ABSTRACT
Hard-tick-borne relapsing fever (HTBRF) is an emerging infectious disease throughout the temperate zone caused by the relapsing-fever spirochete Borrelia miyamotoi. Antibiotic treatment of HTBRF is empirically based on the treatment of Lyme borreliosis; however, the antibiotic susceptibility of B. miyamotoi has not been studied to date. Thus, we set out to determine the in vitro antimicrobial susceptibility of B. miyamotoi. A microdilution method with 96-well microtiter plates was used to determine the antibiotic susceptibilities of two B. miyamotoi strains isolated on two different continents (Asia and North America), two Borrelia burgdorferi sensu lato strains, and one Borrelia hermsii isolate for purposes of comparison. The MIC and minimal bactericidal concentration (MBC) were determined by both microscopy and colorimetric assays. We were able to show that relative to the B. burgdorferi sensu lato isolates, both B. miyamotoi strains and B. hermsii demonstrated greater susceptibility to doxycycline and azithromycin, equal susceptibility to ceftriaxone, and resistance to amoxicillin in vitro. The MIC and MBC of amoxicillin for B. miyamotoi evaluated by microscopy were 16 to 32 mg/liter and 32 to 128 mg/liter, respectively. Since B. miyamotoi is susceptible to doxycycline, azithromycin, and ceftriaxone in vitro, our data suggest that these antibiotics can be used for the treatment of HTBRF. Oral amoxicillin is currently used as an alternative for the treatment of HTBRF; however, since we found that the B. miyamotoi strains tested were resistant to amoxicillin in vitro, this issue warrants further study.
KEYWORDS: hard-tick-borne relapsing fever, relapsing-fever borrelia, Borrelia miyamotoi, Borrelia miyamotoi disease, antibiotic susceptibility, antimicrobials
INTRODUCTION
The relapsing-fever spirochete Borrelia miyamotoi was first described in Japan in 1995 (1). While it is phylogenetically closely related to other relapsing-fever (RF) spirochetes, such as Borrelia hermsii, Borrelia turicatae (transmitted by soft ticks), and Borrelia recurrentis (transmitted by body lice), it is transmitted by hard-bodied Ixodes ticks across the temperate zone (2). Ixodes ticks concomitantly transmit spirochetes belonging to the Borrelia burgdorferi sensu lato group, which are known to cause Lyme borreliosis (3). The incidence of B. miyamotoi in Ixodes ticks is lower than that of B. burgdorferi sensu lato, with infection rates ranging from 0 to 15.4% in the United States and as high as 4% in Europe and Japan (2, 4, 5). RF Borrelia spirochetes cause a variety of diseases, which are characterized by episodes of high fever separated by periods of relative well-being. The clinical presentation of disease caused by B. miyamotoi, however, appears to differ from that of classical RF, since relapsing-fever episodes have been observed only in 10% of patients infected with B. miyamotoi, and their levels of spirochetemia are calculated to be low (6, 7). Since most patients with B. miyamotoi infection are usually treated with antibiotics, this might be an underestimation of the naturally occurring relapses. The disease caused by B. miyamotoi is therefore considered a separate clinical entity and has been designated both Borrelia miyamotoi disease (BMD) (7) and hard-tick-borne relapsing fever (HTBRF) (8). Clinical cases of HTBRF were first described in Russia in 2011 (6), followed by cases in the United States and Japan (9–11). On average, fever episodes last for 3 days and are accompanied by flu-like symptoms, such as headache, chills, abdominal discomfort, arthralgia, and myalgia. Meningoencephalitis caused by B. miyamotoi has been described in three patients receiving B-cell-depleting therapy in the Netherlands, Germany, and the United States (9, 10, 12).
Treatment of HTBRF is currently empirically based on standard regimens used for the treatment of Lyme borreliosis, although no clinical guidelines exist. The antimicrobial susceptibility of B. miyamotoi has not yet been elucidated, due to difficulties with the cultivation of B. miyamotoi spirochetes in vitro, which has been reported only recently (13–15). In contrast, the in vitro susceptibilities of the causative agents of Lyme borreliosis and several RF Borrelia spirochetes have been extensively studied (16–19). The antimicrobial susceptibility of spirochetes is conventionally shown as MICs—measured either by direct (dark-field) microscopy or by colorimetric assays—or measurement of the minimal bactericidal concentrations (MBCs) after 72 h of exposure to antibiotics (18, 20, 21). As part of the current study, we aimed to determine the in vitro antibiotic susceptibility of B. miyamotoi in comparison to those of B. burgdorferi sensu lato and B. hermsii, by determining the MICs and MBCs of the antibiotics most commonly used in the treatment of Lyme borreliosis by both dark-field microscopy and colorimetric assays. This is important because it will help guide future antibiotic treatment of HTBRF.
RESULTS
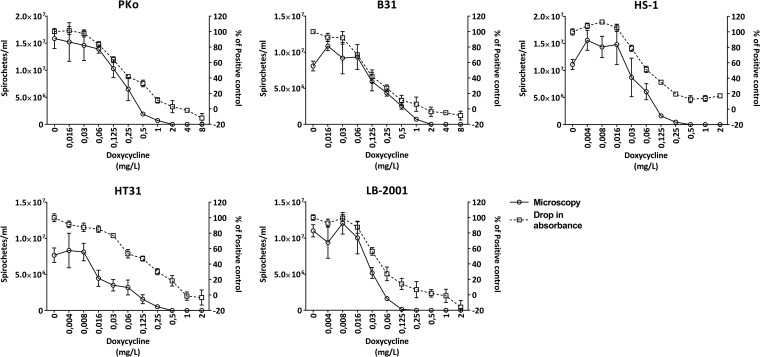
The antibiotic susceptibilities of all Borrelia strains tested, expressed as MICs determined by colorimetric assays and as MICs and MBCs determined by dark-field microscopy, are shown in Tables 1 and 2. In short, the colorimetric MIC1 and MIC2 were similar to the microscopic MICs or one to three 2-fold dilutions lower. Figure 1 shows, as an example, the correlation between microscopy and the drop in absorbance (compared to that for the positive control) with doxycycline. The correlation between normalized MIC2 values and microscopy results is shown in Fig. S2 in the supplemental material. The MICs of the various antimicrobials for the B. burgdorferi sensu lato strains and for the RF Borrelia strains were comparable (within two 2-fold dilutions of each other), with the exception of amikacin, which had a MIC for B. miyamotoi LB-2001 (512 to 1,024 mg/liter) that was much higher than the MICs for B. miyamotoi HT31 and B. hermsii HS1 (32 to 64 mg/liter). As expected, all Borrelia strains tested were resistant to amikacin. In addition, both B. miyamotoi strains demonstrated greater sensitivity to doxycycline and azithromycin, equal susceptibility to ceftriaxone, and relative resistance to amoxicillin compared to B. burgdorferi sensu lato strains (Table 1). In contrast to those for both B. burgdorferi sensu lato strains tested, the amoxicillin MICs for B. miyamotoi isolates HT31 and LB-2001, and for B. hermsii HS1, were above the clinical breakpoint of ≤4 mg/liter, indicating that the RF Borrelia strains tested should be regarded as resistant to amoxicillin in vitro. To exclude the possibility that this difference was due to the addition of fetal calf serum (FCS) to modified Kelly-Pettenkofer (MKP) medium, which is needed for relapsing-fever Borrelia strains, we tested the amoxicillin MIC using Borrelia afzelii (PKo) in both MKP medium alone and MKP medium supplemented with 10% heat-inactivated FCS (MKP-F medium), compared to B. hermsii (HS1) in MKP-F medium. We showed that there was no difference in the susceptibility of PKo to amoxicillin, as determined by microscopy (data not shown) and colorimetry (see Fig. S1 in the supplemental material), whether it was tested in MKP or MKP-F medium. Furthermore, the difference in the amoxicillin MIC between PKo and B. hermsii HS1 was also observed when MKP-F medium was used. The MBCs of all antimicrobial agents were one to two dilution steps above the microscopic MICs except for amikacin, for which the MICs and MBCs were identical (Table 2). In some cases, growth was observed at the highest available concentration; therefore, the exact MBC could not be determined and was marked as greater than the highest concentration tested.
TABLE 1.
MICs of different antimicrobial agents for selected Borrelia burgdorferi sensu lato and relapsing-fever Borrelia strainsa
| Antimicrobial and method | MIC rangeb (mg/liter) |
||||
|---|---|---|---|---|---|
|
B. burgdorferi sensu lato |
Relapsing-fever Borrelia strain |
||||
| PKo | B31 | HT31 | LB-2001 | HS1 | |
| Amoxicillin | |||||
| Microscopy | 4 | 1–2 | 32 | 16–32 | 16–32 |
| MIC1 | 2 | 0.5 | 8 | 8 | 8 |
| MIC2 | 2 | 2 | 16 | 8 | 8 |
| Doxycycline | |||||
| Microscopy | 2 | 2 | 0.5 | 0.125–0.25 | 0.5 |
| MIC1 | 1 | 0.125 | 0.25 | 0.0625 | 0.25 |
| MIC2 | 1 | 0.5 | 0.5 | 0.125 | 0.25 |
| Ceftriaxone | |||||
| Microscopy | 0.125 | 0.06–0.125 | 0.0625–0.25 | 0.25–0.5 | 0.25–0.5 |
| MIC1 | 0.0625 | 0.03125 | 0.03125 | 0.03125 | 0.125 |
| MIC2 | 0.0625 | 0.03125 | 0.0625 | 0.125 | 0.125 |
| Azithromycin | |||||
| Microscopy | 0.0128 | 0.0128 | 0.003–0.006 | 0.003 | 0.0128 |
| MIC1 | 0.0128 | 0.003 | 0.003 | 0.003 | 0.0128 |
| MIC2 | 0.0128 | 0.0256 | 0.003 | 0.003 | 0.006 |
| Amikacin | |||||
| Microscopy | 256 | 512 | 64 | 512 | 32–64 |
| MIC1 | 512 | 512 | 32 | 512 | 64 |
| MIC2 | 256 | 512 | 64 | 1,024 | 64 |
PKo, B. afzelii reference strain; B31, B. burgdorferi sensu stricto reference strain; HT31, B. miyamotoi tick isolate (Japan); LB-2001, B. miyamotoi tick isolate (United States); HS1, B. hermsii reference strain.
Ranges are results of tests that were conducted in quadruplicate.
TABLE 2.
MBCs of different antimicrobial agents for selected Borrelia burgdorferi sensu lato and relapsing-fever Borrelia strainsa
| Antimicrobial | MBC rangeb (mg/liter) |
||||
|---|---|---|---|---|---|
|
B. burgdorferi sensu lato |
Relapsing-fever Borrelia strains |
||||
| PKo | B31 | HT31 | LB-2001 | HS1 | |
| Amoxicillin | 16–32 | 64 | 128 | 32 | >64 |
| Doxycycline | 8–16 | >16 | 4 | 1–2 | >4 |
| Ceftriaxone | >0.5 | 0.25–>1 | >1 | 1–2 | 2 |
| Azithromycin | 0.025 | >0.05 | >0.0125 | 0.0125 | ≥0.05 |
| Amikacin | 256–512 | 512 | 64 | 512 | 32–64 |
MBCs, minimum bactericidal concentrations. PKo, B. afzelii reference strain; B31, B. burgdorferi sensu stricto reference strain; HT31, B. miyamotoi tick isolate (Japan); LB-2001, B. miyamotoi tick isolate (United States); HS1, B. hermsii reference strain.
Ranges are results of tests that were conducted in quadruplicate.
FIG 1.
Correlation between microscopy and the drop in absorbance with doxycycline. Shown is the correlation between microscopy results and the drop in absorbance (A562/A630)—as a percentage of that of the positive control (MIC2)—after 72 h of incubation with a medium containing a 2-fold dilution series of doxycycline. The microscopy results, expressed as the number of spirochetes per milliliter, are given along the left y axis. The drop in absorbance, as a percentage of the decrease in absorbance of the positive control, is given along the right y axis and was calculated as [(Et0 − Et72)/(EPOS,t0 − EPOS,t72)] × 100. Symbols and error bars represent means ± standard deviations. PKo, B. afzelii reference strain; B31, B. burgdorferi sensu stricto reference strain; HS1, B. hermsii reference strain; HT31, B. miyamotoi tick isolate from Japan; LB-2001, B. miyamotoi tick isolate from the United States.
DISCUSSION
In vitro antibiotic susceptibility.
In this study, we describe, to our knowledge for the first time, the in vitro susceptibilities of B. miyamotoi to the antibiotics most commonly used in the treatment of HTBRF and Lyme borreliosis. Microdilution-based methods have been extensively studied by others and have been shown to be robust methods for standardized testing of the antimicrobial susceptibility of Borrelia species (18, 20, 21). Using similar methods, we demonstrated that B. miyamotoi strains HT31 and LB-2001 are susceptible to azithromycin, doxycycline, and ceftriaxone but—compared to the B. burgdorferi sensu lato strains tested—resistant to amoxicillin in vitro. Although multiple pharmacokinetic and dynamic features are important for the efficacy of an antibiotic to eradicate B. miyamotoi, it should be noted that the MICs of ceftriaxone, azithromycin, and doxycycline for B. miyamotoi isolates are well below the serum trough levels (22) that are reached by the dosages of these antibiotics recommended in international guidelines (23). In contrast, amoxicillin MICs for B. miyamotoi isolates (8 to 32 mg/liter) were comparable to, or higher than, the peak serum levels (8 to 10 mg/liter) reached by the dosage of amoxicillin recommended in international guidelines (22, 23). Since no clinical guidelines exist for the treatment of HTBRF, and clinical experience is limited, our in vitro findings are of great interest and warrant further study, including in vivo studies.
Of note, the susceptibilities of B. afzelii and B. burgdorferi sensu stricto isolates in this study to various antibiotics, including amoxicillin, were comparable to those determined in previous studies (Table 3) (17, 18, 24, 25). Also, the susceptibilities of B. hermsii and B. miyamotoi to doxycycline, ceftriaxone, and azithromycin that we report here are in line with previous studies, which showed similar susceptibilities of other RF Borrelia species to tetracyclines, cephalosporins, and macrolides (16, 19, 26–29). It should be mentioned that only a few studies have described the antimicrobial sensitivities of RF Borrelia spirochetes, such as the louse-borne relapsing-fever spirochete B. recurrentis and the soft-tick-borne relapsing-fever spirochetes B. hermsii and B. turicatae (16, 19, 26). Previous studies reported susceptibility of B. hermsii, B. recurrentis, and B. turicatae to antibiotics of the beta-lactam group (19, 26, 27); however, neither of these studies tested amoxicillin. Interestingly, one study did show that B. hermsii was less susceptible than B. burgdorferi sensu stricto to ampicillin (29), supporting our findings. It is known that the effect of beta-lactam antibiotics is temperature dependent, and previous studies revealed a 16-fold-lower efficacy of penicillin during incubation at 36°C than at 38°C and showed that loss of activity during incubation is attributable to chemical instability (30). This might suggest that the in vitro resistance of RF Borrelia spirochetes to amoxicillin that we describe here could be attributed partially to experimental conditions (17). However, the difference in susceptibility to amoxicillin between the RF Borrelia strains tested and to the B. burgdorferi sensu lato strains cannot be explained by the experimental conditions (Table 1; also Fig. S1 in the supplemental material). Nonetheless, our findings do not allow us to suggest that relapsing-fever Borrelia spirochetes are resistant to treatment with beta-lactam antibiotics, such as amoxicillin, in vivo. First, an immunocompromised patient with B. miyamotoi meningoencephalitis was successfully treated using penicillin G (10). Second, since we tested only two B. miyamotoi strains, albeit from two different continents, and one B. hermsii strain, caution should be used in predicting a general pattern of susceptibility of B. miyamotoi or relapsing-fever Borrelia spirochetes.
TABLE 3.
MICs of different antimicrobial agents for Borrelia burgdorferi sensu lato in the literature and the current study
| Study | Strain(s) (no.) | Method | MIC range (mg/liter) |
||||
|---|---|---|---|---|---|---|---|
| Amoxicillin | Doxycycline | Ceftriaxone | Azithromycin | Amikacin | |||
| Hunfeld et al. (20) | Various | Review | 0.03–2 | 0.06–2 | <0.01–0.125 | 0.003–0.03 | 32–>128 |
| Veinović et al. (18) | B. burgdorferi sensu stricto (9) | Microscopy | 0.125–2 | 0.125–2 | 0.03–0.25 | 0.027–0.22 | 32–512 |
| Ruzić-Sabljić et al. (24) | B. afzelii (10) | Microscopy | 1–4 | 1–4 | 0.063–4,0 | 0.0138–0.0275 | 64–128 |
| Baradaran-Dilmaghani and Stanek (16) | B31 | Microscopy | 0.2 | 0.4 | 0.013 | 0.006 | NDa |
| Hunfeld et al. (21) | PKo and B31 | Microscopy | 0.125–0.5b | 0.25 | 0.03 | <0.01 | ND |
| Boerner et al. (29) | B31 and N34 | Microscopy | 0.06–1b | 0.125–2 | 0.03–0.06 | <0.016–0.03 | 8–32c |
| This study | PKo and B31 | Microscopy | 1–4 | 2 | 0.06–0.125 | 0.0128 | 256–512 |
| Colorimetric assay | 0.5–2 | 0.125–1 | 0.03–0.06 | 0.003–0.0256 | 256–512 | ||
| Hunfeld et al. (21) | PKo and B31 | Colorimetric assay | 0.015–2b | 0.06–1 | <0.01–0.06 | <0.01–0.06 | ND |
| Boerner et al. (29) | B31 and N34 | Colorimetric assay | 0.06–2b | 0.125–1 | <0.016–0.125 | <0.016 | 4–32c |
| Morgenstern et al. (25) | B31 | Colorimetric assay | 0.5 | 0.25 | 0.03 | 0.03d | ND |
ND, not determined.
Penicillin G was used instead of amoxicillin.
Gentamicin was used instead of amikacin.
Erythromycin was used instead of azithromycin.
Colorimetric antibiotic susceptibility testing.
As part of this study, we tested the in vitro susceptibilities of several Borrelia strains to various antibiotics by dark-field microscopy, but we also used an existing colorimetric microdilution method, by Hunfeld et al. (21) (yielding MIC1), in order to strive for a more standardized procedure, as well as a newly described variant to this colorimetric method (yielding MIC2). The two colorimetric methods used in this study demonstrated largely similar MIC values. Of interest, a significant correlation (P < 0.01) was found between the colorimetric MIC2 measurement and the number of motile spirochetes as determined by microscopy after 72 h of incubation (Fig. S2 in the supplemental material). However, colorimetric assays are known to be affected by laboratory conditions, such as the type of liquid medium used (e.g., Barbour-Stoenner-Kelly [BSK] medium, modified Kelly-Pettenkofer [MKP] medium, or MKP medium supplemented with 10% heat-inactivated fetal calf serum (MKP-F medium), incubation temperatures, and differences in anaerobic or aerobic cultivation. Indeed, we found that in colorimetric assays, the color of the medium of the microtiter wells, including the negative controls, changes in the first 48 h, possibly due to changes in the solubility of CO2, a volatile acid, at increasing temperatures (31). The resulting change in absorbance affects the outcome of the colorimetric measurement. In the current study, we circumvented this nonspecific color shift in the first 48 h by preincubating the cultivation medium for 72 h at 33°C, as described in Materials and Methods, and by correcting the decrease in absorbance after 72 h for the change in absorbance of the negative control. Importantly, by comparing color shifts to those of the positive control, the newly described MIC2 is less dependent on experimental conditions, limiting intra- and interexperimental differences, Moreover, especially for Borrelia strains with lower replication rates (B31, HT31, and LB-2001), the MIC2 correlated better to the microscopic MIC. We therefore propose that this method should be used for determining in vitro antibiotic susceptibility using a colorimetric change.
Microscopic testing of antibiotic susceptibility.
Not unexpectedly, both MIC1 and MIC2 were mostly a few dilution steps below the MICs determined by dark-field microscopy. This can be explained by the fact that the colorimetric change reflects a significant decrease in borrelial growth or replication rather than indicating the absolute absence of viable spirochetes. Therefore, colorimetric MIC measurement can be considered a non-labor-intensive, robust, and easy-to-standardize method for testing spirochetal antibiotic susceptibility; however, dark-field microscopy remains the gold standard for the determination of motile spirochetes and MICs. Minimal bactericidal concentrations (MBCs) were determined by inoculating a small amount of medium containing spirochetes that were exposed to antibiotics for 72 h into fresh medium. Our culture methods allow for successful cultivation of as little as 1 spirochete (data not shown), suggesting that this is a sensitive method even for small amounts of viable spirochetes. As shown in Table 2, MBCs in general were 1 to 3 dilution steps higher than the MIC values we found; however, in some cases, they exceeded the highest concentration tested, so that we could not determine the exact MBC for each Borrelia strain and antibiotic tested.
Antibiotic treatment of HTBRF.
Treatment for HTBRF is currently empirically based on standard antibiotic therapy for Lyme borreliosis and treatment of other relapsing-fever Borrelia infections, assuming that the organism has the same antibiotic susceptibilities as these Borrelia spp. (8). However, no clinical guideline for the treatment of HTBRF exists yet. Based on our findings and practical considerations, doxycycline (100 mg orally twice daily) seems to be the preferred initial therapy for patients suspected of having a B. miyamotoi infection. This would also be an effective treatment against Lyme disease and human granulocytic anaplasmosis, which might be in the differential diagnosis. Doxycycline is contraindicated for children under the age of 9 years and for pregnant and nursing women, and therefore, amoxicillin has been recommended as an alternative oral therapy to tetracyclines. For HTBRF, intravenous therapy with ceftriaxone has been used for immunocompromised patients with central nervous system disease (e.g., meningoencephalitis) (9, 12) or immunocompetent patients (6), and penicillin G has been used successfully as an alternative (10). The outcome of antibiotic therapy for HTBRF has been described only in a few case reports and case series (6, 9, 10, 32), which reported only minor treatment failures. Of interest, recently, one U.S. case report described the complete recovery of a patient with HTBRF even without antibiotic treatment (33). Treatment of 51 U.S. patients with fever after a tick bite, who were retrospectively shown by PCR to have had HTBRF, consisted of doxycycline, ceftriaxone, and, for 7 patients, oral amoxicillin (7). Of these patients, three reported residual fatigue or other symptoms several months after the completion of antibiotic treatment, but whether these patients had received oral amoxicillin or another antibiotic regime was not mentioned. Penicillins, including aminopenicillins such as amoxicillin, have displayed no more than moderate-to-good in vitro activity against B. burgdorferi sensu lato; nonetheless, amoxicillin is clinically effective in the treatment of Lyme borreliosis. We show that B. miyamotoi strains HT31 and LB-2001 are less susceptible to amoxicillin than B. burgdorferi sensu lato isolates in vitro. Whether these findings are due, for instance, to alterations in known penicillin-binding proteins (PBPs) in RF Borrelia spirochetes or to the production of additional PBPs with a lower affinity to penicillins remains to be investigated, as does the in vivo translation of our findings.
In conclusion, we demonstrate poor in vitro activity of amoxicillin against B. miyamotoi strains from two continents and one B. hermsii isolate, with MICs and MBCs ranging from 8 to 32 mg/liter and 32 to 128 mg/liter, respectively. This finding contrasts sharply with the results for the B. burgdorferi sensu stricto and B. afzelii strains tested, for which MICs were 0.5 to 2 mg/liter and 2 to 4 mg/liter, respectively. Therefore, amoxicillin might have to be used with caution in the treatment of HTBRF. Further research should focus on (i) the occurrence of poor in vitro activity of amoxicillin against clinical B. miyamotoi isolates, (ii) the mechanism underlying the possible resistance of B. miyamotoi to amoxicillin, (iii) the in vivo efficacy of beta-lactams in animal studies, and (iv) the clinical efficacy of the treatment of HTBRF with beta-lactam antibiotics.
MATERIALS AND METHODS
Bacterial strains and culture medium.
The Borrelia strains investigated in this study were PKo (a B. afzelii skin isolate from Germany), B31 (B. burgdorferi sensu stricto; ATCC 35210; tick isolate, United States), LB-2001 (B. miyamotoi, murine isolate; Yale University, United States), HT31 (B. miyamotoi, tick isolate; Japan) and HS1 (B. hermsii, tick isolate; United States). Glycerol stocks of low-passage-number spirochete isolates (<5 passages) were thawed and were cultured in a regular incubator (Memmert, Schwabach, Germany) at 33°C for 4 days to reach the exponential, mid-log phase of growth. Spirochetes were enumerated using a Petroff-Hausser counting chamber and dark-field microscopy. B. burgdorferi sensu stricto B31 and B. afzelii PKo were cultured using modified Kelly-Pettenkofer (MKP) medium, and B. miyamotoi HT31, B. miyamotoi LB-2001, and B. hermsii HS1 were cultured using modified Kelly-Pettenkofer medium with the addition of 10% heat-inactivated fetal calf serum (MKP-F medium), as described previously (14).
Microdilution assays and antimicrobial drugs.
The antibiotics tested belonged to five different classes: penicillins, tetracyclines, macrolides, cephalosporins, and aminoglycosides. These antibiotics are used in standard regimens for the treatment of Lyme borreliosis. Amikacin was used as a negative control, since Borrelia spirochetes are known to be resistant to this antibiotic agent (18, 24, 34). Detailed information is shown in Table 4. MICs were determined by a broth microdilution method using 96-well flat-bottom polystyrene microtiter trays (M0687; Greiner) as described by others (18, 21). Four-day spirochete cultures were centrifuged at 10,000 × g for 10 min, resuspended in MKP (B. burgdorferi sensu lato) or MKP-F (RF Borrelia strains) medium containing extra phenol red (25 mg/liter) as a growth indicator, enumerated, and adjusted to 5 × 107 cells/ml. MKP and MKP-F media with extra phenol red were preincubated at 33°C for 72 h in 50-ml polystyrene tubes with an approximately 30% volume column of air above the medium and were sealed with screw caps to achieve a steady equilibrium of the phenol red color indicator in the media. After preincubation, 2-fold serial dilutions of antibiotics were made in 180 μl MKP and MKP-F media with extra phenol red. Twenty microliters of the spirochete suspension was added to wells, achieving a final concentration of 5 × 106 spirochetes/ml in each well. All antimicrobial agents, as well as a negative control (no spirochetes) and a positive control (no antibiotics), were tested in quadruplicate. The microtiter trays were sealed with adhesive plastic and were incubated in a regular incubator (Memmert, Schwabach, Germany) at 33°C for 72 h. Each day, the microtiter wells were gently mixed to disperse the spirochetes evenly throughout the medium.
TABLE 4.
Tested antimicrobial agents and dilution ranges
| Antimicrobial | Companya | Order no. | Dilution range (mg/liter) |
Breakpoint (mg/liter)b | Stock concn (mg/ml), diluentc | |
|---|---|---|---|---|---|---|
| B. burgdorferi sensu lato | Relapsing-fever Borrelia strains | |||||
| Amoxicillin | Centrafarm | 14029596 | 0.25–128 | 0.25–128 | ≤4 | 8.89, PBS |
| Doxycycline | Sigma-Aldrich | D3447 | 0.03–8 | 0.004–2 | ≤4 | 44.4, DMSO |
| Ceftriaxone | Sigma-Aldrich | PHR1382 | 0.008–4 | 0.008–4 | ≤8 | 4.44, PBS |
| Azithromycin | Sigma-Aldrich | PHR1088 | 0.0001–0.05 | 0.0001–0.05 | ≤2 | 5.56, DMSO |
| Amikacin | Sigma-Aldrich | A1774 | 2–1,024 | 2–1,024 | ≤16 | 11.26, MKP |
Locations: Centrafarm, Etten-Leur, the Netherlands; Sigma-Aldrich, St. Louis, MO, USA.
According to the Clinical and Laboratory Standards Institute.
PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide; MKP, modified Kelly-Pettenkofer medium. The DMSO concentrations used in our studies did not influence Borrelia viability (data not shown).
Colorimetric MIC measurements.
Absorbance was measured at 562 nm and 630 nm at 0, 24, 48, and 72 h using a commercially available enzyme-linked immunosorbent assay (ELISA) reader (PowerWave 200; Bio-Tek Instruments, USA), and absorbance values were calculated by dividing the absorbance at 562 nm by the absorbance at 630 nm. Colorimetric change was calculated by comparing the absorbance after 72 h (Et72) and initial absorbance values (Et0) for each concentration of the antimicrobial agent corrected for the absorbance of the negative control (by subtracting the change in absorbance found in the wells of the negative control from the change in absorbance in each well). Two calculations of the colorimetric MIC were used and compared. MIC1, as described by others, is defined as the lowest concentration of antibiotics where (Et0 − Et72) is <10% of Et0 (21). It has been established that the absence of borrelial growth in microtiter wells results in a decrease in absorbance of <5 to 10% compared to the initial absorbance values of the microtiter well after 72 h (17, 20, 21, 35). However, this measurement is also dependent on the initial absorbance, which is independent of borrelial growth and can differ between experiments. Also, low replication rates of some Borrelia strains lead to a smaller decrease in absorbance compared to the initial absorbance values. Therefore, we also applied an alternative calculation, MIC2, in which the MIC was calculated by comparing the drop in absorbance (Et0 − Et72) to the drop in absorbance of the positive control (no antibiotics) (EPOS,t0 − EPOS,t72), and the MIC was set arbitrarily at 25%. Thus, MIC2 was defined as the lowest concentration of antibiotics where (Et0 − Et72) is <25% of (EPOS,t0 − EPOS,t72). This method is solely dependent on borrelial growth and is independent of the initial absorbance and replication rate.
MIC measurement by dark-field microscopy.
Spirochetes were counted in all wells after 72 h by dark-field microscopy, as described previously (14). Briefly, 5 μl from each well was put on a microscopy slide and covered by a coverslip. Slides were blinded, and motile spirochetes were counted in six separate microscopy fields. The MIC was defined as the lowest concentration of the antimicrobial agent at which no motile spirochetes were observed by dark-field microscopy. One spirochete per microscopy field equals a concentration of 2.5 × 105/ml. Since we counted 6 separate fields, the lower detection limit using this method was 4 × 104 spirochetes/ml.
Determination of MBCs.
MBCs were determined by taking medium from wells one concentration below the MIC (as determined by dark-field microscopy) up to two wells above the MIC (in which minimal spirochetal growth could still be discerned) and diluting these samples 1:75 to a total volume of 1.35 ml of fresh MKP or MKP-F medium in 2-ml screw-cap tubes, as described by others (20). These samples were evaluated for growth after 3 weeks. The MBC was defined as the lowest concentration of the antimicrobial agent at which no motile spirochetes could be detected by dark-field microscopy. The lower detection limit using this counting method was approximately 4 ×104 spirochetes/ml, as described above.
Statistical analysis.
A Pearson correlation was used to calculate the correlation between normalized (0 to 1) MIC2 results and normalized (0 to 1) microscopy results. All analyses were performed using PASW Statistics software, version 19.0 (SPSS Inc., Chicago, IL, USA), and a P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank both Linda Bockenstedt (Department of Internal Medicine, Yale School of Medicine, New Haven, CT, USA) and Durland Fish (Department of Epidemiology and Public Health, Yale School of Medicine, New Haven, CT, USA) for providing B. miyamotoi strain LB-2001, and both Barbara Johnson (Centers for Disease Control and Prevention, USA) and Volker Fingerle (German National Reference Centre for Borrelia) for providing B. miyamotoi strain HT31.
All authors declare no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00535-17.
REFERENCES
- 1.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol 45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 2.Wagemakers A, Staarink PJ, Sprong H, Hovius JW. 2015. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol 31:260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Hardin JA, Malawista SE. 1977. Erythema chronicum migrans and Lyme arthritis: cryoimmunoglobulins and clinical activity of skin and joints. Science 196:1121–1122. doi: 10.1126/science.870973. [DOI] [PubMed] [Google Scholar]
- 4.Crowder CD, Carolan HE, Rounds MA, Honig V, Mothes B, Haag H, Nolte O, Luft BJ, Grubhoffer L, Ecker DJ, Schutzer SE, Eshoo MW. 2014. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg Infect Dis 20:1678–1682. doi: 10.3201/eid2010.131583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takano A, Toyomane K, Konnai S, Ohashi K, Nakao M, Ito T, Andoh M, Maeda K, Watarai M, Sato K, Kawabata H. 2014. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS One 9:e104532. doi: 10.1371/journal.pone.0104532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. 2011. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molloy PJ, Telford SR III, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. 2015. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med 163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 8.Krause PJ, Barbour AG. 2015. Borrelia miyamotoi: the newest infection brought to us by deer ticks. Ann Intern Med 163:141–142. doi: 10.7326/M15-1219. [DOI] [PubMed] [Google Scholar]
- 9.Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, Jahfari S, Pals ST, Horlings HM, Fikrig E, Sprong H, van Oers MH. 2013. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gugliotta JL, Goethert HK, Berardi VP, Telford SR III. 2013. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med 368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Takano A, Konnai S, Nakao M, Ito T, Koyama K, Kaneko M, Ohnishi M, Kawabata H. 2014. Human infections with Borrelia miyamotoi, Japan. Emerg Infect Dis 20:1391–1393. doi: 10.3201/eid2008.131761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boden K, Lobenstein S, Hermann B, Margos G, Fingerle V. 2016. Borrelia miyamotoi-associated neuroborreliosis in immunocompromised person. Emerg Infect Dis 22:1617–1620. doi: 10.3201/eid2209.152034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. 2009. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg 81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. 2014. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit Vectors 7:418. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margos G, Stockmeier S, Hizo-Teufel C, Hepner S, Fish D, Dautel H, Sing A, Dzaferovic E, Rieger M, Jungnick S, Binder K, Straubinger RK, Fingerle V. 2015. Long-term in vitro cultivation of Borrelia miyamotoi. Ticks Tick Borne Dis 6:181–184. doi: 10.1016/j.ttbdis.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Baradaran-Dilmaghani R, Stanek G. 1996. In vitro susceptibility of thirty Borrelia strains from various sources against eight antimicrobial chemotherapeutics. Infection 24:60–63. doi: 10.1007/BF01780660. [DOI] [PubMed] [Google Scholar]
- 17.Hunfeld KP, Brade V. 2006. Antimicrobial susceptibility of Borrelia burgdorferi sensu lato: what we know, what we don't know, and what we need to know. Wien Klin Wochenschr 118:659–668. doi: 10.1007/s00508-006-0693-z. [DOI] [PubMed] [Google Scholar]
- 18.Veinović G, Cerar T, Strle F, Lotric-Furlan S, Maraspin V, Cimperman J, Ruzić-Sabljić E. 2013. In vitro susceptibility of European human Borrelia burgdorferi sensu stricto strains to antimicrobial agents. Int J Antimicrob Agents 41:288–291. doi: 10.1016/j.ijantimicag.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Barbour AG, Todd WJ, Stoenner HG. 1982. Action of penicillin on Borrelia hermsii. Antimicrob Agents Chemother 21:823–829. doi: 10.1128/AAC.21.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunfeld KP, Kraiczy P, Kekoukh E, Schafer V, Brade V. 2002. Standardised in vitro susceptibility testing of Borrelia burgdorferi against well-known and newly developed antimicrobial agents—possible implications for new therapeutic approaches to Lyme disease. Int J Med Microbiol 291(Suppl 33):S125–S137. doi: 10.1016/S1438-4221(02)80024-8. [DOI] [PubMed] [Google Scholar]
- 21.Hunfeld KP, Kraiczy P, Wichelhaus TA, Schafer V, Brade V. 2000. New colorimetric microdilution method for in vitro susceptibility testing of Borrelia burgdorferi against antimicrobial substances. Eur J Clin Microbiol Infect Dis 19:27–32. doi: 10.1007/s100960050005. [DOI] [PubMed] [Google Scholar]
- 22.Grayson ML, Crowe SM, McCarthy JS, Mills J, Mouton JW, Norrby SR, Paterson DL, Pfaller MA. 2010. Kucers' the use of antibiotics: a clinical review of antibacterial, antifungal and antiviral drugs, 6th ed CRC Press, Boca Raton, FL. [Google Scholar]
- 23.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 24.Ruzić-Sabljić E, Podreka T, Maraspin V, Strle F. 2005. Susceptibility of Borrelia afzelii strains to antimicrobial agents. Int J Antimicrob Agents 25:474–478. doi: 10.1016/j.ijantimicag.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Morgenstern K, Baljer G, Norris DE, Kraiczy P, Hanssen-Hubner C, Hunfeld KP. 2009. In vitro susceptibility of Borrelia spielmanii to antimicrobial agents commonly used for treatment of Lyme disease. Antimicrob Agents Chemother 53:1281–1284. doi: 10.1128/AAC.01247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutler SJ, Fekade D, Hussein K, Knox KA, Melka A, Cann K, Emilianus AR, Warrell DA, Wright DJ. 1994. Successful in vitro cultivation of Borrelia recurrentis. Lancet 343:242. doi: 10.1016/S0140-6736(94)91032-4. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RC, Kodner CB, Jurkovich PJ, Collins JJ. 1990. Comparative in vitro and in vivo susceptibilities of the Lyme disease spirochete Borrelia burgdorferi to cefuroxime and other antimicrobial agents. Antimicrob Agents Chemother 34:2133–2136. doi: 10.1128/AAC.34.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dever LL, Jorgensen JH, Barbour AG. 1992. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J Clin Microbiol 30:2692–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boerner J, Failing K, Wittenbrink MM. 1995. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: influence of test conditions on minimal inhibitory concentration (MIC) values. Zentralbl Bakteriol 283:49–60. doi: 10.1016/S0934-8840(11)80890-X. [DOI] [PubMed] [Google Scholar]
- 30.Reisinger E, Wendelin I, Gasser R, Halwachs G, Wilders-Truschnig M, Krejs G. 1996. Antibiotics and increased temperature against Borrelia burgdorferi in vitro. Scand J Infect Dis 28:155–157. doi: 10.3109/00365549609049067. [DOI] [PubMed] [Google Scholar]
- 31.Carroll JJ, Slupsky JD, Mather AE. 1991. The solubility of carbon dioxide in water at low pressure. J Phys Chem 20:1201–1209. [Google Scholar]
- 32.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. 2013. Human Borrelia miyamotoi infection in the United States. N Engl J Med 368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sudhindra P, Wang G, Schriefer ME, McKenna D, Zhuge J, Krause PJ, Marques AR, Wormser GP. 2016. Insights into Borrelia miyamotoi infection from an untreated case demonstrating relapsing fever, monocytosis and a positive C6 Lyme serology. Diagn Microbiol Infect Dis 86:93–96. doi: 10.1016/j.diagmicrobio.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunfeld KP, Kraiczy P, Wichelhaus TA, Schafer V, Brade V. 2000. Colorimetric in vitro susceptibility testing of penicillins, cephalosporins, macrolides, streptogramins, tetracyclines, and aminoglycosides against Borrelia burgdorferi isolates. Int J Antimicrob Agents 15:11–17. doi: 10.1016/S0924-8579(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 35.Rödel R, Freyer A, Bittner T, Schafer V, Hunfeld KP. 2007. In vitro activities of faropenem, ertapenem, imipenem and meropenem against Borrelia burgdorferi s.l. Int J Antimicrob Agents 30:83–86. doi: 10.1016/j.ijantimicag.2007.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.