ABSTRACT
The pharmacokinetics (PK) of drugs are known to be significantly altered in patients receiving extracorporeal membrane oxygenation (ECMO). However, clinical studies of the PK of drugs administered during ECMO are scarce, and the proper dosing adjustment has yet to be established. We developed a population PK model for teicoplanin, investigated covariates influencing teicoplanin exposure, and suggested an optimal dosing regimen for ECMO patients. Samples for PK analysis were collected from 10 adult patients, and a population PK analysis and simulations were performed to identify an optimal teicoplanin dose needed to provide a >50% probability of target attainment at 72 h using a trough concentration target of >10 μg/ml for mild to moderate infections and a trough concentration target of >15 μg/ml for severe infections. Teicoplanin was well described by a two-compartment PK model with first-order elimination. The presence of ECMO was associated with a lower central volume of distribution, and continuous renal replacement therapy (CRRT) was associated with a higher peripheral volume of distribution. For mild to moderate infections, an optimal dose was a loading dose (LD) of 600 mg and a maintenance dose (MD) of 400 mg for ECMO patients not receiving CRRT and an LD of 800 mg and an MD of 600 mg for those receiving CRRT. For severe infections, an optimal dose was an LD of 1,000 mg and an MD of 800 mg for ECMO patients not receiving CRRT and an LD of 1,200 mg and an MD of 1,000 mg for those receiving CRRT. In conclusion, doses higher than the standard doses are needed to achieve fast and appropriate teicoplanin exposure during ECMO. (This study has been registered at ClinicalTrials.gov under identifier NCT02581280.)
KEYWORDS: teicoplanin, population pharmacokinetics, pharmacokinetics, cardiogenic shock, extracorporeal membrane oxygenation
INTRODUCTION
Venoarterial extracorporeal membrane oxygenation (VA ECMO) is a mechanical cardiopulmonary bypass that serves a role in bridging patients to cardiac recovery after refractory cardiogenic shock (1). Patients on ECMO are prone to severe hemodynamic instability and require the prolonged use of multiple invasive devices (e.g., an ECMO cannula, endotracheal tube, and central venous catheter), which render them at high risk of infection (2–4). Therefore, antibiotics are frequently used for both prophylaxis and treatment of infections during ECMO (5). Among them, teicoplanin is a glycopeptide antibiotic effective against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (6). In the summary of product characteristics (SPC) (7), achievement of a trough concentration (Ctrough) of >10 μg/ml, when measured by high-performance liquid chromatography (HPLC), or >15 μg/ml, when measured by fluorescence polarization immunoassay (FPIA) on day 3 of therapy, is recommended for most Gram-positive bacterial infections. In the case of more severe infections, such as endocarditis, a higher Ctrough (>15 μg/ml by HPLC or >30 μg/ml by FPIA) is required.
Significant alterations in the pharmacokinetic (PK) characteristics of drugs are anticipated during ECMO as the circulating volume increases and the drugs, depending on their physiochemical properties (e.g., molecular size, degree of ionization, and lipophilicity), may be inactivated, adsorbed, and sequestered to various degrees by the various ECMO circuit components (8–11). In addition, critical illness itself is often accompanied by marked physiological changes, such as systemic inflammatory responses, multiple organ dysfunction, and drug interactions, which further complicate the prediction of drug disposition (12, 13). At present, limited clinical PK studies have been conducted in ECMO patients, and no clear guidelines regarding selection of the proper dose in the setting of ECMO exist. This prospective population PK study was carried out to characterize the PK of teicoplanin in critically ill patients with cardiogenic shock undergoing VA ECMO, to identify and quantify the sources of PK variability, and to suggest optimal dosing regimens in order to achieve prompt and adequate exposure to teicoplanin.
RESULTS
Patient characteristics.
Ten patients were enrolled in the present study from December 2014 to February 2016. The demographic information and baseline characteristics of the patients are shown in Table 1. The patients' median age was 62.5 years (range, 19 to 77 years), and the median body weight was 67.5 kg (range, 41 to 87 kg). Seven patients were male. The reasons for undergoing ECMO included acute myocardial infarction (n = 7), myocarditis (n = 2), and valvular heart disease (n = 1). The mean duration of ECMO was 6.82 days (range, 1.88 to 12.1 days), and the mean ECMO blood flow rate was 2,256 ml/min (range, 1,650 to 2,520 ml/min). Five patients received concomitant continuous renal replacement therapy (CRRT). All 10 patients provided samples for PK analysis while they were on ECMO. Among them, four patients who survived after they were weaned from ECMO were able to provide samples for PK analysis while they were off of ECMO to serve as controls. Overall, a total of 99 samples were collected and used in the analysis.
TABLE 1.
Demographic information and baseline characteristics of all enrolled patientsa
| Patient no. | Age (yr) | Sex | Body wt (kg) | Indication for VA ECMO | Duration of VA ECMO (days) | VA ECMO blood flow rate (ml/min) | SCr level (mg/dl) | Use of CRRT |
|---|---|---|---|---|---|---|---|---|
| 1 | 68 | Male | 70 | Myocardial infarction | 10.4 | 2,060 | 1.5 | Yes |
| 2 | 71 | Male | 61 | Valvular heart disease | 1.88 | 1,650 | 1.7 | Yes |
| 3 | 62 | Male | 65 | Myocardial infarction | 5.21 | 2,420 | 0.9 | No |
| 4 | 55 | Male | 81 | Myocardial infarction | 10.3 | 2,520 | 2.3 | Yes |
| 5 | 23 | Female | 56 | Myocarditis | 12.1 | 2,520 | 0.6 | Yes |
| 6 | 60 | Male | 83 | Myocardial infarction | 6.38 | 2,520 | 2.1 | No |
| 7 | 76 | Female | 41 | Myocardial infarction | 2.38 | 2,350 | 1.2 | Yes |
| 8 | 63 | Male | 70 | Myocardial infarction | 5.79 | 2,290 | 1.7 | No |
| 9 | 19 | Male | 87 | Myocarditis | 3.79 | 1,950 | 3.5 | No |
| 10 | 77 | Female | 59 | Myocardial infarction | 9.92 | 2,280 | 1.1 | No |
Data are for 10 patients. VA ECMO, venoarterial extracorporeal membrane oxygenation; SCr, serum creatinine; CRRT, continuous renal replacement therapy.
Population pharmacokinetic analysis.
The PK profile of teicoplanin was well described by a two-compartment model with first-order elimination and with combined (additive and proportional) residual variability and interindividual variability in clearance from the central compartment (CL), the central volume of distribution (V1), and intercompartmental (central-peripheral) clearance (Q). Among the covariates tested, the inclusion of ECMO in V1 and Q using a proportional model (change in objective function value [ΔOFV] = −8.94 and −7.64, respectively) and the inclusion of CRRT in the peripheral volume of distribution (V2) using a power model (ΔOFV = −5.44) significantly improved the model fit. No significant covariate influenced the CL of teicoplanin. The final model was as follows: CL (in liters per hour) = 0.95, V1 (in liters) = 15.7 × (1 − 0.34 × ECMO), Q (in liters per hour) = 5.57 × (1 − 0.5 × ECMO), and V2 (in liters) = 71.7 × (1.5)CRRT.
The typical population values for CL, V1, Q, and V2 derived from the final model were 0.95 liters/h, 15.7 liters, 5.57 liters/h, and 71.7 liters, respectively. These values generally agreed with the median estimates obtained by the bootstrap method (CL, 1.02 liters/h; V1, 16.2 liters; Q, 6.22 liters/h; V2, 74.9 liters) and were contained within the 95% confidence interval (CI) of the bootstrap results (Table 2), corroborating the stability of the model. The presence of ECMO significantly influenced the V1 and Q of teicoplanin, such that V1 was 34% lower in the presence of ECMO (10.4 liters) than in the absence of ECMO (15.7 liters) and Q was 50% lower in the presence of ECMO (2.79 liters/h) than in the absence of ECMO (5.57 liters/h). CRRT was associated with a 50% higher V2 (107.6 liters with CRRT versus 71.7 liters without CRRT).
TABLE 2.
Final population PK model parameter estimates for teicoplanin and bootstrap resultsa
| Parameter | Population estimate (% RSE) | Bootstrap median (2.5th, 97.5th percentile) |
|---|---|---|
| Fixed effects | ||
| CL (liters/h) | 0.95 (29.6) | 1.02 (0.58, 1.57) |
| V1 (liters) | 15.7 (17.3) | 16.2 (12.2, 21.0) |
| Q (liters/h) | 5.57 (43.1) | 6.22 (3.06, 10.2) |
| V2 (liters) | 71.7 (28.1) | 74.9 (65.8, 93.5) |
| θECMO on V1 | −0.34 (16.3) | −0.35 (−0.41, −0.26) |
| θECMO on Q | −0.50 (74.6) | −0.53 (−0.72, −0.15) |
| θCRRT on V2 | 1.50 (25.3) | 1.39 (0.87, 2.24) |
| Random effects | ||
| Interindividual variability | ||
| CL | 0.34 (463) | 0.31 (0.04, 0.64) |
| V1 | 0.13 (54.7) | 0.10 (0.02, 0.19) |
| Q | 0.15 (52.3) | 0.13 (0.02, 0.28) |
| Residual variability | ||
| Additive (μg/ml) | 3.32 (84.3) | 4.91 (0.87, 8.32) |
| Proportional (%) | 24.3 (4.12) | 19.9 (10.3, 26.4) |
CL, clearance from the central compartment; V1, central volume of distribution; Q, intercompartmental (central-peripheral) clearance; V2, peripheral volume of distribution; θECMO, effect of extracorporeal membrane oxygenation; θCRRT, effect of continuous renal replacement therapy; RSE, relative standard error.
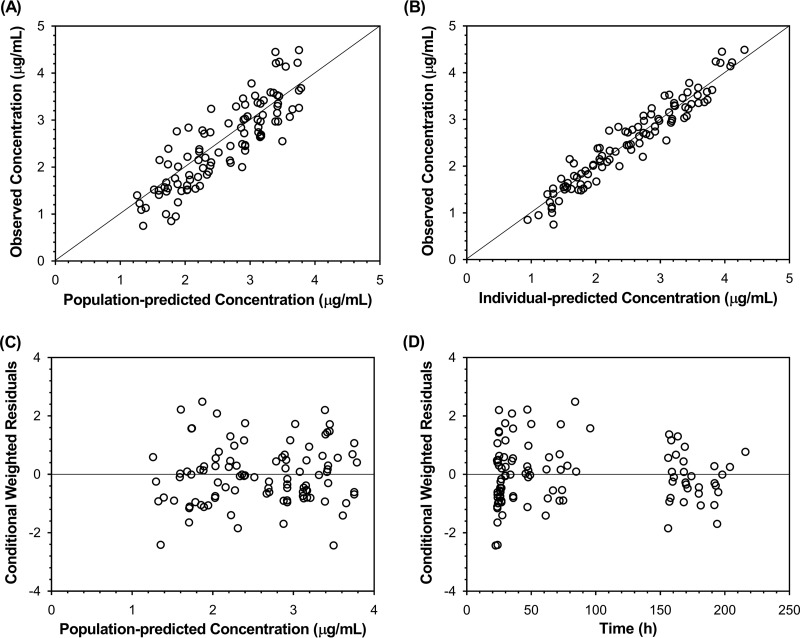
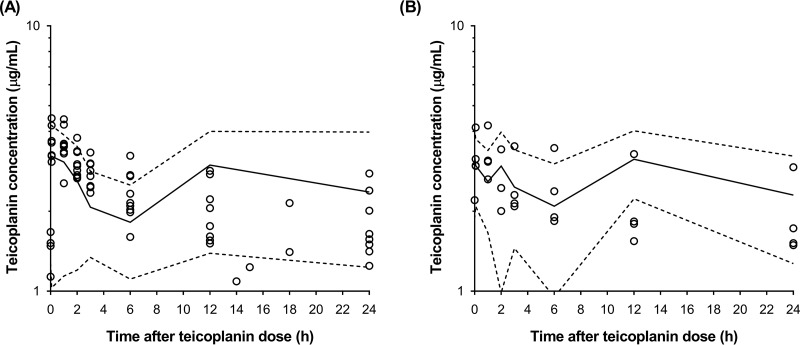
The basic goodness-of-fit plots (Fig. 1) showed that the final model was acceptable, as the predicted population and predicted individual concentrations were generally in agreement with the observed concentrations. Additionally, most conditional weighted residual values were evenly distributed in a random manner around the line of unity (±2 standard deviations of the mean), which indicated the suitability of the error model. The results of the visual predictive check (VPC) showed that the 5th to 95th percentiles of the simulated data overlaid most of the observed data, supporting the predictive performance of the model (Fig. 2).
FIG 1.
Goodness-of-fit plots of the final population pharmacokinetic model for teicoplanin in 10 patients who received venoarterial extracorporeal membrane oxygenation. Observed teicoplanin concentrations versus population predicted concentrations (A) and individual predicted concentrations (B) and conditional weighted residuals versus population predicted concentrations (C) and time (D) are shown.
FIG 2.
Visual predictive check of the final population pharmacokinetic model for teicoplanin in 10 patients during venoarterial extracorporeal membrane oxygenation (A) and after discontinuation of venoarterial extracorporeal membrane oxygenation (B). Open circles, observed teicoplanin concentrations; solid line, the median; lower and upper dashed lines, 5th and 95th percentiles of the simulated data, respectively.
Monte Carlo simulations.
Table 3 shows the probability of target attainment (PTA; in percent) at 72 h after ECMO initiation and at 72 h after ECMO discontinuation using the eight different dosing regimens, stratified by the use of CRRT. The Ctrough target was set at >10 μg/ml for mild to moderate infections and >15 μg/ml for severe infections. Overall, a trend toward an increase in the PTA with increasing teicoplanin doses was noted. Also, the PTA was higher in the absence of CRRT than in the presence of CRRT. For mild to moderate infections, the standard dosing regimen (regimen A) resulted in a PTA of 22.8% during ECMO, which increased to 63.1% after ECMO cessation in patients without CRRT. However, in patients receiving CRRT, the same dosing regimen resulted in a lower PTA of 5.62% during ECMO and 58.6% after ECMO. For mild to moderate infections, the optimal dosing regimen was regimen B for patients without CRRT (PTA, 50.1% during ECMO and 65.4% after ECMO) and regimen D for patients with concomitant CRRT (PTA, 56.1% during ECMO and 83.2% after ECMO).
TABLE 3.
PTA at 72 h after VA ECMO initiation and at 72 h after VA ECMO discontinuation, using eight different dosing regimens, stratified by use of CRRTa
| Dosing regimenb | Use of CRRT | PTA (%) at 72 h after ECMO initiation |
PTA (%) at 72 h after ECMO discontinuation |
||
|---|---|---|---|---|---|
| Mild to moderate infections | Severe infections | Mild to moderate infections | Severe infections | ||
| A (LD, 400; MD, 400) | Yes | 5.62 | 0.30 | 58.6 | 26.8 |
| No | 22.8 | 3.16 | 63.1 | 36.0 | |
| B (LD, 600; MD, 400) | Yes | 24.6 | 3.14 | 62.9 | 32.9 |
| No | 50.1 | 15.2 | 65.4 | 39.7 | |
| C (LD, 600; MD, 600) | Yes | 34.8 | 5.74 | 82.0 | 58.5 |
| No | 59.5 | 22.9 | 82.6 | 63.0 | |
| D (LD, 800; MD, 600) | Yes | 56.1 | 17.6 | 83.2 | 61.7 |
| No | 75.5 | 41.6 | 83.2 | 64.6 | |
| E (LD, 800; MD, 800) | Yes | 64.3 | 23.3 | 91.6 | 76.3 |
| No | 81.0 | 49.1 | 91.2 | 77.5 | |
| F (LD, 1,000; MD, 800) | Yes | 78.2 | 39.9 | 92.0 | 77.3 |
| No | 87.7 | 62.7 | 91.3 | 78.1 | |
| G (LD, 1,000; MD, 1,000) | Yes | 82.5 | 45.8 | 95.7 | 86.2 |
| No | 90.3 | 68.3 | 95.0 | 86.3 | |
| H (LD, 1,200; MD, 1,000) | Yes | 88.6 | 58.9 | 95.8 | 86.9 |
| No | 93.4 | 77.4 | 95.1 | 86.5 | |
The Ctrough target was set at >10 μg/ml for mild to moderate infections and >15 μg/ml for severe infections. PTA, probability of target attainment; ECMO, extracorporeal membrane oxygenation; LD, loading dose; MD, maintenance dose; CRRT, continuous renal replacement therapy.
The loading doses (in milligrams) were administered q12h, and the maintenance doses (in milligrams) were administered q24h.
For severe infections, the standard dosing regimen (regimen A) resulted in a PTA of 3.16% during ECMO, which increased to 36.0% after ECMO cessation in patients without CRRT. Using the same dosing regimen, almost none of the patients (0.30%) would reach the target during ECMO with concomitant CRRT. The optimal teicoplanin dosing regimen for severe infections both during and after ECMO was regimen F for patients without CRRT (PTA, 62.7% during ECMO and 78.1% after ECMO) and regimen H for patients with concomitant CRRT (PTA, 58.9% during ECMO and 86.9% after ECMO).
DISCUSSION
To our knowledge, this is the first prospective population PK study of teicoplanin in patients receiving VA ECMO. In our study, the patients were used as their own controls to compare the PK parameters during and after ECMO to potentially minimize the interindividual variability. A two-compartment model with first-order elimination reasonably fitted the concentration-time data for teicoplanin. The typical population values for the PK parameters of teicoplanin derived from the final model are similar to those that reported previously in 26 critically ill patients (CL, 0.69 liters/h; V1, 25.3 liters; Q, 3.93 liters/h; V2, 86.5 liters) (14).
In our study, ECMO was associated with a 34% lower V1 and a 50% lower Q of teicoplanin and CRRT was associated with a 50% higher V2 of teicoplanin. Our results are different from those of other studies, in which the volume of distribution was generally increased and the clearance was decreased for drugs administered during ECMO (8, 15–17). Several explanations for our findings are possible. First, teicoplanin is a hydrophilic drug, which makes the sequestration of teicoplanin in the ECMO circuit less likely than that of lipophilic drugs, which show an increased volume of distribution during ECMO due to the substantial sequestration in the circuit (10, 18). For teicoplanin, factors such as hemodilution, altered protein binding, and other pathophysiologic changes that occur during ECMO may influence the PK more significantly (19). Moreover, since the previous studies were mostly conducted in neonates or an ex vivo system, the same findings may not be applicable to adult patients in our study. In fact, studies involving adult ECMO patients demonstrated no significant alterations in the PK of oseltamivir, piperacillin-tazobactam, and tigecycline (20–22). For vancomycin, another glycopeptide antibiotic frequently used during ECMO (5), no differences in the volume of distribution or clearance were seen between adult patients with ECMO and their matched controls (23). Even a trend for a slightly lower volume of distribution has been reported for meropenem and azithromycin (22, 24). In addition, no significant impact of the patients' creatinine clearance (CLCR) on the teicoplanin CL was found in our study. The reasons may be multifactorial, including the small sample size, the large interindividual variability of CL, and the undetected amount of teicoplanin excreted nonrenally (through CRRT).
In our study, despite the lower V1 and no apparent changes in the CL of teicoplanin during ECMO, PTA was lower during ECMO than after ECMO for all eight levels of simulated dosing. This indicates large PK fluctuations and variability among patients, which is also reflected in the high relative standard error (RSE) of the interindividual variability of CL. When the final population PK model and its variability are taken together, the dosing simulations resulted in a gradual increase in PTA over time from 72 h after ECMO initiation to 72 h after ECMO cessation. Based on the results of our study, we propose the following dosing regimens for mild to moderate infections: for ECMO patients not on CRRT, an LD of 600 mg every 12 h (q12h) for the first three doses followed by an MD of 400 mg every 24 h (q24h), and for ECMO patients on CRRT, we propose an LD of 800 mg q12h for the first three doses followed by an MD of 600 mg q24h. For severe infections, for ECMO patients not on CRRT, we propose an LD of 1,000 mg q12h for the first three doses followed by an MD of 800 mg q24h, and for ECMO patients on CRRT, we propose an LD of 1,200 mg q12h for the first three doses followed by an MD of 1,000 mg q24h. Our recommendation is in line with a growing body of evidence in the literature that advocates the use of higher teicoplanin doses in critically ill patients (25–27). Close monitoring of the teicoplanin plasma concentrations and clinical status of the patients is crucial to provide effective protection against infection and reduce the risk of microbiological resistance.
Our study has some limitations. First, the number of patients was relatively small, and therefore, the data might not have been sufficient to provide robust PK parameter estimates for the overall ECMO patient population. Also, the use of concomitant medications, such as inotropes, vasopressors, and diuretics, which could impact the PK of teicoplanin, was not evaluated. Nevertheless, our study may serve as the first step toward understanding the PK characteristics of teicoplanin in adult patients receiving VA ECMO and toward further improving patient outcomes through the use of an optimal dosing strategy. Future studies are needed to validate the current findings and to evaluate the clinical efficacy and safety of the recommended dosing regimen.
MATERIALS AND METHODS
Study patients and ECMO system.
This study was conducted at the cardiac intensive care unit in Severance Hospital, a university-affiliated tertiary care hospital in Seoul, Republic of Korea. The study protocol was approved by the institutional review board (IRB) of Yonsei University (IRB no. 4-2014-0919) and was registered at ClinicalTrials.gov (NCT02581280). Patients were eligible for inclusion in the study if they were aged 19 years or older, undergoing VA ECMO secondary to severe cardiogenic shock, and concomitantly receiving teicoplanin. Written informed consent to participate in the study was obtained from the patients' legal representatives. The VA ECMO system used comprised a centrifugation pump with a pump controller (Capiox SP-101; Terumo Inc., Tokyo, Japan) and an ECMO circuit (Capiox EBS Circuit with X coating; Terumo Inc., Tokyo, Japan). It was percutaneously installed via femoral vein-femoral artery peripheral cannulation, with a drainage cannula being positioned in the femoral vein to remove the deoxygenated blood and an infusion cannula being positioned in the femoral artery to supply the oxygenated blood to the patient (retrograde blood flow to the heart).
Study procedures.
According to the Severance Hospital protocol, teicoplanin (Targocid; Sanofi-Aventis Co., Ltd.) was initiated on day 1 of VA ECMO in all study patients for infection prophylaxis. Teicoplanin was administered by intravenous bolus injection at the standard loading dose (LD) of 400 mg q12h for the first three doses followed by the maintenance dose (MD) of 400 mg q24h. If needed, venovenous hemodiafiltration (Prismaflex; Gambro Inc., Meyzieu, France) was applied as continuous renal replacement therapy (CRRT). On day 2 of VA ECMO, samples (2 ml) for PK analysis were collected via an existing arterial line at 0 min (predosing), 5 min, and 1, 2, 3, 6, 12, and 24 h of teicoplanin administration. If the patient survived and was able to be weaned off ECMO, the collection of samples for PK analysis was repeated on day 2 of ECMO discontinuation at 0 min (predose), 5 min, and 1, 2, 3, 6, 12, and 24 h of teicoplanin administration. Variations in the sampling time were allowed to minimize interruptions in patient care. All samples were collected in tubes containing EDTA and were immediately centrifuged at 1,500 × g for 10 min (4°C) to separate the plasma, which was stored at −80°C until the drug assay. All routine clinical management was carried out at the discretion of the treating physician and was not influenced by the study procedures.
Teicoplanin assay.
Teicoplanin concentrations were measured following protein precipitation using a validated HPLC system coupled with a Shimadzu LCMS-8050 triple quadrupole liquid chromatograph-mass spectrometer (Shimadzu Inc., Kyoto, Japan). HPLC was performed on a Phenomenex Luna C18 analytical column (100 by 2 mm; particle size, 3 μm; Phenomenex, Torrance, CA, USA) with a mobile phase consisting of 0.1% formic acid in acetonitrile and water (20:80, vol/vol) at a flow rate of 0.3 ml/min. All analytical procedures were conducted in accordance with the guidance for industry on bioanalytical method validation issued by the U.S. Food and Drug Administration (28). The lower limit of quantification for teicoplanin was 2.0 μg/ml. The calibration curve was linear from 2 to 150 μg/ml, and the regression coefficient was >0.988. The coefficients of variation at the concentration used to produce the calibration curve and the four quality control samples (2, 6, 12, and 120 mg/liter) were <15%.
Population pharmacokinetic analysis.
A population PK analysis was performed using the nonlinear mixed-effect modeling software NONMEM (version 7.3; Icon Development Solutions, Elliot City, MD, USA) with the aid of the Perl-speaks-NONMEM toolkit (29), Pirana (version 2.9.2) software (30), and Xpose (version 4.0) software (31) contained in the programming language R (version 3.2.1; http://www.r-project.org/). First-order conditional estimation with the interaction approach was used throughout the model-building process. Teicoplanin concentration data were log transformed for analysis and were assumed to follow a two-compartment model with first-order elimination on the basis of the findings of previous studies (14, 25, 32–34). The PK parameters estimated included clearance from the central compartment (CL), the central volume of distribution (V1), the peripheral volume of distribution (V2), and intercompartmental (central-peripheral) clearance (Q). The interindividual variability of the PK parameters was evaluated using an exponential model. Covariance between interindividual variability was estimated using a variance-covariance matrix. Residual variability was described using the following combined additive and proportional model:
and
where Cobs,ij and Cpred,ij represent the jth observed and predicted concentrations for the ith subject, respectively, ε is the residual variability with a mean of zero and a variance of σ2, and θ is the additive/proportional component of the residual variability.
The following clinically plausible patient covariates were tested for their influence on the PK parameters of teicoplanin: age; sex; body weight; serum albumin concentration; serum creatinine (SCr) concentration; blood urea nitrogen (BUN) concentration; creatinine clearance (CLCR), estimated using the Cockcroft-Gault method (35); the ECMO blood flow rate; and presence of CRRT (1 for patients on CRRT and 0 for patients not on CRRT). The presence of ECMO was also evaluated as a potential covariate, with patients serving as their own controls (1 for records during ECMO and 0 for records after ECMO discontinuation). Among the covariates, continuous covariates were centered at their median values and were tested using a power, exponential, and linear model; categorical covariates were tested using a power, exponential, and proportional model. The covariates that, upon addition, resulted in a statistically significant improvement (P < 0.05) in the log likelihood of the model (expressed as an objective function value [OFV]), reduced the residual variability, and/or improved the goodness-of-fit plots were incorporated to build the final model.
The validity of the final model was assessed by visual inspection of goodness-of-fit plots, including observed concentrations versus individual predictions (IPRED), observed concentrations versus population predictions (PRED), and conditional weighted residuals (CWRES) versus population predictions and time. Next, the accuracy and stability of the final model were evaluated using a nonparametric bootstrap method. The medians and 95% confidence intervals of the PK parameter estimates were obtained from the 1,000 bootstrap runs. The model was considered stable if the typical population values for the PK parameters of the final model were within the 95% confidence interval (CI) of the bootstrap results. Furthermore, a visual predictive check (VPC) was performed with 1,000 data set simulations, after which the 5th to 95th percentiles of the simulated teicoplanin concentrations were overlaid with the observed data to assess the predictive performance of the final model.
Monte Carlo simulations.
Monte Carlo simulations using the parameter estimates from the final population PK model were performed to assess the probability of target attainment (PTA) of Ctrough at 72 h after the initiation of ECMO as well as at 72 h after the discontinuation of ECMO. We chose the Ctrough at 72 h since it is considered useful in the clinical monitoring of teicoplanin therapy (26, 36). The Ctrough target was set at >10 μg/ml for mild to moderate infections and >15 μg/ml for severe infections, as recommended by the SPC (7). Five thousand patient simulations were performed for each of the following dosing regimens: regimen A, consisting of an LD of 400 mg q12h for 3 doses and an MD of 400 mg q24h thereafter; regimen B, consisting of an LD of 600 mg q12h and an MD of 400 mg q24h; regimen C, consisting of an LD of 600 mg q12h and an MD of 600 mg q24h; regimen D, consisting of an LD of 800 mg q12h and an MD of 600 mg q24h; regimen E, consisting of an LD of 800 mg q12h and an MD of 800 mg q24h; regimen F, consisting of an LD of 1,000 mg q12h and an MD of 800 mg q24h; regimen G, consisting of an LD of 1,000 mg q12h and an MD of 1,000 mg q24h; and regimen H, consisting of an LD of 1,200 mg q12h and an MD of 1,000 mg q24h. In order to provide the patients with adequate antibiotic coverage throughout the course of teicoplanin therapy, that is, during ECMO as well as after ECMO, an optimal dosing regimen was chosen to be the lowest dose required to maintain a PTA of >50% at 72 h after the start of ECMO and continuously at 72 h after weaning off of ECMO (34). An ECMO duration of 7 days was assumed for simulation purposes, since the mean duration of ECMO in our study patients was 6.82 days.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We thank the nursing staff at the cardiac intensive care unit of the Severance Hospital for cooperation in the study.
REFERENCES
- 1.Allen S, Holena D, McCunn M, Kohl B, Sarani B. 2011. A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med 26:13–26. doi: 10.1177/0885066610384061. [DOI] [PubMed] [Google Scholar]
- 2.Hsu MS, Chiu KM, Huang YT, Kao KL, Chu SH, Liao CH. 2009. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J Hosp Infect 73:210–216. doi: 10.1016/j.jhin.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill JM, Schutze GE, Heulitt MJ, Simpson PM, Taylor BJ. 2001. Nosocomial infections during extracorporeal membrane oxygenation. Intensive Care Med 27:1247–1253. doi: 10.1007/s001340101029. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt M, Brechot N, Hariri S, Guiguet M, Luyt CE, Makri R, Leprince P, Trouillet JL, Pavie A, Chastre J, Combes A. 2012. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis 55:1633–1641. doi: 10.1093/cid/cis783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao LS, Fleming GM, Escamilla RJ, Lew DF, Lally KP. 2011. Antimicrobial prophylaxis and infection surveillance in extracorporeal membrane oxygenation patients: a multi-institutional survey of practice patterns. ASAIO J 57:231–238. doi: 10.1097/MAT.0b013e31820d19ab. [DOI] [PubMed] [Google Scholar]
- 6.Cobo J, Fortun J. 1996. The comparative efficacy and safety of teicoplanin and vancomycin. J Antimicrob Chemother 38:1113–1114. doi: 10.1093/jac/38.6.1113. [DOI] [PubMed] [Google Scholar]
- 7.Electronic Medicines Compendium. 2014. Targocid 200 mg—summary of product characteristics (SPC). http://www.medicines.org.uk/emc/ Accessed 1 March 2017.
- 8.Buck ML. 2003. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet 42:403–417. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 9.Mulla H, Lawson G, Firmin R, Upton DR IV. 2001. Drug disposition during extracorporeal membrane oxygenation (ECMO). Paediatr Perinatal Drug Ther 4:109–120. [Google Scholar]
- 10.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. 2007. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med 33:1018–1024. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 11.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. 2010. Determinants of drug absorption in different ECMO circuits. Intensive Care Med 36:2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda T, Takesue Y, Nakajima K, Ichki K, Wada Y, Tsuchida T, Takahashi Y, Ishihara M, Tatsumi S, Kimura T, Ikeuchi H, Uchino M. 2012. Evaluation of teicoplanin dosing designs to achieve a new target trough concentration. J Infect Chemother 18:296–302. doi: 10.1007/s10156-011-0325-z. [DOI] [PubMed] [Google Scholar]
- 13.Boucher BA, Wood GC, Swanson JM. 2006. Pharmacokinetic changes in critical illness. Crit Care Clin 22:255–271, vi. doi: 10.1016/j.ccc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Whitehouse T, Cepeda JA, Shulman R, Aarons L, Nalda-Molina R, Tobin C, MacGowan A, Shaw S, Kibbler C, Singer M, Wilson AP. 2005. Pharmacokinetic studies of linezolid and teicoplanin in the critically ill. J Antimicrob Chemother 55:333–340. doi: 10.1093/jac/dki014. [DOI] [PubMed] [Google Scholar]
- 15.Amaker RD, DiPiro JT, Bhatia J. 1996. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother 40:1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodge WF, Jelliffe RW, Zwischenberger JB, Bellanger RA, Hokanson JA, Snodgrass WR. 1994. Population pharmacokinetic models: effect of explicit versus assumed constant serum concentration assay error patterns upon parameter values of gentamicin in infants on and off extracorporeal membrane oxygenation. Ther Drug Monit 16:552–559. doi: 10.1097/00007691-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ahsman MJ, Wildschut ED, Tibboel D, Mathot RA. 2010. Pharmacokinetics of cefotaxime and desacetylcefotaxime in infants during extracorporeal membrane oxygenation. Antimicrob Agents Chemother 54:1734–1741. doi: 10.1128/AAC.01696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildschut ED, van Saet A, Pokorna P, Ahsman MJ, Van den Anker JN, Tibboel D. 2012. The impact of extracorporeal life support and hypothermia on drug disposition in critically ill infants and children. Pediatr Clin North Am 59:1183–1204. doi: 10.1016/j.pcl.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shekar K, Fraser JF, Smith MT, Roberts JA. 2012. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care 27:741.e9–e18. doi: 10.1016/j.jcrc.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Mulla H, Peek GJ, Harvey C, Westrope C, Kidy Z, Ramaiah R. 2013. Oseltamivir pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation support. Anaesth Intensive Care 41:66–73. [DOI] [PubMed] [Google Scholar]
- 21.Veinstein A, Debouverie O, Gregoire N, Goudet V, Adier C, Robert R, Couet W. 2012. Lack of effect of extracorporeal membrane oxygenation on tigecycline pharmacokinetics. J Antimicrob Chemother 67:1047–1048. doi: 10.1093/jac/dkr550. [DOI] [PubMed] [Google Scholar]
- 22.Donadello K, Antonucci E, Cristallini S, Roberts JA, Beumier M, Scolletta S, Jacobs F, Rondelet B, de Backer D, Vincent JL, Taccone FS. 2015. β-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: a case-control study. Int J Antimicrob Agents 45:278–282. doi: 10.1016/j.ijantimicag.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Donadello K, Roberts JA, Cristallini S, Beumier M, Shekar K, Jacobs F, Belhaj A, Vincent JL, de Backer D, Taccone FS. 2014. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care 18:632. doi: 10.1186/s13054-014-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner RB, Rouse S, Elbarbry F, Wanek S, Grover V, Chang E. 2016. Azithromycin pharmacokinetics in adults with acute respiratory distress syndrome undergoing treatment with extracorporeal-membrane oxygenation. Ann Pharmacother 50:72–73. doi: 10.1177/1060028015612105. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa R, Kobayashi S, Sasaki Y, Makimura M, Echizen H. 2013. Population pharmacokinetic and pharmacodynamic analyses of teicoplanin in Japanese patients with systemic MRSA infection. Int J Clin Pharmacol Ther 51:357–366. doi: 10.5414/CP201739. [DOI] [PubMed] [Google Scholar]
- 26.Pea F, Viale P, Candoni A, Pavan F, Pagani L, Damiani D, Casini M, Furlanut M. 2004. Teicoplanin in patients with acute leukaemia and febrile neutropenia: a special population benefiting from higher dosages. Clin Pharmacokinet 43:405–415. doi: 10.2165/00003088-200443060-00004. [DOI] [PubMed] [Google Scholar]
- 27.Hiraki Y, Yasumori N, Nagano M, Inoue D, Tsuji Y, Kamimura H, Karube Y. 2015. Optimal loading regimen and achievement of trough concentrations for teicoplanin using Japanese population parameters. Int J Antimicrob Agents 45:87–88. doi: 10.1016/j.ijantimicag.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. 2001. Guidance for industry: bioanalytical method validation. U.S. Food and Drug Administration, Rockville, MD. [Google Scholar]
- 29.Lindbom L, Pihlgren P, Jonsson EN. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. 2011. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed 101:72–79. doi: 10.1016/j.cmpb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson EN, Karlsson MO. 1999. Xpose—an S-Plus based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64. [DOI] [PubMed] [Google Scholar]
- 32.Soy D, Lopez E, Ribas J. 2006. Teicoplanin population pharmacokinetic analysis in hospitalized patients. Ther Drug Monit 28:737–743. doi: 10.1097/01.ftd.0000249942.14145.ff. [DOI] [PubMed] [Google Scholar]
- 33.Yu DK, Nordbrock E, Hutcheson SJ, Lewis EW, Sullivan W, Bhargava VO, Weir SJ. 1995. Population pharmacokinetics of teicoplanin in patients with endocarditis. J Pharmacokinet Biopharm 23:25–39. doi: 10.1007/BF02353784. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W, Zhang D, Storme T, Baruchel A, Decleves X, Jacqz-Aigrain E. 2015. Population pharmacokinetics and dosing optimization of teicoplanin in children with malignant haematological disease. Br J Clin Pharmacol 80:1197–1207. doi: 10.1111/bcp.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 36.Niwa T, Imanishi Y, Ohmori T, Matsuura K, Murakami N, Itoh Y. 2010. Significance of individual adjustment of initial loading dosage of teicoplanin based on population pharmacokinetics. Int J Antimicrob Agents 35:507–510. doi: 10.1016/j.ijantimicag.2009.12.018. [DOI] [PubMed] [Google Scholar]