin seemed to be more effective than teicoplanin in inducing reepithelialization, and the level of EGFR expression increased in this group. Our data support the suggestion that EGFR stimulates cellular events critical to wound healing. The hyperproliferative and hyperkeratotic epidermis reflects the inability of keratinocytes to properly execute either of two processes: activation or differentiation. Indeed, in untreated wounds we observed that reepithelialization was still immature and EGFR staining was modest and was also localized in the cytoplasm. EGFR cytoplasmic staining seems to be associated with poor wound healing (29). According to our results, it is experimentally proved that the absence or modest expression of EGFR causes delayed wound healing, delayed epithelialization, marked edema, and a long-lasting and more prominent scar (30).
bFGF or FGF-2 is a multifunctional polypeptide that promotes the growth and differentiation of a broad spectrum of cell types, including dermal fibroblasts, keratinocytes, endothelial cells, and melanocytes (13). Although little is known about the physiological function of endogenous FGF-2, in vitro and in vivo studies have identified FGF-2 to be a potent mitogen, chemoattractant, and angiogenic agent (31). A study by Xie et al. showed that FGF-2 has the potential to accelerate wound healing and improve the quality of scars by regulating the balance of ECM synthesis and degradation; moreover, FGF-2 is an agent stimulating granulation tissue formation (32).
In our study, FGF-2 staining of the uninfected wound was faint and sporadic in the dermis and epidermis and more prominent in the basal cells. Significant FGF-2 expression was observed in the regenerated dermis of the treated infected and uninfected wounds, in particular, when daptomycin was administered, while in infected untreated wounds, its expression was decreased. FGF-2 seems to promote the healing of burn wounds. Kibe et al. (33) demonstrated dynamic changes in the distribution or reactivity of both FGF-2-positive cells and structures in the process of wound healing. According to our results, FGF-2 immunostaining in the normal epidermis was weak and sporadic in the basal cell layer and was intense throughout the regenerated epidermis, including basal cells, during the proliferative stage. The staining of the regenerated epidermis changed to that of normal skin concomitantly with wound closure. FGF-2 may affect the proliferation and differentiation of epidermal keratinocytes as well as neovascularization in the dermis via the FGF receptor 1 (FGFR-1) expressed during wound healing (33). Besides, in the later stages of wound repair, FGF-2 has an antifibrotic role and promotes healing by downregulating transforming growth factor β-induced collagen production; increasing the amounts of matrix-degrading enzymes, such as matrix metalloprotein-1; and inducing myofibroblast apoptosis (34). In fact, in several studies, exogenous FGF-2 has been used to treat hypertrophic scar and keloid (34–36).
In conclusion, our results support the hypothesis that daptomycin acts as an important modulator of wound repair. Daptomycin is a highly efficient antibiotic that targets the bacterial cell membrane, and in a recent study, it has been suggested that daptomycin's mechanism is due to interference with the lipid organization of the cell membrane, affecting overall bacterial membrane fluidity (37). The beneficial activity of daptomycin may encompass both direct antimicrobial activity on MRSA and its virulence factors, which prevents infection that would delay healing, and an effect on burn tissue repair, possibly by reducing hypertrophic burn scar formation, a major long-term disfiguring morbidity of burn injury. This suggestion warrants further investigation.
MATERIALS AND METHODS
Organisms.
Commercially available methicillin-resistant S. aureus ATCC 43300 was used.
Animals.
Male Wistar rats weighing 240 to 300 g were selected for this study. All animals were housed in individual cages under constant temperature (22°C) and humidity with a 12-h light and a 12-h dark cycle, and they had access to food and water ad libitum throughout the study. The experiments were repeated twice.
The procedures and facilities followed the requirements of Commission Directive 86/609/EEC concerning the protection of animals used for experimental and other scientific purposes. Italian legislation is defined in D.L. no. 116 of 27 January 1992. The experimental protocols were also approved by the Institutional Animal Care Committee of the Ministry of Health of Italy and by the Animal Research Ethics Committee of the Istituto di Ricovero e Cura a Carattere Scientifico-Istituto Nazionale di Riposo e Cura per Anziani (IRCCS-INRCA).
Drugs.
Daptomycin (Novartis, Rome, Italy) and teicoplanin (Aventis Pharma S.p.A., Rome, Italy) were diluted according to the manufacturers' recommendations, yielding 10-mg/ml stock solutions. Solutions were made fresh on the day of assay or stored at −80°C in the dark for short periods. The concentration range assayed for determination of the MIC was 0.25 to 256 mg/liter.
MIC determination.
MICs were determined according to CLSI guidelines (38). For daptomycin, the growth medium was supplemented with Ca2+ to a final concentration of 50 mg/liter. Experiments were performed in triplicate.
Preparation of inoculum.
Bacteria were grown in brain heart infusion broth. When the bacteria were in the log phase of growth, the suspension was centrifuged at 1,000 × g for 15 min, the supernatant was discarded, and the bacteria were resuspended and diluted into sterile saline to achieve a concentration of approximately 5 × 107 CFU/ml.
Experimental protocol.
Animals were randomized and assigned to five groups. Animals were further assigned to subgroups according to the time of observation (7, 14, and 21 days; 10 animals for each subgroup) for a total of 120 rats. The study included two uninfected control groups treated and not treated with daptomycin (7 mg/kg every 24 h [q24h]), an infected group that did not receive any treatment, and two infected groups treated, respectively, with intraperitoneal daptomycin (7 mg/kg q24h) and teicoplanin (7 mg/kg every 12 h) for 21 days. Drug dosages were determined as described in our previous studies and according to pharmacokinetic and pharmacodynamic information from other experimental studies (39–42).
The rats were anesthetized by intramuscular injection of ketamine-xylazine (40 mg/kg and 13 mg/kg, respectively), and the back surface of their bodies was shaved and washed with povidone iodine-propanol solution. A copper bar (12 by 12 mm) heated in boiling water (100°C for 10 min) was placed on the paraspinal site of each animal for 40 s without pressure. Only the weight of the block was used to create the burns. After reheating of the probe in boiling water, a second burn was made as symmetrically as possible on the other side of the back, resulting in two full-thickness burns. In order to avoid variations in the creation of the burns, one person (F.O.) created all burns. A small gauze was placed over each burn and then inoculated with 5 × 107 CFU of S. aureus ATCC 43300. The pocket was closed by means of skin clips (43). This procedure resulted in a local infection at 24 h. After 24 h, in control animals the burn was opened, the gauze was removed for quantitative bacterial culture, and treatment was initiated. Antibiotics were administered intraperitoneally daily for 21 days.
During surgery, the body temperature (36 or 37°C) of the animals were maintained and controlled using a homeothermic blanket (Harvard Apparatus). The rats were then resuscitated with 1 ml of Ringer's lactate solution administered by intraperitoneal injection. The injury was protected with a strip of cloth. The animals were then returned to their individual cages and thoroughly examined daily.
To mimic the clinical situation in burned patients, surgical debridement was performed 48 h after the injury. The skin and subcutis of the animals, which underwent general anesthesia with isoflurane, were excised in the dorsal area near the edge of the burn, avoiding the panniculus carnosum and the muscle layer. This was done to reduce the high risk of wound colonization and minimize the high risk of hypertrophic scarring. The wounds were left to heal by secondary intention (i.e., the wound edges were not closed by sutures). To protect the wounds from outside contamination and infection, a sterile hydrated gauze was used. The animals were returned to their individual cages and thoroughly examined daily. Each wound was gauged every 3 or 4 days according to the method described by Morton and Malone (44). Each dressing change was made while the animals were under general anesthesia with isoflurane. After each measurement, the dressing was removed and the wound was flushed with sterile solution. When the healing was completed, tissue specimens were collected from each treated area and samples were processed for morphological analysis. At the end of the experiment, all the rats were sacrificed with excess anesthetic. Skin samples were divided into two pieces. One piece was used for histological examination (see below), and the other was homogenized in 1 ml phosphate-buffered saline (PBS) using a stomacher. Quantitation of viable bacteria was performed by culturing serial dilutions (0.1 ml) of the bacterial suspension on blood agar plates. All plates were incubated at 37°C for 48 h and evaluated for the presence of bacteria. The organisms were quantitated by counting the number of CFU per plate. The limit of detection for this method was approximately 10 CFU/g. Toxicity was evaluated on the basis of the presence of any drug-related adverse effects, behavioral alterations, and local signs of inflammation. In particular, all the animals were evaluated for the correct consumption of food and drinking water, general locomotor activity, and the display of kyphosis.
Body weights were measured once a day during the experimental period. The amounts of drinking water and food supplied and the residual amounts of drinking water and food were weighed daily in order to calculate the average daily water and food consumption through the entire treatment period. Those animals who were sick, did not adequately eat or drink, or were crouching in the corner were excluded from the study.
Skin biopsy specimens.
Skin samples surgically removed from the wounds at the time of euthanasia at 7, 14, and 21 days after burn injury were frozen in liquid nitrogen and stored at −70°C. Then 6-μm tissue sections were cut with a microtome, air dried overnight, and fixed in cold acetone for 10 min. Skin biopsy specimens included the epidermis, the dermis, and the subcutaneous panniculus carnosus muscle.
Histological and immunohistochemical analysis.
We evaluated the grade of wound healing by histological evaluation of sections stained with hematoxylin and eosin according to wound repair scores (Table 4) and a system reported previously (45, 46). In particular, we analyzed some morphological features, such as the degree of reepithelialization, granulation tissue formation, and collagen organization.
TABLE 4.
Score of morphological features
| Score | Feature for each process |
||
|---|---|---|---|
| Reepithelialization | Granulation tissue formation | Collagen deposition | |
| 0 | Trace and focal migrating | Trace | None |
| 1 | Migrating | Hypocellular and no vessels | Trace |
| 2 | Partial | Many cells and few vessels | Slight |
| 3 | Hypertrophic and partial stratum corneum | Many fibroblasts, some fibers, and some vessels | Moderate |
| 4 | Complete | More fibers and few cells | Marked |
Immunohistochemistry was performed for all samples at 21 days after burn injury. Six-micrometer tissue sections were rinsed in PBS for 10 min and incubated overnight at 4°C with the following polyclonal antibodies: anti-EGFR (clone 1005; dilution, 1:100; Santa Cruz Biotechnologies, Santa Cruz, CA) and anti-FGF-2 (dilution, 1:100; Santa Cruz Biotechnologies, Santa Cruz, CA). Then they were immunostained using the streptavidin-biotin peroxidase technique (Envision universal peroxidase kit; Dako Cytomation, Milan, Italy) as described in our previous studies (42, 45). All evaluations were performed simultaneously by two investigators (G.L., R.D.P.) blind to the groups with the use of a double-headed light microscope equipped with a Nikon DS-Vi1 digital camera. The k value (understanding interobserver agreement) was >0.80, showing a substantial agreement between the two observers and among the different observations of the same observer. For the counting of stained cells, NIS Elements BR (version 3.22) imaging software (Nikon Instruments) was used. Stained cells in at least 10 fields per sample were counted (field, 0.07 mm2; magnification, ×400) and quantified as a percentage of the total number of counted cells.
Statistical analysis.
All results are presented as means ± standard deviations (SDs). Statistical analysis was performed using analysis of variance (ANOVA) and the Bonferroni test. Statistical analyses were performed using the SPSS (version 16) package (SPSS Inc., Chicago, IL, USA). Significance was accepted when the P value was <0.05.
ACKNOWLEDGMENTS
This study was supported by internal funding.
We have no conflicts of interest to declare.
REFERENCES
- 1.Altoparlak U, Erol S, Akcay MN, Celebi F, Kadanali A. 2004. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 30:660–664. doi: 10.1016/j.burns.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Barlow YT. 1994. Lymphocytes and immunosuppression in the burned patient: a review. Burns 20:487–490. doi: 10.1016/0305-4179(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 3.Ben-David D, Mermel LA, Parenteau S. 2008. Methicillin-resistant Staphylococcus aureus transmission: the possible importance of unrecognized health care worker carriage. Am J Infect Control 36:93–97. doi: 10.1016/j.ajic.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Abbasi-Montazeri E, Khosravi AD, Feizabadi MM, Goodarzi H, Khoramrooz SS, Mirzaii M, Kalantar E, Darban-Sarokhalil D. 2013. The prevalence of methicillin-resistant Staphylococcus aureus (MRSA) isolates with high-level mupirocin resistance from patients and personnel in a burn center. Burns 39:650–654. doi: 10.1016/j.burns.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Wearn CM, Hardwicke J, Moiemen N. 2015. Burns in the elderly: mortality is still a relevant outcome. Burns 41:1617–1618. doi: 10.1016/j.burns.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Eisenbeis J, Peisker H, Backes CS, Bur S, Hölters S, Thewes N, Greiner M, Junker C, Schwarz EC, Hoth M, Junker K, Preissner KT, Jacobs K, Herrmann M, Bischoff M. 2017. The extracellular adherence protein (Eap) of Staphylococcus aureus acts as a proliferation and migration repressing factor that alters the cell morphology of keratinocytes. Int J Med Microbiol 307:116–125. doi: 10.1016/j.ijmm.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Kirker KR, James GA, Fleckman P, Olerud JE, Stewart PS. 2012. Differential effects of planktonic and biofilm MRSA on human fibroblasts. Wound Repair Regen 20:253–261. doi: 10.1111/j.1524-475X.2012.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaji S, Watson CL, Ranjan R, King A, Bollyky PL, Keswani SG. 2015. Chemokine involvement in fetal and adult wound healing. Adv Wound Care (New Rochelle) 4:660–672. doi: 10.1089/wound.2014.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonetti O, Lucarini G, Cirioni O, Zizzi A, Orlando F, Provinciali M, Di Primio R, Giacometti A, Offidani A. 2013. Delayed wound healing in aged skin rat models after thermal injury is associated with an increased MMP-9, K6 and CD44 expression. Burns 39:776–787. doi: 10.1016/j.burns.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. 2008. Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 11.Oda K, Matsuoka Y, Funahashi A, Kitano H. 2005. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol 1:2005.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, Hanakawa Y, Ohmoto H, Yoshino K, Shirakata Y, Matsuzawa Y, Hashimoto K, Taniguchi N. 2000. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol 151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sogabe Y, Abe M, Yokoyama Y, Ishikawa O. 2006. Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. Wound Repair Regen 14:457–462. doi: 10.1111/j.1743-6109.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Ruiz A, Seaton RA, Hamed K. 2016. Daptomycin: an evidence-based review of its role in the treatment of Gram-positive infections. Infect Drug Resist 9:47–58. doi: 10.2147/IDR.S99046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbel L, Marahiel MA. 2010. Daptomycin, a bacterial lipopeptide synthesized by a nonribosomal machinery. J Biol Chem 285:27501–27508. doi: 10.1074/jbc.R110.128181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverman JA, Perlmutter NG, Shapiro HM. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 47:2538–2544. doi: 10.1128/AAC.47.8.2538-2544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bubalo JS, Munar MY, Cherala G, Hayes-Lattin B, Maziarz R. 2009. Daptomycin pharmacokinetics in adult oncology patients with neutropenic fever. Antimicrob Agents Chemother 53:428–434. doi: 10.1128/AAC.00943-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He W, Zhang Y, Chen H, Zhao C, Wang H. 2014. Efficacy and safety of daptomycin for the treatment of infectious disease: a meta-analysis based on randomized controlled trials. J Antimicrob Chemother 69:3181–3189. doi: 10.1093/jac/dku277. [DOI] [PubMed] [Google Scholar]
- 19.Pace JL, Yang G. 2006. Glycopeptides: update on an old successful antibiotic class. Biochem Pharmacol 71:968–980. doi: 10.1016/j.bcp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Issler-Fisher AC, McKew G, Fisher OM, Harish V, Gottlieb T, Maitz PK. 2015. Risk factors for, and the effect of MRSA colonization on the clinical outcomes of severely burnt patients. Burns 41:1212–1220. doi: 10.1016/j.burns.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Seaton RA. 2008. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother 62(Suppl 3):iii15–iii23. doi: 10.1093/jac/dkn368. [DOI] [PubMed] [Google Scholar]
- 22.Lawson W, Nathwani D, Eckmann C, Corman S, Stephens J, Solem C, Macahilig C, Li J, Baillon-Plot N, Charbonneau C, Haider S. 2015. Weight-based antibiotic dosing in a real-world European study of complicated skin and soft-tissue infections due to methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 21(Suppl 2):S40–S46. doi: 10.1016/j.cmi.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Senneville E, Caillon J, Calvet B, Jehl F. 2016. Towards a definition of daptomycin optimal dose: lessons learned from experimental and clinical data. Int J Antimicrob Agents 47:12–19. doi: 10.1016/j.ijantimicag.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob Agents Chemother 50:3245–3249. doi: 10.1128/AAC.00247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trilomis T. 2014. Daptomycin and its immunomodulatory effect: consequences for antibiotic treatment of methicillin-resistant Staphylococcus aureus wound infections after heart surgery. Front Immunol 5:97. doi: 10.3389/fimmu.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner S, Grose R. 2003. Regulation of wound healing by growth factors and cytokines. Physiol Rev 83:835–870. [DOI] [PubMed] [Google Scholar]
- 27.Nanney LB, Paulsen S, Davidson MK, Cardwell NL, Whitsitt JS, Davidson JM. 2000. Boosting epidermal growth factor receptor expression by gene gun transfection stimulates epidermal growth in vivo. Wound Repair Regen 8:117–127. doi: 10.1046/j.1524-475x.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 28.Stoll S, Benedict M, Mitra R, Hiniker A, Elder JT, Nuñez G. 1998. EGF receptor signaling inhibits keratinocyte apoptosis: evidence for mediation by Bcl-XL. Oncogene 16:1493–1499. doi: 10.1038/sj.onc.1201657. [DOI] [PubMed] [Google Scholar]
- 29.Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Rosenberg H, Tomic-Canic M. 2007. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med 13:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. 2004. EGFR enhances early healing after cutaneous incisional wounding. J Investig Dermatol 123:982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- 31.O'Keefe EJ, Chiu ML, Payne RE. 1988. Stimulation of growth of keratinocytes by basic fibroblast growth factor. J Investig Dermatol 90:767–769. doi: 10.1111/1523-1747.ep12560956. [DOI] [PubMed] [Google Scholar]
- 32.Xie JL, Bian HN, Qi SH, Chen HD, Li HD, Xu YB, Li TZ, Liu XS, Liang HZ, Xin BR, Huan Y. 2008. Basic fibroblast growth factor (bFGF) alleviates the scar of the rabbit ear model in wound healing. Wound Repair Regen 16:576–581. doi: 10.1111/j.1524-475X.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- 33.Kibe Y, Takenaka H, Kishimoto S. 2000. Spatial and temporal expression of basic fibroblast growth factor protein during wound healing of rat skin. Br J Dermatol 143:720–727. doi: 10.1046/j.1365-2133.2000.03824.x. [DOI] [PubMed] [Google Scholar]
- 34.Shi HX, Lin C, Lin BB, Wang ZG, Zhang HY, Wu FZ, Cheng Y, Xiang LJ, Guo DJ, Luo X, Zhang GY, Fu XB, Bellusci S, Li XK, Xiao J. 2013. The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS One 8:e59966. doi: 10.1371/journal.pone.0059966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eto H, Suga H, Aoi N, Kato H, Doi K, Kuno S, Tabata Y, Yoshimura K. 2012. Therapeutic potential of fibroblast growth factor-2 for hypertrophic scars: upregulation of MMP-1 and HGF expression. Lab Invest 92:214–223. doi: 10.1038/labinvest.2011.127. [DOI] [PubMed] [Google Scholar]
- 36.Ban MJ, Park JH, Kim JW, Park KN, Lee JY, Kim HK, Lee SW. 27 September 2016. The efficacy of fibroblast growth factor for the treatment of chronic vocal fold scarring: from animal model to clinical application. Clin Exp Otorhinolaryngol. doi: 10.21053/ceo.2016.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller A, Wenzel M, Strahl H, Grein F, Saaki TN, Kohl B, Siersma T, Bandow JE, Sahl HG, Schneider T, Hamoen LW. 24 October 2016. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc Natl Acad Sci U S A. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 39.Cirioni O, Silvestri C, Pierpaoli E, Barucca A, Kamysz W, Ghiselli R, Scalise A, Brescini L, Castelli P, Orlando F, Kamysz E, Guerrieri M, Giacometti A, Provinciali M. 2013. IB-367 pretreatment improves the in vivo efficacy of teicoplanin and daptomycin in an animal model of infected wounds with meticillin-resistant Staphylococcus aureus. J Med Microbiol 62:1552–1558. doi: 10.1099/jmm.0.057414-0. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri C, Cirioni O, Arzeni D, Ghiselli R, Simonetti O, Orlando F, Ganzetti G, Staffolani S, Brescini L, Provinciali M, Offidani A, Guerrieri M, Giacometti A. 2012. In vitro activity and in vivo efficacy of tigecycline alone and in combination with daptomycin and rifampin against Gram-positive cocci isolated from surgical wound infection. Eur J Clin Microbiol Infect Dis 31:1759–1764. doi: 10.1007/s10096-011-1498-1. [DOI] [PubMed] [Google Scholar]
- 41.Niska JA, Shahbazian JH, Ramos RI, Pribaz JR, Billi F, Francis KP, Miller LS. 2012. Daptomycin and tigecycline have broader effective dose ranges than vancomycin as prophylaxis against a Staphylococcus aureus surgical implant infection in mice. Antimicrob Agents Chemother 56:2590–2597. doi: 10.1128/AAC.06291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonetti O, Cirioni O, Ghiselli R, Goteri G, Orlando F, Monfregola L, De Luca S, Zizzi A, Silvestri C, Veglia G, Giacometti A, Guerrieri M, Offidani A, Scaloni A. 2012. Antimicrobial properties of distinction in an experimental model of MRSA-infected wounds. Eur J Clin Microbiol Infect Dis 31:3047–3055. doi: 10.1007/s10096-012-1663-1. [DOI] [PubMed] [Google Scholar]
- 43.Kugelberg E, Norström T, Petersen TK, Duvold T, Andersson DI, Hughes D. 2005. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob Agents Chemother 49:3435–3441. doi: 10.1128/AAC.49.8.3435-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton JJP, Malone MH. 1972. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacodyn 196:117–126. [PubMed] [Google Scholar]
- 45.Simonetti O, Cirioni O, Goteri G, Ghiselli R, Kamysz W, Kamysz E, Silvestri C, Orlando F, Barucca C, Scalise A, Saba V, Scalise G, Giacometti A, Offidani A. 2008. Temporin A is effective in MRSA-infected wounds through bactericidal activity and acceleration of wound repair in a murine model. Peptides 29:520–528. doi: 10.1016/j.peptides.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Hebda PA, Whaley D, Kim HG, Wells A. 2003. Absence of inhibition of cutaneous wound healing in mice by oral doxycycline. Wound Repair Regen 11:373–379. doi: 10.1046/j.1524-475X.2003.11510.x. [DOI] [PubMed] [Google Scholar]