ABSTRACT
We determined imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibactam MICs against 100 CRE isolates that underwent whole-genome sequencing. Klebsiella pneumoniae carbapenemases (KPCs) were the most common carbapenemases. Forty-six isolates carried extended-spectrum β-lactamases (ESBLs). With the addition of relebactam, imipenem susceptibility increased from 8% to 88%. With the addition of avibactam, ceftazidime susceptibility increased from 0% to 85%. Neither imipenem-relebactam nor ceftazidime-avibactam was active against metallo-β-lactamase (MBL) producers. Ceftazidime-avibactam (but not imipenem-relebactam) was active against OXA-48-like producers, including a strain not harboring any ESBL. Major OmpK36 porin mutations were independently associated with higher imipenem-relebactam MICs (P < 0.0001) and showed a trend toward independent association with higher ceftazidime-avibactam MICs (P = 0.07). The presence of variant KPC-3 was associated with ceftazidime-avibactam resistance (P < 0.0001). In conclusion, imipenem-relebactam and ceftazidime-avibactam had overlapping spectra of activity and niches in which each was superior. Major OmpK36 mutations in KPC-K. pneumoniae may provide a foundation for stepwise emergence of imipenem-relebactam and ceftazidime-avibactam resistance.
KEYWORDS: imipenem-relabactam, ceftazidime-avibactam, Enterobacteriaceae, porins, mechanisms of resistance, CRE, KPC, drug resistance mechanisms
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae (CRE) are major health care threats. The production of carbapenemases, particularly Klebsiella pneumoniae carbapenemases (KPCs), remains the most common mechanism of carbapenem resistance among Enterobacteriaceae. In addition, porin mutations, efflux pumps, aminoglycoside-modifying enzymes, extended-spectrum β-lactamases (ESBLs), and AmpC β-lactamases may also contribute to resistance phenotypes (1–7).
Ceftazidime-avibactam was recently approved by the U.S. Food and Drug Administration and has activity against multidrug-resistant Enterobacteriaceae (8). Avibactam, a novel β-lactamase inhibitor, is active against class A (e.g., KPC), class C (e.g., AmpC), and certain class D (e.g., OXA-48) carbapenemases, but not class B metallo-β-lactamases (MBLs) (e.g., VIM, IMP, and NDM) (9, 10). Unfortunately, ceftazidime-avibactam resistance has emerged during treatment of KPC-producing K. pneumoniae (KPC-K. pneumoniae) infections (11, 12). Resistance has been linked to specific mutations in the blaKPC-3 gene that result in variant KPC-3 enzymes (13, 14), porin mutations, and increased expression of wild-type blaKPC-3 (15). Variant KPC-3 enzymes that confer ceftazidime-avibactam resistance no longer function as carbapenemases, but rather as ESBLs (13, 14).
Relebactam is a novel β-lactamase inhibitor currently being evaluated for use with imipenem for the treatment of Gram-negative bacterial infections (16, 17). Like avibactam, it inhibits class A and C β-lactamases and has no activity against MBLs. Unlike avibactam, however, relebactam does not have activity against class D carbapenemases (18, 19). In a recent phase 2 dose-ranging study of patients with complicated intra-abdominal infections (13% of whom were infected with imipenem-resistant Gram-negative organisms), clinical response rates for imipenem-relebactam were >96% (16). An earlier study established the in vitro efficacy of imipenem-relebactam against Gram-negative pathogens from New York City, including KPC-producing organisms (20). However, not all the isolates were CRE, and whole-genome-sequencing (WGS) data were available for only a minority of isolates. In the present study, we determined the in vitro activities of imipenem-relebactam and ceftazidime-avibactam against genetically diverse international CRE isolates, and we used WGS data to identify predictors of elevated MICs.
RESULTS
Genetic description of CRE isolates.
One hundred isolates underwent testing: 72 K. pneumoniae, 17 Enterobacter cloacae complex, 9 Escherichia coli, and Klebsiella oxytoca and Enterobacter aerogenes (1 each) isolates (Table 1). The K. pneumoniae sequence types (ST) were ST258 (n = 46), ST147 (n = 5), ST11 (n = 4), and others (n = 17). Ninety-seven isolates harbored a carbapenemase. KPCs were the most common carbapenemases (n = 88), followed by MBLs (n = 7) and OXA-48-like carbapenemases (n = 4). Two isolates produced both KPC and MBL (1 each with NDM-1 and VIM-1). Two K. pneumoniae and 1 E. cloacae isolate did not produce carbapenemases but were still carbapenem resistant and harbored both ESBLs and mutations within OmpK35 and OmpK36 porins. Six K. pneumoniae isolates carried mutated blaKPC-3 (Table 1). We previously identified 5 of these isolates as ceftazidime-avibactam resistant (13, 14), including isolates carrying KPC-3 variants with a glycine-for-valine substitution at Ambler position 240 (V240G [or KPC-8]; n = 1), a tyrosine-for-aspartic-acid substitution at position 179 (D179Y; n = 2), and both D179Y and a methionine-for-threonine substitution at position 243 (D179Y/T243M; n = 2). The sixth isolate was a K. pneumoniae isolate that carried three mutations within KPC-3 (L7P, A177E, and D179Y). The KPC, OXA, and MBL subtypes are shown in Table 1.
TABLE 1.
Description of isolates included in this study
| Organism (n) | No. of isolates |
||||||
|---|---|---|---|---|---|---|---|
| MBLa (n = 7) | OXA-48-like carbapenemaseb (n = 4) | KPC (n = 88) |
No carbapenemase (n = 3) | ||||
| KPC-2c (n = 40) | KPC-3 (n = 41) | Mutated KPC-3d (n = 6) | KPC-4 (n = 1) | ||||
| K. pneumoniae (72) | 4c | 4 | 31c | 26 | 6 | 0 | 2 |
| K. oxytoca (1) | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| E. cloacae (17) | 1e | 0 | 5 | 10e | 0 | 1 | 1f |
| E. aerogenes (1) | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| E. coli (9) | 2 | 0 | 3 | 4 | 0 | 0 | 0 |
Class B carbapenemases: NDM-1 (5 isolates), NDM-6 (1 isolate), and VIM-1 (1 isolate).
Class D OXA-carbapenemases: OXA-48 (2 isolates), OXA-181 (1 isolate), and OXA-232 (1 isolate) (see Table 2).
One isolate carried both class B carbapenemase (VIM-1) and KPC-2.
Six isolates carried mutated blaKPC-3 genes that had previously been identified in ceftazidime-avibactam-resistant isolates among patients from our center: 2 resulting in a tyrosine-for-aspartic acid substitution at Ambler position 179 and a methionine-for-threonine substitution at Ambler position 243 of KPC-3 (D179Y/T243M), 2 resulting in a tyrosine-for-aspartic acid substitution at position 179 (D179Y, and 1 resulting in a glycine-for-valine substitution at position 240 (V240G, also known as KPC-8).
One isolate carried both class B carbapenemase (NDM-1) and KPC-3.
Genetic profile for this isolate: CTX-M-15, ACT-16, TEM-1, and mutations within Omp35 and Omp36.
Forty-six isolates carried ESBLs, including SHV (n = 28), CTX-M (n = 19), and TEM (n = 2). Three isolates had >1 ESBL. Thirty-seven isolates harbored both KPC and ESBL. Five isolates harbored MBL and ESBL, and three isolates harbored both OXA-48-like carbapenemase and ESBL (Table 2).
TABLE 2.
Description of K. pneumoniae isolates producing OXA-48-like carbapenemases (n = 4 isolates)
| OXA, ESBL, and porin pattern | MIC (μg/ml) |
|||
|---|---|---|---|---|
| OXA-48, no ESBL, wild-type porins | OXA-48, ESBL, wild-type porins | OXA-181, ESBL, wild-type porins | OXA-232, ESBL, OmpK35, OmpK36 porin mutations | |
| Imipenem | 4 | 2 | 2 | 8 |
| Imipenem-relebactam | 2 | 2 | 8 | 1 |
| Ceftazidime | 128 | 128 | 256 | 32 |
| Ceftazidime-avibactam | 1 | 1 | 1 | 0.5 |
Susceptibility patterns for all isolates.
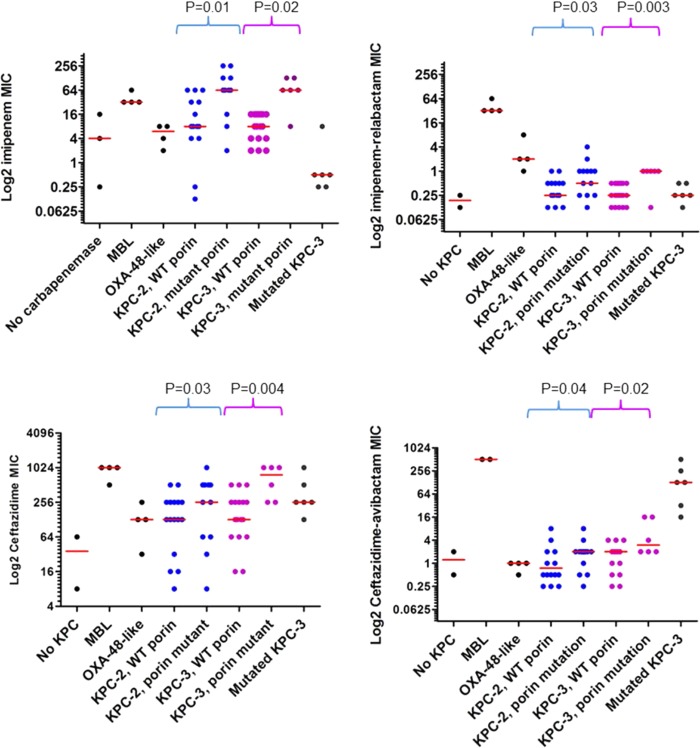
Eight isolates were susceptible to imipenem (MICs ≤ 1 μg/ml); 5 of these carried a variant KPC-3, 2 carried KPC-2, and 1 carried an ESBL and a major OmpK36 mutation (Fig. 1). The MIC50 and MIC90 against all the isolates were 8 and 64 μg/ml, respectively, for imipenem; 0.25 and 2 μg/ml for imipenem-relebactam; 256 and 1,024 μg/ml for ceftazidime; and 2 and 128 μg/ml for ceftazidime-avibactam. All the isolates were resistant to ceftazidime (MIC > 4 μg/ml). With the addition of relebactam, imipenem MICs were reduced by a median of 32-fold (range, 0.5- to 256-fold); with the addition of avibactam, ceftazidime MICs were reduced by a median of 128-fold (range, 0- to 2,048-fold). There were weak correlations between imipenem and imipenem-relebactam MICs (Spearman r = 0.41; P < 0.0001) and ceftazidime and ceftazidime-avibactam MICs (r = 0.55; P < 0.0001). The addition of relebactam improved imipenem susceptibility rates from 8% to 88%; the addition of avibactam improved ceftazidime susceptibility rates from 0% to 85%.
FIG 1.
Distributions of imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibacam MICs, stratified by type of carbapenemase and porin status. The horizontal red lines represent median MICs.
As expected, neither imipenem-relebactam nor ceftazidime-avibactam had in vitro activity against CRE isolates that produced MBLs. Ceftazidime-avibactam was active against all 4 isolates with OXA-48-like carbapenemases, 3 of which also carried ESBLs. These isolates were ceftazidime resistant; ceftazidime-avibactam MICs were significantly lower than ceftazidime MICs (median, 1 versus 128 μg/ml; P = 0.02). Imipenem-relebactam did not have activity against 3 of 4 isolates with OXA-48-like carbapenemases (Fig. 1 and Table 2). There was no significant difference in imipenem-relebactam versus imipenem MICs against OXA-48-like carbapenemase producers (median, 2 versus 6 μg/ml; P = 0.28).
Susceptibility patterns for 62 KPC-producing K. pneumoniae isolates.
Since KPC-K. pneumoniae strains are the most common CRE and two major mutations within the KPC-K. pneumoniae OmpK36 porin (guanine and alanine insertions at amino acids 135 and 136 [ins AA135-136 GD] or IS5 promoter insertion) have been linked to high-level carbapenem resistance (7, 21), we focused on these isolates for the remainder of the study. An isolate coproducing KPC and MBL was excluded. Thirty-five percent (22/62) of KPC-K. pneumoniae isolates harbored a major OmpK36 mutation. Eighty-one percent (50/62) of the isolates had a premature stop codon within the OmpK35 porin. Fifty-eight percent (29/50) of OmpK35 mutant isolates had major OmpK36 mutations versus only 8% (1/12) of isolates with wild-type OmpK35 (P = 0.04).
MIC50 and MIC90 against KPC-K. pneumoniae were 8 and 64 μg/ml, respectively, for imipenem; 0.25 and 2 μg/ml for imipenem-relebactam; 256 and 1,024 μg/ml for ceftazidime; and 2 and 128 μg/ml for ceftazidime-avibactam. Overall, the presence of KPC-2 or KPC-3 did not significantly impact imipenem, imipenem-relebactam, ceftazidime, or ceftazidime-avibactam MICs. In contrast, KPC-3 variants were associated with lower imipenem MICs by univariate analysis (P = 0.0007) (Table 3). Our ability to accurately assess the association between KPC-3 variants and ceftazidime susceptibility was limited because the median MICs against isolates with wild-type and mutant blaKPC-3 were within a 2-fold dilution of the highest concentration of drug tested (512 μg/ml). The presence of ESBL or a major OmpK36 mutation was associated with higher MICs of both imipenem and ceftazidime by univariate analysis (Table 3). The presence of a major OmpK36 mutant was associated with higher imipenem-relebactam MICs, whereas the presence of a KPC-3 variant was associated with higher ceftazidime-avibactam MICs. OmpK35 mutations were not associated with MICs of any agent.
TABLE 3.
Associations between imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibactam MICs against KPC-K. pneumoniae isolates and presence of ESBLs, KPC-3 variants, and major OmpK36 mutationsa
| Factor | Median MIC (μg/ml) (range) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imipenem |
Imipenem-relebactam |
Ceftazidime |
Ceftazidime-avibactam |
|||||||||
| Factor absent | Factor present | P value | Factor absent | Factor present | P value | Factor absent | Factor present | P value | Factor absent | Factor present | P value | |
| ESBL | 8 (0.125–256) | 16 (2–128) | 0.03 | 0.25 (0.125–2) | 0.5 (0.125–4) | 0.39 | 128 (8–1,024) | 256 (8–1,024) | 0.002 | 2 (0.25–512) | 1 (0.25–4) | 0.03 |
| KPC-3 variant | 16 (0.125–256) | 0.5 (0.25–8) | 0.0007 | 0.5 (0.125–4) | 0.25 (0.125–0.5) | 0.31 | 256 (8–1,024) | 256 (128–1,024) | 0.26 | 2 (0.25–16) | 128 (16–512) | 0.0001 |
| Major OmpK36 mutations | 8 (0.125–64) | 64 (2–256) | 0.0001 | 0.25 (0.125–1) | 1 (0.125–4) | 0.0002 | 128 (8–1,024) | 384 (8–1,024) | 0.007 | 1.5 (0.25–256) | 2 (0.25–512) | 0.09 |
Isolates that carried MBL and OXA-carbapenemases were excluded from the analysis.
Multivariate analyses were performed to identify factors independently associated with drug MICs (Table 4). Factors included in the model were presence of ESBL, KPC-3 variant, and major OmpK36 mutations. Of note, none of the KPC-3 variants carried OmpK36 porin mutations. A major OmpK36 mutation was the sole factor independently associated with increased imipenem and imipenem-relebactam MICs (P < 0.0001 for both). ESBL, KPC-3 variants, and major OmpK36 mutations were independently associated with increased ceftazidime MICs. The KPC-3 variant was the sole factor associated with higher ceftazidime-avibactam MICs. There was a trend toward an association between major OmpK36 mutation and ceftazidime-avibactam MICs that did not achieve statistical significance (P = 0.07). There was no significant colinearity between variables included in either of the models (all variance inflation factor [VIF] values were <1.1).
TABLE 4.
Factors independently associated with imipenem, imipenem-relebactam, ceftazidime, and ceftazidime-avibactam MICs against KPC-K. pneumoniae isolates by multivariate analysisa
| Factor |
P value |
|||
|---|---|---|---|---|
| Imipenem | Imipenem-relebactam | Ceftazidime | Ceftazidime-avibactam | |
| ESBL | 0.84 | 0.44 | 0.002 | 0.90 |
| KPC-3 variant | 0.21 | 0.74 | 0.01 | <0.0001 |
| Major OmpK36 mutation | <0.0001 | <0.0001 | 0.001 | 0.07 |
Isolates that carried MBL and OXA-carbapenemase were excluded from the analysis.
DISCUSSION
In this study, we utilized unique CRE isolates that were characterized by WGS to identify potential therapeutic niches for imipenem-relebactam and ceftazidime-avibactam. The use of WGS data allowed us to identify genetic factors that correlated with drug MICs and also assured us that our results were not biased by the testing of clonal isolates. Three findings were particularly notable. First, the addition of relebactam improved the susceptibility of CRE isolates to imipenem. Likewise, the addition of avibactam improved ceftazidime susceptibility. Second, imipenem-relebactam was active against ceftazidime-avibactam-resistant K. pneumoniae isolates that carried variant KPC-3. In turn, ceftazidime-avibactam was active against imipenem-relebactam-resistant K. pneumoniae isolates that carried OXA-48-type carbapenemase. Finally, major OmpK36 porin mutations among KPC-producing K. pneumoniae isolates were independently associated with higher imipenem-relebactam, imipenem, and ceftazidime MICs. Major OmpK36 mutations also showed a trend toward an independent association with higher ceftazidime-avibactam MICs. Overall, imipenem-relebactam and ceftazidime-avibactam are promising additions to the current antibiotic armamentarium, providing broad activity against CRE, including Klebsiella spp., Enterobacter spp., and E. coli (18, 20). The drugs have overlapping spectra of activity, as well as niches in which each is superior.
Relebactam restored imipenem activity against CRE isolates regardless of KPC type or ESBL. We recently reported that KPC-3 mutations, in particular D179Y, can emerge and confer ceftazidime-avibactam resistance following treatment of carbapenem-resistant K. pneumoniae infections (13, 14, 22). Imipenem MICs in the present study were significantly lower against K. pneumoniae with variant KPC-3 than against K. pneumoniae with wild-type KPCs. One KPC-3 variant-carrying isolate was resistant to both ceftazidime-avibactam and imipenem (MICs = 16 and 8 μg/ml, respectively). However, the isolate was susceptible to imipenem-relebactam (MIC = 0.5 μg/ml), suggesting that this agent may be more reliably active than imipenem against infections caused by ceftazidime-avibactam-resistant Enterobacteriaceae. We recently demonstrated that meropenem resistance emerged in ceftazidime-avibactam-resistant K. pneumoniae isolates during in vitro passage at subinhibitory meropenem concentrations, often in association with reversion of mutant blaKPC-3 to wild-type blaKPC-3 (22). Therefore, studies are needed to determine the roles of imipenem-relebactam in treating infections caused by CRE that carry mutant KPC-3 enzymes and in suppressing carbapenem resistance among these isolates.
Not surprisingly, relebactam had little to no effect on imipenem MICs against 3 of 4 CRE isolates that carried OXA-48 or OXA-48-like carbapenemases (OXA-181 and -232) (Table 2). Median imipenem MICs against these isolates were lower than those against CRE with KPC-2 or wild-type KPC-3 (6 versus 16 μg/ml; P = 0.057), consistent with the fact that OXA-48 exerts low-level carbapenem hydrolysis (23). Unlike relebactam, avibactam has potent activity against OXA-48-like carbapenemases due to the formation of a strong covalent complex (9, 18, 19). Three of our four isolates that produced OXA-48-like carbapenemases also produced ESBL, and addition of avibactam to ceftazidime caused the isolates to revert from ceftazidime resistant to susceptible. Since ceftazidime is not hydrolyzed or only weakly hydrolyzed by OXA-48, our data confirm the activity of avibactam against ESBLs (23). Interestingly, one isolate producing only OXA-48 (and not ESBL) was resistant to ceftazidime (MIC, 128 μg/ml) and susceptible to ceftazidime-avibactam (MIC, 1 μg/ml). As expected, neither ceftazidime-avibactam nor imipenem-relebactam was active against CRE carrying MBLs due to the formation of a strong covalent complex.
Porins are outer membrane channels that allow β-lactams and other antibiotics to diffuse into periplasmic active sites (24). Outer membrane impermeability due to porin mutations is well recognized as the Achilles heel of new antibiotics (24). The associations we found between major OmpK36 mutations and higher MICs corroborate the findings of earlier studies (20, 25, 26). A distinct minority of isolates with major OmpK36 mutations were resistant to imipenem-relebactam (n = 2, both carrying KPC-2) or ceftazidime-avibactam (n = 2, both carrying KPC-3). However, OmpK36 mutations may provide a platform for the future stepwise emergence of imipenem-relebactam and ceftazidime-avibactam resistance (25). Our finding that OmpK35 mutations were not associated with differences in MICs was consistent with previous studies by our group (21).
Although we tested isolates from around the world, it is important to acknowledge that the majority were from North America. As clinicians contemplate how to incorporate new agents like imipenem-relebactam and ceftazidime-avibactam into the management strategies against CRE infections, it will be necessary to understand local epidemiology and strain genetics. At centers such as ours that are experiencing the emergence of ceftazidime-avibactam resistance, particularly among KPC-3-producing K. pneumoniae isolates, imipenem-relebactam may afford an option for primary or salvage treatment. In contrast, ceftazidime-avibactam may occupy a valuable therapeutic niche at centers with high prevalence of OXA-48 or OXA-48-like carbapenemases. A pressing question is whether using these agents as part of combination regimens will best preserve their effectiveness and limit resistance. The present study demonstrates that nuanced approaches to therapeutic decision making, likely in conjunction with rapid molecular assays, will be essential in optimizing the utility of anti-CRE agents.
MATERIALS AND METHODS
Characterization of isolates.
One hundred clinical CRE isolates were selected from previous studies (14, 27) or sequenced in the current study. The latter were obtained from repositories at the University of Pittsburgh Medical Center [UPMC] and Rutgers-New Jersey Medical School. The geographic distribution was as follows: United States (88), India (3), China (3), Belgium (1), Brazil (1), Canada (1), Colombia (1), Greece (1), and Turkey (1). The isolates were subcultured at least twice on Mueller-Hinton agar before testing. Ceftazidime was purchased from the UPMC pharmacy. Imipenem and relebactam were provided by Merck & Co.; avibactam was kindly provided by AstraZeneca. Stock solutions of antimicrobial agents were prepared and stored at −80°C. MICs of imipenem with and without relebactam (at a fixed concentration of 4 μg/ml) and ceftazidime with and without avibactam (at a fixed concentration of 4 μg/ml) were determined by standard broth microdilution methods. Pseudomonas aeruginosa ATCC 27853 was used as a reference strain for quality control of the in vitro susceptibility study. Susceptibility to these antibiotics was defined in accordance with Clinical and Laboratory Standards Institute breakpoints (28). CRE was defined according to revised CDC criteria (29).
Genomic DNA was prepared from overnight cultures using the Wizard Genomic DNA purification kit (Promega, Madison, WI). Genome libraries were prepared using the Nextera DNA library preparation kit and sequenced using the Illumina NextSeq platform (paired-end reads of 150 bp), as previously described by our group (14). In studies of patients infected by multilocus sequence type ST258 K. pneumoniae, we found that intrapatient and between-patient differences in strains were usually <15 and <25 core genome single nucleotide polymorphisms (SNPs) (per 5 Mbp), respectively (14, 30). Therefore, to reduce the likelihood of testing clonal isolates, all the isolates in the present study were recovered from unique patients and differed by >50 core genome SNPs.
Statistical analysis.
Graphics were created and statistical analyses were performed using Microsoft (Redmond, WA) Excel, GraphPad (La Jolla, CA) Prism software, and STATA (STATA Corp., College Station, TX). Comparisons were made by Fisher's exact test for categorical variables and the Kruskal-Wallis rank test for continuous variables. All tests were two tailed; a P value of ≤0.05 was considered significant. Multiple linear regression analyses were performed to identify factors that were independently associated with drug MICs. It was also decided a priori that three factors would be forced into the model: presence of ESBL, blaKPC-3 mutation, and major OmpK36 porin mutation (ins AA135–136 GD or IS5 promoter insertion). Collinearity among variables was assessed using VIFs. VIF values of >3 suggest significant collinearity.
Accession number(s).
The sequences from this study have been deposited in GenBank BioProject under accession no. PRJNA353361.
ACKNOWLEDGMENTS
This work was funded, in part, by grants from the National Institutes of Health (R21AI111037 to C.J.C., R21 AI128338 to M.H.N., K08AI114883 to R.K.S., R21AI117338 to L.C., and R01AI090155 to B.N.K.).
We declare no conflicts of interest.
REFERENCES
- 1.Haidar G, Alkroud A, Cheng S, Churilla TM, Churilla BM, Shields RK, Doi Y, Clancy CJ, Nguyen MH. 2016. Association between the presence of aminoglycoside-modifying enzymes and in vitro activity of gentamicin, tobramycin, amikacin, and plazomicin against Klebsiella pneumoniae carbapenemase- and extended-spectrum-beta-lactamase-producing Enterobacter species. Antimicrob Agents Chemother 60:5208–5214. doi: 10.1128/AAC.00869-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacoby GA. 2009. AmpC beta-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson DL, Bonomo RA. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo D, Silveira F, Hujer AM, Bonomo RA, Hujer KM, Marsh JW, Bethel CR, Doi Y, Deeley K, Paterson DL. 2006. Outer membrane protein changes and efflux pump expression together may confer resistance to ertapenem in Enterobacter cloacae. Antimicrob Agents Chemother 50:2833–2835. doi: 10.1128/AAC.01591-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. 2015. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 7.Hong JH, Clancy CJ, Cheng S, Shields RK, Chen L, Doi Y, Zhao Y, Perlin DS, Kreiswirth BN, Nguyen MH. 2013. Characterization of porin expression in Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae identifies isolates most susceptible to the combination of colistin and carbapenems. Antimicrob Agents Chemother 57:2147–2153. doi: 10.1128/AAC.02411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackel M, Kazmierczak KM, Hoban DJ, Biedenbach DJ, Bouchillon SK, de Jonge BL, Stone GG. 2016. Assessment of the in vitro activity of ceftazidime-avibactam against multidrug-resistant Klebsiella spp. collected in the INFORM global surveillance study, 2012 to 2014. Antimicrob Agents Chemother 60:4677–4683. doi: 10.1128/AAC.02841-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against Class A, C, and D beta-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. 2015. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 59:6605–6607. doi: 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. 2017. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob Agents Chemother 61:e02534-16. doi: 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphries RM, Hemarajata P. 2017. Resistance to ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother 61:e00537-17. doi: 10.1128/AAC.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucasti C, Vasile L, Sandesc D, Venskutonis D, McLeroth P, Lala M, Rizk ML, Brown ML, Losada MC, Pedley A, Kartsonis NA, Paschke A. 2016. Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother 60:6234–6243. doi: 10.1128/AAC.00633-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lob SH, Hackel MA, Kazmierczak KM, Hoban DJ, Young K, Motyl MR, Karlowsky JA, Sahm DF. 2017. In vitro activity of imipenem-relebactam against gram-negative bacilli isolated from patients with lower respiratory tract infections in the United States in 2015: results from the SMART global surveillance program. Diagn Microbiol Infect Dis 88:171–176. doi: 10.1016/j.diagmicrobio.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Livermore DM, Warner M, Mushtaq S. 2013. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 68:2286–2290. doi: 10.1093/jac/dkt178. [DOI] [PubMed] [Google Scholar]
- 19.Bush K. 2015. A resurgence of beta-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens. Int J Antimicrob Agents 46:483–493. doi: 10.1016/j.ijantimicag.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Lapuebla A, Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Quale J. 2015. Activity of imipenem with relebactam against Gram-negative pathogens from New York City. Antimicrob Agents Chemother 59:5029–5031. doi: 10.1128/AAC.00830-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clancy CJ, Chen L, Hong JH, Cheng S, Hao B, Shields RK, Farrell AN, Doi Y, Zhao Y, Perlin DS, Kreiswirth BN, Nguyen MH. 2013. Mutations of the ompK36 porin gene and promoter impact responses of sequence type 258, KPC-2-producing Klebsiella pneumoniae strains to doripenem and doripenem-colistin. Antimicrob Agents Chemother 57:5258–5265. doi: 10.1128/AAC.01069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. In vitro selection of meropenem resistance among ceftazidime-avibactam resistant, meropenem susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother 61:e00079-17. doi: 10.1128/AAC.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 24.Pages JM, James CE, Winterhalter M. 2008. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol 6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 25.Shields RK, Clancy CJ, Hao B, Chen L, Press EG, Iovine NM, Kreiswirth BN, Nguyen MH. 2015. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum beta-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae. Antimicrob Agents Chemother 59:5793–5797. doi: 10.1128/AAC.00548-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clancy CJ, Hao B, Shields RK, Chen L, Perlin DS, Kreiswirth BN, Nguyen MH. 2014. Doripenem, gentamicin, and colistin, alone and in combinations, against gentamicin-susceptible, KPC-producing Klebsiella pneumoniae strains with various ompK36 genotypes. Antimicrob Agents Chemother 58:3521–3525. doi: 10.1128/AAC.01949-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavda KD, Chen L, Fouts DE, Sutton G, Brinkac L, Jenkins SG, Bonomo RA, Adams MD, Kreiswirth BN. 2016. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. mBio 7:e02093-16. doi: 10.1128/mBio.02093-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement (M100-S25). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Centers for Disease Control and Prevention. Accessed January 26, 2017. FAQs about choosing and implementing a CRE definition. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/hai/organisms/cre/definition.html. [Google Scholar]
- 30.Lynch T, Chen L, Peirano G, Gregson DB, Church DL, Conly J, Kreiswirth BN, Pitout JD. 2016. Molecular evolution of a Klebsiella pneumoniae ST278 isolate harboring blaNDM-7 and involved in nosocomial transmission. J Infect Dis 214:798–806. doi: 10.1093/infdis/jiw240. [DOI] [PMC free article] [PubMed] [Google Scholar]