ABSTRACT
Open-access drug discovery provides a substantial resource for diseases primarily affecting the poor and disadvantaged. The open-access Pathogen Box collection is comprised of compounds with demonstrated biological activity against specific pathogenic organisms. The supply of this resource by the Medicines for Malaria Venture has the potential to provide new chemical starting points for a number of tropical and neglected diseases, through repurposing of these compounds for use in drug discovery campaigns for these additional pathogens. We tested the Pathogen Box against kinetoplastid parasites and malaria life cycle stages in vitro. Consequently, chemical starting points for malaria, human African trypanosomiasis, Chagas disease, and leishmaniasis drug discovery efforts have been identified. Inclusive of this in vitro biological evaluation, outcomes from extensive literature reviews and database searches are provided. This information encompasses commercial availability, literature reference citations, other aliases and ChEMBL number with associated biological activity, where available. The release of this new data for the Pathogen Box collection into the public domain will aid the open-source model of drug discovery. Importantly, this will provide novel chemical starting points for drug discovery and target identification in tropical disease research.
KEYWORDS: high-throughout screening, in vitro, Leishmania, MMV Pathogen Box, plasmodium, trypanosoma, drug discovery, open-source drug discovery
INTRODUCTION
The face of drug discovery is continually changing and never more so than in the last 5 to 10 years where early-stage drug discovery has transitioned to extend beyond the traditional domain of large pharmaceutical companies. Open drug discovery has evolved as a component of this transition and is a major contributor to combatting diseases primarily affecting the poor through identifying and optimizing potential compounds for a range of pathogens (1–10). The open drug discovery approach can be described by several models as recently defined by Wells et al. (Medicines for Malaria Venture [MMV]) in which malaria drug discovery is discussed in the open arena (11). The success of this approach is not limited to malaria (12–16) and includes a number of diseases, such as tuberculosis (17, 18), schistosomiasis (19–21), diseases caused by kinetoplastids (22), toxoplasmosis (23), and cryptosporidiosis (24); in addition, treatments for diseases caused by other viral and bacterial pathogens have also benefited. The open-access drug discovery model, whereby free access and supply of compounds, such as the MMV Malaria Box and subsequently the MMV Pathogen Box (PBox), is made available to researchers with the condition that data obtained are shared with the wider community, has rapidly taken hold.
The first open-access compound set provided by MMV was the Malaria Box (25). More than 200 research groups were involved in a worldwide collaboration to evaluate these compounds against a diverse array of disease indications, with 236 different in vitro screens performed. The outcome of this consolidated effort is the continuation of approximately 30 projects where compounds were demonstrated to exhibit activity for different pathogens, as well as for cancers (26).
Following the success of the Malaria Box, MMV has provided the PBox (http://www.pathogenbox.org/) as a second open-access compound collection. The 376 compounds selected for the PBox demonstrate activity against a range of different pathogens, predominantly Plasmodium, Mycobacterium, and kinetoplastid parasites (Trypanosoma brucei, Leishmania spp., and Trypanosoma cruzi). In addition, a smaller number of compounds was selected with activity against a range of other pathogens, including Schistosoma, Toxoplasma, Cryptosporidium, helminths, and dengue. Also included within the PBox is a set of 26 reference compounds with activity associated with one or many pathogens. As part of the PBox compound package, MMV has provided the biological activity of compounds from allied screening platforms (ChEMBL-NTD [https://www.ebi.ac.uk/chemblntd]), along with the plate layout and compound details (structure, trivial name, salt form, and cLogP) in the form of an Excel spreadsheet, referred to here as the PBox supporting information.
The first successes from PBox have recently been published with the identification of Tolfenpyrad (MMV688934), a pyrazole-5-carboxamide based insecticide with activity against the helminth, barber's pole worm (27). Second, the demonstration of inhibitory activity against Candida albicans biofilm formation by MMV688768 (an original indication for schistosomiasis) (28).
Our extensive well-established and validated image-based assays for neglected tropical disease drug discovery provided the ideal platform to enable biological profiling and direct comparative analysis of the PBox against a number of pathogens simultaneously. In close collaboration with both MMV and the Drugs for Neglected Diseases Initiative, innovative image-based assays for the following parasites and life cycle stages have been established. Plasmodium falciparum asexual blood stage (ABS) (29) and late-stage gametocyte (LSG) (14) assays supporting numerous hit identification, hit-to-lead, and lead optimization projects have been developed. The development of a 384-well surrogate assay for human African trypanosomiasis (HAT), which utilizes the parasite Trypanosoma brucei brucei (30), was the first of its kind to be published. Image-based assays for both Trypanosoma cruzi (31) and Leishmania donovani provide essential support for compound profiling for hit-to-lead and lead optimization, contributing valuable insights into mechanism of action of these lead molecules. The P. falciparum, T. brucei brucei, and T. cruzi assays are well validated and have been used extensively for a number of years in the identification and characterization of compounds active for these particular pathogens (31–47).
The PBox compounds are each provided as 10 μl of 10 mM dimethyl sulfoxide (DMSO) stock solutions. In order to maximize this resource and ensure reproducible, high-quality data, liquid handling and compound management expertise, in combination with well-validated highly reproducible assay formats, is required. Compounds Australia (48) is a world class compound storage and handling facility, providing compound management research logistics within Australia and internationally to support diverse open and closed drug discovery programs. This enabling infrastructure and expertise allows for small-volume compound handling and therefore the ability to test not only the dose response at concentrations higher than 1 μM (as suggested by MMV) but also against multiple parasites from an initial 10-μl volume.
The compounds within PBox are categorized into disease indication subsets by MMV and partners, where compounds demonstrated a minimum of 5-fold selectivity for the pathogen over mammalian cells (www.pathogenbox.org/about-pathogen-box/supporting-information). Excluding the reference set, 33% of the PBox collection is comprised of compounds with activity against Plasmodium, with another 30% active against tuberculosis and 18% active against kinetoplastid species. The remaining 19% of the compounds are distributed through the other pathogen disease indications. Rather than testing only compounds with no antiplasmodial or antikinetoplastid activity, as defined by the PBox supporting information, all compounds were tested in multiple-parasite assays in order to provide comparative data from a second screening platform versus that originally used to generate the initial PBox biological data. The assays consisted of four high-content-imaging platforms, namely, P. falciparum asexual blood stage (ABS) and late-stage gametocytes (LSG), plus the intracellular forms of the kinetoplastid pathogens T. cruzi and L. donovani, as well as a viability assay for T. brucei brucei utilizing the fluorescent metabolic indicator resazurin.
In order to thoroughly assess the suitability of selectively active compounds as potential chemical starting points for hit-to-lead, lead optimization, or target identification programs, an extensive review of the scientific literature and activity databases was performed and collated with the biological activity data we generated. This combined information has provided the means to select compounds both within their respective disease indication set (especially within the kinetoplastid set) and also within those of other disease indications. By both confirming and identifying potential chemical starting points for drug discovery programs for malaria, HAT, Chagas disease, and leishmaniasis and the publishing of the data generated in free access databases, the open-source model will be greatly advanced by the availability of these data.
We believe that the activity data generated, the information gathered, and the indication of new chemical starting points will ultimately facilitate more successful open-source drug discovery programs fueling the identification of new drug candidates for neglected and tropical diseases worldwide.
RESULTS
The complete screening data set for all five assays is presented in Supplemental File 1, with the data represented as an activity heat map. The details for all compounds, excluding the reference compound set from the PBox supporting information Excel file, are aligned and merged with the biological activity obtained from the evaluation undertaken within Discovery Biology. This in turn was aligned with the biological activity of the kinetoplastid, Plasmodium, and HepG2 activity data provided within the PBox supporting information file.
The primary screening data for each Discovery Biology assay was used to color code activity and subsequently applied to both PBox data and Discovery Biology IC50s (see Supplemental File 1; the second tab contains the color code for the activity heat map). Evaluation of the compounds for their suitability for repurposing or for progression into hit-to-lead or target identification studies was performed using searches on ChEMBL and PubMed, along with SciFinder and Chemspider. All information gathered, including literature citations, alternative aliases, ChEMBL numbers, ChEMBL biological activity data (in the case of malaria), and commercial availabilities (Table 1) is incorporated into Dataset S1 in the supplemental material.
TABLE 1.
Percentages of compounds for each pathogen for which a commercial supply of the compound was identified
| Pathogen set | No. of compounds |
% with a commercial supplier |
|
|---|---|---|---|
| In PBox | Commercial supplier identified | ||
| Cryptosporidiosis | 13 | 2 | 13 |
| Dengue | 5 | 0 | 0 |
| Hookworm | 1 | 1 | 100 |
| Kinetoplastids | 70 | 25 | 36 |
| Lymphatic filariasis | 3 | 1 | 34 |
| Malaria | 125 | 84 | 67 |
| Onchocerciasis | 11 | 3 | 27 |
| Schistosomiasis | 13 | 9 | 69 |
| Toxoplasmosis | 15 | 2 | 13 |
| Trichuriasis | 1 | 0 | 0 |
| Tuberculosis | 116 | 83 | 71 |
| Wolbachia LF | 3 | 0 | 0 |
Commercial supplier.
Using the SMILE string from the PBox information file, we performed as search for chemical suppliers for all of the compounds within PBox, primarily with Chemspider and SciFinder (http://www.cas.org/products/scifinder). Supplier availability is presented as a percentage of the total number of compounds per disease set (Table 1).
Reference compound set.
The PBox contains 26 compounds used as internal references for evaluations. The reference compound set was tested directly in an 11-point dose-response format used to calculate the 50% inhibitory concentrations (IC50s), as described in Supplemental File 2. The data are presented in Table 2. The compounds were tested twice in the L. donovani assay due to insolubility problems with certain active compounds, such as amphotericin B, miltefosine, and buparvaquone. The activities of many of these compounds against Plasmodium and kinetoplastid parasites have been published previously (49, 50).
TABLE 2.
Reference compound IC50 data
| Compound | IC50 (μM)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
P. falciparum |
Mammalian cell cytotoxicity |
T. cruzi |
T. brucei brucei |
L. donovani |
|||||
| 3D7 ABS | NF54 LSG | HEK293 | Intracellular amastigotes | 3T3 host cells | Emax | Trypomastigotes | n = 1 | n = 2 | |
| Doxycycline | 90% | 77% | IA | IA | IA | IA | IA | ||
| Mefloquine | <0.016 | 5.61 | 4.7 | IA | 99 | 0.33 | 5.17 | 6.58 | |
| Primaquine | 91% | 96% | 94% | 73% | 91% | 33% | 79% | ||
| Pentamidine | 0.01 | 1.43 | 75% | IA | IA | <0.02D | IA | IA | |
| Sitamaquine | 2.03 | 0.982 | 100% | IA | IA | 91% | IA | IA | |
| Nifurtimox | IA | 39% | 1.42A | IA | 100 | 5.38 | IA | IA | |
| α-Difluoromethylornithine | IA | IA | IA | IA | IA | IA | IA | ||
| Praziquantel | IA | IA | IA | IA | IA | IA | IA | ||
| Diethylcarbamazine | IA | IA | IA | IA | IA | IA | IA | ||
| Mebendazole | IA | IA | IA | IA | IA | IA | IA | ||
| Suramin | IA | IA | IA | IA | 0.11 | IA | IA | ||
| Amikacin | IA | IA | IA | IA | IA | IA | IA | ||
| Levofloxacin (–)-ofloxacin | IA | IA | IA | IA | IA | IA | IA | ||
| Clofazimine | 84% | IA | IA | IA | IA | IA | IA | ||
| Ethambutol | IA | 42% | IA | IA | IA | IA | IA | ||
| Linezolid | IA | IA | IA | IA | IA | IA | IA | ||
| Benznidazole | IA | IA | 94% | IA | IA | IA | IA | ||
| Posaconazole | 4.1 | 63% | 0.172B | IA | 73 | IA | 64% | 60% | |
| Rifampin | 2.23 | 78% | IA | IA | IA | IA | IA | IA | |
| Auranofin | 0.70 | 0.55 | 2.20 | 3.02 | 4.90 | 0.24 | 0.63 | 2.00 | |
| Miltefosine | IA | IA | IA | IA | IA | 11.69 | 2.26 | ||
| Nitazoxanide | IA | IA | IA | IA | 41% | 44% | 41% | ||
| Streptomycin | IA | 41% | IA | IA | IA | IA | IA | ||
| Amphotericin B | 87% | IA | IA | IA | IA | 100%C | IAC | ||
| Buparvaquone | 0.14 | 5.3 | IA | 4.92 | IA | 98.5 | IA | 1.88 | 60% |
| Bedaquiline | 91% | 75% | IA | IA | 59% | IA | IA | ||
The activities of all 26 compounds are presented for all assays plus HEK cytotoxicity. IA, inactive at the top screening dose (20 μM for P. falciparum, L. donovani, and T. brucei brucei; 16.4 μM for T. cruzi). Percentage values, where specified in the body of the table, indicate the percent inhibition at the top screening dose (20 μM for P. falciparum, L. donovani, and T. brucei brucei; 16.4 μM for T. cruzi). Standard in-house assay controls are indicated by superscript capital letters: A, 1.23 μM; B, 0.001 μM; C, 0.41 and 0.30 μM; D, <0.001 μM. Emax is the maximal % inhibition plateau generated in compound dose response curve analysis.
Whereas one of the replicates for amphotericin B contained within the PBox compound set was not active against L. donovani when tested at 20 μM, the amphotericin used as an in-house control in the same assay was active, as expected. Given the poor solubility of this compound in water, which was used to generate an intermediate dilution plate, it is possible that at high concentrations the compound did not solubilize completely. Pentamidine received in the PBox compound collection also did not show activity at 20 μM against L. donovani. It is reported that this drug reduces the number of parasites per macrophage in in vitro experiments but not the number of infected macrophages as assessed here (51).
Evaluation of individual parasite selectivity for the kinetoplastid disease indication compound set.
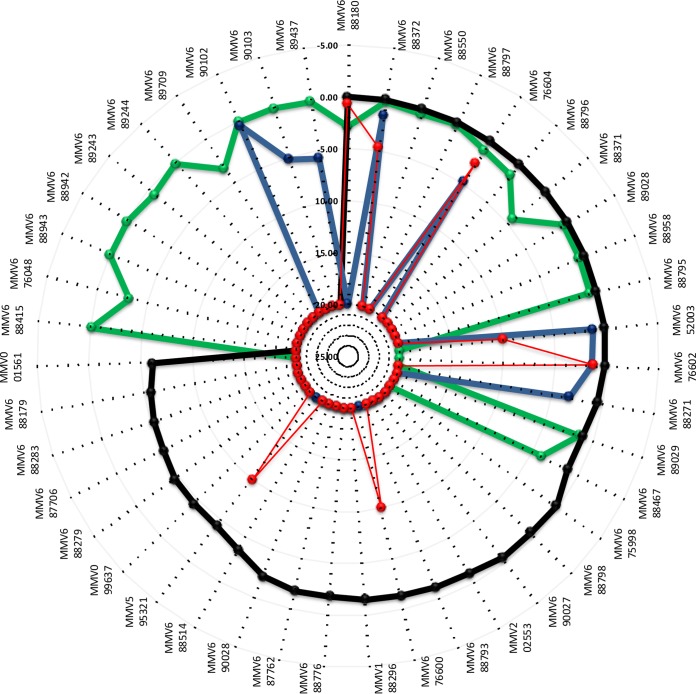
Seventy compounds within the PBox have demonstrated published activity against L. donovani, T. brucei brucei, and/or T. cruzi or various dually active combinations and are clustered within the single kinetoplastid set within the PBox supporting information. A large number of these compounds had no cytotoxicity data provided within the PBox supporting information. In order to classify compounds as individually parasite selective, all compounds demonstrating activity within the Discovery Biology kinetoplastid assays, namely, 43 of the 70, were also evaluated for cytotoxicity using the HEK293 cell line. Selectivity of >5-fold (as used by MMV) for the parasite over HEK293 cytotoxicity was used to determine whether compound activity was parasite selective. A summary of the activity data is provided in Fig. 1.
FIG 1.
Radar activity plot of IC50s (μM) for MMV Pathogen Box compounds: T. brucei brucei (black), T. cruzi (green), L. donovani (blue), and HEK293 mammalian cell cytotoxicity (red). The activity scale is IC50s in μM.
Three-quarters of the kinetoplastid compounds identified as active by the Discovery Biology assays were active against T. brucei brucei, with approximately one-quarter also active against T. cruzi and only approximately one-fifth active against L. donovani. Three of the eight compounds that displayed activity against L. donovani also exhibited similar activity in the HEK293 cytotoxicity assay and were therefore classified as not selective.
Comparison between Discovery Biology and PBox data sets for kinetoplastid and Plasmodium disease indication compounds.
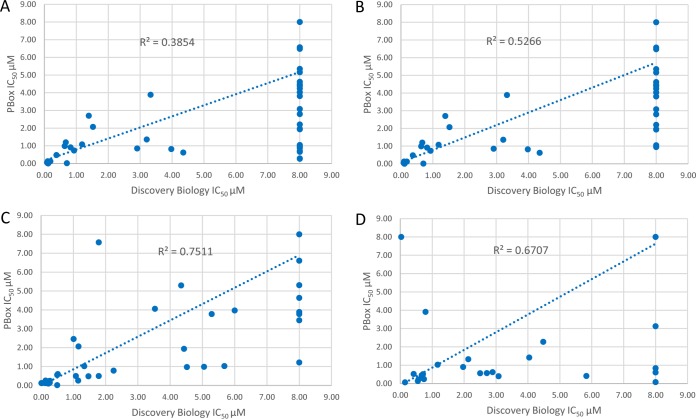
To accommodate the screening of all pathogens from the same compound set provided, 5 mM concentrations of compound stocks were prepared. Thus, due to compound limitations, IC50s greater than 8 μM could not be determined since the maxiumum effect (Emax) values were not obtainable. To perform a comparison with the PBox activity data, all compounds with activity greater than 8 μM, for either assay, were assigned an IC50 of 8 μM in order to perform comparisons of the compounds within biologically relevant levels of activity. These data are presented in Fig. 2.
FIG 2.
Correlation of Discovery Biology data for compounds tested against T. cruzi, T. brucei brucei, and P. falciparum gametocyte versus respective PBox activity data provided. (A) T cruzi (all compounds); (B) T. cruzi (5 discrepant compounds were removed); (C) T. brucei brucei; (D) P. falciparum gametocytes.
Of the 70 compounds within the kinetoplastid pathogen set, 65 were compared for T. cruzi activity, and the results are presented in Fig. 2A. Five compounds from the PBox were not tested against T. cruzi in the supporting information file; thus, a comparison could not be made. The activity data comparison for all 65 compounds has an r2 value of 0.385, which is low. However, with the removal of five compounds (MMV652006, MMV688271, MMV688776, MMV688179, and MMV658988) that had no activity at 8 μM in the Discovery Biology assay and <1 μM in the PBox supporting information file, the r2 value increased to 0.527. It would appear that a small number of compounds have different activities between the two assays but that, in general, activity against T. cruzi correlated well with the PBox data (Fig. 2A and B). There were 25 compounds (30%) with demonstrated IC50s against T. cruzi (as high as 25 μM IC50s) in the PBox supporting information file (undertaken at the Laboratory of Microbiology, Parasitology, and Hygiene [LMPH], University of Antwerp) for which an IC50 could not be determined. Eleven of these compounds displayed >50% inhibitory activity at 18.3 μM (top dose tested); however, none showed >50% activity at 9.15 μM (second dose), suggesting theoretical IC50s higher than 8 to 9 μM.
The variance in the activity of these compounds may be attributed to differences in the assay formats. The LMPH assay utilized a C4 β-galactosidase reporter gene-transfected parasite in human lung fibroblast (MRC 5SV2) host cells exposed to compound for 7 days compared to 2 days of exposure for the Discovery Biology assay using 3T3 host cells infected with nontransfected parasites. It is possible that these compounds are either slow acting and therefore not detected in the shorter 48-h exposure assay or the transgenic parasite and MRC5SV2 host cells were contributing factors to differences in compound activity. Also, without details of any corresponding effects on the host cells by the compounds, it is impossible to determine whether parasite selectivity over the MRC5SV2 host cells was impacted upon and thus would be more evident during the longer incubation period.
A correlation of Discovery Biology T. brucei brucei versus PBox T. brucei brucei is presented in Fig. 2C. Sixty-four of the compounds within the kinetoplastid set were available for comparison. There was a high degree of correlation (r2 = 0.751) between the Discovery Biology T. brucei brucei activity compared to PBox (Fig. 2C), particularly for compounds that possess potent activity (IC50 < 2 μM), but a higher degree of deviation was observed for compounds with IC50s of >4 μM.
The PBox contains 125 compounds with antiplasmodial activity. For 91% of these compounds, the P. falciparum ABS and LSG assays from Discovery Biology and the data contained within the PBox supporting information Excel file aligned well (r2 = 0.67 for LSG). The ABS correlation between the two screening platforms cannot be made for IC50s, since these compounds were only taken for IC50 evaluation if the degree of activity at primary testing was considerably different from that provided in the PBox supporting information file. A high degree of correlation was observed between assays with most of the compounds.
A correlation of the Discovery Biology stage IV/V gametocyte assay with the PBox stage V gametocyte assay is presented in Fig. 2D. One hundred and twenty compounds from the 125 malaria compound set were compared. The correlation of activity between the Discovery Biology LSG assay (stage IV) to that of stage V gametocyte data (saponin-lysis sexual stage assay [SaLSSA]) (52) within the PBOX supporting information file indicates good correlation between these two assays (r2 = 0.67). A high level of correlation for the majority of compounds was observed, though five had differing levels of activity between the two assays (MMV023233, MMV023985, MMV1019989, MMV1029203, and MMV1037162). In general, the PBox stage V assay showed higher activity for these compounds than those determined by the Discovery Biology stage IV imaging assay when we tested the compounds the MMV PBox supplied. Seven of the compounds present within PBox with low ABS activity and greater activity against stage V gametocytes (MMV062221, MMV1019989, MMV1029203, MMV1030799, MMV1037162, and MMV1088520) had been previously identified from an HTS campaign of a malaria-naive library of 80,000 compounds performed by Discovery Biology (53). On testing freshly solubilized stocks of MMV1019989 (diaminotriazine), MMV1037162, and MMV1029203 (pyrdiyl thienopyrimidine) it has been noted (S. Duffy, unpublished observations) that the compound-treated assay wells had a glassy and/or shiny appearance and, under light microscopy, red blood cell (RBC) lysis was witnessed in the ABS assay and, to a lesser extent, the gametocyte assays at screening concentrations of ≥2 μM. Images of artemisinin-treated wells compared to MMV1037162-treated wells are presented in Supplemental File 2, which shows the destruction of the RBCs at the end of the 72-h assay incubation. The activity of these three compounds was found to be highly variable upon repeat testing by the Discovery Biology imaging assays (Duffy, unpublished).
MMV009135 has no activity data within the PBox supporting information file. This compound is the regioisomer of the compound initially identified as active (54, 55) and did not show any activity within the Discovery Biology antiplasmodial assays. MMV016838 also has no activity data within the PBox supporting information file, but the activity is indicated as “known to be active” (ktba) against both gametocytes and liver-stage in vitro assays. However, this compound was found not to be active in either the Discovery Biology ABS or gametocyte assays. MMV676270 also demonstrated no activity against either the ABS or LSG Discovery Biology assays in comparison to literature ABS activity data (0.99 μM) (54). The PBox supporting information file has no activity provided for direct comparison of this batch of compound supplied. MMV011229, according to the PBox supporting information, is ABS active (0.38 μM) and reported as “ktba” for gametocytes and the liver stage. However, although the ABS data are in agreement, the stage IV Discovery Biology gametocyte assay did not find this compound to be active. MMV006901 had ABS activity confirmed with that from the PBox supporting information, but the Discovery Biology gametocyte assay found this compound to be only moderately active against stage IV gametocytes at primary testing, and thus an IC50 was not determined. Nine compounds (MMV007133, MMV007625, MMV007920, MMV019742, MMV020291, MMV02388, MMV676358, MMV676442, and MMV687794) demonstrated variable LSG activity to that contained within the PBox supporting information file. Overall, however, the Plasmodium activity data were generally consistent between Discovery Biology and the PBox.
It should also be noted that aspects other than assay technology come into play in these multicenter compound screening approaches. Assay medium composition and protein binding of the compounds may also affect the activity of some compounds more than others (25). Compound solubility, stability in DMSO solution over time, plate storage conditions, and dilution protocols can all contribute to variances in activities between screening platforms and between laboratories.
New and novel parasite biological activity for potential repurposing of compounds.
Table 3 lists 13 compounds from the other disease indication sets with antikinetoplastid activity where biological activity is new to the PBox activity data.
TABLE 3.
Compounds from other disease sets with antikinetoplastid activity
| Compound ID | ChEMBL DB reference | Disease set within pathogen box | New selective indication (PBox) | IC50 (μM) for new indication | Compound class | Predicted cellular target of associated class with new disease indication | New disease reference | Mammalian cellular target of associated class | Reference for mammalian cellular target of associated class |
|---|---|---|---|---|---|---|---|---|---|
| MMV687776 | Lymphatic filariasis | T. cruzi | 2.39 | Benzoxaborole warhead | No hits | 57, 58 | |||
| MMV022478 | 54 | Malaria | T. brucei brucei | 1.45 | Pyrazolo[1,5-a]pyrimidine | No hits | No hits | NADPH oxidase 4 | 56 |
| MMV022029 | 54 | Malaria | T. brucei brucei | 2.38 | Biaryl sulfonamide | No hits | No hits | No hits | |
| MMV028694 | 54 | Malaria | T. brucei brucei | 2.39 | 2,4-Disbustituted pyrimidine | No hits | No hits | No hits | |
| MMV006901 | 54, 55 | Malaria | T. brucei brucei | 3.36 | 2,4-Aminoquinoline | No hits | No hits | No hits | |
| MMV010576 | 54 | Malaria | L. donovani | 5.18 | 2-Amino-3,5-diaryl pyridine | No hits | No hits | Kinase | Numerous |
| MMV688768 | Schistosomiasis | T. brucei brucei | 1.50 | 2,3-Disubstituted Indole | No hits | No hits | Alanyl aminopeptidase and dipeptidyl peptidase | 61 | |
| MMV688417 | 68 | Toxoplasmosis | T. cruzi | 1.43 | Pyrazolo[3,4-d]pyrimidin-4-amine | Kinase | 64 | PI3K/AKT/mTOR pathway | Numerous |
| MMV637229 | Trichuriasis | T. cruzi | 1.22 | Diarylmethane ether | No hits | No hits | Antihistamine | 62 | |
| MMV687248 | 18 | Tuberculosis | T. brucei brucei, T. cruzi | 1.05, 4.00 | 3,5-Disubstituted pyridine | No hits | No hits | No hits | |
| MMV687273 | 69–73 | Tuberculosis | T. brucei brucei, L. donovani | 2.28, 3.47 | Acyclic monoterpene | HMG-CoA reductase pathway? | 65 | No hits | |
| MMV153413 | Tuberculosis | T. brucei brucei | 2.99 | Tetrasubstituted thiophene | No hits | No hits | SIRT2 | 61 | |
| MMV021013 | 18 | Tuberculosis | T. cruzi, L. donovani | 1.67, 1.48 | 2-Pyridyl-4-aminopyrimidine | Methionine aminopeptidase | 66, 67 | Methionine aminopeptidase; nuclear factor κB ligand (RANKL) | 62, 63 |
Table 4 lists the 31 compounds identified from screening the PBox against the Discovery Biology antiplasmodial assays which are from other disease indication compound sets. The activity identified is new to the PBox data set and, after chemical class identification and literature review, 10 compounds were identified as having novel activity against P. falciparum.
TABLE 4.
Compounds from “other disease indication” sets demonstrating antiplasmodial activity
| Compound ID | Disease set within PBox | Antiplasmodial IC50 (μM) | Compound class | Predicted cellular target of associated class with new disease indication | New disease reference | Mammalian cellular target of associated class | Reference for mammalian cellular target of associated class | Novel antimalarial activity |
|---|---|---|---|---|---|---|---|---|
| MMV675968 | Cryptosporidiosis | 0.03 (ABS) | Quinazoline-2,4-diamine | Dihydrofolate reductase | 76 | Folate pathway | 74 | No |
| MMV688547 | Kinetoplastids | 1.02 (ABS) | Bisarylamidine | DNA-targeted agent; DNA minor groove binding at AT-rich DNA sequences | 77 | No hits | No | |
| MMV688362 | Kinetoplastids | 0.59 (ABS) | Bisarylamidine | As above | 77 | No hits | No | |
| MMV688179 | Kinetoplastids | 0.59 (ABS) | Bisarylguanidinium | As above | 77 | No hits | No | |
| MMV688271 | Kinetoplastids | 1.21 (ABS) | Bisarylguanidinium | As above | 77 | No hits | No | |
| MMV687749 | Tuberculosis | 2.08 (ABS) | 2-Amino-4-aryl ether pyrimdine | No hits | No hits | Burtons tyrosine kinase | 75 | Yes |
| MMV687765 | Tuberculosis | 2.01 (ABS) | 2-Amino-4-aryl ether pyrimdine | No hits | No hits | Burtons tyrosine kinase | 75 | Yes |
| MMV659004 | Kinetoplastids | 1.86 (ABS) | 2-Pyridyl-4-aminopyrimidine | Methionine aminopeptidase | 66, 67 | Methionine aminopeptidase; nuclear factor kappa-B ligand (RANKL) | 62, 63 | No |
| MMV658988 | Kinetoplastids | 2.18 (ABS) | 2-Pyridyl-4-aminopyrimidine | As above | 66, 67 | Methionine aminopeptidase; nuclear factor κB ligand (RANKL) | 62, 63 | No |
| MMV659010 | Kinetoplastids | 1.71 (GAM) | 2-Pyridyl-4-aminopyrimidine | Methionine aminopeptidase | 66, 67 | Methionine aminopeptidase; nuclear factor κB ligand (RANKL) | 62, 63 | No |
| MMV688122 | Tuberculosis | 2.11 (ABS), 0.11 (GAM) | 2- Pyridyl thieno[3,2]pyrimidine | Methionine aminopeptidase? | 66, 67 | Methionine aminopeptidase; Receptor activator of nuclear factor κB ligand (RANKL)? | 62, 63 | No |
| MMV661713 | Tuberculosis | 1.49 (ABS) | 4-Pyridyl-2-aryl pyrimidine | No hits | No hits | Glutaminyl cyclase | 78 | Yes |
| MMV652003 | Kinetoplastids | 0.92 (ABS) | Benzoxaborole warhead | Leucyl-tRNA synthetase | 79 | No hits | No | |
| MMV021057 | Malaria (GAM) | 0.03 (GAM) | β-Methoxyacrylate analogue (azoxystrobin, a known fungicide) | bc1 complex(?) | 80 | No hits | No | |
| MMV688754 | Kinetoplastids (GAM) | 0.14 (GAM) | Oxime analogue (trifloxystrobin, a known fungicide) | No hits | 80 | No hits | No | |
| MMV671636 | Onchocerciasis | 1.01 (ABS) | Quinolone | Mitochondrial cytochrome bc1 complex | 36 | No hits | No | |
| MMV688279 | Kinetoplastids | 0.32 (ABS), 2.35 (GAM) | Dihydroquinazoline | No hits | 81, 82 | No hits | Yes/no? | |
| MMV687703 | Tuberculosis | 1.33 (ABS), 3.83 (GAM) | 2-Aryl imidazole | No hits | No hits | No hits | Yes | |
| MMV688550 | Kinetoplastids | 2.48 (ABS) | Imidazo[1,2]purine | No hits | No hits | No hits | Yes | |
| MMV688283 | Kinetoplastids | 0.66 (ABS), 4.10 (GAM) | 4-Amino quinoline | No hits β-hematin? | 86 | No hits | No | |
| MMV676383 | Tuberculosis | 4.43 (ABS), 1.37 (GAM) | 2-Substituted benzooxazole | No hits | No hits | No hits | Yes | |
| MMV688703 | Toxoplasmosis | 3.16 (ABS) | Trisubstituted pyrrole | cGMP-dependent protein kinase | 87, 88 | p38 kinase | 83 | No |
| MMV688766 | Schistosomiasis | 0.85 (ABS), 1.31 (GAM) | Trisubstituted isooxazole | No hits | No hits | PPAR agonist (?) | 84 | Yes |
| MMV676388 | Tuberculosis | 4.93 (ABS), 2.99 (GAM) | 5-Sulfonyl tetrazole | No hits | No hits | Thioredoxin reductase | 85 | Yes |
| MMV023969 | Tuberculosis | 0.59 (ABS) | Isoquinoline | No hits | No hits | No hits | No* | |
| MMV024311 | Tuberculosis | 0.75 (ABS) | 2,3-Disubstituted indole | No hits | No hits | No hits | No* | |
| MMV021660 | Tuberculosis | 0.16 (ABS) | 2,4-Diamino pyrimidine | Folate pathway | 81, 89–92 | Too numerous | No |
Twelve compounds from the kinetoplastid disease indication set were identified, with two demonstrating novel antiplasmodial activity: MMV688550 and MMV68827. The tuberculosis disease indication set resulted in the greatest number of novel antiplasmodial active compounds. From 14 compounds identified from the tuberculosis compound set, 7 were deemed as novel for activity against P. falciparum (MMV661713, MMV687703, MMV687273, MMV687749, and MMV687765 having activity less than 2 μM, with MMV676383 and MMV676388 demonstrating only moderate activity between 4 and 5 μM). Although one compound each was identified from four other disease indication sets (cryptosporidiosis, schistosomiasis, toxoplasmosis, and onchocerciasis), only one schistosomiasis compound (MMV688766) demonstrated novel antiplasmodial activity. However, a number of compounds, although not novel in their antiplasmodial activity, do not have a known drug target and therefore provide interesting starting points for target elucidation and validation studies.
DISCUSSION
The PBox presents a significant opportunity for identification of compounds for progression into hit-to-lead and target identification projects for a variety of pathogens. Detailed information regarding the compound suitability for repurposing or for taking forward into hit-to-lead or target identification studies has been gathered. Searches were performed using ChEMBL and Scifinder, and the information gathered was collated, including the kinetoplastid and Plasmodium supporting information from PBox. All the literature search information was incorporated into supporting information (see Dataset S1 in the supplemental material). The third worksheet within the Excel file contains the citations for the pathogen activity present in ChEMBL.
All the assays performed in this evaluation demonstrated good to reasonable (r2 = 0.52 to 0.75) correlations with the data within the PBox supporting information; however, some compounds displayed differences in the activity/inactivity status or the level of activity obtained. The complete set of activity data is presented in the form of a heat map, including IC50s and the level of activity (a heat map color code is presented in Dataset S1 in the supplemental material). This information and alignment of data for the compounds against Plasmodium and the kinetoplastids (T. brucei brucei. L. donovani, and T. cruzi) is a valuable tool for researchers in the field of drug discovery.
Twenty-six reference compounds were also included in PBox, although activity data are not provided within the PBox supporting literature. The data provided here are therefore a valuable reference tool for determining variances within assay performance between laboratories. For projects run across numerous laboratories, it is essential to be able to compare the activity of reference compound sets to monitor assay performance. The activity data for the references are presented in Table 2.
Potential chemical starting points for drug discovery. (i) New and novel compounds identified against kinetoplastids.
Of the compounds not within the Pbox defined kinetoplastid set, 21 were identified with activity against L. donovani, T. cruzi, or T. brucei brucei. However, if the selectivity criteria of >5-fold (as used by MMV for inclusion within PBox) over either HEK293 or HEPG2 is applied, only 13 (57%) of the compounds identified by the Discovery Biology assays displayed selective, antiparasitic activity (Table 3).
Five compounds from the malaria disease indication set were identified as selective: four for T. brucei brucei (MMV022478, MMV022029, MMV028694, and MMV006901, IC50s of 1.45 to 3.36 μM) and one for L. donovani (MMV010576, IC50 of 5.18 μM). The activity for these compounds is novel for T. brucei brucei. A literature search revealed that these active compounds were not associated with a mechanism of action apart from a potential link to trypanothionine reductase for MMV022478. The pyrazolo[1,5-a]pyrimidine class, to which MMV022478 belongs, has been reported to inhibit mammalian NADPH oxidase 4 (56). In T. brucei brucei it has been proposed that the enzyme trypanothione reductase (TryR) may function as an NADPH oxidase (57). TryR is essential and a potential drug target in T. brucei brucei (58, 59). However, despite the numerous inhibitors that have been designed to target TryR, none have progressed past in vitro studies (60–62).
The tuberculosis PBox subset yielded 10 compounds with activity against kinetoplastids; however, only 4 compounds (MMV687248, MMV687273, MMV021013, and MMV153413) displayed >5-fold selectivity over mammalian cell cytotoxicity. MMV687273, the clinical candidate SQ-109, was active against T. brucei brucei, T. cruzi, and L. donovani, as well as P. falciparum. Based on the HEK293 activity, the compound was selective for all four parasites but only showed a marginal window with respect to HEPG2 activity. SQ-109 has been extensively studied for T. cruzi, T. brucei brucei, and L. donovani; therefore, further studies on this compound may be of limited benefit (63, 64). MMV687248, a 3,5-disubstituted pyridine with an IC50 of 1.05 μM against T. brucei brucei with a selectivity index (SI) of >20 against both HEK293 and HEPG2, is an interesting novel starting point for HAT drug discovery. MMV021013, a 2-pyridyl-4-aminopyrimidine, was active against all three parasites but demonstrates greater activity against T. cruzi (1.67 μM) and L. donovani (1.48 μM) than against T. brucei brucei (3.51 μM). Activity against Plasmodium and Leishmania has been reported (65, 66); however, activity against T. cruzi has not been recorded within the scientific literature. Based on chemical structure, the cellular target of these compounds in Plasmodium is proposed to be methionine aminopeptidase. Therefore, these compounds could also target peptidases in T. cruzi, although this could need to be determined. MMV153413, a tetrasubstituted thiophene, demonstrated moderate activity against T. brucei brucei (2.99 μM) and, interestingly, against P. falciparum stage IV gametocytes but not ABS. The activity of this compound in mammalian cells has been related to SIRT2 [(NAD)-dependent deacetylase], which functions as an intracellular regulatory protein (67).
One compound from the schistosomiasis compound set, MMV688768, a 2,3-disubstituted indole, displayed activity against T. brucei brucei with an IC50 of 1.5 μM and SIs of >13 and >6, respectively, for HEK293 and HEPG2 cells. This compound has demonstrated dual activity against both alanyl aminopeptidase and dipeptidyl peptidase in mammals, with patent literature suggesting a range of medical indications, including autoimmune disease, allergies, and psoriasis (68). A number of dipeptidases have been shown to be essential virulence factors in T. brucei brucei infections and thus potential drug targets (69, 70). Inhibitors of T. brucei brucei serine peptidases have been identified, and the currently used antitrypanosomal drugs suramin, pentamidine, and diminazene all inhibit T. brucei brucei oligopeptidase B, although it is not clear whether this accounts for the antitrypanosomal activity of these drugs (69). Since MMV688768 has low micromolar (1.5 μM) activity and good selectivity against T. brucei brucei, further studies with this compound are warranted to determine the target and whether activity is retained against the human infective subspecies.
One compound from the lymphatic filariasis set, MMV687776 (which possesses a benzoxaborole warhead), and one compound from the toxoplasmosis set, MMV688417 (pyrazolo[3,4-d]pyrimidin-4-amine), displayed T. cruzi selectivity in relation to HEK293 cytotoxicity, with IC50s of 2.39 and 1.43 μM, respectively. MMV688417 was developed as a calcium-dependent protein kinase inhibitor against T. gondii (72). However, this compound displayed subefficacious activity against T. cruzi amastigotes in the Discovery Biology assay, which suggests a slow-acting mode of action against the parasite. MMV687776 was solely active against T. cruzi based on the testing undertaken in these studies. This compound did not display activity against HEK293; however, based on the PBox data, it did show activity against HEPG2 cells, with an IC50 of 10.9 μM and an SI of only 4.3 compared to >8-fold for HEK293 cytotoxicity. Although the activity of benzoxaboroles against trypanosomes and plasmodia has been cited (73–75), their activity solely against T. cruzi is of interest from a target and chemical starting point for hit-to-lead purposes. MMV637229, a diarylmethane ether (clemastine), is active against T. cruzi (1.22 μM) and P. falciparum ABS (2.2 μM). However, the activity of clemastine against T. cruzi, particularly with respect to in vitro and in vivo drug combinations, has been published (50).
(ii) New and novel compounds identified against Plasmodium.
When comparing the activity data obtained from Discovery Biology with the PBox supporting information activity data, a number of compounds demonstrated potent antiplasmodial activity not represented within PBox supporting information file. Upon searching for activity within ChEMBL, it was observed that many of the active compounds have already demonstrated antiplasmodial activity. A number of these compounds are not reported within the PBox supporting information file to have antiplasmodial activity, and these findings are therefore considered new data. A number of the compounds from pathogen disease indication sets, including, cryptosporidiosis, kinetoplastids, onchocerciasis, schistosomiasis, toxoplasmosis, trichuriasis, and tuberculosis, have demonstrated new antiplasmodial data for the PBox. These compounds are presented in Table 4.
After clustering by compound class and database searches for antiplasmodial activity, the majority of the compound classes are known to possess activity against P. falciparum. After filtering out known antimalarial actives, several compounds were identified that were not previously known to possess antimalarial activity. Some of these compounds displayed modest activity against ABS P. falciparum parasites, such as MMV687749 and MMV687765, both with the 2-amino-4-aryl ether pyrimidine chemotype from the tuberculosis set, with an IC50 of 2.0 μM. The most potent ABS compounds were related to known fungicides used in agriculture. MMV021057, a β-methoxyacrylate analogue of azoxystrobin present in the malaria set, and MMV688754, an oxime analogue of trifloxystrobin from the kinetoplastid set, had IC50s of 0.04 and 0.19 μM, respectively. Although the ABS data are not novel for the parent compounds, the identification of potent activity against stage IV gametocytes (0.03 and 0.14 μM, respectively) is novel data. However, determination of whether the parent compounds or their potential metabolites are the active components needs to be undertaken to ascertain whether these compounds are to be taken forward for further optimization (76). Other compounds with potent ABS antimalarial activity—MMV688279, a dihydroquinazoline, and MMV021660, a 2,4-diamino pyrimidine (with IC50s of 0.320 and 0.160 μM) from the kinetoplastid and tuberculosis sets, respectively—are more promising from an optimization viewpoint. MMV688279 also possesses modest LSG activity (IC50 of 2.4 μM).
Of note is the activity of MMV688547, MMV688362 (bisarylamidine), MMV688179, and MMV688271 (bisarylguanidinium) compounds, which have been identified as binding to the DNA minor groove at AT-rich regions of DNA (77–79). Bisarylamidine and bisarylguanidinium both demonstrate activity against P. falciparum 3D7, but the bisarylamidine compounds in the kinetoplast collection do not have antikinetoplastid activity against the parasites tested either from the PBox supporting information file or the Discovery Biology data, apart from T. brucei rhodesiense. Compounds of this chemotype, but not the specific compound itself, have been reported to have antitrypanosomal activity (79). The bisarylguanidinium compounds MMV688179 and MMV688271 both demonstrate activity for all pathogens within the kinetoplastid set, as represented by the PBox supporting information. Of note is the lack of activity for MMV688179 and MMV688271 within the Discovery Biology T. cruzi assay. It has been mentioned previously that the Discovery Biology T. cruzi assay has a 2-day incubation period compared to the 7-day incubation period for the PBox assay. This could indicate a delayed mechanism of action for this compound. However, additional parameters, such as medium components, compound stability/solubility, or compound processing (such as concentration, diluent, or storage), could contribute to differences observed in compound activity.
Several compounds that possessed P. falciparum ABS activity also possessed LSG activity. MMV659004 (ABS:LSG, 1.86:0.67 μM), MMV658988 (ABS:LSG, 2.18:0.30 μM), MMV021013 (ABS:LSG, 0.46:0.19 μM), MMV688122 (ABS:LSG, 2.11:0.11 μM) and MMV659010 (ABS:LSG, >8:1.71 μM), all from the 2-pyridyl-4-aminopyrimidine class, possessed LSG activities that were ≥2-fold those of the ABS activities. Two other compounds, MMV153413 (1.06 μM) and MMV688768 (4.70 μM), also exhibited LSG activity but not ABS activity, suggesting that these compounds are likely targeting cellular proteins and pathways that are essential in the gametocyte stage and not in the asexual stage. It must be noted that this is only a single determination of the IC50; however, compounds that display LSG activity represent suitable starting points for developing transmission blocking antimalarial agents.
Compounds with activity against the P. falciparum parasite from the Discovery Biology data may be expected to also display activity against either Cryptosporidium or Toxoplasma spp., since they are all from the Apicomplexa phylum. However, of the 24 compounds in the PBox described as targeting Toxoplasma or Cryptosporidium, only 2, MMV688703, and MMV675968, possessed antiplasmodial activity. The mechanism of action is known for both the Apicomplexa active compounds. MMV675968 is known to target Cryptosporidium dihydrofolate reductase (DHFR) (80) and is structurally similar to antiplasmodials that are known to target DHFR (81), and it is therefore likely that MMV675968 targets Plasmodium DHFR. MMV688703 is known to be a potent inhibitor of Toxoplasma cGMP-dependent protein kinase, involved in the regulation of calcium critical for signaling in Toxoplasma (82). It has also been shown that MMV688703 targets the Plasmodium cGMP-dependent protein kinase (PfPKG) and that this kinase plays an important role in asexual-stage parasite and gametocyte development (83, 84). Although this compound potently inhibits PfPKG (IC50 = 8.5 nM), it has a modest IC50 in a low micromolar range against the ABS P. falciparum parasite, a finding consistent with the IC50 determined here (3.16 μM). The function of PfPKG has been shown to be important in early gametocytogenesis (85) but not late-stage gametocytogenesis, corroborating the data here to suggest that MMV688703 does not affect this stage of gametocytogenesis.
Potential chemical starting points for kinetoplastid drug discovery programs from the PBox “kinetoplastid disease indication” compound set.
Of the 70 compounds within the kinetoplastid compound set, a number interesting as potential starting points for hit-to-lead and target identification purposes. Seventy percent of the kinetoplastid set of compounds were active against one or more of the three kinetoplastid pathogens tested in this study. Below, we classified the compounds into selective (based on an MMV selection index applied to compounds contained within PBox of >5-fold) individual parasite activities and those which are active across more than one parasite. An SI of 5 is lower than generally accepted by this laboratory as a starting point for chemistry optimization; however, for normalization with the PBox compound selection criteria we have also used 5-fold as a measure of selectivity. Greater selectivity for mammalian cells (at least 10-fold for kinetoplasts and 50-fold for malaria) is desirable for compound progression.
T. brucei brucei and T. cruzi.
MMV688550, an imidazo[1,2]purine, displayed good selectivity for both T. brucei brucei and T. cruzi, with SIs of >154 and >31, respectively, compared to HEK293 cell growth inhibition and represents a suitable starting point for targeting both T. brucei brucei and T. cruzi parasites. MMV688372, a substituted 2-phenylimidazopyridine, showed SIs of 24 for T. cruzi and 230 for T. brucei brucei in relation to HEK293 cells. Although MMV688372 demonstrated an IC50 of 1.15 μM against L. donovani, the selectivity over HEK293 cytotoxicity was not >5-fold. MMV688372 was identified through the development of an oxazolopyridine series and was shown to possess potent activity against T. brucei brucei, with an example from this series demonstrating efficacy in a mouse model of trypanosomiasis (86). A separate study also found representatives from the related oxazol-2-ylanilide to be active against T. brucei brucei (33), and in a metabolomics study an analogue was discovered to target sphingolipid metabolism in T. brucei brucei (87). In addition, a structurally related azabenzoxazole has recently been identified as a proteasome inhibitor in all three kinetoplastid parasites as a part of the Novartis Research Foundation Genomics Institute kinetoplastid screening campaign (88).
MMV688797, MMV688958, and MMV688795 are all structurally related to the 2-aryl oxazole chemotype, which is reported to display activity against T. brucei rhodesiense (89), and activity was identified against T. brucei brucei and T. cruzi from a hit-to-lead program targeting T. brucei brucei (33). Although the antikinetoplastid activity of these compounds is not novel, the target is unknown and remains to be elucidated. MMV688467 is a butyl sulfanilamide and a known inhibitor of microtubule formation in T. brucei brucei (90). MMV688467 also demonstrated modest activity against T. cruzi (IC50 of 3.98 μM). MMV689028, a benzyl piperazine, originated from the GSK Kinetoplastid Screening program (91) and exhibited potent activity against both T. brucei brucei (Discovery Biology, IC50 of 0.24 μM; PBox, 0.14 of μM) and T. cruzi (Discovery Biology, IC50 of 0.67 μM; PBox, IC50 of 1.20 μM). MMV689028 provides a suitable starting point for target identification studies for T. brucei brucei and T. cruzi.
MMV688796 (a 2,4-substituted furan), MMV689028 and MMV689029 (both benzyl piperazines), and MMV688371 (a benzamide) present suitable starting points for both T. brucei brucei and T. cruzi. All of these compounds also demonstrated activity against the HAT infective species T. brucei rhodesiense, supporting the potential for these compounds as novel and attractive targets for further development.
L. donovani and T. cruzi.
MMV690102, MMV690103, and MMV689437, which belong to the pyrimido[4,5-d]pyrimidine-2,4,7-triamine chemotype, all demonstrated activity against L. donovani and T. cruzi, with no activity displayed in the HEK293 assay at the concentrations tested. All of the compounds were previously identified from the GSK kinetoplastid screen. Structurally related compounds have been found to be inhibitors of DHFR isolated from mammals (81), which is potentially the target in these parasites. It should also be noted that these compounds are not active against malaria or T. brucei brucei, for which DHFR is a recognized drug target. However, MMV675968 (cryptosporidiosis set), a structurally related diaminoquinazoline, has potent activity against the asexual stage of P. falciparum (3D7 IC50, 0.03 μM; SI HEK, 7.4; SI HepG2, 113), and DHFR has been shown to be the cellular target (92). This suggests a structural divergence between DHFR orthologues expressed by these species of parasites. MMV690102, MMV690103, and MMV68943 are considered promising starting points for hit-to-lead optimization; however, their activity against the L. donovani and T. cruzi DHFR remains to be confirmed.
For many of the compounds displaying activity against kinetoplastid parasites, there was a lack of activity against L. donovani intracellular amastigotes. This could be associated with environmental factors within the acidic vacuole, where the parasite resides (93), or indicate that division of the intracellular form is slow (94). In contrast to T. brucei brucei, there is also an additional host membrane which the compounds must cross to have affect the Leishmania parasite. Previous studies have shown a poor correlation of compound activity against axenic amastigotes and intracellular amastigotes (94). Data were provided for a selection of compounds from the PBox tested against L. donovani axenic amastigotes; however, many other compounds were not tested. Further evaluation of PBox compounds against axenic forms of L. donovani and also the promastigote forms could provide insights into the differences observed between the kinetoplastids and expand our understanding of compound action against the intracellular form of the parasite.
Activity against T. brucei brucei and L. donovani.
MMV688271 and MMV688179 are bisaryl guanidinium analogues that have been identified with activity against L. donovani, T. cruzi (77), and T. brucei brucei. DNA affinity of guanidinium-like derivatives has been reported in parasites as the potential cellular target (78), and therefore the mammalian selectivity of this compound class should be monitored due to the potential mode of action.
Activity against T. cruzi only.
Of seven compounds demonstrating activity against T. cruzi alone, MMV688942, MMV688415, MMV688943, MMV689244, MMV689243, and MMV676048 had a subefficacious effect (less than 100% Emax as demonstrated by posaconazole from Table 2) in the Discovery Biology assay in that they did not clear 100% of the parasite from host cells. This property has been associated with the activity of azole antifungals and T. cruzi cytochrome P450 inhibitors (T. cruzi CYP51) against the parasite in the Discovery Biology image-based assay (31) and also two other assay formats reported in the literature (95, 96). These compounds are therefore likely to be associated with CYP51 or sterol biosynthesis activity, and several have been confirmed in the literature, such as, for example, Bitertanol (MMV688942) or difenoconazole (MMV688943), both azole antifungals targeting CYP51 in common fungal pathogens. Considering the recent failure rate of CYP51 inhibitors against T. cruzi in preclinical and clinical trials (97), these are not considered attractive starting points for targeting T. cruzi. MMV689709, a 3-substituted indazole, is a suitable starting point, with a modest IC50 of 3.32 μM.
Activity against T. brucei brucei only.
Three-quarters of the compounds active in the kinetoplastid set were T. brucei brucei active, with 54% demonstrating activity only against T. brucei brucei. Of the 18 compounds that demonstrate activity against T. brucei brucei specifically, 16 were selective for the parasite, whereas two (MMV595321 and MMV67660) demonstrated comparable activity against mammalian HEK293 cells.
MMV690028 and MMV690027, both hexahydrophthalazinones, have reported activity against trypanosomal phosphodiesterase (PDE) B1 (98). MMV690027 has been shown to inhibit human PDE4 and has been repurposed as a potent inhibitor of T. brucei brucei PDE B1 (99). The activity data for MMV690028 against L. donovani and for MMV690027 against T. cruzi and L. donovani has not been reported previously.
MMV202553 and MMV688793, both 2-pyridyl benzamides, exhibited modest activity against T. brucei brucei (IC50s of 1.0 and 1.2 μM, respectively); however, they are likely to be difficult to optimize since this chemotype is commonly found in serine protease factor Xa inhibitors (100). MMV188296, a 2-indolinecarboxamide, was previously optimized to potently inhibit and selectively target T. brucei brucei (101). MMV688776, a pyrazoloquinazoline, previously considered for treatment of mycobacteria but not trypanosomes (102), is potentially a suitable starting point for optimization against HAT, due to its demonstrated activity against the human infective form.
Potential chemical starting points for malaria drug discovery programs from the PBox antiplasmodial compound set.
Extensive screening campaigns for the identification of antiplasmodial compounds have been completed over the past 5 to 10 years (35, 54, 55), with reports of between 4 and 12 million compounds tested thus far, reducing, but not completely negating, the likelihood of identification of new antiplasmodial compound classes. Compounds within the malaria set constitute one-third of the PBox, with approximately 100 ABS active compounds with no known mechanism of action. Compounds that exhibit antiplasmodial activity with an unknown mechanism of action not only offer a suitable starting point for medicinal chemistry optimization but also offer the potential to be used as a chemical probe to reveal previous unstudied targets and mechanisms essential for Plasmodium parasite survival. Future testing of PBox against some of the malaria target and pathway specific assays (16, 40, 103–111) available throughout the global research community will highlight potential targets for these orphan compounds. Classes with novel mechanisms of action are in dire demand in order to attempt to combat resistance generation to numerous small-molecule agents that have the same mechanism of action.
There are 13 compounds from the malaria set with activity across ABS, gametocytes, and the liver stage. These compounds are of high priority for malaria drug discovery programs. MMV634140 and MMV667494, quinolone 4-carboxamides, have recently been identified as inhibitors of the Plasmodium translational elongation factor 2 (elF2) (42), while MMV010576 ((2-amino pyridine), the “hit” molecule (112) from which the antimalarial drug candidate MMV390048 arose, and MMV085499 (2-amino pyrazine) (113) are expected to have phosphatidylinositol 4-kinase (PI4K) activity based on the elucidated target for clinical candidates MMV390048 (114) and MMV642943 (drug candidate UCT943). MMV024443 (an indole-2-carboxamide) has been shown to target CDPK1 from Plasmodium parasites (115), along with MMV023985 and MMV010545, for which CDPK1 or PK7 have been proposed as the target (114, 116–120). The compounds above with PI4K, elF2, CDPK1, or PK7 targets in Plasmodium parasites have shown no activity in the kinetoplast assays undertaken by Discovery Biology.
The remaining six compounds (MMV026356, MMV019189, MMV023860, MMV019721, MMV022029, and MMV024035) do not have any associated target reported in the literature and, although they are only moderately active (ABS, 0.2 to 7 μM), they are therefore considered to be very interesting starting points for malaria drug discovery. There are also eight compounds (MMV023233, MMV020081, MMV022478, MMV062221, MMV108852, MMV560185, MMV1028806, and MMV1030799) that demonstrate activity against stage V gametocytes (and stage IV as determined here); these compounds are either active or inactive against the ABS and provide potential hits for malaria transmission blocking strategies.
This is the first comprehensive report to canvas the activity of the PBox compound collection against kinetoplastid and malaria parasites with a thorough analysis of the novelty of hits and the potential for either further biological characterization or target identification in the drug discovery arena. The simultaneous screening of the PBox against these parasite species has resulted in the identification of new and novel compounds, which offer potential starting points in early drug discovery campaigns. The outcome from this investigation will allow researchers from around the world to prioritize compounds or complement their studies based upon the information provided here. The data provided in this study support the MMV open-access initiative to build upon the knowledge that is publicly available for these compounds, with our data to be shared in ChEMBL.
MATERIALS AND METHODS
Pathogen Box collection.
From the Pathogen Box website (http://www.pathogenbox.org/) a link to a supporting information Excel file that contains the following information is provided. The compound plate layout, which compounds are in each “pathogen set,” the chemical structure and formula, SMILE string, salt, molecular weight, cLogP, and the confirmed biological activity against the respective pathogen set are included. HepG2 cytotoxicity data (CC20 or CC50) are also provided for approximately three-quarters of the compounds. Acknowledgments to the testing platforms for confirmation of biological activity are provided; however, no details of the assay specifics are recorded.
Compound handling.
The PBox was received as five 96-well plates containing 10 μl of 10 mM stocks of 400 compounds. Compounds Australia processed the compounds by the addition of 10 μl of DMSO to all compound-containing wells to give a total volume of 20 μl, which was then transferred and compressed to make two identical sets of low-dispense-volume plates (384-well), each containing 10 μl of compounds in a 5 mM DMSO stock solution.
Australian biosecurity level 2 (BC2) and physical containment level 2 (PC2) restrictions associated with working with these specific pathogens and different assay formats dictated the preparation of the assay plates. In the case of P. falciparum, assay-ready plates were prepared with nanoliter acoustic dispensing directly into imaging plates. For all other assays involving adherent infected cell lines, nanoliter to microliter acoustic dispensing was performed in sterile intermediate plates (Fig. 3). All compound plates were then transferred to the appropriate assay platform in a BC2/PC2 facility.
FIG 3.
Compound and plate processing of PBox by Compounds Australia.
Assays.
The PBox was tested in P. falciparum (Pf3D7 was obtained from BEI Resources Manassas, VA, and PfNF54-pfs16-LUC-GFP was kindly supplied by David Fidock, Columbia University, New York, NY) ABS and LSG assays. Also tested were the kinetoplastid pathogens T. cruzi (kindly provided by Frederick Buckner, University of Washington, Seattle, WA), L. donovani (American Type Culture Collection [ATCC], Manassas, VA), and T. brucei brucei (kindly provided by Achim Schnaufer, University of Edinburgh, Edinburgh, Scotland), and a resazurin metabolic viability assay was tested for mammalian cell cytotoxicity utilizing HEK293 cells (ATCC). (All published assays are briefly described in Supplemental File 2.)
Leishmania donovani intracellular amastigote assay. (i) Cell culture.
L. donovani MHOM/IN/80/DD8 (ATCC 50212) promastigote parasites were maintained in modified M199 Hanks salt medium (pH 6.8) supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 27°C. Parasites were subcultured every 7 days at a concentration of 105 cells/ml. THP-1 (ATCC TIB202) cells were maintained in RPMI medium and 10% FBS at 37°C/5% CO2. The cells were subcultured every 2 to 3 days in order to maintain a cell density between 2 × 105 and 1 × 106 cells/ml.
(ii) Assay protocol.
THP-1 cells were seeded in 384-well cell carrier imaging plates (Perkin-Elmer, Waltham, MA) using a Bravo automated liquid handling platform (Agilent Technologies, Santa Clara, CA) at a concentration of 12,500 cells per well in RMPI plus 10% fetal calf serum (FCS) medium containing 25 ng of phorbol 12-myristate 13-acetate (PMA)/ml in order to induce differentiation of the THP-1 cells. Assay plates were incubated at room temperature for 30 min to allow cells to adhere before being incubator at 37°C and 5% CO2 for 24 h. After incubation, the PMA was removed by discarding the medium within wells and washing the assay plates three times in phosphate-buffered saline (PBS) on an EL405 plate washer (Biotek Instruments, Winooski, VT). After washing, 40 μl of fresh RMPI and 10% FBS medium were added to the assay plates, followed by incubation for 48 h at 37°C and 5% CO2.
The number of metacyclic promastigotes present in a 7-day-old L. donovani DD8 promastigote culture was subsequently determined, and parasites were added to the assay plates containing the transformed THP-1 cells (72 h after initial cell seeding) at a multiplicity of infection of 1:5 (ratio of host cells to parasites). Assay plates were incubated at room temperature for 30 min, followed by 24 h incubation at 37°C and 5% CO2. Noninternalized parasites were subsequently removed by aspirating the medium within wells and washing the assay plates six times in PBS on a EL405 plate washer before the addition of 45 μl of fresh RMPI (10% FBS and 25 ng/ml PMA). Controls consisted of positive wells containing a final assay concentration of 2 μM amphotericin B, and negative wells containing 0.4% DMSO were used as in-plate controls for all experiments. Then, 1 μl of each compound, prepared by Compounds Australia, was diluted by the addition of 24 μl of RPMI medium containing no FCS. Next, 5-μl portions of this dilution were dispensed via a Bravo liquid handler to assay plates to give final assay concentrations of 20, 10, 5, and 0.5 μM (at 0.4% DMSO). For IC50 confirmation, the final assay concentrations ranged from 20 to 0.001 μM. Assay plates were incubated for 96 h at 37°C and 5% CO2 before being fixed with 4% paraformaldehyde and stained with SYBR green and CellMask Deep Red (ThermoFisher Scientific, Wlatham, MA).
Images were acquired on an Opera high-content imaging system (Perkin-Elmer). Healthy host (THP-1) cells were identified based on the CellMask Deep Red cytoplasmic and SYBR green nuclear area and intensities. Segmentation of nuclear and cell boundaries was used to identify the region of host cell cytoplasm. Intracellular parasites were then identified within this region based on spot detection algorithms of the SYBR green staining (with size and intensity measurements used to define parasite nucleus of kinetoplast) to determine the number of parasites present within THP-1 host cells. An infected cell was defined as a host cell containing >3 parasites within the cytoplasm boundary. The compound activity was determined based on the number of infected cells normalized to the positive (2 μM amphotericin B) and negative (0.4% DMSO) controls. Nonlinear sigmoidal dose-response curves with no constraints were plotted, and IC50s were calculated using GraphPad Prism 6. The IC50s were calculated from two independent experiments.
Pathogen Box primary screening.
The PBox was initially tested at four doses (20, 10, 5, and 0.5 μM). Active compounds taken for IC50 determination (i.e., the concentration at which 50% of the parasites were killed) were selected individually for each of the parasite assays performed based on the level of activity, correlation with data presented within the PBox supporting information, and whether the observed activity was novel to a pathogen other than that already determined within PBox supporting information file.
IC50 determination. (i) Kinetoplastids.
Compounds demonstrating > 50% inhibition at 10 μM for L. donovani and T. cruzi were progressed for IC50 evaluation, while all compounds demonstrating >50% activity at 5 μM for T. brucei brucei were taken for IC50 determination.
(ii) Plasmodium.
Compounds with activity greater than 60% at 5 μM observed for either ABS or LSG assays were considered for IC50 determination. Compounds from the subset of the PBox designated “malaria disease indication” had IC50s determined only if there were discrepancies with the data provided within the PBox supporting information file or if LSG activity was observed. All compounds from “other pathogen disease indication” compound subsets with activity greater than 60% for ABS or LSG at 5 μM underwent IC50 determination. Simultaneous testing of compounds in ABS, LSG, and HEK293 cytotoxicity assays were performed.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Australian Research Council (LP120200557 and LP140100560 awarded to V.M.A.), the National Health and Medical Research Council (APP1067728 awarded to V.M.A.), a Wellcome Trust Pathfinder Award (WT109662MA awarded to B.E.S.), and a Victorian State Government Operational Infrastructure Support (B.E.S.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank the Australian Red Cross Blood Bank for the provision of fresh red blood cells, without which this research could not have been performed. We thank Medicines Malaria Venture (MMV) for the supply of the MMV Pathogen Box. We also appreciate the contributions of Kate Parsons, Elspeth Johnson, and Elouise Gaylard, who assisted with parasite and mammalian cell culture and assays.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00379-17.
REFERENCES
- 1.Maurer SM, Rai A, Sali A. 2004. Finding cures for tropical diseases: is open source an answer? PLoS Med 1:e56. doi: 10.1371/journal.pmed.0010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLano WL. 2005. The case for open-source software in drug discovery. Drug Discov Today 10:213–217. doi: 10.1016/S1359-6446(04)03363-X. [DOI] [PubMed] [Google Scholar]
- 3.Munos B. 2006. Can open-source R&D reinvigorate drug research? Nat Rev Drug Discov 5:723–729. doi: 10.1038/nrd2131. [DOI] [PubMed] [Google Scholar]
- 4.Gunther S, Kuhn M, Dunkel M, Campillos M, Senger C, Petsalaki E, Ahmed J, Urdiales EG, Gewiess A, Jensen LJ, Schneider R, Skoblo R, Russell RB, Bourne PE, Bork P, Preissner R. 2008. SuperTarget and Matador: resources for exploring drug-target relationships. Nucleic Acids Res 36:D919–D922. doi: 10.1093/nar/gkm862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S. 2008. India takes an open source approach to drug discovery. Cell 133:201–203. doi: 10.1016/j.cell.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Orti L, Carbajo RJ, Pieper U, Eswar N, Maurer SM, Rai AK, Taylor G, Todd MH, Pineda-Lucena A, Sali A, Marti-Renom MA. 2009. A kernel for open source drug discovery in tropical diseases. PLoS Negl Trop Dis 3:e418. doi: 10.1371/journal.pntd.0000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazanetz MP, Marmon RJ, Reisser CB, Morao I. 2012. Drug discovery applications for KNIME: an open source data mining platform. Curr Top Med Chem 12:1965–1979. doi: 10.2174/156802612804910331. [DOI] [PubMed] [Google Scholar]
- 8.Ardal C, Rottingen JA. 2012. Open source drug discovery in practice: a case study. PLoS Negl Trop Dis 6:e1827. doi: 10.1371/journal.pntd.0001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies M, Nowotka M, Papadatos G, Dedman N, Gaulton A, Atkinson F, Bellis L, Overington JP. 2015. ChEMBL web services: streamlining access to drug discovery data and utilities. Nucleic Acids Res 43:W612–W620. doi: 10.1093/nar/gkv352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichman M, Simpson PB. 2016. Open innovation in early drug discovery: roadmaps and roadblocks. Drug Discov Today 21:779–788. doi: 10.1016/j.drudis.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Wells TN, Willis P, Burrows JN, van Huijsduijnen RH. 2016. Open data in drug discovery and development: lessons from malaria. Nat Rev Drug Discov 15:661–662. doi: 10.1038/nrd.2016.154. [DOI] [PubMed] [Google Scholar]
- 12.Lucantoni L, Duffy S, Adjalley SH, Fidock DA, Avery VM. 2013. Identification of MMV malaria box inhibitors of Plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob Agents Chemother 57:6050–6062. doi: 10.1128/AAC.00870-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. 2014. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the malaria box. Antimicrob Agents Chemother 58:811–819. doi: 10.1128/AAC.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malaria J 12:408. doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. 2014. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother 58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hain AU, Bartee D, Sanders NG, Miller AS, Sullivan DJ, Levitskaya J, Meyers CF, Bosch J. 2014. Identification of an Atg8-Atg3 protein-protein interaction inhibitor from the medicines for Malaria Venture Malaria Box active in blood- and liver-stage Plasmodium falciparum parasites. J Med Chem 57:4521–4531. doi: 10.1021/jm401675a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhardwaj A, Scaria V, Raghava GP, Lynn AM, Chandra N, Banerjee S, Raghunandanan MV, Pandey V, Taneja B, Yadav J, Dash D, Bhattacharya J, Misra A, Kumar A, Ramachandran S, Thomas Z, Brahmachari SK. 2011. Open source drug discovery: a new paradigm of collaborative research in tuberculosis drug development. Tuberculosis (Edinb) 91:479–486. doi: 10.1016/j.tube.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Ballell L, Bates RH, Young RJ, Alvarez-Gomez D, Alvarez-Ruiz E, Barroso V, Blanco D, Crespo B, Escribano J, Gonzalez R, Lozano S, Huss S, Santos-Villarejo A, Martin-Plaza JJ, Mendoza A, Rebollo-Lopez MJ, Remuinan-Blanco M, Lavandera JL, Perez-Herran E, Gamo-Benito FJ, Garcia-Bustos JF, Barros D, Castro JP, Cammack N. 2013. Fueling open-source drug discovery: 177 small-molecule leads against tuberculosis. ChemMedChem 8:313–321. doi: 10.1002/cmdc.201200428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todd MH, Coaker H. 2015. Using an open source model to accelerate schistosomiasis drug research. Future Med Chem 7:689–692. doi: 10.4155/fmc.15.28. [DOI] [PubMed] [Google Scholar]
- 20.Ingram-Sieber K, Cowan N, Panic G, Vargas M, Mansour NR, Bickle QD, Wells TN, Spangenberg T, Keiser J. 2014. Orally active antischistosomal early leads identified from the open access malaria box. PLoS Negl Trop Dis 8:e2610. doi: 10.1371/journal.pntd.0002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramamoorthi R, Graef KM, Dent J. 2015. Repurposing pharma assets: an accelerated mechanism for strengthening the schistosomiasis drug development pipeline. Future Med Chem 7:727–735. doi: 10.4155/fmc.15.26. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser M, Maes L, Tadoori LP, Spangenberg T, Ioset JR. 2015. Repurposing of the open access malaria box for kinetoplastid diseases identifies novel active scaffolds against trypanosomatids. J Biomol Screen 20:634–645. doi: 10.1177/1087057115569155. [DOI] [PubMed] [Google Scholar]
- 23.Boyom FF, Fokou PV, Tchokouaha LR, Spangenberg T, Mfopa AN, Kouipou RM, Mbouna CJ, Donfack VF, Zollo PH. 2014. Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob Agents Chemother 58:5848–5854. doi: 10.1128/AAC.02541-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessoff K, Spangenberg T, Foderaro JE, Jumani RS, Ward GE, Huston CD. 2014. Identification of Cryptosporidium parvum active chemical series by repurposing the open access malaria box. Antimicrob Agents Chemother 58:2731–2739. doi: 10.1128/AAC.02641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Voorhis WC, Adams JH, Adelfio R, Ahyong V, Akabas MH, Alano P, Alday A, Aleman Resto Y, Alsibaee A, Alzualde A, Andrews KT, Avery SV, Avery VM, Ayong L, Baker M, Baker S, Ben Mamoun C, Bhatia S, Bickle Q, Bounaadja L, Bowling T, Bosch J, Boucher LE, Boyom FF, Brea J, Brennan M, et al. 2016. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog 12:e1005763. doi: 10.1371/journal.ppat.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celik H, Hong SH, Colon-Lopez DD, Han J, Kont YS, Minas TZ, Swift M, Paige M, Glasgow E, Toretsky JA, Bosch J, Uren A. 2015. Identification of novel ezrin inhibitors targeting metastatic osteosarcoma by screening open access malaria box. Mol Cancer Ther 14:2497–2507. doi: 10.1158/1535-7163.MCT-15-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preston S, Jiao Y, Jabbar A, McGee SL, Laleu B, Willis P, Wells TN, Gasser RB. 2016. Screening of the “Pathogen Box” identifies an approved pesticide with major anthelmintic activity against the barber's pole worm. Int J Parasitol Drugs Drug Resist 6:329–334. doi: 10.1016/j.ijpddr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vila T, Lopez-Ribot JL. 2016. Screening the “Pathogen Box” for the identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother 61:e02006-16. doi: 10.1128/AAC.02006-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy S, Avery VM. 2012. Development and optimization of a novel 384-well anti-malarial imaging assay validated for high-throughput screening. Am J Trop Med Hyg 86:84–92. doi: 10.4269/ajtmh.2012.11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sykes ML, Avery VM. 2009. Development of an Alamar Blue viability assay in 384-well format for high-throughput whole-cell screening of Trypanosoma brucei brucei bloodstream form strain 427. Am J Trop Med Hyg 81:665–674. doi: 10.4269/ajtmh.2009.09-0015. [DOI] [PubMed] [Google Scholar]
- 31.Sykes ML, Avery VM. 2015. Development and application of a sensitive, phenotypic, high-throughput image-based assay to identify compound activity against Trypanosoma cruzi amastigotes. Int J Parasitol Drugs Drug Resist 5:215–228. doi: 10.1016/j.ijpddr.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avery VM, Bashyam S, Burrows JN, Duffy S, Papadatos G, Puthukkuti S, Sambandan Y, Singh S, Spangenberg T, Waterson D, Willis P. 2014. Screening and hit evaluation of a chemical library against blood-stage Plasmodium falciparum. Malaria J 13:190. doi: 10.1186/1475-2875-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sykes ML, Baell JB, Kaiser M, Chatelain E, Moawad SR, Ganame D, Ioset JR, Avery VM. 2012. Identification of compounds with anti-proliferative activity against Trypanosoma brucei brucei strain 427 by a whole-cell viability based HTS campaign. PLoS Negl Trop Dis 6:e1896. doi: 10.1371/journal.pntd.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sykes ML, Avery VM. 2013. Approaches to protozoan drug discovery: phenotypic screening. J Med Chem 56:7727–7740. doi: 10.1021/jm4004279. [DOI] [PubMed] [Google Scholar]
- 35.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edlin CD, Morgans G, Winks S, Duffy S, Avery VM, Wittlin S, Waterson D, Burrows J, Bryans J. 2012. Identification and in-vitro ADME assessment of a series of novel anti-malarial agents suitable for hit-to-lead chemistry. ACS Med Chem Lett 3:570–573. doi: 10.1021/ml300091c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsen A, Lacrue AN, White KL, Forquer IP, Cross RM, Marfurt J, Mather MW, Delves MJ, Shackleford DM, Saenz FE, Morrisey JM, Steuten J, Mutka T, Li Y, Wirjanata G, Ryan E, Duffy S, Kelly JX, Sebayang BF, Zeeman AM, Noviyanti R, Sinden RE, Kocken CH, Price RN, Avery VM, Angulo-Barturen I, Jimenez-Diaz MB, Ferrer S, Herreros E, Sanz LM, Gamo FJ, Bathurst I, Burrows JN, Siegl P, Guy RK, Winter RW, Vaidya AB, Charman SA, Kyle DE, Manetsch R, Riscoe MK. 2013. Quinolone-3-diarylethers: a new class of antimalarial drug. Sci Transl Med 5:177ra37. doi: 10.1126/scitranslmed.3005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Manach C, Gonzalez Cabrera D, Douelle F, Nchinda AT, Younis Y, Taylor D, Wiesner L, White KL, Ryan E, March C, Duffy S, Avery VM, Waterson D, Witty MJ, Wittlin S, Charman SA, Street LJ, Chibale K. 2014. Medicinal chemistry optimization of antiplasmodial imidazopyridazine hits from high-throughput screening of a SoftFocus kinase library: part 1. J Med Chem 57:2789–2798. doi: 10.1021/jm500098s. [DOI] [PubMed] [Google Scholar]
- 39.Ramachandran S, Hameed PS, Srivastava A, Shanbhag G, Morayya S, Rautela N, Awasthy D, Kavanagh S, Bharath S, Reddy J, Panduga V, Prabhakar KR, Saralaya R, Nanduri R, Raichurkar A, Menasinakai S, Achar V, Jimenez-Diaz MB, Martinez MS, Angulo-Barturen I, Ferrer S, Sanz LM, Gamo FJ, Duffy S, Avery VM, Waterson D, Lee MC, Coburn-Flynn O, Fidock DA, Iyer PS, Narayanan S, Hosagrahara V, Sambandamurthy VK. 2014. N-Aryl-2-aminobenzimidazoles: novel, efficacious, antimalarial lead compounds. J Med Chem 57:6642–6652. doi: 10.1021/jm500715u. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez-Diaz MB, Ebert D, Salinas Y, Pradhan A, Lehane AM, Myrand-Lapierre ME, O'Loughlin KG, Shackleford DM, Justino de Almeida M, Carrillo AK, Clark JA, Dennis AS, Diep J, Deng X, Duffy S, Endsley AN, Fedewa G, Guiguemde WA, Gomez MG, Holbrook G, Horst J, Kim CC, Liu J, Lee MC, Matheny A, Martinez MS, Miller G, Rodriguez-Alejandre A, Sanz L, Sigal M, Spillman NJ, Stein PD, Wang Z, Zhu F, Waterson D, Knapp S, Shelat A, Avery VM, Fidock DA, Gamo FJ, Charman SA, Mirsalis JC, Ma H, Ferrer S, Kirk K, Angulo-Barturen I, Kyle DE, DeRisi JL, Floyd DM, Guy RK. 2014. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of plasmodium. Proc Natl Acad Sci U S A 111:E5455–E5462. doi: 10.1073/pnas.1414221111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato N, Comer E, Sakata-Kato T, Sharma A, Sharma M, Maetani M, Bastien J, Brancucci NM, Bittker JA, Corey V, Clarke D, Derbyshire ER, Dornan GL, Duffy S, Eckley S, Itoe MA, Koolen KM, Lewis TA, Lui PS, Lukens AK, Lund E, March S, Meibalan E, Meier BC, McPhail JA, Mitasev B, Moss EL, Sayes M, et al. 2016. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 538:344–349. doi: 10.1038/nature19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baragana B, Norcross NR, Wilson C, Porzelle A, Hallyburton I, Grimaldi R, Osuna-Cabello M, Norval S, Riley J, Stojanovski L, Simeons FR, Wyatt PG, Delves MJ, Meister S, Duffy S, Avery VM, Winzeler EA, Sinden RE, Wittlin S, Frearson JA, Gray DW, Fairlamb AH, Waterson D, Campbell SF, Willis P, Read KD, Gilbert IH. 2016. Discovery of a quinoline-4-carboxamide derivative with a novel mechanism of action, multistage antimalarial activity, and potent in vivo efficacy. J Med Chem 59:9672–9685. doi: 10.1021/acs.jmedchem.6b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norcross NR, Baragana B, Wilson C, Hallyburton I, Osuna-Cabello M, Norval S, Riley J, Stojanovski L, Simeons FR, Porzelle A, Grimaldi R, Wittlin S, Duffy S, Avery VM, Meister S, Sanz L, Jimenez-Diaz B, Angulo-Barturen I, Ferrer S, Martinez MS, Gamo FJ, Frearson JA, Gray DW, Fairlamb AH, Winzeler EA, Waterson D, Campbell SF, Willis P, Read KD, Gilbert IH. 2016. Trisubstituted pyrimidines as efficacious and fast-acting antimalarials. J Med Chem 59:6101–6120. doi: 10.1021/acs.jmedchem.6b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrins L, Gazdik M, Rahmani R, Varghese S, Sykes ML, Jones AJ, Avery VM, White KL, Ryan E, Charman SA, Kaiser M, Bergstrom CA, Baell JB. 2014. Pyridyl benzamides as a novel class of potent inhibitors for the kinetoplastid Trypanosoma brucei. J Med Chem 57:6393–6402. doi: 10.1021/jm500191u. [DOI] [PubMed] [Google Scholar]
- 45.Rahmani R, Ban K, Jones AJ, Ferrins L, Ganame D, Sykes ML, Avery VM, White KL, Ryan E, Kaiser M, Charman SA, Baell JB. 2015. 6-Arylpyrazine-2-carboxamides: a new core for Trypanosoma brucei inhibitors. J Med Chem 58:6753–6765. doi: 10.1021/acs.jmedchem.5b00438. [DOI] [PubMed] [Google Scholar]
- 46.Manos-Turvey A, Watson EE, Sykes ML, Jones AJ, Baell JB, Kaiser M, Avery VM, Payne RJ. 2015. Synthesis and evaluation of phenoxymethylbenzamide analogues as anti-trypanosomal agents. MedChemComm 6:403–406. doi: 10.1039/C4MD00406J. [DOI] [Google Scholar]
- 47.Russell S, Rahmani R, Jones AJ, Newson HL, Neilde K, Cotillo I, Rahmani Khajouei M, Ferrins L, Qureishi S, Nguyen N, Martinez-Martinez MS, Weaver DF, Kaiser M, Riley J, Thomas J, De Rycker M, Read KD, Flematti GR, Ryan E, Tanghe S, Rodriguez A, Charman SA, Kessler A, Avery VM, Baell JB, Piggott MJ. 2016. Hit-to-lead optimization of a novel class of potent, broad-spectrum trypanosomacides. J Med Chem 59:9686–9720. doi: 10.1021/acs.jmedchem.6b00442. [DOI] [PubMed] [Google Scholar]
- 48.Simpson M, Poulsen SA. 2014. An overview of Australia's compound management facility: the Queensland Compound Library. ACS Chem Biol 9:28–33. doi: 10.1021/cb400912x. [DOI] [PubMed] [Google Scholar]
- 49.Kaiser M, Maser P, Tadoori LP, Ioset JR, Brun R. 2015. Antiprotozoal activity profiling of approved drugs: a starting point toward drug repositioning. PLoS One 10:e0135556. doi: 10.1371/journal.pone.0135556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Planer JD, Hulverson MA, Arif JA, Ranade RM, Don R, Buckner FS. 2014. Synergy testing of FDA-approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl Trop Dis 8:e2977. doi: 10.1371/journal.pntd.0002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang MZ, Zhu XH, Srivastava A, Liu Q, Sweat JM, Pandharkar T, Stephens CE, Riccio E, Parman T, Munde M, Mandal S, Madhubala R, Tidwell RR, Wilson WD, Boykin DW, Hall JE, Kyle DE, Werbovetz KA. 2010. Novel arylimidamides for treatment of visceral leishmaniasis. Antimicrob Agents Chemother 54:2507–2516. doi: 10.1128/AAC.00250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plouffe DM, Wree M, Du AY, Meister S, Li F, Patra K, Lubar A, Okitsu SL, Flannery EL, Kato N, Tanaseichuk O, Comer E, Zhou B, Kuhen K, Zhou Y, Leroy D, Schreiber SL, Scherer CA, Vinetz J, Winzeler EA. 2016. High-throughput assay and discovery of small molecules that interrupt malaria transmission. Cell Host Microbe 19:114–126. doi: 10.1016/j.chom.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duffy S, Loganathan S, Holleran JP, Avery VM. 2016. Large-scale production of Plasmodium falciparum gametocytes for malaria drug discovery. Nat Protoc 11:976–992. doi: 10.1038/nprot.2016.056. [DOI] [PubMed] [Google Scholar]
- 54.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 55.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A 105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borbély G, Szabadkai I, Horváth Z, Markó P, Varga Z, Breza N, Baska F, Vántus T, Huszár M, Geiszt M, Hunyady L, Buday L, Őrfi L, Kéri G. 2010. Small-molecule inhibitors of NADPH oxidase 4. J Med Chem 53:6758–6762. doi: 10.1021/jm1004368. [DOI] [PubMed] [Google Scholar]
- 57.Lu J, Vodnala SK, Gustavsson AL, Gustafsson TN, Sjoberg B, Johansson HA, Kumar S, Tjernberg A, Engman L, Rottenberg ME, Holmgren A. 2013. Ebsulfur is a benzisothiazolone cytocidal inhibitor targeting the trypanothione reductase of Trypanosoma brucei. J Biol Chem 288:27456–27468. doi: 10.1074/jbc.M113.495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hunter WN, Bailey S, Habash J, Harrop SJ, Helliwell JR, Aboagye-Kwarteng T, Smith K, Fairlamb AH. 1992. Active site of trypanothione reductase: a target for rational drug design. J Mol Biol 227:322–333. doi: 10.1016/0022-2836(92)90701-K. [DOI] [PubMed] [Google Scholar]
- 59.Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C. 2000. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol Microbiol 35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- 60.Spinks D, Shanks EJ, Cleghorn LA, McElroy S, Jones D, James D, Fairlamb AH, Frearson JA, Wyatt PG, Gilbert IH. 2009. Investigation of trypanothione reductase as a drug target in Trypanosoma brucei. ChemMedChem 4:2060–2069. doi: 10.1002/cmdc.200900262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson JL, Nett IR, Jones DC, Abdille MH, Gilbert IH, Fairlamb AH. 2009. Improved tricyclic inhibitors of trypanothione reductase by screening and chemical synthesis. ChemMedChem 4:1333–1340. doi: 10.1002/cmdc.200900097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beig M, Oellien F, Garoff L, Noack S, Krauth-Siegel RL, Selzer PM. 2015. Trypanothione reductase: a target protein for a combined in vitro and in silico screening approach. PLoS Negl Trop Dis 9:e0003773. doi: 10.1371/journal.pntd.0003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li K, Wang Y, Yang G, Byun S, Rao G, Shoen C, Yang H, Gulati A, Crick DC, Cynamon M, Huang G, Docampo R, No JH, Oldfield E. 2015. Oxa, thia, heterocycle, and carborane analogues of SQ109: bacterial and protozoal cell growth inhibitors. ACS Infect Dis 1:215–221. doi: 10.1021/acsinfecdis.5b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veiga-Santos P, Li K, Lameira L, de Carvalho TM, Huang G, Galizzi M, Shang N, Li Q, Gonzalez-Pacanowska D, Hernandez-Rodriguez V, Benaim G, Guo RT, Urbina JA, Docampo R, de Souza W, Oldfield E. 2015. SQ109, a new drug lead for Chagas disease. Antimicrob Agents Chemother 59:1950–1961. doi: 10.1128/AAC.03972-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, Chong CR, Shi L, Yoshimoto T, Sullivan DJ Jr, Liu JO. 2006. Inhibitors of Plasmodium falciparum methionine aminopeptidase 1b possess antimalarial activity. Proc Natl Acad Sci U S A 103:14548–14553. doi: 10.1073/pnas.0604101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musonda CC, Whitlock GA, Witty MJ, Brun R, Kaiser M. 2009. Synthesis and evaluation of 2-pyridyl pyrimidines with in vitro antiplasmodial and antileishmanial activity. Bioorg Med Chem Lett 19:401–405. doi: 10.1016/j.bmcl.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 67.Aleksey G. December 2008. Compositions and methods for modulating sirtuin activity. Google patent WO2008011476 A3.
- 68.Ansorge S, Bank U, Nordhoff K, Striggow F, Taeger M. June 2006. Dual alanyl aminopeptidase and dipeptidyl peptidase IV inhibitors for functionally influencing different cells and for treating immunological, inflammatory, neuronal, and other diseases. Google patent WO2005034940 A8.
- 69.Moss CX, Brown E, Hamilton A, Van der Veken P, Augustyns K, Mottram JC. 2015. An essential signal peptide peptidase identified in an RNAi screen of serine peptidases of Trypanosoma brucei. PLoS One 10:e0123241. doi: 10.1371/journal.pone.0123241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morty RE, Troeberg L, Powers JC, Ono S, Lonsdale-Eccles JD, Coetzer TH. 2000. Characterisation of the antitrypanosomal activity of peptidyl alpha-aminoalkyl phosphonate diphenyl esters. Biochem Pharmacol 60:1497–1504. doi: 10.1016/S0006-2952(00)00459-7. [DOI] [PubMed] [Google Scholar]
- 71.Reference deleted .
- 72.Johnson SM, Murphy RC, Geiger JA, DeRocher AE, Zhang Z, Ojo KK, Larson ET, Perera BG, Dale EJ, He P, Reid MC, Fox AM, Mueller NR, Merritt EA, Fan E, Parsons M, Van Voorhis WC, Maly DJ. 2012. Development of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitors with potent anti-toxoplasma activity. J Med Chem 55:2416–2426. doi: 10.1021/jm201713h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobs RT, Nare B, Wring SA, Orr MD, Chen D, Sligar JM, Jenks MX, Noe RA, Bowling TS, Mercer LT, Rewerts C, Gaukel E, Owens J, Parham R, Randolph R, Beaudet B, Bacchi CJ, Yarlett N, Plattner JJ, Freund Y, Ding C, Akama T, Zhang YK, Brun R, Kaiser M, Scandale I, Don R. 2011. SCYX-7158, an orally active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl Trop Dis 5:e1151. doi: 10.1371/journal.pntd.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs RT, Plattner JJ, Nare B, Wring SA, Chen D, Freund Y, Gaukel EG, Orr MD, Perales JB, Jenks M, Noe RA, Sligar JM, Zhang YK, Bacchi CJ, Yarlett N, Don R. 2011. Benzoxaboroles: a new class of potential drugs for human African trypanosomiasis. Future Med Chem 3:1259–1278. doi: 10.4155/fmc.11.80. [DOI] [PubMed] [Google Scholar]
- 75.Ding D, Zhao Y, Meng Q, Xie D, Nare B, Chen D, Bacchi CJ, Yarlett N, Zhang Y-K, Hernandez V, Xia Y, Freund Y, Abdulla M, Ang K-H, Ratnam J, McKerrow JH, Jacobs RT, Zhou H, Plattner JJ. 2010. Discovery of novel benzoxaborole-based potent antitrypanosomal agents. ACS Med Chem Lett 1:165–169. doi: 10.1021/ml100013s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laird WJ, Gledhill AJ, Lappin GJ. 2003. Metabolism of methyl-(E)-2-[2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl]-3-methoxyacrylate (azoxystrobin) in rat. Xenobiotica 33:677–690. doi: 10.1080/0049825031000105020. [DOI] [PubMed] [Google Scholar]
- 77.Stephens CE, Brun R, Salem MM, Werbovetz KA, Tanious F, Wilson WD, Boykin DW. 2003. The activity of diguanidino and “reversed” diamidino 2,5-diarylfurans versus Trypanosoma cruzi and Leishmania donovani. Bioorg Med Chem Lett 13:2065–2069. doi: 10.1016/S0960-894X(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 78.Nagle PS, Rodriguez F, Nguyen B, Wilson WD, Rozas I. 2012. High DNA affinity of a series of peptide linked diaromatic guanidinium-like derivatives. J Med Chem 55:4397–4406. doi: 10.1021/jm300296f. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez F, Rozas I, Kaiser M, Brun R, Nguyen B, Wilson WD, Garcia RN, Dardonville C. 2008. New bis(2-aminoimidazoline) and bisguanidine DNA minor groove binders with potent in vivo antitrypanosomal and antiplasmodial activity. J Med Chem 51:909–923. doi: 10.1021/jm7013088. [DOI] [PubMed] [Google Scholar]
- 80.Popov VM, Chan DC, Fillingham YA, Atom Yee W, Wright DL, Anderson AC. 2006. Analysis of complexes of inhibitors with Cryptosporidium hominis DHFR leads to a new trimethoprim derivative. Bioorg Med Chem Lett 16:4366–4370. doi: 10.1016/j.bmcl.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 81.Gebauer MG, McKinlay C, Gready JE. 2003. Synthesis of quaternised 2-aminopyrimido[4,5-d]pyrimidin-4(3H)-ones and their biological activity with dihydrofolate reductase. Eur J Med Chem 38:719–728. doi: 10.1016/S0223-5234(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 82.Zhang C, Ondeyka JG, Herath KB, Guan Z, Collado J, Pelaez F, Leavitt PS, Gurnett A, Nare B, Liberator P, Singh SB. 2006. Highly substituted terphenyls as inhibitors of parasite cGMP-dependent protein kinase activity. J Nat Prod 69:710–712. doi: 10.1021/np0505418. [DOI] [PubMed] [Google Scholar]
- 83.Diaz CA, Allocco J, Powles MA, Yeung L, Donald RG, Anderson JW, Liberator PA. 2006. Characterization of Plasmodium falciparum cGMP-dependent protein kinase (PfPKG): antiparasitic activity of a PKG inhibitor. Mol Biochem Parasitol 146:78–88. doi: 10.1016/j.molbiopara.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 84.Panchal D, Bhanot P. 2010. Activity of a trisubstituted pyrrole in inhibiting sporozoite invasion and blocking malaria infection. Antimicrob Agents Chemother 54:4269–4274. doi: 10.1128/AAC.00420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, Polley SD, Billker O, Baker DA. 2008. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol 6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tatipaka HB, Gillespie JR, Chatterjee AK, Norcross NR, Hulverson MA, Ranade RM, Nagendar P, Creason SA, McQueen J, Duster NA, Nagle A, Supek F, Molteni V, Wenzler T, Brun R, Glynne R, Buckner FS, Gelb MH. 2014. Substituted 2-phenylimidazopyridines: a new class of drug leads for human African trypanosomiasis. J Med Chem 57:828–835. doi: 10.1021/jm401178t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stoessel D, Nowell CJ, Jones AJ, Ferrins L, Ellis KM, Riley J, Rahmani R, Read KD, McConville MJ, Avery VM, Baell JB, Creek DJ. 2016. Metabolomics and lipidomics reveal perturbation of sphingolipid metabolism by a novel anti-trypanosomal 3-(oxazolo[4,5-b]pyridine-2-yl)anilide. Metabolomics 12:126. [Google Scholar]
- 88.Khare S, Nagle AS, Biggart A, Lai YH, Liang F, Davis LC, Barnes SW, Mathison CJ, Myburgh E, Gao MY, Gillespie JR, Liu X, Tan JL, Stinson M, Rivera IC, Ballard J, Yeh V, Groessl T, Federe G, Koh HX, Venable JD, Bursulaya B, Shapiro M, Mishra PK, Spraggon G, Brock A, Mottram JC, Buckner FS, Rao SP, Wen BG, Walker JR, Tuntland T, Molteni V, Glynne RJ, Supek F. 2016. Proteasome inhibition for treatment of leishmaniasis, Chagas disease, and sleeping sickness. Nature 537:229–233. doi: 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patrick DA, Wenzler T, Yang S, Weiser PT, Wang MZ, Brun R, Tidwell RR. 2016. Synthesis of novel amide and urea derivatives of thiazol-2-ethylamines and their activity against Trypanosoma brucei rhodesiense. Bioorg Med Chem 24:2451–2465. doi: 10.1016/j.bmc.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.George TG, Endeshaw MM, Morgan RE, Mahasenan KV, Delfín DA, Mukherjee MS, Yakovich AJ, Fotie J, Li C, Werbovetz KA. 2007. Synthesis, biological evaluation, and molecular modeling of 3,5-substituted-N1-phenyl-N4,N4-di-n-butylsulfanilamides as antikinetoplastid antimicrotubule agents. Bioorg Med Chem 15:6071–6079. doi: 10.1016/j.bmc.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pena I, Pilar Manzano M, Cantizani J, Kessler A, Alonso-Padilla J, Bardera AI, Alvarez E, Colmenarejo G, Cotillo I, Roquero I, de Dios-Anton F, Barroso V, Rodriguez A, Gray DW, Navarro M, Kumar V, Sherstnev A, Drewry DH, Brown JR, Fiandor JM, Julio Martin J. 2015. New compound sets identified from high-throughput phenotypic screening against three kinetoplastid parasites: an open resource. Sci Rep 5:8771. doi: 10.1038/srep08771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansch C, Fukunaga JY, Jow PY. 1977. Quantitative structure-activity relationships of antimalarial and dihydrofolate reductase inhibition by quinazolines and 5-substituted benzyl-2,4-diaminopyrimidines. J Med Chem 20:96–102. doi: 10.1021/jm00211a020. [DOI] [PubMed] [Google Scholar]
- 93.Real F, Mortara RA. 2012. The diverse and dynamic nature of Leishmania parasitophorous vacuoles studied by multidimensional imaging. PLoS Negl Trop Dis 6:e1518. doi: 10.1371/journal.pntd.0001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Rycker M, Hallyburton I, Thomas J, Campbell L, Wyllie S, Joshi D, Cameron S, Gilbert IH, Wyatt PG, Frearson JA, Fairlamb AH, Gray DW. 2013. Comparison of a high-throughput high-content intracellular Leishmania donovani assay with an axenic amastigote assay. Antimicrob Agents Chemother 57:2913–2922. doi: 10.1128/AAC.02398-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moraes CB, Giardini MA, Kim H, Franco CH, Araujo-Junior AM, Schenkman S, Chatelain E, Freitas-Junior LH. 2014. Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development. Sci Rep 4:4703. doi: 10.1038/srep04703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Rycker M, Thomas J, Riley J, Brough SJ, Miles TJ, Gray DW. 2016. Identification of trypanocidal activity for known clinical compounds using a new Trypanosoma cruzi hit-discovery screening cascade. PLoS Negl Trop Dis 10:e0004584. doi: 10.1371/journal.pntd.0004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Molina I, Prat JGI, Salvador F, Trevino B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sanchez-Montalva A, Vidal X, Pahissa A. 2014. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N Engl J Med 370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- 98.Orrling KM, Jansen C, Vu XL, Balmer V, Bregy P, Shanmugham A, England P, Bailey D, Cos P, Maes L, Adams E, van den Bogaart E, Chatelain E, Ioset JR, van de Stolpe A, Zorg S, Veerman J, Seebeck T, Sterk GJ, de Esch IJP, Leurs R. 2012. Catechol pyrazolinones as trypanocidals: fragment-based design, synthesis, and pharmacological evaluation of nanomolar inhibitors of trypanosomal phosphodiesterase B1. J Med Chem 55:8745–8756. doi: 10.1021/jm301059b. [DOI] [PubMed] [Google Scholar]
- 99.Van der Mey M, Hatzelmann A, Van Klink GP, Van der Laan IJ, Sterk GJ, Thibaut U, Ulrich WR, Timmerman H. 2001. Novel selective PDE4 inhibitors. 2. Synthesis and structure-activity relationships of 4-aryl-substituted cis-tetra- and cis-hexahydrophthalazinones. J Med Chem 44:2523–2535. [DOI] [PubMed] [Google Scholar]
- 100.Zhu B-Y, Scarborough RM. 2000. Factor Xa inhibitors: recent advances in anticoagulant agents. Annu Rep Med Chem 35:83–102. doi: 10.1016/S0065-7743(00)35010-2. [DOI] [Google Scholar]
- 101.Cleghorn LA, Albrecht S, Stojanovski L, Simeons FR, Norval S, Kime R, Collie IT, De Rycker M, Campbell L, Hallyburton I, Frearson JA, Wyatt PG, Read KD, Gilbert IH. 2015. Discovery of indoline-2-carboxamide derivatives as a new class of brain-penetrant inhibitors of Trypanosoma brucei. J Med Chem 58:7695–7706. doi: 10.1021/acs.jmedchem.5b00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Vita D, Pandolfi F, Cirilli R, Scipione L, Di Santo R, Friggeri L, Mori M, Fiorucci D, Maccari G, Arul Christopher RS, Zamperini C, Pau V, De Logu A, Tortorella S, Botta M. 2016. Discovery of in vitro antitubercular agents through in silico ligand-based approaches. Eur J Med Chem 121:169–180. doi: 10.1016/j.ejmech.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 103.Paiardini A, Bamert RS, Kannan-Sivaraman K, Drinkwater N, Mistry SN, Scammells PJ, McGowan S. 2015. Screening the medicines for malaria venture “malaria box” against the Plasmodium falciparum aminopeptidases, M1, M17 and M18. PLoS One 10:e0115859. doi: 10.1371/journal.pone.0115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fletcher S, Avery VM. 2014. A novel approach for the discovery of chemically diverse anti-malarial compounds targeting the Plasmodium falciparum coenzyme A synthesis pathway. Malaria J 13:343. doi: 10.1186/1475-2875-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spillman NJ, Kirk K. 2015. The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int J Parasitol Drugs Drug Resist 5:149–162. doi: 10.1016/j.ijpddr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu W, Herrera Z, Ebert D, Baska K, Cho SH, DeRisi JL, Yeh E. 2015. A chemical rescue screen identifies a Plasmodium falciparum apicoplast inhibitor targeting MEP isoprenoid precursor biosynthesis. Antimicrob Agents Chemother 59:356–364. doi: 10.1128/AAC.03342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ortiz D, Guiguemde WA, Johnson A, Elya C, Anderson J, Clark J, Connelly M, Yang L, Min J, Sato Y, Guy RK, Landfear SM. 2015. Identification of selective inhibitors of the Plasmodium falciparum hexose transporter PfHT by screening focused libraries of anti-malarial compounds. PLoS One 10:e0123598. doi: 10.1371/journal.pone.0123598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Creek DJ, Chua HH, Cobbold SA, Nijagal B, Macrae JI, Dickerman BK, Gilson PR, Ralph SA, McConville MJ. 2016. Metabolomics-based screening of the Malaria Box reveals both novel and established mechanisms of action. Antimicrob Agents Chemother 60:6650–6663. doi: 10.1128/AAC.01226-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kher SS, Penzo M, Fulle S, Ebejer JP, Finn PW, Blackman MJ, Jirgensons A. 2015. Quinoxaline-based inhibitors of malarial protease PfSUB1*. Chem Heterocycl Compd 50:1457–1463. doi: 10.1007/s10593-014-1610-4. [DOI] [Google Scholar]
- 110.Fong KY, Sandlin RD, Wright DW. 2015. Identification of beta-hematin inhibitors in the MMV Malaria Box. Int J Parasitol Drugs Drug Resist 5:84–91. doi: 10.1016/j.ijpddr.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahyong V, Sheridan CM, Leon KE, Witchley JN, Diep J, DeRisi JL. 2016. Identification of Plasmodium falciparum specific translation inhibitors from the MMV Malaria Box using a high-throughput in vitro translation screen. Malaria J 15:173. doi: 10.1186/s12936-016-1231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Younis Y, Douelle F, Feng T-S, Cabrera DG, Manach CL, Nchinda AT, Duffy S, White KL, Shackleford DM, Morizzi J, Mannila J, Katneni K, Bhamidipati R, Zabiulla KM, Joseph JT, Bashyam S, Waterson D, Witty MJ, Hardick D, Wittlin S, Avery V, Charman SA, Chibale K. 2012. 3,5-Diaryl-2-aminopyridines as a novel class of orally active antimalarials demonstrating single dose cure in mice and clinical candidate potential. J Med Chem 55:3479–3487. doi: 10.1021/jm3001373. [DOI] [PubMed] [Google Scholar]
- 113.Le Manach C, Nchinda AT, Paquet T, Gonzalez Cabrera D, Younis Adam Y, Han Z, Bashyam S, Zabiulla M, Taylor D, Lawrence N, White KL, Charman SA, Waterson D, Witty MJ, Wittlin S, Botha ME, Nondaba SH, Reader J, Birkholtz LM, Jimenez-Diaz MB, Martinez-Martinez MS, Ferrer-Bazaga S, Angulo-Barturen I, Meister S, Antonova-Koch Y, Winzeler EA, Street LJ, Chibale K. 2016. Identification of a potential anti-malarial drug candidate from a series of 2-aminopyrazines by optimization of aqueous solubility and potency across the parasite life-cycle. J Med Chem 59:9890–9905. doi: 10.1021/acs.jmedchem.6b01265. [DOI] [PubMed] [Google Scholar]
- 114.Ghidelli-Disse S, Lafuente-Monasterio MJ, Waterson D, Witty M, Younis Y, Paquet T, Street LJ, Chibale K, Gamo-Benito FJ, Bantscheff M, Drewes G. 2014. Identification of Plasmodium PI4 kinase as target of MMV390048 by chemoproteomics. Malaria J 13:P38. doi: 10.1186/1475-2875-13-S1-P38. [DOI] [Google Scholar]
- 115.Crowther GJ, Hillesland HK, Keyloun KR, Reid MC, Lafuente-Monasterio MJ, Ghidelli-Disse S, Leonard SE, He P, Jones JC, Krahn MM, Mo JS, Dasari KS, Fox AM, Boesche M, El Bakkouri M, Rivas KL, Leroy D, Hui R, Drewes G, Maly DJ, Van Voorhis WC, Ojo KK. 2016. Biochemical screening of five protein kinases from Plasmodium falciparum against 14,000 cell-active compounds. PLoS One 11:e0149996. doi: 10.1371/journal.pone.0149996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chapman TM, Osborne SA, Wallace C, Birchall K, Bouloc N, Jones HM, Ansell KH, Taylor DL, Clough B, Green JL, Holder AA. 2014. Optimization of an imidazopyridazine series of inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1). J Med Chem 57:3570–3587. doi: 10.1021/jm500342d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chapman TM, Osborne SA, Bouloc N, Large JM, Wallace C, Birchall K, Ansell KH, Jones HM, Taylor D, Clough B, Green JL, Holder AA. 2013. Substituted imidazopyridazines are potent and selective inhibitors of Plasmodium falciparum calcium-dependent protein kinase 1 (PfCDPK1). Bioorg Med Chem Lett 23:3064–3069. doi: 10.1016/j.bmcl.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lemercier G, Fernandez-Montalvan A, Shaw JP, Kugelstadt D, Bomke J, Domostoj M, Schwarz MK, Scheer A, Kappes B, Leroy D. 2009. Identification and characterization of novel small molecules as potent inhibitors of the plasmodial calcium-dependent protein kinase 1. Biochemistry 48:6379–6389. doi: 10.1021/bi9005122. [DOI] [PubMed] [Google Scholar]
- 119.Chapman T, Osborne S, Wallace C. September 2012. Imidazo[1,2b]pyridazine derivatives as cdpk1 inhibitors. Google patent WO2012127212 A1.
- 120.Osborne S, Chapman T, Large J, Bouloc N, Wallace C. August 2011. Fused heterocyclic compounds for use in the treatment of malaria. Google patent WO2011101640 A1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.