ABSTRACT
Breakthrough Acinetobacter bacteremia during carbapenem therapy is not uncommon, and it creates therapeutic dilemmas for clinicians. This study was conducted to evaluate the clinical and microbiological characteristics of breakthrough Acinetobacter bacteremia during carbapenem therapy and to assess the efficacy of various antimicrobial therapies. We analyzed 100 adults who developed breakthrough Acinetobacter bacteremia during carbapenem therapy at 4 medical centers over a 6-year period. Their 30-day mortality rate was 57.0%, and the carbapenem resistance rate of their isolates was 87.0%. Among patients with carbapenem-resistant Acinetobacter bacteremia, breakthrough bacteremia during carbapenem therapy was associated with a significantly higher 14-day mortality (51.7% versus 37.4%, respectively; P = 0.025 by bivariate analysis) and a higher 30-day mortality (P = 0.037 by log rank test of survival analysis) than in the nonbreakthrough group. For the treatment of breakthrough Acinetobacter bacteremia during carbapenem therapy, tigecycline-based therapy was associated with a significantly higher 30-day mortality (80.0%) than those with continued carbapenem therapy (52.5%) and colistin-based therapy (57.9%) by survival analysis (P = 0.047 and 0.045 by log rank test, respectively). Cox regression controlling for confounders, including severity of illness indices, demonstrated that treatment with tigecycline-based therapy for breakthrough Acinetobacter bacteremia was an independent predictor of 30-day mortality (hazard ratio, 3.659; 95% confidence interval, 1.794 to 7.465; P < 0.001). Patients with breakthrough Acinetobacter bacteremia during carbapenem therapy posed a high mortality rate. Tigecycline should be used cautiously for the treatment of breakthrough Acinetobacter bacteremia that develops during carbapenem therapy.
KEYWORDS: Acinetobacter, bacteremia, carbapenem, breakthrough, tigecycline
INTRODUCTION
Acinetobacter species have become major nosocomial pathogens associated with high mortality in immunocompromised hosts (1). Carbapenems, such as imipenem, meropenem, and doripenem, are preferred agents for treating serious Acinetobacter infections (2, 3). However, the emergence of carbapenem-resistant Acinetobacter spp. threatens the efficacy of these agents for the treatment of health care-associated infections (2, 4). In addition, carbapenem treatment itself is a risk factor for the development of infections caused by carbapenem-resistant Acinetobacter species (2, 5, 6).
Clinical and microbiological features of breakthrough Gram-negative bacteremia during carbapenem therapy have been reported (7), but clinical data specific for Acinetobacter spp. are limited. The recommended therapy for carbapenem-resistant Acinetobacter spp. was combinations of carbapenem and colistin (2). However, it is unknown whether these regimens or those with other antimicrobials, such as tigecycline, that are active against carbapenem-resistant Acinetobacter spp. are appropriate for treating breakthrough Acinetobacter bacteremia during carbapenem therapy. Furthermore, the determinants of carbapenem resistance among the causative microorganisms have not yet been elucidated. Therefore, this study was conducted to evaluate the clinical and microbiological features of breakthrough Acinetobacter bacteremia during carbapenem therapy and to assess the clinical efficacy of various antimicrobial regimens for breakthrough Acinetobacter bacteremia.
RESULTS
We reviewed the charts and medical records of 1,352 patients who had Acinetobacter bacteremia during the study period. Of these, 100 patients met the inclusion criteria, after excluding 1,252 patients for various reasons (see Fig. S1 in the supplemental material). The study population included 53 patients who received meropenem, 44 patients who received imipenem, and 3 patients who received doripenem therapy for more than 48 h before the onset of Acinetobacter bacteremia and who had a viable first isolate. All patients received carbapenem therapy with a dosage appropriate for end-organ(s) function. The treatment durations with imipenem, meropenem, and doripenem before the onset of Acinetobacter bacteremia were 9.8 ± 10.7, 10.2 ± 6.2, and 12.0 ± 6.5 days, respectively (P = 0.589). The infections that were treated with carbapenems prior to Acinetobacter bacteremia were caused by Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and Enterobacter, Citrobacter, and Serratia species, which were all susceptible to carbapenems. None were caused by Acinetobacter species. There was no significant difference in 14-day or 30-day mortality after the onset of Acinetobacter bacteremia based on the bacterial species that caused infections prior to the Acinetobacter bacteremia.
The carbapenem-resistant rate was high (87.0%) among Acinetobacter isolates that caused breakthrough bacteremia during carbapenem therapy, and it may have been a confounding factor that influenced patient outcomes (8). Thus, we sought to compare the patients with breakthrough Acinetobacter bacteremia during carbapenem therapy caused by carbapenem-resistant strains (n = 87) and nonbreakthrough Acinetobacter bacteremia caused by carbapenem-resistant strains (n = 265) (Table 1). Patients with breakthrough bacteremia were more likely to receive appropriate antimicrobial therapy for their carbapenem-resistant Acinetobacter bacteremia than the nonbreakthrough group. They received a carbapenem in combination with colistin or tigecycline as an effective regimen more frequently than the nonbreakthrough group. The Acute Physiology and Chronic Health Evaluation (APACHE) II scores and 14-day mortality rate were significantly higher in the breakthrough group than in the nonbreakthrough group, but there was no significant difference in 30-day mortality rates between the two patient groups by bivariate analysis. Survival analysis revealed that the breakthrough group had a significantly higher 30-day mortality than the nonbreakthrough group (P = 0.037, by log rank test; Fig. 1). Breakthrough bacteremia during carbapenem therapy is an independent risk factor for 14-day mortality (Table S1), but not for 30-day mortality (odds ratio [OR], 1.551; 95% confidence interval [CI], 0.864 to 2.783; P = 0.141), among patients with carbapenem-resistant Acinetobacter bacteremia. Carbapenem-resistant Acinetobacter isolates causing breakthrough bacteremia had resistance rates of commonly used antimicrobials similar to those causing nonbreakthrough bacteremia, except for a significantly lower rate of sulbactam resistance, and they were less likely to carry the carbapenemase gene-associated ISAba1-blaOXA-23-like genetic structure (Table 1). The imipenem and meropenem MICs were not significantly different between the 2 groups (P = 0.321 and 0.871, respectively). Among carbapenem-resistant isolates of Acinetobacter baumannii, the isolates causing breakthrough bacteremia were more likely to carry the ISAba1-blaOXA-51-like structure than those causing nonbreakthrough bacteremia (Table 1). The tigecycline MICs were not significantly different between the 2 groups (P = 0.424). For the treatment of carbapenem-resistant Acinetobacter bacteremia, none of the antimicrobial regimens was associated with significantly higher or lower 14-day and 30-day mortality (Table S2), and none of the antimicrobial regimens was an independent risk factor associated with 14-day or 30-day mortality by the logistic regression model (14-day mortality, Table S1; 30-day mortality, data not shown) or Cox regression model (data not shown) in the multivariable analysis. Subgroup analysis among patients with nonbreakthrough carbapenem-resistant Acinetobacter bacteremia yielded similar results (data not shown).
TABLE 1.
Univariate comparison between patients with breakthrough Acinetobacter bacteremia during carbapenem therapy and nonbreakthrough Acinetobacter bacteremia caused by carbapenem-resistant strains
| Characteristica | Breakthrough carbapenem resistant (n = 87) | Nonbreakthrough carbapenem resistant (n = 265) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age (median [IQR]) (yr) | 69 (53–80) | 72 (58–81) | 0.192 |
| Male sex | 51 (58.6) | 186 (70.2) | 0.062 |
| Recent ICU stay | 49 (56.3) | 124 (46.8) | 0.156 |
| Bacteremia acquired in ICU | 60 (69.0) | 120 (45.3) | <0.001 |
| Length of hospitalization before bacteremia (median [IQR]) (days) | 21 (13–34) | 22 (10–39) | 0.624 |
| Comorbid conditions | |||
| Alcoholism | 3 (3.4) | 13 (4.9) | 0.770 |
| Liver cirrhosis | 8 (9.2) | 44 (16.6) | 0.130 |
| Chronic obstructive pulmonary disease | 28 (32.2) | 48 (18.1) | 0.009 |
| Chronic kidney disease | 32 (36.8) | 101 (38.1) | 0.924 |
| Type 2 diabetes mellitus | 31 (35.6) | 97 (36.6) | 0.972 |
| Hypertension | 37 (42.5) | 108 (40.8) | 0.868 |
| Coronary artery disease | 14 (16.1) | 31 (11.7) | 0.379 |
| Congestive heart failure | 21 (24.1) | 50 (18.9) | 0.363 |
| Cerebrovascular accident | 18 (20.7) | 51 (19.2) | 0.890 |
| Collagen vascular disease | 3 (3.4) | 8 (3.0) | 0.737 |
| Immunosuppressant therapy | 11 (12.6) | 26 (9.8) | 0.585 |
| Solid tumor | 13 (14.9) | 71 (26.8) | 0.035 |
| Hematological malignancy | 8 (9.2) | 8 (3.0) | 0.032 |
| Chemotherapy | 7 (8.0) | 14 (5.3) | 0.494 |
| Neutropenia | 7 (8.0) | 11 (4.2) | 0.165 |
| Trauma | 4 (4.6) | 9 (3.4) | 0.743 |
| Burn | 1 (1.1) | 5 (1.9) | 1.000 |
| Recent surgery | 21 (24.1) | 57 (21.5) | 0.716 |
| Charlson comorbidity index (median [IQR]) | 4 (2–6) | 4 (2–6) | 0.919 |
| Invasive procedures | |||
| Arterial catheter | 40 (46.0) | 108 (40.8) | 0.465 |
| Central venous catheter | 49 (56.3) | 139 (52.5) | 0.614 |
| Ventilator use | 70 (80.5) | 163 (61.5) | 0.002 |
| Hemodialysis | 18 (20.7) | 58 (21.9) | 0.932 |
| Thoracic drain | 10 (11.5) | 18 (6.8) | 0.239 |
| Abdominal drain | 11 (12.6) | 25 (9.4) | 0.514 |
| Sources of bacteremia | |||
| Pneumonia | 42 (48.3) | 99 (37.4) | 0.094 |
| Catheter | 18 (20.7) | 46 (17.4) | 0.590 |
| Urinary tract infection | 1 (1.1) | 10 (3.8) | 0.305 |
| Intra-abdominal infection | 6 (6.9) | 16 (6.0) | 0.975 |
| Wound | 2 (2.3) | 11 (4.2) | 0.532 |
| Primary bacteremia | 18 (20.7) | 83 (31.3) | 0.077 |
| Antimicrobial therapy after bacteremia onset | |||
| Appropriate antimicrobial therapy | 32 (36.8) | 56 (21.1) | 0.005 |
| Effective regimensb | |||
| Colistin | 18 (56.3) | 43 (75.4) | 0.102 |
| Tigecycline | 12 (37.5) | 15 (26.3) | 0.389 |
| Fluoroquinolone | 7 (21.9) | 9 (15.8) | 0.667 |
| Sulbactam | 2 (6.3) | 4 (7.0) | 1.000 |
| Carbapenem + colistin | 16 (50.0) | 11 (19.3) | 0.005 |
| Carbapenem + tigecycline | 6 (18.8) | 2 (3.5) | 0.023 |
| Carbapenem + sulbactam | 2 (6.3) | 1 (1.8) | 0.293 |
| Colistin + tigecycline | 4 (12.5) | 12 (21.1) | 0.471 |
| Outcome | |||
| Shock | 29 (33.3) | 89 (33.6) | 1.000 |
| APACHE II score (median [IQR]) | 26 (19–32) | 24 (17–30) | 0.023 |
| 14-day mortality | 45 (51.7) | 99 (37.4) | 0.025 |
| 30-day mortality | 54 (62.1) | 132 (49.8) | 0.062 |
| Species causing bacteremia | |||
| A. baumannii | 52 (59.8) | 184 (69.4) | 0.125 |
| A. nosocomialis | 24 (27.6) | 66 (24.9) | 0.722 |
| A. pittii | 10 (11.5) | 8 (3.0) | 0.004 |
| A. soli | 0 (0.0) | 4 (1.5) | 0.576 |
| Microbiological characteristics of causative microorganisms | |||
| Nonsusceptibility to: | |||
| Amikacin | 46 (52.9) | 117 (44.2) | 0.196 |
| Ampicillin-sulbactam | 52 (59.8) | 192 (72.3) | 0.036 |
| Cefepime | 75 (86.2) | 246 (92.9) | 0.094 |
| Ceftazidime | 78 (89.7) | 250 (94.3) | 0.208 |
| Piperacillin-tazobactam | 83 (95.4) | 258 (97.4) | 0.475 |
| Ciprofloxacin | 67 (77.0) | 223 (84.2) | 0.176 |
| Levofloxacin | 70 (80.5) | 221 (83.4) | 0.642 |
| Colistin | 0 (0.0) | 6 (2.3) | 0.343 |
| Tigecycline | 31 (35.6) | 94 (35.5) | 1.000 |
| Isolates harboring ISAba1-blaOXA-51-like | 20 (23.0) | 38 (14.3) | 0.085 |
| A. baumannii isolates harboring ISAba1-blaOXA-51-like/total no. of isolates harboring ISAba1-blaOXA-51-like (%) | 18/52 (36.7) | 32/184 (17.4) | 0.013 |
| Isolates harboring ISAba1-blaOXA-23-like | 37 (42.5) | 168 (63.4) | 0.001 |
| A. baumannii isolates harboring ISAba1-blaOXA-23-like/total no. of isolates harboring ISAba1-blaOXA-23-like (%) | 21/52 (40.4) | 147/184 (79.9) | <0.001 |
| Isolates harboring IS1008 (or IS1006)-ΔISAba3-blaOXA-58-like | 6 (6.9) | 8 (3.0) | 0.120 |
| Isolates harboring blaOXA-24-like | 11 (12.6) | 16 (6.0) | 0.076 |
| Isolates harboring blaIMP-like | 5 (5.7) | 5 (1.9) | 0.072 |
| Isolates harboring blaVIM-like | 3 (3.4) | 5 (1.9) | 0.414 |
Data are presented as the number (%), unless otherwise indicated. IQR, interquartile range; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II.
Each item denotes the corresponding antimicrobial agent alone or in combination with other antimicrobial agent(s). For example, “colistin” denotes “colistin alone or in combination with other antimicrobial agent(s).” The numbers in parentheses denote the percentage of patients who received the corresponding antimicrobial agent alone or in combination with other antimicrobial agent(s) among the patients who received appropriate antimicrobial therapy.
FIG 1.
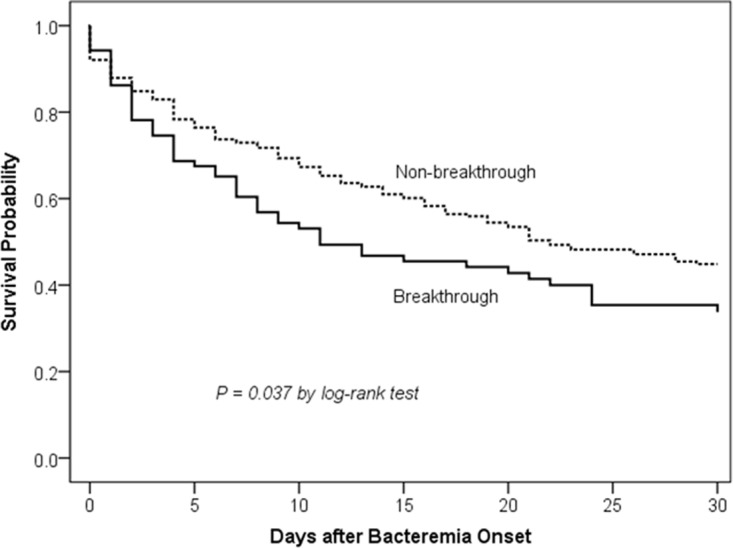
Comparison of Kaplan-Meier survival curves at 30 days among patients with breakthrough and nonbreakthrough carbapenem-resistant Acinetobacter bacteremia during carbapenem therapy.
The overall 30-day mortality rate of breakthrough Acinetobacter bacteremia during carbapenem therapy was 57.0%. The baseline demographics, clinical, and microbiological characteristics of survivors and nonsurvivors at 30 days after breakthrough Acinetobacter bacteremia are shown in Table 2. There were no significant differences between survivors and nonsurvivors in terms of comorbid conditions, the regimen and length of carbapenem therapy before bacteremia, and the appropriateness of antimicrobial therapy after the onset of bacteremia. Among the 20 patients with catheter-related infections as the source of bacteremia, early removal of the catheter within 48 h of bacteremia onset was not associated with a lower 30-day mortality (P = 0.921). A Cox proportional regression analysis was performed to see if any regimen was associated with a better or worse outcome (Table 3). It revealed that tigecycline-based therapy (hazard ratio [HR], 3.659; 95% CI, 1.794 to 7.465; P < 0.001), higher APACHE II score at bacteremia onset (HR, 1.049; 95% CI, 1.020 to 1.080; P = 0.001), and catheter-related infection as a source of bacteremia (HR, 1.984; 95% CI, 1.075 to 3.660; P = 0.028) were independent risk factors associated with 30-day mortality. Patients receiving colistin and tigecycline combination therapy with or without other antimicrobial(s) were excluded from the following analysis that compared tigecycline-based and colistin-based therapies. The Kaplan-Meier survival analysis revealed that the 30-day mortality rate was significantly higher in patients receiving tigecycline-based therapy than in those continuing carbapenem therapy without any concomitant antimicrobial(s) (P = 0.047, by log rank test) and those receiving colistin-based therapy (P = 0.045, by log rank test) (Fig. 2). The APACHE II scores were not significantly different among patients receiving tigecycline-based therapy, continued carbapenem therapy, and colistin-based therapy (P = 0.828 in a comparison of 3 therapies; tigecycline-based therapy versus continued carbapenem therapy, P = 0.554; tigecycline-based therapy versus colistin-based therapy, P = 0.861) (Table S3). In the tigecycline-based therapy group, most patients (11/15) received concomitant antimicrobial(s) with tigecycline. Among 100 Acinetobacter isolates causing breakthrough bacteremia, 40.0% were inhibited at 1 mg/liter and 71.0% at 2 mg/liter tigecycline (MIC50, 2 mg/liter; MIC90, 4 mg/liter). The case patients were treated continuously with a carbapenem without combination with other antimicrobial agent(s) after the onset of breakthrough Acinetobacter bacteremia due to the following reasons. First, some of the causative Acinetobacter isolates of breakthrough Acinetobacter bacteremia were susceptible to carbapenems. Second, the case patients may have been treated continuously with a carbapenem before the blood culture reported carbapenem-resistant Acinetobacter spp. Third, the case patients may have been treated continuously with a carbapenem even though the blood culture reported carbapenem-resistant Acinetobacter spp. because they improved after receiving carbapenem therapy. There was no significant difference in patient outcomes based on the reasons for continuous treatment with carbapenem monotherapy after the onset of breakthrough Acinetobacter bacteremia.
TABLE 2.
Univariate comparison between 30-day survivors and nonsurvivors in patients with breakthrough Acinetobacter bacteremia during carbapenem therapy
| Characteristica | All (n = 100) | Survivors (n = 43) | Nonsurvivors (n = 57) | P value |
|---|---|---|---|---|
| Demographical characteristics | ||||
| Age (median [IQR]) (yr) | 70.5 (53.25–80.75) | 75 (55–83) | 66 (52–79.5) | 0.215 |
| Male sex | 62 (62.0) | 22 (51.2) | 40 (70.2) | 0.083 |
| Recent ICU stay | 58 (58.0) | 26 (60.5) | 32 (56.1) | 0.819 |
| Bacteremia acquired in ICU | 66 (66.0) | 27 (62.8) | 39 (68.4) | 0.707 |
| Length of hospitalization before bacteremia (median [IQR]) (days) | 21.5 (13.25–36.5) | 25 (15–37) | 21 (11.5–36) | 0.477 |
| Comorbid conditions | ||||
| Alcoholism | 4 (4.0) | 0 (0.0) | 4 (7.0) | 0.132 |
| Liver cirrhosis | 9 (9.0) | 4 (9.3) | 5 (8.8) | 1.000 |
| Chronic obstructive pulmonary disease | 32 (32.0) | 12 (27.9) | 20 (35.1) | 0.585 |
| Chronic kidney disease | 38 (38.0) | 18 (41.9) | 20 (35.1) | 0.629 |
| Type 2 diabetes mellitus | 34 (34.0) | 14 (32.6) | 20 (35.1) | 0.959 |
| Hypertension | 42 (42.0) | 19 (44.2) | 23 (40.4) | 0.857 |
| Coronary artery disease | 17 (17.0) | 10 (23.3) | 7 (12.3) | 0.239 |
| Congestive heart failure | 24 (24.0) | 12 (27.9) | 12 (21.1) | 0.577 |
| Cerebrovascular accident | 19 (19.0) | 11 (25.6) | 8 (14.0) | 0.230 |
| Collagen vascular disease | 4 (4.0) | 3 (7.0) | 1 (1.8) | 0.312 |
| Immunosuppressant therapy | 12 (12.0) | 3 (7.0) | 9 (15.8) | 0.302 |
| Solid tumor | 16 (16.0) | 8 (18.6) | 8 (14.0) | 0.733 |
| Hematological malignancy | 10 (10.0) | 4 (9.3) | 6 (10.5) | 1.000 |
| Chemotherapy | 8 (8.0) | 4 (9.3) | 4 (7.0) | 0.722 |
| Neutropenia | 8 (8.0) | 2 (4.7) | 6 (10.5) | 0.460 |
| Trauma | 4 (4.0) | 3 (7.0) | 1 (1.8) | 0.312 |
| Burn | 1 (1.0) | 1 (2.3) | 0 (0.0) | 0.430 |
| Recent surgery | 25 (25.0) | 14 (32.6) | 11 (19.3) | 0.200 |
| Charlson comorbidity index (median [IQR]) | 4 (2–5.75) | 4 (2–6) | 4 (2–5) | 0.816 |
| Invasive procedures | ||||
| Arterial catheter | 43 (43.0) | 19 (44.2) | 24 (42.1) | 0.997 |
| Central venous catheter | 55 (55.0) | 26 (60.5) | 29 (50.9) | 0.453 |
| Ventilator use | 76 (76.0) | 30 (69.8) | 46 (80.7) | 0.303 |
| Hemodialysis | 20 (20.0) | 9 (20.9) | 11 (19.3) | 1.000 |
| Thoracic drain | 11 (11.0) | 2 (4.7) | 9 (15.8) | 0.109 |
| Abdominal drain | 14 (14.0) | 8 (18.6) | 6 (10.5) | 0.389 |
| Sources of bacteremia | ||||
| Pneumonia | 49 (49.0) | 21 (48.8) | 28 (49.1) | 1.000 |
| Catheter | 20 (20.0) | 6 (14.0) | 14 (24.6) | 0.289 |
| Urinary tract infection | 1 (1.0) | 1 (2.3) | 0 (0.0) | 0.430 |
| Intra-abdominal infection | 6 (6.0) | 2 (4.7) | 4 (7.0) | 0.697 |
| Wound | 2 (2.0) | 2 (4.7) | 0 (0.0) | 0.182 |
| Primary bacteremia | 22 (22.0) | 11 (25.6) | 11 (19.3) | 0.612 |
| Carbapenem therapy before bacteremia | ||||
| Imipenem | 44 (44.0) | 23 (53.5) | 21 (36.8) | 0.145 |
| Meropenem | 53 (53.0) | 18 (41.9) | 35 (61.4) | 0.083 |
| Doripenem | 3 (3.0) | 2 (4.7) | 1 (1.8) | 0.576 |
| Length of carbapenem therapy before bacteremia (median [IQR]) (days) | 9 (5–13) | 9 (4–13) | 9 (5–14) | 0.829 |
| Antimicrobial therapy after bacteremia onsetb | ||||
| Appropriate antimicrobial therapy | 44 (44.0) | 23 (53.5) | 22 (38.6) | 0.201 |
| Continued carbapenem monotherapy | 59 (59.0) | 28 (65.1) | 31 (54.4) | 0.382 |
| Colistin-based therapyc | 19 (19.0) | 8 (18.6) | 11 (19.3) | 1.000 |
| Tigecycline-based therapy | 15 (15.0) | 3 (7.0) | 12 (21.1) | 0.095 |
| Fluoroquinolone-based therapy | 9 (9.0) | 3 (7.0) | 6 (10.5) | 0.728 |
| Sulbactam-based therapy | 5 (5.0) | 3 (7.0) | 2 (3.5) | 0.649 |
| Carbapenem- and colistin-based therapyc | 17 (17.0) | 7 (16.3) | 10 (17.5) | 1.000 |
| Outcome | ||||
| Shock | 34 (34.0) | 11 (25.6) | 23 (40.4) | 0.183 |
| APACHE II score (median [IQR]) | 26 (18–31.75) | 22 (17–29) | 28 (21–33) | 0.014 |
| Species causing bacteremia | ||||
| A. baumannii | 53 (53.0) | 17 (39.5) | 36 (63.2) | 0.032 |
| A. nosocomialis | 30 (30.0) | 15 (34.9) | 15 (26.3) | 0.481 |
| A. pittii | 13 (13.0) | 9 (20.9) | 4 (7.0) | 0.080 |
| A. soli | 2 (2.0) | 1 (2.3) | 1 (1.8) | 1.000 |
| Microbiological characteristics of causative microorganisms | ||||
| Isolates harboring ISAba1-blaOXA-51-like | 22 (22.0) | 8 (18.6) | 13 (22.8) | 0.793 |
| Isolates harboring ISAba1-blaOXA-23-like | 37 (37.0) | 11 (25.6) | 26 (45.6) | 0.065 |
| Isolates harboring IS1008 (or IS1006)-ΔISAba3-blaOXA-58-like | 6 (6.0) | 0 (0.0) | 6 (10.5) | 0.036 |
| Isolates harboring blaOXA-24-like | 11 (11.0) | 6 (14.0) | 5 (8.8) | 0.523 |
| Isolates harboring blaIMP-like | 5 (5.0) | 4 (9.3) | 1 (1.8) | 0.162 |
| Isolates harboring blaVIM-like | 4 (4.0) | 2 (4.7) | 2 (3.5) | 1.000 |
Data are presented as the number (%), unless otherwise indicated. IQR, interquartile range; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II.
An antimicrobial agent (or antimicrobial agents)-based therapy denotes the corresponding antimicrobial agent(s) alone or in combination with other antimicrobial agent(s).
Only intravenous colistin was included. Inhaled colistin was not included.
TABLE 3.
Cox regression analyses of prognostic factors associated with 30-day mortality among patients with breakthrough Acinetobacter bacteremia during carbapenem therapy
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI)a | P | HR (95% CI)a | P | |
| APACHE II scoreb | 1.044 (1.015–1.074) | 0.003 | 1.049 (1.020–1.080) | 0.001 |
| Recent surgery | 0.508 (0.262–0.983) | 0.044 | ||
| Catheter-related infection | 1.842 (1.003–3.384) | 0.049 | 1.984 (1.075–3.660) | 0.028 |
| Bacteremia due to A. baumannii | 1.866 (1.086–3.205) | 0.024 | ||
| Bacteremia due to A. pittii | 0.422 (0.153–1.167) | 0.097 | ||
| Tigecycline-based therapy | 2.142 (1.124–4.082) | 0.021 | 3.659 (1.794–7.465) | <0.001 |
| Appropriate therapy | 0.637 (0.373–1.088) | 0.099 | ||
HR, hazard ratio; CI, confidence interval.
APACHE II, Acute Physiology and Chronic Health Evaluation II.
FIG 2.
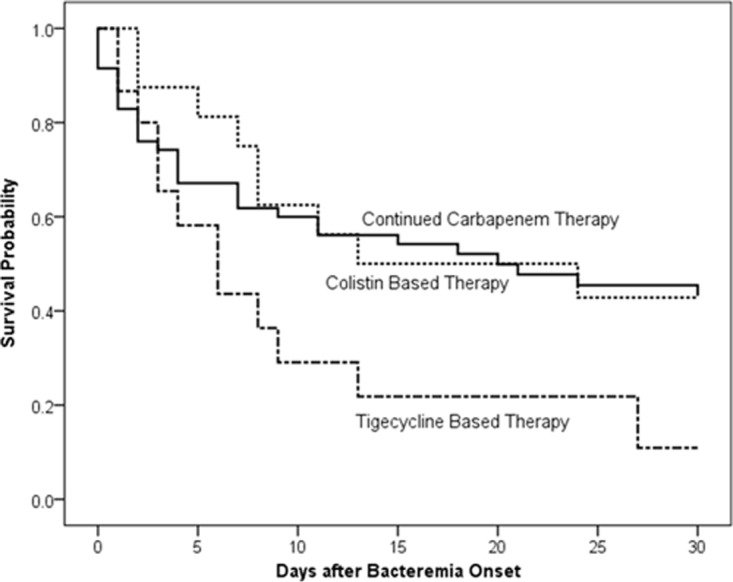
Comparison of Kaplan-Meier survival curves at 30 days among patients who received continued carbapenem therapy, colistin-based therapy, and tigecycline-based therapy for their breakthrough Acinetobacter bacteremia (tigecycline-based therapy versus continued carbapenem therapy, P = 0.047 by log rank test; tigecycline-based therapy versus colistin-based therapy, P = 0.045 by log rank test).
Tigecycline-based therapy was independently associated with a poor outcome in patients with breakthrough Acinetobacter bacteremia during carbapenem therapy but not in patients with carbapenem-resistant Acinetobacter bacteremia or nonbreakthrough carbapenem-resistant Acinetobacter bacteremia. Among patients with carbapenem-resistant Acinetobacter bacteremia who were treated with tigecycline-based therapy, the breakthrough group had a significantly higher 14-day mortality (78.6% [11/14] versus 36.4% [12/33], respectively; P = 0.020) and a higher 30-day mortality but without reaching statistical significance (78.6% [11/14] versus 42.4% [14/33], respectively; P = 0.051) than the nonbreakthrough group. There were no significant differences in demographic characteristics, underlying diseases, Charlson comorbidity index (P = 0.831), invasive procedures, sources of bacteremia, including pneumonia (50.0% [7/14] versus 51.5% [17/33]; P = 1.000), APACHE II scores (P = 0.369), bacterial species and tigecycline MICs (P = 0.654) of causative pathogens, or the percentage and regimens of combination therapy between the 2 groups.
DISCUSSION
This multicenter study was designed to assess the clinical features of breakthrough Acinetobacter bacteremia during carbapenem therapy and to evaluate the clinical outcomes among patient groups receiving different antimicrobial therapies. Breakthrough Acinetobacter bacteremia during carbapenem therapy was associated with a high mortality rate and high carbapenem resistance rate. Among patients with carbapenem-resistant Acinetobacter bacteremia, the breakthrough group was associated with significantly higher 14-day mortality than the nonbreakthrough group, even though the breakthrough group was more likely to receive appropriate antimicrobial therapy. For the treatment of patients with breakthrough bacteremia, tigecycline-based therapy was independently associated with a poor outcome.
Breakthrough Acinetobacter bacteremia during carbapenem therapy is not uncommon in patients. However, its clinical impact has not yet been determined. In addition, patients with breakthrough bacteremia during carbapenem therapy are sometimes excluded from the study population of carbapenem-resistant Acinetobacter bloodstream infections in outcome analysis (9), despite the high prevalence of carbapenem resistance among their Acinetobacter isolates. This study provides the first data on the clinical significance of breakthrough Acinetobacter bacteremia during carbapenem therapy. We found that in the carbapenem-resistant subgroup, breakthrough bacteremia was associated with a higher 14-day mortality than with nonbreakthrough bacteremia. For 30-day mortality, survival analysis revealed that the breakthrough group had a higher mortality rate. Overall, the breakthrough group was associated with a poorer outcome than the nonbreakthrough group in carbapenem-resistant Acinetobacter bacteremia. The unfavorable outcome is not a result of inappropriate antimicrobial therapy, because patients with breakthrough bacteremia are more likely to receive appropriate antimicrobial therapy for their carbapenem-resistant Acinetobacter bacteremia than those in the nonbreakthrough group, such as a carbapenem in combination with colistin or tigecycline. Since they had already received a carbapenem, it was reasonable to add colistin or tigecycline when symptoms/signs of bacteremia occurred.
Tigecycline is often used for the treatment of carbapenem-resistant Acinetobacter infections or as a salvage therapy for Acinetobacter infections with carbapenem treatment failure. However, our results do not support the use of a tigecycline-based regimen for the treatment of breakthrough Acinetobacter bacteremia during carbapenem therapy. The similarity of APACHE II scores among patient groups receiving different regimens and the finding that tigecycline-based therapy remains an independent mortality risk factor after controlling for severity of illness indices exclude disease severity as a confounder to explain the difference in mortality. Possible explanations include the bacteriostatic property of tigecycline, the relatively high MICs of tigecycline of our study isolates that were unachievable by the currently approved dose of tigecycline in serum (10), a low AUC/MIC ratio when the currently approved dose is used (11–16), and a high prevalence of hospital-acquired pneumonia as a source of bacteremia in breakthrough Acinetobacter bacteremia during carbapenem therapy (14, 17). Since there were no differences in patient characteristics, tigecycline MICs, and the percentage of pneumonia between breakthrough and nonbreakthrough groups of carbapenem-resistant Acinetobacter bacteremia, the reasons for the association of tigecycline-based therapy with more unfavorable outcomes in the breakthrough group require further investigation.
Although all the study isolates were susceptible to colistin, colistin alone or in combination with other antimicrobial agents was still associated with a high mortality rate. It is suggested that susceptibility to colistin cannot ensure successful treatment. The current colistin susceptibility breakpoint of 2 mg/liter may not be adequate, based on its pharmacokinetic properties, such as inadequate plasma levels and potential for development of resistance (2). Whether colistin is effective for certain subgroups of patients and whether colistin combined with other antimicrobials, such as rifampin, can improve patient outcomes are yet to be determined. In addition, only patients who received the standard dose of carbapenem therapy were included in the current study. Maximizing carbapenem dosing or prolonging infusion may be associated with better patient outcomes, since these strategies have improved the probability of attaining pharmacodynamic targets (3, 18). Further studies are needed to evaluate if these strategies can prevent or treat breakthrough Acinetobacter bacteremia during carbapenem therapy.
Among the mechanisms of carbapenem resistance in Acinetobacter spp., the most notable is the expression of class D carbapenemases (1, 2). Although carbapenem-resistant Acinetobacter isolates causing breakthrough bacteremia during carbapenem therapy were less likely to carry the ISAba1-blaOXA-23-like structure than the nonbreakthrough group, the ISAba1-blaOXA-23-like structure was still the most prevalent carbapenem resistance determinant in both groups. The ISAba1-blaOXA-23-like genetic structure was often contained in transposons which were carried by conjugative plasmids, facilitating its widespread in Acinetobacter isolates in recent years (19, 20). Of greater interest is that the carbapenem-resistant A. baumannii isolates causing breakthrough bacteremia are more likely to carry the ISAba1-blaOXA-51-like structure. It has been suggested that carbapenem therapy may be a risk factor for rapid acquisition of A. baumannii isolates harboring the ISAba1-blaOXA-51-like structure. The universal chromosomal location of blaOXA-51-like genes in A. baumannii (21) and the wide distribution of the insertion sequence ISAba1 in the A. baumannii genome (22) may facilitate the transposition of ISAba1 upstream of blaOXA-51-like genes to confer a high level of carbapenem resistance (23).
The major limitations of this study are its retrospective design and intrinsic selection bias. The strengths of this study are the inclusion of a large number of patients from multiple medical centers located in representative regions of Taiwan using stringent inclusion criteria, recent isolates, and detailed characterization of resistance markers among breakthrough and nonbreakthrough carbapenem-resistant isolates. Our findings provide clinicians with outcome data of breakthrough Acinetobacter bacteremia during carbapenem therapy.
In conclusion, patients with breakthrough Acinetobacter bacteremia during carbapenem therapy posed a high mortality rate. Compared to continued carbapenem- or colistin-based therapy, tigecycline-based therapy was associated with higher mortality. Further studies are required to determine the optimal treatment of breakthrough Acinetobacter bacteremia during carbapenem therapy.
MATERIALS AND METHODS
Hospital setting and study population.
This retrospective study was conducted from January 2010 to December 2015 at 4 medical centers in Taiwan: Changhua Christian Hospital (CCH; 1,676 beds) in central Taiwan, Mackay Memorial Hospital (MMH; 2,055 beds) in northern Taiwan, Taipei Veterans General Hospital (TVGH; 2,900 beds) in northern Taiwan, and Tri-Service General Hospital (TSGH; 1,712 beds) of the National Defense Medical Center in northern Taiwan. Patients with at least one positive blood culture for Acinetobacter spp. who had symptoms and signs of infection were recruited into the study. For patients with ≥2 positive blood cultures, only the first blood culture was included. Patients <20 years of age and those with incomplete medical records were excluded. Case patients were defined as individuals whose blood cultures grew Acinetobacter spp. and who had been receiving a type II carbapenem (e.g., imipenem, meropenem, or doripenem) as monotherapy for at least 48 h before breakthrough bacteremia. The case patients were treated with a carbapenem before the onset of breakthrough Acinetobacter bacteremia as definite antimicrobial treatment for infections which were not caused by Acinetobacter spp. and were caused by carbapenem-susceptible microorganisms, such as pneumonia caused by carbapenem-susceptible Pseudomonas aeruginosa, or urosepsis caused by extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Patients who received ertapenem and those whose blood cultures yielded the same Acinetobacter spp. prior to breakthrough bacteremia were excluded. Among the patients with carbapenem-resistant Acinetobacter bacteremia, the nonbreakthrough group was defined as those who did not receive any type II carbapenem therapy within 48 h before the onset of bacteremia. All patients who fulfilled the criteria were included in the study. The protocol was approved by the hospitals' institutional review boards (IRB) (CCH, IRB no. 140514; MMH, IRB no. 14MMHIS125; TVGH, IRB no. 2014-07-006CC; and TSGH, IRB no. 1-103-05-100).
Data collection and definitions.
The medical records of the patients were reviewed retrospectively and analyzed. Patients were assessed for demographic characteristics, duration of hospitalization, stay in the intensive care unit (ICU), comorbidities, invasive procedures at the time of bacteremia onset, and time of receipt, dose, and route of therapy with individual antimicrobial drugs. Recent stay in the ICU was defined as being within 2 weeks of the first positive blood culture. Episodes of bloodstream infection were considered to be acquired in the ICU if they appeared beyond 48 h after ICU admission. Immunosuppressive therapy was defined as use of immunosuppressive agents within 2 weeks or use of corticosteroids at a dosage equivalent to or higher than 15 mg of prednisolone daily for 1 week within 4 weeks before the onset of bacteremia. Chemotherapy was defined as administration of cytotoxic agents within 6 weeks before onset of bacteremia. Recent surgery was defined as operations performed within 4 weeks before the onset of bacteremia. The source of bacteremia was determined according to the definitions of the U.S. Centers for Disease Control and Prevention (24). The severity of infection was evaluated using the Acute Physiology and Chronic Health Evaluation (APACHE) II score within 24 h before the onset of bacteremia. Appropriate antimicrobial therapy was defined as administration of at least one antimicrobial agent to which the causative pathogen was susceptible in vitro within 24 h after the onset of bacteremia for a minimum of 24 h, with an approved route and dosage appropriate for end-organ(s) function. Antimicrobial therapy that did not meet this definition was considered inappropriate. Monotherapy with an aminoglycoside was not considered an appropriate therapy. An antimicrobial agent (or antimicrobial agents)-based therapy was defined as treatment with the antimicrobial agent(s) alone or in combination with another antimicrobial agent(s). Continued carbapenem therapy was defined as maintaining treatment with the carbapenem that the patient had received before the onset of breakthrough Acinetobacter bacteremia without any concomitant antimicrobial agent(s). The dose of colistin was 5 mg/kg colistin base activity loading, followed by 5 mg/kg/day colistin base activity divided over 8 or 12 h in patients with normal renal function. For those with impaired renal function, the dosage was adjusted according to renal function, as previously described (25, 26). The loading dose of tigecycline was 100 mg, followed by a maintenance dose of 50 mg every 12 h. The all-cause 14-day and 30-day mortality rates were used as the endpoints and were defined as death occurring within 14 and 30 days after the date of bacteremia onset, respectively. For patients who were discharged before the 30-day limit, the status was determined by a review of outpatient records or by contacting the patient directly.
Bacterial identification, clonal study, antimicrobial susceptibility testing, and detection of carbapenem resistance determinants.
The initial isolate was used for the microbiological studies. The bacteria were phenotypically identified as Acinetobacter spp. using the Vitek 2 system (bioMérieux, Marcy l'Étoile, France). Acinetobacter baumannii was identified by a multiplex PCR method (10). Isolates identified as non-baumannii Acinetobacter spp. were further identified to the genomic species level by 16S-23S ribosomal DNA intergenic spacer sequence analysis, as previously described (27). The MICs of carbapenems, tigecycline, and colistin and the antimicrobial susceptibilities of other agents were determined by agar dilution according to the Clinical and Laboratory Standards Institute (CLSI) (28). Multidrug resistance (MDR) was defined as resistance to any one agent in at least 3 of the following classes of antimicrobials: aminoglycosides, carbapenems, antipseudomonal cephalosporins, β-lactam–β-lactamase inhibitor combinations, and fluoroquinolones.
Multiplex PCR assays were performed to detect the carbapenem-hydrolyzing class D β-lactamase (CHDL) genes (blaOXA-23-like, blaOXA-24-like, blaOXA-51-like, blaOXA-58-like, and blaOXA-143-like) (29). Metallo-β-lactamases were detected by phenotypic methods and PCR assays, including the multiplex PCR with primers specific for the blaIMP, blaVIM, blaSIM, blaSPM, and blaGIM-1 genes (30), and the PCR assay detecting the presence of blaNDM-1 (31). The upstream locations of insertion sequences (ISs) ISAba1 of the blaOXA-51-like or blaOXA-23-like gene and IS1008 or IS1006 upstream of the blaOXA-58-like gene were analyzed by PCR mapping (23, 30, 32–34).
Statistical analysis.
PASW for Windows version 18 (SPSS, Chicago, IL, USA) was used for all data analyses. The χ2 test with Yates correction or Fisher's exact test was used to compare categorical data. Continuous variables were analyzed using the Mann-Whitney U test or two-sample t test. The Wilcoxon signed-rank test was used to determine statistically significant differences between paired samples. The time to mortality, defined as the interval between the onset of bacteremia and death, was analyzed using the Kaplan-Meier survival analysis, and the log rank test was used to compare univariable survival distributions between different groups of patients. A logistic regression model was used to explore independent prognostic factors associated with 14-day and 30-day mortality of patients with Acinetobacter bacteremia caused by carbapenem-resistant strains. A Cox proportional hazard regression model was used to explore independent prognostic factors associated with the 30-day mortality of patients with breakthrough Acinetobacter bacteremia during carbapenem therapy. Univariable analyses were performed separately for each risk factor to ascertain the odds ratio (OR) or hazard ratio (HR) and 95% confidence interval (CI). All biologically plausible variables with a P value of <0.10 in the univariable analysis were considered for inclusion in the logistic regression model or Cox regression model in the multivariable analysis. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Taipei Veterans General Hospital (grants V105B-005 and V106B-002 [to Y.-T.L.]), Tri-Service General Hospital (grants TSGH-C105-112 [to Y.-C.W.], TSGH-105-113 [to Y.-S.Y.], and TSGH-C106-096 [to Y.-S.Y.]), the National Defense Medical Center (grants MAB-106-076 [to Y.-C.W.] and MAB-106-098 [to Y.-S.Y.]), and the Ministry of Science and Technology (grants MOST 104-2314-B-075-043-MY3 [to Y.-T.L.], MOST 105-2314-B-016-039-MY3 [to Y.-C.W.], and MOST 105-2628-B-016-003-MY2 [to Y.-S.Y.]). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare no relevant conflicts of interest related to this article.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00931-17.
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med 36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin Infect Dis 51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 4.Liang-Yu C, Kuo SC, Liu CY, Luo BS, Huang LJ, Lee YT, Chen CP, Chen TL, Fung CP. 2011. Difference in imipenem, meropenem, sulbactam, and colistin nonsusceptibility trends among three phenotypically undifferentiated Acinetobacter baumannii complex in a medical center in Taiwan, 1997–2007. J Microbiol Immunol Infect 44:358–363. doi: 10.1016/j.jmii.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, He YQ, Chen JH. 2013. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am J Infect Control 41:e59–63. doi: 10.1016/j.ajic.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Chen TL, Lee YT, Lee MH, Kuo SC, Yu KW, Dou HY, Fung CP. 2014. Risk factors for imipenem-nonsusceptible Acinetobacter nosocomialis bloodstream infection. J Microbiol Immunol Infect 47:311–317. doi: 10.1016/j.jmii.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Kang CI, Ko JH, Lee WJ, Seok HR, Park GE, Cho SY, Ha YE, Chung DR, Lee NY, Peck KR, Song JH. 2016. Clinical features and risk factors for development of breakthrough Gram-negative bacteremia during carbapenem therapy. Antimicrob Agents Chemother 60:6673–6678. doi: 10.1128/AAC.00984-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemos EV, de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castaneda C, Kawai K. 2014. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 20:416–423. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 9.Bass SN, Bauer SR, Neuner EA, Lam SW. 2015. Impact of combination antimicrobial therapy on mortality risk for critically ill patients with carbapenem-resistant bacteremia. Antimicrob Agents Chemother 59:3748–3753. doi: 10.1128/AAC.00091-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen TL, Siu LK, Wu RC, Shaio MF, Huang LY, Fung CP, Lee CM, Cho WL. 2007. Comparison of one-tube multiplex PCR, automated ribotyping and intergenic spacer (ITS) sequencing for rapid identification of Acinetobacter baumannii. Clin Microbiol Infect 13:801–806. doi: 10.1111/j.1469-0691.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng A, Chuang YC, Sun HY, Sheng WH, Yang CJ, Liao CH, Hsueh PR, Yang JL, Shen NJ, Wang JT, Hung CC, Chen YC, Chang SC. 2015. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med 43:1194–1204. doi: 10.1097/CCM.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 12.Bhavnani SM, Rubino CM, Hammel JP, Forrest A, Dartois N, Cooper CA, Korth-Bradley J, Ambrose PG. 2012. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother 56:1065–1072. doi: 10.1128/AAC.01615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrose PG, Hammel JP, Bhavnani SM, Rubino CM, Ellis-Grosse EJ, Drusano GL. 2012. Frequentist and Bayesian pharmacometric-based approaches to facilitate critically needed new antibiotic development: overcoming lies, damn lies, and statistics. Antimicrob Agents Chemother 56:1466–1470. doi: 10.1128/AAC.01743-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liou BH, Lee YT, Kuo SC, Liu PY, Fung CP. 2015. Efficacy of tigecycline for secondary Acinetobacter bacteremia and factors associated with treatment failure. Antimicrob Agents Chemother 59:3637–3640. doi: 10.1128/AAC.04987-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein GE, Babinchak T. 2013. Tigecycline: an update. Diagn Microbiol Infect Dis 75:331–336. doi: 10.1016/j.diagmicrobio.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Vardakas KZ, Rafailidis PI, Falagas ME. 2012. Effectiveness and safety of tigecycline: focus on use for approved indications. Clin Infect Dis 54:1672–1674. doi: 10.1093/cid/cis239. [DOI] [PubMed] [Google Scholar]
- 17.Chuang YC, Cheng CY, Sheng WH, Sun HY, Wang JT, Chen YC, Chang SC. 2014. Effectiveness of tigecycline-based versus colistin-based therapy for treatment of pneumonia caused by multidrug-resistant Acinetobacter baumannii in a critical setting: a matched cohort analysis. BMC Infect Dis 14:102. doi: 10.1186/1471-2334-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdul-Aziz MH, Lipman J, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Dulhunty J, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Roberts JA, DALI Study Group. 2016. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort J Antimicrob Chemother 71:196–207. doi: 10.1093/jac/dkv288. [DOI] [PubMed] [Google Scholar]
- 19.Nigro SJ, Hall RM. 2016. Structure and context of Acinetobacter transposons carrying the OXA-23 carbapenemase gene. J Antimicrob Chemother 71:1135–1147. doi: 10.1093/jac/dkv440. [DOI] [PubMed] [Google Scholar]
- 20.Ku WW, Kung CH, Lee CH, Tseng CP, Wu PF, Kuo SC, Chen TL, Lee YT, Wang FD, Fung CP. 2015. Evolution of carbapenem resistance in Acinetobacter baumannii: an 18-year longitudinal study from a medical center in northern Taiwan. J Microbiol Immunol Infect 48:57–64. doi: 10.1016/j.jmii.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Evans BA, Amyes SGB. 2014. OXA beta-lactamases. Clin Microbiol Rev 27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugnier PD, Poirel L, Nordmann P. 2009. Functional analysis of insertion sequence ISAba1, responsible for genomic plasticity of Acinetobacter baumannii. J Bacteriol 191:2414–2418. doi: 10.1128/JB.01258-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen TL, Lee YT, Kuo SC, Hsueh PR, Chang FY, Siu LK, Ko WC, Fung CP. 2010. Emergence and distribution of plasmids bearing the blaOXA-51-like gene with an upstream ISAba1 in carbapenem-resistant Acinetobacter baumannii isolates in Taiwan. Antimicrob Agents Chemother 54:4575–4581. doi: 10.1128/AAC.00764-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 25.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogue JM, Ortwine JK, Kaye KS. 2017. Clinical considerations for optimal use of the polymyxins: a focus on agent selection and dosing. Clin Microbiol Infect 23:229–233. doi: 10.1016/j.cmi.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Chang HC, Wei YF, Dijkshoorn L, Vaneechoutte M, Tang CT, Chang TC. 2005. Species-level identification of isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex by sequence analysis of the 16S-23S rRNA gene spacer region. J Clin Microbiol 43:1632–1639. doi: 10.1128/JCM.43.4.1632-1639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CLSI. 2017. Performance standards for antimicrobial susceptibility testing; 27th informational supplement. CLSI document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Higgins PG, Lehmann M, Seifert H. 2010. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 35:305. doi: 10.1016/j.ijantimicag.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Lee YT, Huang LY, Chiang DH, Chen CP, Chen TL, Wang FD, Fung CP, Siu LK, Cho WL. 2009. Differences in phenotypic and genotypic characteristics among imipenem-non-susceptible Acinetobacter isolates belonging to different genomic species in Taiwan. Int J Antimicrob Agents 34:580–584. doi: 10.1016/j.ijantimicag.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Huang TW, Lauderdale TL, Liao TL, Hsu MC, Chang FY, Chang SC, Khong WX, Ng OT, Chen YT, Kuo SC, Chen TL, Mu JJ, Tsai SF. 2015. Effective transfer of a 47 kb NDM-1-positive plasmid among Acinetobacter species. J Antimicrob Chemother 70:2734–2738. doi: 10.1093/jac/dkv191. [DOI] [PubMed] [Google Scholar]
- 32.Lee YT, Turton JF, Chen TL, Wu RC, Chang WC, Fung CP, Chen CP, Cho WL, Huang LY, Siu LK. 2009. First identification of blaOXA-51-like in non-baumannii Acinetobacter spp. J Chemother 21:514–520. doi: 10.1179/joc.2009.21.5.514. [DOI] [PubMed] [Google Scholar]
- 33.Chen TL, Chang WC, Kuo SC, Lee YT, Chen CP, Siu LK, Cho WL, Fung CP. 2010. Contribution of a plasmid-borne blaOXA-58 gene with its hybrid promoter provided by IS1006 and an ISAba3-like element to β-lactam resistance in Acinetobacter genomic species 13TU. Antimicrob Agents Chemother 54:3107–3112. doi: 10.1128/AAC.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL. 2008. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob Agents Chemother 52:2573–2580. doi: 10.1128/AAC.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


