ABSTRACT
The Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) offer different recommendations for carbapenem MIC susceptibility breakpoints for Acinetobacter species. In addition, the clinical efficacy of the intermediate category remains uncertain. This study was designed to determine the optimal predictive breakpoints based on the survival of patients with Acinetobacter bacteremia treated with a carbapenem. We analyzed the 30-day mortality rates of 224 adults who received initial carbapenem monotherapy for the treatment of Acinetobacter bacteremia at 4 medical centers over a 5-year period, according to the carbapenem MICs of the initial isolates. The 30-day mortality was about 2-fold greater in patients whose isolates had carbapenem MICs of ≥8 mg/liter than in those with isolates with MICs of ≤4 mg/liter. The differences were significant by bivariate analysis (53.1% [60/113] versus 25.2% [28/111], respectively; P < 0.001) and on survival analysis by the log rank test (P < 0.001). Classification and regression tree analysis revealed a split between MICs of 4 and 8 mg/liter and predicted the same difference in mortality, with a P value of <0.001. Carbapenem treatment for Acinetobacter bacteremia caused by isolates with carbapenem MICs of ≥8 mg/liter was an independent predictor of 30-day mortality (odds ratio, 4.218; 95% confidence interval, 2.213 to 8.039; P < 0.001). This study revealed that patients with Acinetobacter bacteremia treated with a carbapenem had a more favorable outcome when the carbapenem MICs of their isolates were ≤4 mg/liter than those with MICs of ≥8 mg/liter.
KEYWORDS: Acinetobacter, carbapenem, MIC, bloodstream infection
INTRODUCTION
Acinetobacter species have become major nosocomial pathogens associated with high mortality in immunocompromised hosts (1, 2). Carbapenems, including imipenem, meropenem, and doripenem, are the preferred agents for the treatment of severe Acinetobacter infections (3). The emergence of carbapenem-resistant Acinetobacter spp. threatens the efficacy of these agents for the treatment of health care-associated infections (2, 3).
Breakpoints are useful to define susceptibility and resistance to antimicrobial agents (4). However, there are some discrepancies between the carbapenem breakpoints set by the two major organizations, the Clinical and Laboratory Standards Institute (CLSI) (5) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (6). The CLSI resistance breakpoints for the MICs of imipenem and meropenem against Acinetobacter spp. are both ≥8 mg/liter (5). The EUCAST breakpoints are both ≥16 mg/liter (6). An MIC of 8 mg/liter indicates resistance according to the CLSI and intermediate susceptibility by the EUCAST guidelines. An MIC of 4 mg/liter is considered intermediate susceptibility by both organizations. An MIC of 4 mg/liter was considered to be susceptible by the CLSI in a previous version of the breakpoints (7). There are no clinical data to support the change from susceptible to intermediate for an MIC of 4 mg/liter.
Susceptibility breakpoints are constructed on the basis of clinical data, MIC distributions, pharmacokinetics/pharmacodynamics (PK/PD) derived from animal exposure-response studies, and Monte Carlo simulations (4, 8). The justification for animal and simulation studies to set carbapenem breakpoints for Acinetobacter species has been addressed by investigators (9–12). Clinical correlations that validate the recommended carbapenem breakpoints in patients with Acinetobacter infection are limited (13). Outcome data from clinical studies are needed to justify these breakpoints, especially when discrepancies exist between different organizations.
We previously performed a single-center study to provide clinical data to support the carbapenem breakpoints for the Acinetobacter baumannii group in patients with bacteremia (13). However, the study was limited by the potential lack of external validity, implausible effect size, and long study period. Therefore, this retrospective chart review study was conducted to evaluate the clinical outcomes of patients with Acinetobacter bacteremia treated with carbapenems whose isolates had different carbapenem MICs at multiple centers from 2011 to 2015. The evidence provided in this report should help optimize the current carbapenem breakpoints for Acinetobacter species.
RESULTS
We reviewed the charts of 1,104 patients who had Acinetobacter bacteremia and their complete medical records during the study period (Fig. 1). Of these, 224 patients met the entry criteria, after the exclusions shown in Fig. 1. The study population included 115 patients who received imipenem and 109 patients who received meropenem monotherapy within 24 h of the onset of bacteremia and had a viable first isolate. The durations of treatment with imipenem and meropenem were 11 ± 8 and 13 ± 8 days, respectively (P = 0.106). After initiation of carbapenem monotherapy, 66 of the 224 patients were switched to other antimicrobial agents or treated with another antimicrobial agent in combination with a carbapenem (see Table S1 in the supplemental material). Among the 66 patients, 13 (19.7%) patients started to receive other active drugs 48 to 72 h after the onset of bacteremia, 36 (54.5%) patients started 72 to 96 h after the onset of bacteremia, and 17 (25.8%) patients started 96 to 120 h after the onset of bacteremia. None of the alternative antimicrobial regimens were associated with a significantly higher or lower 30-day mortality. There was no significant difference in survival based on the timing of the additional agents. A total of 147 (65.6%) patients had received antimicrobial agents prior to carbapenem therapy. Most of these patients (128/147 [87.1%]) had received antimicrobial agents that were inactive against Acinetobacter species. This group did not have a higher 30-day mortality than those who did not receive prior antimicrobial therapy. There was no significant difference in survival based on the class of antimicrobial agent used prior to carbapenem therapy.
FIG 1.
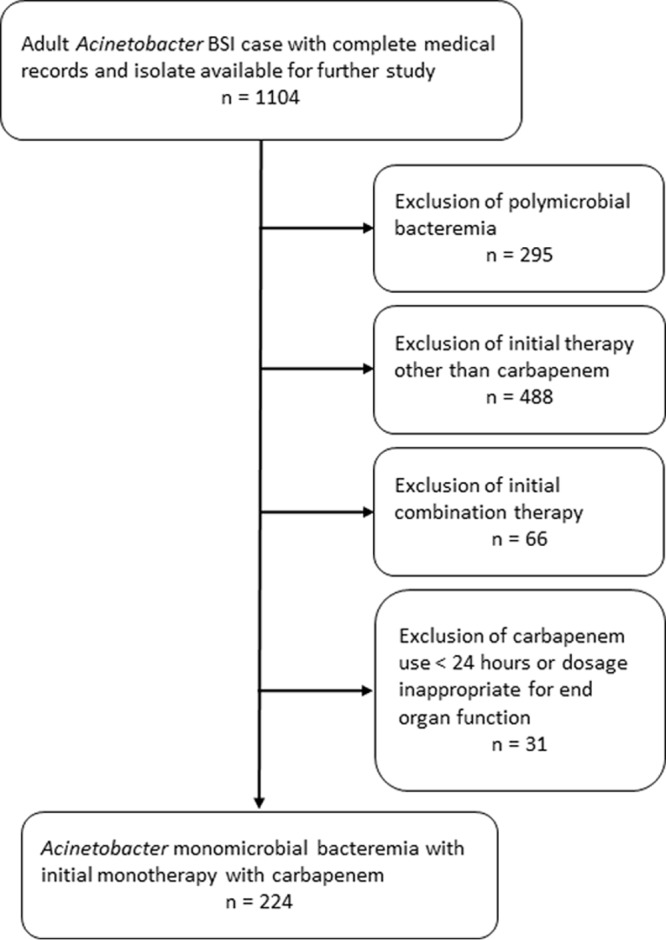
Methodology for application of exclusion criteria. BSI, bloodstream infection.
The Acinetobacter isolates (one from each patient) were identified as A. baumannii (121 isolates, 63 clones), Acinetobacter pittii (19 isolates, 12 clones), Acinetobacter nosocomialis (73 isolates, 37 clones), Acinetobacter soli (2 isolates, 2 clones), and other Acinetobacter spp. (9 isolates, 9 clones). The MICs for meropenem or imipenem for the same isolate were not always the same, and there were no significant differences between the MICs for imipenem and those for meropenem (P = 0.297). Therefore, only the MIC of the carbapenem that the patient received was presented in this study. There was no significantly difference in the 30-day mortality rates among patients infected with A. baumannii, A. pittii, A. nosocomialis, and other Acinetobacter spp. by survival analysis (Fig. S1). In addition, the 30-day mortality rates between patients receiving either imipenem or meropenem were also not significantly different, as shown by survival analysis (Fig. S2) or by bivariate analysis in different susceptibility categories (including MICs of ≤2, 4, 8 or ≥16 mg/liter). Therefore, we included all of the Acinetobacter species and the two carbapenems in our analysis.
The 30-day mortality rates varied in relation to the carbapenem MICs of the Acinetobacter isolates (Fig. 2). The mortality rates did not differ significantly between the patients with isolates with MICs of ≤2 and 4 mg/liter (25.5% versus 23.5%, respectively; P = 1.000). We then compared the clinical outcomes of patients with isolates with an MIC of 4 mg/liter, which indicated intermediate susceptibility according to the CLSI and EUCAST guidelines, to those with isolates with an MIC of 8 mg/liter, which indicated resistance and intermediate susceptibility according to the CLSI and EUCAST guidelines, respectively. Patients with isolates with an MIC of ≥8 mg/liter had significantly higher 30-day mortality than those with isolates with an MIC of 4 mg/liter (53.1% versus 23.5%, respectively; P = 0.044). The 30-day mortality rates of patients with isolates with an MIC of 8 mg/liter did not differ significantly from those with isolates with an MIC of ≥16 mg/liter (42.9% versus 55.4%, respectively; P = 0.424). However, those who acquired isolates with an MIC of ≥8 mg/liter had significantly higher 30-day mortality (about 2-fold) than those who acquired isolates with an MIC of ≤4 mg/liter (53.1% versus 25.2%, respectively; P < 0.001).
FIG 2.
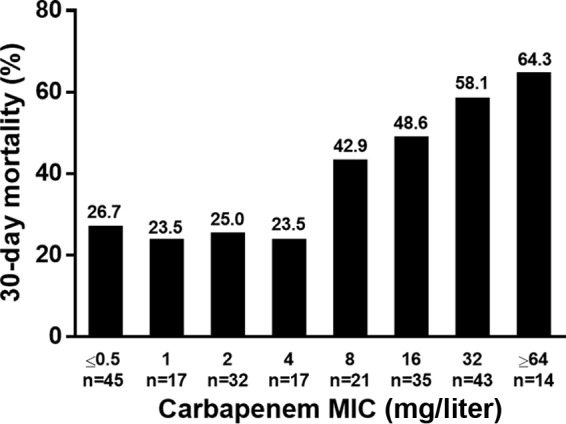
Thirty-day mortality rate of patients with Acinetobacter bacteremia in different susceptibility categories. The rate was significantly lower in those with a carbapenem MIC of ≤4 mg/liter than in those with a carbapenem MIC of ≥8 mg/liter.
Classification and regression tree (CART) analysis was performed to determine the carbapenem MIC breakpoint that maximized the difference in 30-day mortality. We found a division in the MICs between 4 and 8 mg/liter that predicted a difference in mortality (P < 0.001) (Fig. S3). The Kaplan-Meier survival analysis also revealed that the 30-day mortality rate was significantly higher in patients for Acinetobacter isolates with carbapenem MICs of ≥8 mg/liter than those with isolates with MICs of ≤4 mg/liter (Fig. 3).
FIG 3.
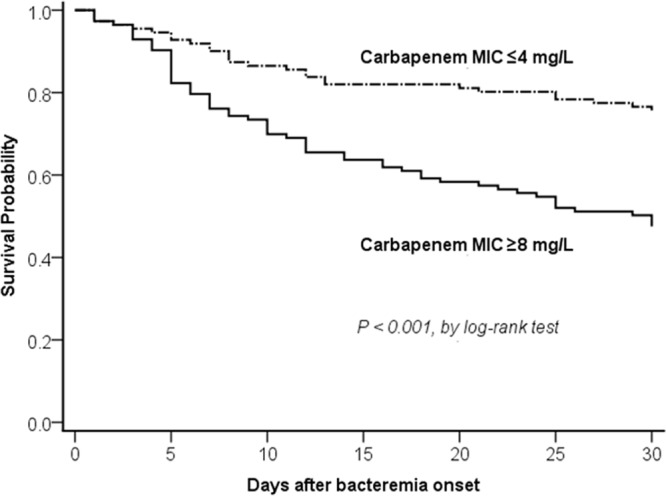
Comparison of Kaplan-Meier survival curves, at 30 days, between patients with Acinetobacter bacteremia caused by isolates having MICs of ≤4 mg/liter and ≥8 mg/liter.
The baseline demographics, clinical characteristics, and microbiologic characteristics of patients, stratified by carbapenem MICs, are shown in Table 1. There were no significant differences in underlying diseases, Charlson comorbidity index values, or Acute Physiology and Chronic Health Evaluation (APACHE) II scores at the onset of bacteremia between patients whose isolates had a carbapenem MIC of ≤4 mg/liter and those with isolates with an MIC of ≥8 mg/liter (Table 1). A multivariate logistic regression analysis was performed to see whether acquisition of isolates with a carbapenem MIC of ≥8 mg/liter was independently associated with 30-day mortality in patients with Acinetobacter bloodstream infections (Table 2). It revealed that the acquisition of Acinetobacter isolates with a carbapenem MIC of ≥8 mg/liter (odds ratio [OR], 4.218; 95% confidence interval [CI], 2.213 to 8.039; P < 0.001), higher APACHE II score at bacteremia onset (OR, 1.055; 95% CI, 1.019 to 1.093; P < 0.001), and shock at bacteremia onset (OR, 4.180; 95% CI, 2.173 to 8.040; P = 0.003) were independent risk factors associated with 30-day mortality. The above-mentioned analyses were performed in the same manner using all-cause 14-day mortality as the endpoint, and the results were similar to those of analysis using all-cause 30-day mortality as the primary outcome measure (date not shown).
TABLE 1.
Univariate comparison between patients acquiring Acinetobacter isolates with carbapenem MICs ≤4 mg/liter and ≥8 mg/liter
| Characteristica | Results by carbapenem MIC |
P value | |
|---|---|---|---|
| ≤4 mg/liter (n = 111) | ≥8 mg/liter (n = 113) | ||
| Demographic characteristics | |||
| Age (median [IQR]) (yr) | 74 (62–82) | 75 (58–82) | 0.676 |
| Males | 80 (72.1) | 74 (65.5) | 0.358 |
| Length of hospitalization before bacteremia (median [IQR]) (days) | 17 (9–34) | 24 (12–38) | 0.032 |
| Recent stay in ICU | 61 (55.0) | 68 (60.2) | 0.512 |
| Acquired in ICU | 53 (47.7) | 81 (71.7) | <0.001 |
| Comorbid condition | |||
| Charlson comorbidity index (median [IQR]) | 3 (2–5) | 4 (2–5) | 0.584 |
| Type 2 diabetes mellitus | 41 (36.9) | 33 (29.2) | 0.276 |
| Hypertension | 43 (38.7) | 39 (34.5) | 0.605 |
| Cerebrovascular accident | 22 (19.8) | 29 (25.7) | 0.377 |
| Chronic obstructive pulmonary disease | 19 (17.1) | 29 (25.7) | 0.163 |
| Coronary artery disease | 17 (15.3) | 18 (15.9) | 1.000 |
| Congestive heart failure | 15 (13.7) | 20 (17.7) | 0.497 |
| Alcoholism | 8 (7.2) | 7 (6.2) | 0.971 |
| Liver cirrhosis | 11 (9.9) | 7 (6.2) | 0.437 |
| Chronic kidney disease | 37 (33.3) | 41 (36.3) | 0.747 |
| Collagen vascular disease | 7 (6.3) | 5 (4.4) | 0.743 |
| Solid tumor | 35 (31.5) | 23 (20.4) | 0.079 |
| Hematological malignancy | 10 (9.0) | 8 (7.1) | 0.775 |
| Chemotherapy | 15 (13.5) | 7 (6.2) | 0.106 |
| Immunosuppressant therapy | 9 (8.1) | 10 (8.8) | 1.000 |
| Neutropenia | 5 (4.5) | 3 (2.7) | 0.497 |
| Recent surgery | 36 (32.4) | 36 (31.9) | 1.000 |
| Trauma | 2 (1.8) | 5 (4.4) | 0.446 |
| Invasive procedure | |||
| Central venous catheter | 58 (52.3) | 72 (63.7) | 0.109 |
| Endotracheal intubation or tracheostomy | 71 (64.0) | 91 (80.5) | 0.009 |
| Ventilator use | 65 (58.6) | 84 (74.3) | 0.018 |
| Hemodialysis | 11 (9.9) | 18 (15.9) | 0.253 |
| Thoracic drain | 7 (6.3) | 13 (11.5) | 0.259 |
| Abdominal drain | 11 (9.9) | 12 (10.6) | 1.000 |
| Sources of bacteremia | |||
| Pneumonia | 62 (55.9) | 60 (53.1) | 0.779 |
| Catheter | 9 (8.1) | 20 (17.7) | 0.053 |
| Urinary tract infection | 6 (5.4) | 4 (3.5) | 0.537 |
| Intra-abdominal infection | 7 (6.3) | 5 (4.4) | 0.743 |
| Wound | 6 (5.4) | 4 (3.5) | 0.537 |
| Primary bacteremia | 24 (21.6) | 21 (18.6) | 0.689 |
| Previous use of antibiotics | 71 (64.0) | 76 (67.3) | 0.705 |
| Aminoglycoside | 13 (11.7) | 9 (8.0) | 0.473 |
| Penicillin | 5 (4.5) | 1 (0.9) | 0.118 |
| β-lactam–β-lactamase inhibitors | 16 (14.4) | 8 (7.1) | 0.119 |
| Nonantipseudomonal cephalosporins | 14 (12.6) | 20 (17.7) | 0.382 |
| Antipseudomonal cephalosporins | 21 (18.9) | 22 (19.5) | 1.000 |
| Antipseudomonal carbapenems | 11 (9.9) | 25 (22.1) | 0.021 |
| Fluoroquinolone | 12 (10.8) | 15 (13.3) | 0.718 |
| Tigecycline | 2 (1.8) | 4 (3.5) | 0.683 |
| Colistin | 1 (0.9) | 6 (5.3) | 0.119 |
| Macrolide | 2 (1.8) | 5 (4.4) | 0.446 |
| Clindamycin | 9 (8.1) | 0 (0) | 0.002 |
| Vancomycin | 2 (1.8) | 6 (5.3) | 0.280 |
| Teicoplanin | 7 (6.3) | 13 (11.5) | 0.259 |
| Duration of carbapenem therapy (median [IQR]) (days) | 13 (7–16) | 9 (5.5–15) | 0.097 |
| Outcome | |||
| Shock | 42 (37.8) | 38 (33.6) | 0.604 |
| APACHE II score (median [IQR]) | 24 (17–30) | 26 (18.5–31) | 0.172 |
| 14-day mortality | 20 (18.0) | 41 (36.3) | 0.003 |
| 30-day mortality | 28 (25.2) | 60 (53.1) | <0.001 |
| Length of stay after bacteremia for survivors (median [IQR]) (days) | 28 (17–45) | 38 (19.75–64.25) | 0.196 |
| Species causative of bacteremia | |||
| A. baumannii | 50 (45.0) | 71 (62.8) | 0.011 |
| A. nosocomialis | 37 (33.3) | 36 (31.9) | 0.813 |
| A. pittii | 13 (11.7) | 6 (5.3) | 0.139 |
| Microbiological characteristics of causative microorganisms | |||
| Multidrug resistance | 47 (42.3) | 92 (81.4) | <0.001 |
| Isolates harboring genetic structure: | |||
| ISAba1-blaOXA-51-like | 16 (14.4) | 27 (23.9) | 0.103 |
| ISAba1-blaOXA-23-like | 1 (0.9) | 38 (33.6) | <0.001 |
| IS1008 (or IS1006)-ΔISAba3-blaOXA-58-like | 1 (0.9) | 12 (10.6) | 0.005 |
| blaOXA-24-like | 1 (0.9) | 8 (7.1) | 0.035 |
| blaIMP-like | 0 (0) | 5 (4.4) | 0.060 |
| blaVIM-like | 1 (0.9) | 8 (7.1) | 0.035 |
Data presented as number (%), unless otherwise specified. IQR, interquartile range; ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II.
TABLE 2.
Logistic regression analysis of prognostic factors associated with 30-day mortality among patients treated with carbapenem for Acinetobacter bacteremia
| Variable | Survivors (n = 136) | Nonsurvivors (n = 88) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| OR (95% CI)a | P | OR (95% CI)a | P | |||
| Bacteremia due to A. baumannii (no. [%]) | 66 (48.5) | 55 (62.5) | 1.768 (1.023–3.055) | 0.041 | ||
| Shock (no. [%]) | 32 (23.5) | 48 (54.5) | 3.900 (2.190–6.945) | <0.001 | 4.180 (2.173–8.040) | 0.003 |
| APACHE II score (median [IQR])b | 23 (17–28) | 27.5 (21–34) | 1.071 (1.036–1.107) | <0.001 | 1.055 (1.019–1.093) | <0.001 |
| Acquisition of isolates with MIC of ≥8 mg/liter (no. [%]) | 53 (39.0) | 60 (68.2) | 3.356 (1.906–5.908) | <0.001 | 4.218 (2.213–8.039) | <0.001 |
OR, odds ratio; CI, confidence interval.
APACHE II, Acute Physiology and Chronic Health Evaluation II.
Patients who acquired isolates with an MIC of ≥8 mg/liter were more likely to have acquired the isolate in the intensive care unit (ICU), had a longer length of hospitalization prior to bacteremia, had endotracheal intubation or tracheostomy and ventilator use at the onset of bacteremia, and had prior use of antipseudomonal carbapenems in the univariate analysis (Table 1). The risk factors that independently predicted the acquisition of Acinetobacter isolates with a carbapenem MIC of ≥8 mg/liter included the acquisition of an Acinetobacter isolate in an ICU (OR, 2.305; 95% CI, 1.295 to 4.103; P = 0.005), bacteremia caused by A. baumannii (OR, 2.194; 95% CI, 1.243 to 3.873; P = 0.007), and previous use of antipseudomonal carbapenems (OR, 2.331; 95% CI, 1.027 to 5.290; P = 0.043) (Table 3).
TABLE 3.
Risk factors associated with acquisition of Acinetobacter isolates with carbapenem MIC of ≥8 mg/liter
| Variablea | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI)b | P | OR (95% CI)b | P | |
| Acquired in ICU | 2.770 (1.593–4.817) | <0.001 | 2.305 (1.295–4.103) | 0.005 |
| Previous use of antipseudomonal carbapenems | 2.583 (1.202–5.549) | 0.015 | 2.331 (1.027–5.290) | 0.043 |
| Solid tumor | 0.555 (0.302–1.020) | 0.058 | ||
| Presence of endotracheal tube or tracheostomy | 2.330 (1.272–4.271) | 0.006 | ||
| Ventilator use | 2.050 (1.163–3.612) | 0.013 | ||
| Catheter as source of bacteremia | 2.437 (1.057–5.620) | 0.037 | ||
| Bacteremia caused by A. baumannii | 2.062 (1.209–3.519) | 0.008 | 2.194 (1.243–3.873) | 0.007 |
ICU, intensive care unit.
OR, odds ratio; CI, confidence interval.
Bloodstream isolates with a carbapenem MIC of ≥8 mg/liter had a significantly higher rate of multidrug resistance than those with a carbapenem MIC of ≤4 mg/liter and were more likely to carry the carbapenemase gene-associated genetic structures, such as the ISAba1-blaOXA-23-like, IS1008 (or IS1006)-ΔISAba3-blaOXA-58-like, blaIMP-like, and blaVIM-like structures (Table 1). Among the 113 isolates with a carbapenem MIC of ≥8 mg/liter, the carbapenemase genes (and associated insertion sequences) associated with carbapenem resistance were detected in 98 (86.0%) isolates (Table 1). Fourteen isolates with a carbapenem MIC of ≥8 mg/liter carried either a blaOXA-23-like gene without upstream ISAba1 or blaOXA-58-like gene with upstream ISAba3 without IS1008 or IS1006 truncation. Only one isolate with a carbapenem MIC of ≥8 mg/liter did not carry any currently known carbapenemase gene. Carbapenemase genes and associated upstream insertion sequences carried by Acinetobacter isolates with different carbapenem MICs are shown in Table 4. Nine (9/32 [28.1%]) and 7 (7/17 [41.2%]) isolates with carbapenem MICs of 2 and 4 mg/liter carried carbapenem resistance-associated genetic structures, respectively. These genetic structures were observed in 16 (16/21 [76.2%]) and 32 (32/35 [91.4%]) isolates with carbapenem MICs of 8 and 16 mg/liter, respectively. The 20 patients who acquired isolates that exhibited carbapenem MICs of ≤4 mg/liter and harbored a carbapenem resistance-associated carbapenemase gene had 30-day mortality similar to that of the 91 patients who acquired isolates that exhibited carbapenem MICs of ≤4 mg/liter and did not harbor carbapenem resistance-associated carbapenemase genes (25.0% [5/20] versus 24.2% [22/91], respectively; P = 1.000); however, the group of 20 patients had significantly lower 30-day mortality than the 113 patients who acquired isolates with carbapenem MICs of ≥8 mg/liter (25.0% [5/20] versus 53.1% [60/113], respectively; P = 0.038).
TABLE 4.
Carbapenemase genes and associated insertion sequences carried by Acinetobacter isolates with different carbapenem MICs
| Genetic structure harbored by isolates | n | 30-day mortality (%) | No. of isolates with carbapenem MIC (mg/liter): |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | |||
| ISAba1-blaOXA-51-like | 43 | 37.2 | 0 | 2 | 8 | 6 | 5 | 5 | 12 | 5 |
| ISAba1-blaOXA-23-like | 39 | 59.0 | 1 | 0 | 0 | 0 | 3 | 15 | 17 | 3 |
| IS1008 (or IS1006)-ΔISAba3-blaOXA-58-like | 13 | 69.2 | 1 | 0 | 0 | 0 | 7 | 5 | 0 | 0 |
| blaOXA-24-like | 9 | 55.6 | 0 | 0 | 1 | 0 | 0 | 2 | 3 | 3 |
| blaIMP-like | 5 | 40.0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 |
| blaVIM-like | 9 | 44.4 | 0 | 0 | 0 | 1 | 1 | 2 | 4 | 1 |
| Total | 118 | 50.0 | 2 | 2 | 9 | 7 | 16 | 32 | 38 | 12 |
To ensure that the results of this study were not driven by the results of the previous single-center study (13), we carried out a sensitivity analysis that excluded the 31 overlapping patients between the two studies. The clinical data of the 193 patients finally included in the sensitivity analysis were analyzed in the same manner as the above-mentioned analysis. The results were similar to the original results (data not shown). In addition, we performed a subgroup analysis of patients with A. baumannii bacteremia (121 patients). The 30-day mortality was significantly higher in patients who had isolates of A. baumannii with carbapenem MICs of ≥8 mg/liter than in those who had isolates with carbapenem MICs of ≤4 mg/liter, as shown by bivariate analysis (54.9% [39/71] versus 32.0% [16/50], respectively; P = 0.021) and survival analysis by the log rank test (P = 0.019). Carbapenem treatment for A. baumannii bacteremia caused by isolates with carbapenem MICs of ≥8 mg/liter was an independent predictor of 30-day mortality (OR, 4.468; 95% CI, 1.783 to 11.198; P = 0.001).
DISCUSSION
This multicenter center study was designed to assess the different carbapenem breakpoints for Acinetobacter spp. set by CLSI and EUCAST and the potential clinical efficacy of the intermediate category of an MIC of 4 mg/liter. We included patients who received carbapenem monotherapy for the treatment of Acinetobacter bacteremia and evaluated the clinical outcomes among patient groups acquiring isolates with different carbapenem MICs. We found that patients with Acinetobacter bacteremia had a more favorable outcome when the carbapenem MICs of their isolates were ≤4 mg/liter than those with isolates with MICs of ≥8 mg/liter when treated with a carbapenem. Isolates with carbapenem MICs of ≥8 mg/liter were independently associated with a poor outcome in patients treated with carbapenems for Acinetobacter bacteremia.
The CLSI and EUCAST guidelines classify an MIC of imipenem or meropenem of 8 mg/liter as resistant and intermediate susceptible, respectively. In the current study, we found that the mortality rate did not differ significantly between the patients who acquired Acinetobacter isolates with carbapenem MICs 8 mg/liter and ≥16 mg/liter. Patients who acquired isolates with carbapenem MICs of ≥8 mg/liter had a significantly higher 30-day mortality than those with isolates with MICs of ≤4 mg/liter. After controlling for confounders, including severity of illness indices, acquisition of isolates with an MIC of ≥8 mg/liter remained an independent risk factor associated with 30-day mortality. In addition, most Acinetobacter isolates carrying carbapenem resistance-associated genetic structures exhibit carbapenem MICs of ≥8 mg/liter. These results provide clinical data and genetic resistance mechanisms to support the use of an MIC of ≥8 mg/liter as carbapenem resistant.
An MIC of 4 mg/liter for imipenem or meropenem is considered intermediate susceptibility by both organizations, but the clinical efficacy in this cutoff remains uncertain. A PK/PD simulation from healthy volunteers and PK/PD data from animal models proposed that the susceptibility breakpoints for both imipenem and meropenem for A. baumannii are ≤4 mg/liter (9, 10). PK/PD data from other groups in both critically ill (14) and healthy patients (15) suggested that standard dosing of carbapenems might be suboptimal when the MIC is over 2 mg/liter. Discrepancies between these PK/PD breakpoints may have resulted from variation in patient populations. An MIC of 4 mg/liter was categorized as susceptible in the previous CLSI version. Clinical studies that include significant numbers of cases, wherein the infectious pathogen has an MIC on either side of the epidemiological and PK/PD cutoff values, are valuable to assist in the clinical validation of final breakpoints (4). In the current study, the mortality rate did not differ significantly between the patients who acquired Acinetobacter isolates with carbapenem MICs of ≤2 and 4 mg/liter. Patients who acquired isolates with carbapenem MICs of ≥8 mg/liter and received the standard dose of carbapenem therapy had a significantly higher 30-day mortality than those who acquired isolates with an MIC of 4 mg/liter or ≤4 mg/liter. These findings support the concept that MICs of ≤4 mg/liter should be considered carbapenem susceptible.
In addition to susceptibility testing, PK/PD, and clinical outcome data, resistance markers are also required for the establishment of appropriate breakpoints (4). Among the mechanisms of carbapenem resistance in Acinetobacter spp., the most notable contribution comes from the expression of class D carbapenemases (1, 2). In the current study, we found that PCR detection of the ISAba1-blaOXA-23-like, IS1008 (or IS1006)-ΔISAba3-blaOXA-58-like, blaIMP-like, and blaVIM-like genetic structures can be used as a tool to predict higher carbapenem MIC in an Acinetobacter isolate. However, the Acinetobacter isolates carrying the ISAba1-blaOXA-51-like genetic structure exhibit carbapenem MICs ranging from 1 to ≥64 mg/liter. A possible explanation for this phenomenon is that clinical variants of OXA-51 enzymes exhibit different hydrolytic activities against carbapenems (16, 17). In addition, the contribution of the ISAba1-blaOXA-51-like structure on carbapenem resistance varied, depending on its plasmid or chromosome location (18). The findings that the outcomes of the patients who acquired isolates with carbapenem MICs of ≤4 mg/liter and with a carbapenemase gene were more similar to those who acquired isolates with carbapenem MICs of ≤4 mg/liter and without a carbapenemase gene than those who acquired isolates with carbapenem MICs of ≥8 mg/liter suggest that a high carbapenem MIC, rather than the presence of carbapenemase gene, is associated with an unfavorable outcome in patients with Acinetobacter bacteremia treated with carbapenems.
Early identification of patients who acquired isolates with a carbapenem MIC of ≥8 mg/liter is important, because carbapenem therapy at standard doses is likely to fail. In addition to PCR detection of the genetic structures associated with carbapenem resistance, the multiplex PCR assay that identifies A. baumannii (19) can also be used as a tool to predict higher carbapenem MICs. Medical centers that are unable to perform PCR for Acinetobacter can use acquisition in an ICU and prior use of antipseudomonal carbapenems as independent risk factors to consider these isolates to have carbapenem MICs of ≥8 mg/liter.
In the current study, we included only those patients with Acinetobacter bacteremia who received the standard dose of carbapenem therapy. Maximizing carbapenem dosing or prolonging infusion may be associated with better patient outcomes, since these strategies have improved the probability of attaining pharmacodynamic targets (3, 20, 21). Further studies are needed to evaluate the effect of various carbapenem dosing strategies on the clinical outcome of patients with Acinetobacter infections.
The major limitations of this study are its retrospective design, intrinsic selection bias, and strict inclusion criteria. The strengths of this study are the inclusion of a large number of patients from multiple medical centers located in representative regions of Taiwan, recent isolates, and detailed characterization of resistance markers among isolates with various carbapenem susceptibilities. Our findings are consistent with the major findings of a previous study (13). The sensitivity analysis excluding the overlapping cases between the previous single-center study and the current multicenter study yielded similar results, which adds credence to the robustness of our results.
In conclusion, patients with Acinetobacter bacteremia and treated with a carbapenem had a more favorable outcome when the carbapenem MICs of the isolates were ≤4 mg/liter than those with an isolate with an MIC of ≥8 mg/liter.
MATERIALS AND METHODS
Study design and patient population.
This retrospective cohort study was conducted at 4 medical centers located in different parts of Taiwan, including (in alphabetical order): Changhua Christian Hospital (CCH; 1,676 beds) in central Taiwan, Mackay Memorial Hospital (MMH; 2,055 beds) in northern Taiwan, Taipei Veterans General Hospital (TVGH; 2,900 beds) in northern Taiwan, and Tri-Service General Hospital (TSGH; 1,712 beds) of the National Defense Medical Center in northern Taiwan. Adult patients age >20 years with Acinetobacter bacteremia between January 2011 and December 2015 were identified from microbiological records. Patients who had monomicrobial growth of Acinetobacter spp. in blood cultures and had received either imipenem or meropenem as initial monotherapy within 24 h of the onset bacteremia for a minimum of 24 h were included in the study. For patients with creatinine clearance (CLCR) of >50 ml/min/1.73 m2, 500 mg of imipenem was administered intravenously every 6 h, and 1 g of meropenem was administered every 8 h. For patients with CLCR of 10 to 50 ml/min/1.73 m2, 250 mg of imipenem was administered every 8 h. For patients with CLCR of 10 to 25 or 25 to 50 ml/min/1.73 m2, 500 mg or 1 g of meropenem was administered every 12 h, respectively. For patients with CLCR of <10 ml/min/1.73 m2, 250 mg of imipenem was administered every 12 h, and 500 mg of meropenem was administered every 24 h. Imipenem/meropenem was normally infused for 30 to 60 min, and a uniform dose/infusion strategy was used across all sites. Patients who received inappropriate dosages of carbapenem for end organ function, and those with incomplete medical records were excluded. The institutional review board (IRB) of each hospital approved the protocol (CCH, IRB no. 140514; MMH, IRB no. 14MMHIS125; TVGH, IRB no. 2014-07-006CC; TSGH, IRB no. 1-103-05-100).
Data collection and definition.
The medical records of the patients were retrospectively reviewed and analyzed. Patients were assessed for demographic characteristics, duration of hospital and ICU stays, comorbidities, the presence of tubes, lines, and drainage catheters at the time of bacteremia onset, and time of receipt, dose, and route of therapy with individual antimicrobial drugs. An episode of Acinetobacter bacteremia was defined as isolation of an Acinetobacter sp. from a blood culture on 1 or more occasions and clinical manifestations compatible with sepsis syndrome. The onset of bacteremia was defined as the day the blood culture was obtained that eventually yielded Acinetobacter species. Recent stay in an ICU was defined as within 2 weeks of the first positive blood culture. Episodes of bloodstream infection were considered acquired in the ICU if they occurred 48 h after ICU admission. Chemotherapy was defined as receipt of cytotoxic agents within 6 weeks before the onset of bacteremia. Immunosuppressive therapy was defined as receipt of an immunosuppressive agent within 2 weeks or corticosteroid use at a dosage equivalent to or higher than 15 mg of prednisolone daily for 1 week within 4 weeks prior to the onset of bacteremia. Recent surgery was defined as an operation performed within 4 weeks before the onset of bacteremia. The source of bacteremia was determined according to the definitions of the U.S. Centers for Disease Control and Prevention (22). Prior use of antimicrobial agents was defined as treatment with these drugs within 30 days prior to the date of onset of bacteremia. The severity of the infection was evaluated using the Acute Physiology and Chronic Health Evaluation (APACHE) II score within 24 h before the onset of bacteremia. The all-cause 30-day mortality rate was used as the endpoint; it was defined as death occurring within 30 days after the date of onset of bacteremia. No follow-up was done after hospital discharge unless the discharge occurred before the 30-day time limit. For those who were discharged before the 30-day limit, their status was determined by directly contacting the patient or by review of their outpatient records; no patients in this group were lost to follow-up.
Laboratory investigations.
The initial isolate was used for the microbiological studies. The bacteria were phenotypically identified as Acinetobacter spp. using the Vitek 2 system (bioMérieux, Marcy l'Etoile, France). Acinetobacter baumannii was identified by a multiplex PCR method (19). Isolates recognized as non-baumannii Acinetobacter spp. were identified to the genomic species level by 16S-23S ribosomal DNA intergenic spacer sequence analysis, as previously described (23). The clonality was determined by pulsed-field gel electrophoresis, as previously described (24). MICs of carbapenems and antimicrobial susceptibilities of other agents were determined by agar dilution, in accordance with the recommendations of the CLSI (5). Multidrug resistance (MDR) was defined as resistance to any agent in at least three of the following classes of antimicrobials: aminoglycosides, antipseudomonal carbapenems, antipseudomonal cephalosporins, β-lactam–β-lactamase inhibitor combinations, and fluoroquinolones.
Multiplex PCR assays were performed to detect the carbapenem-hydrolyzing class D β-lactamase (CHDL) genes (blaOXA-23-like, blaOXA-24-like, blaOXA-51-like, blaOXA-58-like, and blaOXA-143-like genes) and metallo-β-lactamase genes (25). The upstream locations of insertion sequences (ISs) ISAba1 of the blaOXA-51-like or blaOXA-23-like gene and the IS1008 or IS1006 upstream of the blaOXA-58-like gene were determined by PCR mapping (18, 25–28).
Statistical analysis.
Data were analyzed using the SPSS software for Windows, version 22.0. A χ2 test or Fisher's exact test was used to compare categorical differences. Continuous variables were analyzed using the Mann-Whitney U test or two-sample t test. The time to mortality was analyzed using the Kaplan-Meier curve and log rank test. Logistic regression models were used to explore independent predictors for mortality. Variables with a P value of <0.10, as determined using univariate analysis, were included in a multiple conditional logistic regression analysis. Classification and regression tree (CART) modeling was utilized to define a split in the carbapenem MIC distribution that maximized the difference in mortality. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We express appreciation to Calvin M. Kunin for his critical review of the manuscript.
This work was supported by grants from Taipei Veterans General Hospital (grants V105B-005 and V106B-002), the Tri-Service General Hospital (grants TSGH-C105-112, TSGH-105-113, and TSGH-C106-096), the National Defense Medical Center (grants MAB-106-076 and MAB-106-098), and the Ministry of Science and Technology (grants MOST 104-2314-B-075-043-MY3, MOST 105-2314-B-016-039-MY3, and MOST 105-2628-B-016-003-MY2).
We declare no relevant conflicts of interest related to this article.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00661-17.
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med 36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin Infect Dis 51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 4.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI. 2017. Performance standards for antimicrobial susceptibility testing; 27th informational supplement. CLSI document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.EUCAST. 2017. Breakpoint tables for interpretation of MICs and zone diameters. Version 7.0. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.0_Breakpoint_Tables.pdf. [Google Scholar]
- 7.CLSI. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Dalhoff A, Ambrose PG, Mouton JW. 2009. A long journey from minimum inhibitory concentration testing to clinically predictive breakpoints: deterministic and probabilistic approaches in deriving breakpoints. Infection 37:296–305. doi: 10.1007/s15010-009-7108-9. [DOI] [PubMed] [Google Scholar]
- 9.Frei CR, Wiederhold NP, Burgess DS. 2008. Antimicrobial breakpoints for Gram-negative aerobic bacteria based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. J Antimicrob Chemother 61:621–628. doi: 10.1093/jac/dkm536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macvane SH, Crandon JL, Nicolau DP. 2014. Characterizing in vivo pharmacodynamics of carbapenems against Acinetobacter baumannii in a murine thigh infection model to support breakpoint determinations. Antimicrob Agents Chemother 58:599–601. doi: 10.1128/AAC.02029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeRyke CA, Kuti JL, Nicolau DP. 2007. Reevaluation of current susceptibility breakpoints for Gram-negative rods based on pharmacodynamic assessment. Diagn Microbiol Infect Dis 58:337–344. doi: 10.1016/j.diagmicrobio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Koomanachai P, Bulik CC, Kuti JL, Nicolau DP. 2010. Pharmacodynamic modeling of intravenous antibiotics against Gram-negative bacteria collected in the United States. Clin Ther 32:766–779. doi: 10.1016/j.clinthera.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Lee YT, Chiang MC, Kuo SC, Wang YC, Lee IH, Chen TL, Yang YS. 2016. Carbapenem breakpoints for Acinetobacter baumannii group: supporting clinical outcome data from patients with bacteremia. PLoS One 11:e0163271. doi: 10.1371/journal.pone.0163271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaruratanasirikul S, Limapichat T, Jullangkoon M, Aeinlang N, Ingviya N, Wongpoowarak W. 2011. Pharmacodynamics of meropenem in critically ill patients with febrile neutropenia and bacteraemia. Int J Antimicrob Agents 38:231–236. doi: 10.1016/j.ijantimicag.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Lee LS, Kinzig-Schippers M, Nafziger AN, Ma L, Sorgel F, Jones RN, Drusano GL, Bertino JS Jr. 2010. Comparison of 30-min and 3-h infusion regimens for imipenem/cilastatin and for meropenem evaluated by Monte Carlo simulation. Diagn Microbiol Infect Dis 68:251–258. doi: 10.1016/j.diagmicrobio.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Schroder EC, Klamer ZL, Saral A, Sugg KA, June CM, Wymore T, Szarecka A, Leonard DA. 2016. Clinical variants of the native class D beta-lactamase of Acinetobacter baumannii pose an emerging threat through increased hydrolytic activity against carbapenems. Antimicrob Agents Chemother 60:6155–6164. doi: 10.1128/AAC.01277-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell JM, Leonard DA. 2014. Common clinical substitutions enhance the carbapenemase activity of OXA-51-like class D beta-lactamases from Acinetobacter spp. Antimicrob Agents Chemother 58:7015–7016. doi: 10.1128/AAC.03651-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TL, Lee YT, Kuo SC, Hsueh PR, Chang FY, Siu LK, Ko WC, Fung CP. 2010. Emergence and distribution of plasmids bearing the blaOXA-51-like gene with an upstream ISAba1 in carbapenem-resistant Acinetobacter baumannii isolates in Taiwan. Antimicrob Agents Chemother 54:4575–4581. doi: 10.1128/AAC.00764-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen TL, Siu LK, Wu RC, Shaio MF, Huang LY, Fung CP, Lee CM, Cho WL. 2007. Comparison of one-tube multiplex PCR, automated ribotyping and intergenic spacer (ITS) sequencing for rapid identification of Acinetobacter baumannii. Clin Microbiol Infect 13:801–806. doi: 10.1111/j.1469-0691.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Aziz MH, Lipman J, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Dulhunty J, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Roberts JA, DALI Study Group. 2016. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J Antimicrob Chemother 71:196–207. doi: 10.1093/jac/dkv288. [DOI] [PubMed] [Google Scholar]
- 21.Kongthavonsakul K, Lucksiri A, Eakanunkul S, Roongjang S, Issaranggoon Na Ayuthaya S, Oberdorfer P. 2016. Pharmacokinetics and pharmacodynamics of meropenem in children with severe infection. Int J Antimicrob Agents 48:151–157. doi: 10.1016/j.ijantimicag.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 23.Chang HC, Wei YF, Dijkshoorn L, Vaneechoutte M, Tang CT, Chang TC. 2005. Species-level identification of isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex by sequence analysis of the 16S-23S rRNA gene spacer region. J Clin Microbiol 43:1632–1639. doi: 10.1128/JCM.43.4.1632-1639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LY, Chen TL, Lu PL, Tsai CA, Cho WL, Chang FY, Fung CP, Siu LK. 2008. Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin Microbiol Infect 14:1010–1019. doi: 10.1111/j.1469-0691.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee YT, Huang LY, Chiang DH, Chen CP, Chen TL, Wang FD, Fung CP, Siu LK, Cho WL. 2009. Differences in phenotypic and genotypic characteristics among imipenem-non-susceptible Acinetobacter isolates belonging to different genomic species in Taiwan. Int J Antimicrob Agents 34:580–584. doi: 10.1016/j.ijantimicag.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Lee YT, Turton JF, Chen TL, Wu RC, Chang WC, Fung CP, Chen CP, Cho WL, Huang LY, Siu LK. 2009. First identification of blaOXA-51-like in non-baumannii Acinetobacter spp. J Chemother 21:514–520. doi: 10.1179/joc.2009.21.5.514. [DOI] [PubMed] [Google Scholar]
- 27.Chen TL, Chang WC, Kuo SC, Lee YT, Chen CP, Siu LK, Cho WL, Fung CP. 2010. Contribution of a plasmid-borne blaOXA-58 gene with its hybrid promoter provided by IS1006 and an ISAba3-like element to beta-lactam resistance in Acinetobacter genomic species 13TU. Antimicrob Agents Chemother 54:3107–3112. doi: 10.1128/AAC.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL. 2008. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob Agents Chemother 52:2573–2580. doi: 10.1128/AAC.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


