ABSTRACT
The pharmacokinetics, safety, and tolerability of intravenous (i.v.) fosfomycin disodium (ZTI-01) and oral fosfomycin tromethamine were evaluated after a single dose in 28 healthy adult subjects. Subjects received a single 1-h i.v. infusion of 1 g and 8 g fosfomycin disodium and a single dose of 3 g oral fosfomycin tromethamine in a phase I, randomized, open-label, three-period crossover study. Serial blood and urine samples were collected before and up to 48 h after dosing. The mean pharmacokinetic parameters ± standard deviations of fosfomycin in plasma after 1 g and 8 g i.v., respectively, were the following: maximum clearance of drug in serum (Cmax), 44.3 ± 7.6 and 370 ± 61.9 μg/ml; time to maximum concentration of drug in serum (Tmax), 1.1 ± 0.05 and 1.08 ± 0.01 h; volume of distribution (V), 29.7 ± 5.7 and 31.5 ± 10.4 liters; clearance (CL), 8.7 ± 1.7 and 7.8 ± 1.4 liters/h; renal clearance (CLR), 6.6 ± 1.9 and 6.3 ± 1.6 liters/h; area under the concentration-time curve from 0 to infinity (AUC0–∞), 120 ± 28.5 and 1,060 ± 192 μg·h/ml; and half-life (t1/2), 2.4 ± 0.4 and 2.8 ± 0.6 h. After oral administration, the parameters were the following: Cmax, 26.8 ± 6.4 μg/ml; Tmax, 2.25 ± 0.4 h; V/F, 204 ± 70.7 liters; CL/F, 17 ± 4.7 liters/h; CLR, 6.5 ± 1.8 liters/h; AUC0–∞, 191 ± 57.6 μg · h/ml; and t1/2, 9.04 ± 4.5 h. The percent relative bioavailability of orally administered fosfomycin was 52.8% in relation to the 1-g i.v. dose. Approximately 74% and 80% of the 1-g and 8-g i.v. doses were excreted unchanged in the urine by 48 h compared to 37% after oral administration, with the majority of this excretion occurring by 12 h regardless of dosage form. No new safety concerns were identified during this study. The results of this study support further investigation of i.v. fosfomycin in the target patient population, including patients with complicated urinary tract infections and pyelonephritis.
KEYWORDS: fosfomycin, ZTI-01, pharmacokinetics, safety, absorption, flip-flop kinetics
INTRODUCTION
The prevalence, morbidity, and mortality associated with multidrug-resistant (MDR) pathogens is increasing, while the number of effective antimicrobial options for these organisms continues to diminish (1). Fosfomycin possesses a unique mechanism of action and maintains broad in vitro antibacterial activity against many clinically significant MDR pathogens, including extended-spectrum β-lactamase (ESBL) and carbapenemase-producing Gram-negative organisms, vancomycin-resistant Enterococcus (VRE), and methicillin-resistant Staphylococcus aureus (MRSA) (2). Oral fosfomycin, in the form of the tromethamine salt, has been approved by the Food and Drug Administration (FDA) and widely used in the United States since 1996, while the intravenous formulation has been restricted to date to outside the U.S. (3). The use of the oral formulation has been studied extensively globally and has demonstrated safety and efficacy in a variety of patient populations, including adult, elderly, and pediatric patients (4, 5). Outside the United States, the parenteral formulation has been used to treat serious infections for more than 40 years (6). ZTI-01 is a phosphonic acid derivative of fosfomycin, formulated as a disodium salt for intravenous (i.v.) administration, and is being considered for addition to the U.S. therapeutic armamentarium for difficult-to-treat infections. Given the dearth of effective treatment options in this era of MDR bacteria, it is crucial to fully understand the pharmacokinetics of antibacterial agents in order to assess the pharmacokinetic/pharmacodynamic parameters associated with efficacy. The purpose of this study was to determine the safety, tolerability, and pharmacokinetics of two single doses of i.v. fosfomycin disodium (1 g and 8 g) and a single dose (3 g) of oral fosfomycin tromethamine in a randomized, three-period crossover study in healthy volunteer subjects.
RESULTS
A total of 30 healthy adult subjects were enrolled in the study. Two subjects were prematurely withdrawn prior to completion of all study procedures due to violations of the study protocol, leaving 28 subjects who completed the study and were included in the pharmacokinetic population. Of the 28 subjects who completed the study, 27 received all three regimens while one subject withdrew consent and only received the oral fosfomycin tromethamine regimen. The baseline demographics of the pharmacokinetically evaluable subjects are presented in Table 1. Overall, the subjects were young and the majority were white, non-Hispanic, non-Latino females.
TABLE 1.
Characteristics of healthy adult subjects receiving intravenous and oral fosfomycin
| Fosfomycin treatment (no. of subjects) | No. (%) of male subjects | No. (%) of white subjects | Value (means ± SD) for: |
||||
|---|---|---|---|---|---|---|---|
| Age (yr) | Height (cm) | Weight (kg) | Body mass index (kg/m2) | CLCRa (ml/min) | |||
| i.v. (n = 27) | 11 (41) | 20 (74) | 27 ± 5 | 169.2 ± 10.3 | 70.5 ± 11.1 | 24.6 ± 2.9 | 139.6 ± 24.4 |
| p.o. (n = 28) | 11 (39) | 21 (75) | 26 ± 5 | 169.2 ± 10.1 | 69.9 ± 11.2 | 24.4 ± 2.9 | 139.3 ± 23.9 |
CLCR, estimated creatinine clearance.
Pharmacokinetics of intravenous fosfomycin.
Mean (± standard deviation [SD]) plasma concentrations of fosfomycin after each study drug administration regimen are displayed in Fig. 1. Figure S1 in the supplemental material also shows mean (±SD) concentration-time profiles of fosfomycin in plasma after a single 1-h i.v. infusion of 1 g and 8 g fosfomycin disodium. The mean (±SD) plasma pharmacokinetic parameters of fosfomycin after i.v. administration are summarized in Table 2. After i.v. administration of 1 g and 8 g, all subjects had quantifiable plasma concentrations within 5 min of the end of the 1-h infusion. After a single i.v. dose of 1 g, almost all (24/27) subjects had measurable plasma concentrations 12 h after the dose, while only one subject had a measurable plasma concentration at 18 h postdose, and none had measurable plasma concentrations at the 24-h sampling point or beyond. After an 8-g i.v. dose, all subjects had measurable plasma concentrations at 18 h and almost half (13/27) had quantifiable plasma concentrations at 24 h postdose. All plasma concentrations at 36 and 48 h were below the limit of quantitation (BLQ). Plasma fosfomycin exposure increased in an approximately dose-proportional manner following i.v. administration of 1 g and 8 g of fosfomycin disodium. The geometric least-squares mean maximum clearance of drug in serum (Cmax) and area under the concentration-time curve from 0 to infinity (AUC0–∞) increased approximately 8.4- and 8.8-fold, respectively, with an 8-fold increase in dose (Table 2).
FIG 1.

Mean (±SD) concentration-versus-time profile of fosfomycin in plasma after intravenous infusion of 1 g and 8 g fosfomycin disodium and a single dose of 3 g oral fosfomycin tromethamine. Intravenous administration of 1 g is illustrated by open circles and a dashed lined, and 8 g is illustrated by open diamonds and a dashed line. Oral administration is illustrated by filled circles and a solid line. The y axis is in the log scale.
TABLE 2.
Pharmacokinetic parameters of fosfomycin in plasma after intravenous and oral administration
| Fosfomycin treatmenta (no. of subjects) | Value (means ± SD) for: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUClast (μg · h/ml) | AUC0–∞ (μg · h/ml) | V or V/F (liters) | CL or CL/F (liters/h) | CLR (liters/h) | t1/2 (h) | |
| 1 g i.v. (n = 27) | 44.3 ± 7.6 | 1.1 ± 0.05 | 117 ± 27.7 | 120 ± 28.5 | 29.7 ± 5.7 | 8.7 ± 1.7 | 6.6 ± 1.9 | 2.4 ± 0.4 |
| 8 g i.v. (n = 27) | 370 ± 61.9 | 1.08 ± 0.01 | 1,056 ± 192 | 1,060 ± 192 | 31.5 ± 10.4 | 7.8 ± 1.4 | 6.3 ± 1.6 | 2.8 ± 0.6 |
| 3 g p.o. (n = 28) | 26.8 ± 6.4 | 2.25 ± 0.4 | 178 ± 49.9b | 191 ± 57.6 | 204 ± 70.7g | 17.0 ± 4.7h | 6.5 ± 1.8 | 9.04 ± 4.5 |
| 26.8 ± 6.4 | 2.25 ± 0.4 | 174 ± 45.1c | 187 ± 52.2 | 184 ± 65.6g | 17.2 ± 4.6h | 6.3 ± 1.7 | 7.93 ± 3.5 | |
| 26.8 ± 6.4 | 2.25 ± 0.4 | 165 ± 39.2d | 179 ± 43.8 | 152 ± 53.5g | 17.8 ± 4.4h | 6.1 ± 1.7 | 6.09 ± 2.1 | |
| 26.8 ± 6.4 | 2.25 ± 0.4 | 156 ± 36.6e | 174 ± 40.1 | 134 ± 54.9g | 18.2 ± 4.2h | NRi | 5.17 ± 2.1 | |
| 26.8 ± 6.4 | 2.25 ± 0.4 | 139 ± 33.6f | 160 ± 37.4 | 109 ± 54.1g | 19.7 ± 4.6h | 6.0 ± 1.7 | 3.91 ± 2.1 | |
Each subject received only one dose of fosfomycin. Pharmacokinetic parameters are displayed for incremental noncompartmental analyses on partial areas from 0 to 12, 0 to 24, 0 to 36, and 0 to 48 h, as delineated.
AUC0–48.
AUC0–36.
AUC0–24.
AUC0–18.
AUC0–12.
V/F.
CL/F.
CLR not reported given the lack of corresponding urine collection interval.
The mean (±SD) cumulative fraction (percent) of fosfomycin dose excreted in urine over time following i.v. administration is displayed in Fig. 2. Table S1 shows the mean (±SD) cumulative fraction (percent) of the fosfomycin dose excreted per urine collection interval. The mean renal clearance of fosfomycin after i.v. administration of 1 and 8 g (6.5 and 6.3 liters/h) approximated normal glomerular filtration (Table 2). Following i.v. dosing of 1 and 8 g, approximately 74% and 80% of the dose, respectively, was excreted unchanged in the urine by 48 h postdose. The largest proportion of this excretion occurred by 12 h postdose, with 70% of the dose being excreted in this time frame for both regimens. The mean (±SD) concentration of fosfomycin in the urine during each collection interval is shown in Table S2, along with the mean (±SD) volume of urine excreted during that interval. After i.v. administration, peak urinary concentrations occurred during the 0- to 4-h collection interval. Eighty-eight percent (24/27) of subjects had measurable urinary concentrations during the 24- to 36-h collection interval after receiving 1 g i.v., compared to only 6 subjects who had measurable urinary concentrations during the 36- to 48-h interval. After 8 g i.v., all subjects had measurable urinary concentrations through the 48-h collection interval.
FIG 2.
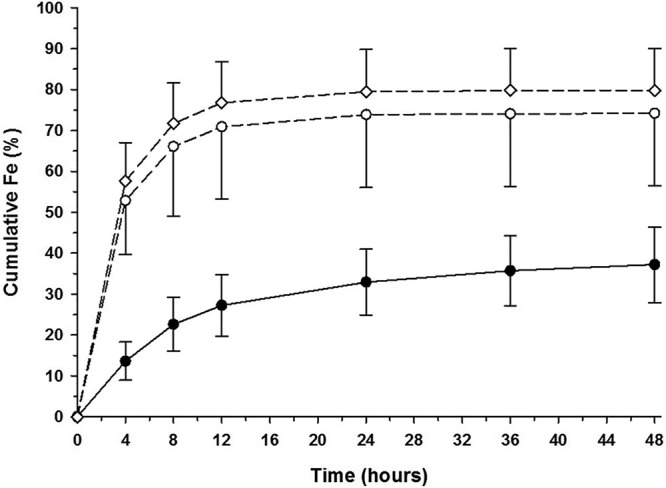
Mean (±SD) cumulative fraction (percent) of the fosfomycin dose excreted in urine over time following oral and intravenous administration. Intravenous administration of 1 g is illustrated by open circles and a dashed lined, and 8 g is illustrated by open diamonds and a dashed line. Oral administration is illustrated by filled circles and a solid line.
Pharmacokinetics of oral fosfomycin tromethamine.
Mean (±SD) plasma concentrations of fosfomycin after each study drug administration regimen are displayed in Fig. 1. Mean (±SD) plasma concentrations of fosfomycin after ingestion of a single 3-g oral dose of fosfomycin tromethamine are displayed in Fig. S2a. The mean (±SD) pharmacokinetic parameters of fosfomycin in plasma after oral administration are summarized in Table 2. After deconvolution from 1 g i.v., the absorption rate constant (ka) of oral fosfomycin was 0.0175 h−1. Following a single oral dose of fosfomycin tromethamine, all subjects had plasma fosfomycin concentrations above the quantifiable limit 1 h after ingestion. The vast majority of subjects (25/28) also had measurable plasma concentrations 24 h after oral administration. Half (14/28) of the subjects had quantifiable plasma fosfomycin concentrations 36 h postdose, while only 6 of 28 had measurable plasma concentrations at 48 h postdose. Given this observed variability in plasma half-life, incremental noncompartmental analyses were performed, including on all plasma fosfomycin concentrations from 0 to 48 h, 0 to 36 h, 0 to 24 h, 0 to 18 h, and 0 to 12 h. The mean (±SD) pharmacokinetic parameters resulting from these analyses are displayed in Table 2. Figure S2b, c, and d in the supplemental material show corresponding mean (±SD) plasma fosfomycin concentration-time curves from 0 to 36 h, 0 to 24 h, and 0 to 12 h, respectively. The geometric mean percent bioavailable (F) of 3 g oral fosfomycin tromethamine relative to the 1-g i.v. dose based on the AUC0–∞ was 52.8%.
The mean (±SD) cumulative fraction (percent) of the dose excreted in urine over time following ingestion of a single 3-g dose of oral fosfomycin tromethamine is displayed in Fig. 2. Table S1 shows the mean (±SD) cumulative fraction (percent) of the dose excreted per urine collection interval. The mean renal clearance of fosfomycin (6.5 liters/h) approximated normal glomerular filtration (Table 2). Following oral dosing, approximately 37% of the dose was excreted unchanged in the urine by 48 h postdose. The largest proportion of this excretion (27%) occurred by 12 h postdose. The observed mean (±SD) concentration of fosfomycin in the urine during each collection interval is shown in Table S2, along with the mean (±SD) volume of urine excreted during that interval. After oral administration of fosfomycin, mean peak urinary concentrations occurred during the 0- to 4-h collection interval, and all subjects had measurable urinary concentrations through the 48-h collection interval.
Safety of intravenous and oral fosfomycin.
Overall, treatment-emergent adverse events (TEAEs) were reported for 24 (80%) subjects in the study. The majority of TEAEs (75%) were experienced after receiving i.v. infusions of fosfomycin disodium, of which a greater proportion occurred after the 8-g dose (19 subjects; 67.9%) compared to the 1-g dose (11 subjects; 39.3%). The most common TEAE after 8-g i.v. infusion was bradycardia (28.6%), defined as heart rate of ≤54 beats per minute, followed by hypocalcemia (10.7%), while hypocalcemia was most common (17.9%) after infusion of 1 g, followed by headache and bradycardia (10.7% each). Six of the 28 subjects with TEAEs experienced after i.v. infusion had events that were considered treatment related, with headache being the most common (10.7%). These treatment-related TEAEs were mild or moderate in intensity and resolved without sequelae. There were no treatment-related TEAEs after oral fosfomycin tromethamine administration. There were no clinical laboratory abnormalities reported as treatment-related TEAEs after either i.v. or oral administration of fosfomycin. All TEAEs except two were considered resolved by the end of the study. After the 8-g i.v. dose, one subject experienced mild hypocalcemia and another experienced mild anemia that were unresolved but considered clinically stable at the end of the study. There were no serious adverse events reported, and no subjects were withdrawn from the study due to TEAEs.
DISCUSSION
This phase I, single-dose, randomized, three-period, crossover study evaluated the pharmacokinetics and safety of fosfomycin in plasma and urine after both i.v. and oral administration in healthy volunteer subjects. The plasma pharmacokinetics of i.v. fosfomycin disodium were roughly linear and proportional between the 1-g and 8-g doses. The administration of 3 g of oral fosfomycin tromethamine resulted in a 1.5-fold higher plasma exposure in terms of mean AUC0–∞ compared to the 1-g i.v. dose due to the longer plasma half-life but a 5.5-fold lower mean AUC0–∞ than that of the 8-g dose. The percent relative bioavailability of orally administered fosfomycin was 52.8% in relation to the 1-g i.v. dose. Approximately 74% and 80% of the 1-g and 8-g i.v. doses were excreted unchanged in the urine by 48 h compared to only 37% after oral administration, with the majority of this excretion occurring by 12 h regardless of dosage form. No new safety concerns were identified during this study. The adverse events observed in this study were similar to those seen in other studies of healthy subjects and across the decades of clinical experience with fosfomycin (7–17).
Few studies evaluating the pharmacokinetics of fosfomycin after i.v. administration have been completed in healthy volunteers. The vast majority of modern pharmacokinetic studies have been completed in patients with various infections and pathophysiological derangements that preclude direct comparison with our study. Even so, the parameters established in these patients administered doses similar to those of our study are comparable (18).
Goto et al. examined seven adult male volunteers administered approximately 1 g (20 mg/kg of body weight) and 2 g (40 mg/kg) of i.v. fosfomycin as a bolus over 5 min (19). The observed Cmax after the 1-g dose was 132.1 ± 31.8 μg/ml, while V, AUC0–∞, CL, and CLR were 0.34 ± 0.08 liters/kg, 167.9 ± 26.4 μg · h/ml, 2.08 ± 0.45 ml/min/kg, and 1.74 ± 0.63 ml/min/kg, respectively. A modern study by Frossard et al. evaluated the distribution of fosfomycin into the plasma and interstitial fluid of healthy volunteers given a single i.v. dose of fosfomycin (20). Six healthy male volunteers were administered 4 and 8 g of i.v. fosfomycin as a 30-min infusion. After an 8-g i.v. dose, the mean (±SD) plasma Cmax and AUC0–8 reported were 395 ± 46 mg/liter and 887 ± 71 μg · h/ml, respectively. These parameters are analogous to the parameters observed in our study after i.v. administration of 1 g and 8 g fosfomycin disodium.
The plasma pharmacokinetic parameters generated in this study after administration of oral fosfomycin tromethamine compare well to previous studies, despite the fact that the vast majority of these analyses were performed in the 1970s and 1980s, prior to advanced sampling and bioanalytical techniques and the recognized need to supplement microbiological assays with glucose-6-phosphate (G6P). Many previous studies also utilize the calcium salt of fosfomycin, which is known to have impaired bioavailability compared to the tromethamine salt. Borsa et al. examined the pharmacokinetics of oral fosfomycin tromethamine in young and elderly adults (21). Thirteen healthy subjects were administered a single oral 25-mg/kg dose (∼2 g) of fosfomycin tromethamine under fasted conditions. The mean (±SD) Cmax and time to maximum concentration of drug in serum (Tmax) in young subjects (26 to 33 years of age; n = 5) were 18.48 ± 10.27 μg/ml and 1.61 ± 0.23 h, respectively. Mean (±SD) V, t1/2, and AUC0–∞ were 2.42 ± 1.68 liters/kg, 5.37 ± 2.56 h, and 102.85 ± 42.1 μg · h/ml, respectively. Total body and renal clearance were determined to be 33.6 ± 14.5 liters/h and 18.6 ± 2.6 liters/h, respectively, and 57.7% ± 30.2% of the administered dose was eliminated renally by 24 h. Other older studies, administering 3 g of oral fosfomycin tromethamine, have demonstrated Cmax values ranging from 22 to 32 μg/ml, Tmax from 2 to 2.5 h, t1/2 from 2.4 to 7.3 h, and AUC from 145 to 228 mg · h/liter (22–24). A recent study examining the plasma pharmacokinetics after administration of 3 g oral fosfomycin tromethamine to 26 mostly older males prior to prostate resection demonstrated a mean (±SD) Cmax, Tmax, t1/2, V, CL, AUC, and AUC0–∞ of 17.9 ± 8 μg/ml, 2.7 ± 1.0 h, 7.1 ± 0.3 h, 169.4 ± 79.3 liters, 15 ± 7.1 liters/h, 236.5 ± 121.8 μg · h/ml, and 247 ± 128.7 μg · h/ml, respectively (25, 26). The pharmacokinetic parameters observed in these studies are all within ranges of the parameters determined in our study considering the differences in study populations, doses, bioanalytical methods, and sampling schemes.
In this study, oral fosfomycin tromethamine displayed unique pharmacokinetic qualities compared to i.v. fosfomycin disodium. Regardless of the number of concentrations included in the noncompartmental analysis in this study, the plasma elimination half-life of oral fosfomycin tromethamine was longer than that after i.v. administration. This may be due to slow absorption of the oral drug into the central compartment, causing the elimination rate constant (ke) to become larger than the ka and, therefore, absorption to become the rate-limiting step to drug removal as opposed to elimination. In this study, the ka for oral fosfomycin was 0.0175 h−1, while the ke was 0.1093 h−1, indicating a slow first-order absorption process. This phenomenon, sometimes known as flip-flop kinetics (27, 28), has been reported with other antimicrobials and may be a result of the saturable carrier-mediated phosphate transport system and nonsaturable first-order absorption processes of fosfomycin in the small intestine (29). All subjects in this study were fasted and free from concomitant medications at the time of study drug administration, therefore factors affecting absorption of oral fosfomycin tromethamine deserve further exploration.
An understanding of the pharmacokinetic/pharmacodynamics (PK/PD) index that links antimicrobial exposure with efficacy is an important step in designing regimens that optimize safety and efficacy. The index dynamically linked to antibacterial activity of fosfomycin in vitro has not been clearly elucidated and has varied among experimental models (30–32). A recent neutropenic murine thigh infection model utilizing fosfomycin disodium for injection demonstrated AUC/MIC to be the PK/PD index most closely associated with efficacy (R2 = 0.7), with average net stasis and 1-log kill ratios for Enterobacteriaceae of 23 and 83, respectively (33). If these data are applied to the mean AUC values (AUC0–8 for i.v., AUC0–24 for oral) observed in our study, a 1-g i.v. or 3-g oral dose of fosfomycin would be expected to achieve net stasis against Enterobacteriaceae isolates with a MIC of ≤4 mg/liter and 1-log kill for a MIC of ≤1 mg/liter. For the 8-g i.v. dose, stasis and 1-log kill could be achieved with a MIC of ≤32 mg/liter and ≤8 mg/liter, respectively. A recently completed phase III randomized controlled trial (ZEUS) utilized fosfomycin disodium at a dose of 6 g i.v. every 8 h for the treatment of patients with complicated urinary tract infections or acute pyelonephritis (ClinicalTrials registration no. NCT02753946). Given the linearity observed in this study, this dosing regimen of 6 g i.v. every 8 h likely would achieve an AUC0–8 of approximately 715 μg · h/ml, allowing for treatment of systemic infections due to Enterobacteriaceae with MIC values up to 16 mg/liter and 8 mg/liter for stasis and 1-log kill, respectively. Ideally, these PK/PD targets should be validated in humans and correlated with clinical outcomes.
In summary, the results of this study provide important information on the time course and magnitude of plasma and urine concentrations of fosfomycin following both oral ingestion of fosfomycin tromethamine and i.v. infusion of fosfomycin disodium. In an era of increasing bacterial resistance, parenteral antimicrobial agents with unique mechanisms of action are needed to treat patients with serious infections. The broad in vitro activity of fosfomycin against Gram-positive and Gram-negative pathogens, including those that are MDR, and the systemic exposure after i.v. administration suggest its potential to be an effective agent for the treatment of serious infections in the United States as it has been for years outside the United States. The adequate pharmacokinetic exposure and safety profile of fosfomycin after oral and i.v. administration support further investigation in the target patient population. Results of the recently completed phase III trial (ClinicalTrials registration no. NCT02753946) will provide support to the suitability of i.v. fosfomycin disodium for the treatment of complicated urinary tract infections and pyelonephritis.
MATERIALS AND METHODS
Study design and subjects.
This was a phase I, randomized, open-label, three-period crossover, single-dose study that evaluated the safety, tolerability, and pharmacokinetics of intravenous (ZTI-01; Zavante Therapeutics, Inc., San Diego, CA) and oral (Monurol; Forest Pharmaceuticals, Inc., St. Louis, MO) fosfomycin in healthy adult subjects. This study was approved by the Western Institutional Review Board and conducted in accordance with Good Clinical Practices at Quintiles Phase One Services (Overland Park, KS). The Division of Microbiology and Infectious Diseases (DMID), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) provided sponsorship and pharmacovigilance and appointed the independent Safety Monitoring Committee (SMC). Written informed consent was obtained from each subject prior to the conduct of any study-related procedures.
Inclusion criteria included healthy male or female subjects between 18 and 45 years of age with no clinically significant findings on medical history, physical examination, vital signs, 12-lead electrocardiogram (ECG), or clinical laboratory evaluation. Subjects of childbearing potential were required to use protocol-defined acceptable methods of birth control. Eligible body weight was >50 kg with a body mass index of >18 and <30 kg/m2. Subjects must not have used nicotine-containing products within the 30 days preceding study day one. Grapefruit-containing products and alcoholic beverages were prohibited in the 48 h preceding study day one.
Exclusion criteria included any surgical or medical condition that could have interfered with study drug absorption, distribution, metabolism, or excretion or placed the subject at increased risk during study participation. Specifically, subjects with any history of or current significant allergic conditions, cancer, or gastrointestinal disease were excluded, along with those screening positive for HIV, hepatitis B, or hepatitis C. Subjects with a history of alcohol abuse in the previous 12 months or a positive urine drug or alcohol screen at enrollment were not eligible. Subjects could not have had a history of intolerance or hypersensitivity to phosphonic acid derivative antibiotics. Prescription and nonprescription drugs (including vitamins and herbal or dietary supplements) were not allowed within 30 and 7 days, respectively, prior to day 1. Subjects could not have donated blood within a 60-day period or consumed more than 300 mg of caffeine within the 7 days prior to study participation.
Subjects were enrolled in study drug administration sequences in parallel so that each subject received all three regimens in a randomized, crossover fashion. The three regimens were the following: regimen A, 1 g i.v.; regimen B, 8 g i.v.; and regimen C, 3 g per os (p.o.). The three administration sequences were the following: 1, regimens A, B, and C; 2, regimens B, C, and A; and 3, regimens C, A, and B. Intravenous doses of fosfomycin were administered as a 1-h infusion, and oral fosfomycin was delivered as a powder sachet in water. Study drug administration was completed under fasted conditions for both i.v. and oral regimens. Subjects were confined to the study center the day before dosing in each sequence through 48 h postdose. Each administration sequence was separated by a 7-day washout period.
Pharmacokinetic samples.
For regimens A and B, blood samples for measurement of fosfomycin concentrations were collected before and at 1.08, 1.25, 1.5, 2, 3, 4, 5, 6, 8, 12, 18, 24, 36, and 48 h after the start of the infusion. Urine samples for measurement of fosfomycin concentrations were collected before and at intervals of 0 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 36, and 36 to 48 h after the start of the infusion. For regimen C, blood samples were collected before and at 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 18, 24, 36, and 48 h after ingestion. Urine samples were collected before and at intervals of 0 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 36, and 36 to 48 h after ingestion. Blood was collected and centrifuged, and plasma was separated for bioanalytical analysis. Plasma samples were flash frozen within 60 min of collection and stored at −70°C until shipment. Urine samples were collected and stored at ≤4°C during collection intervals. After completion of the collection interval, 3-ml aliquots of urine were extracted, flash frozen, and stored at −70°C until shipment.
Bioanalytical procedures for determination of fosfomycin concentrations.
Concentrations of fosfomycin in plasma and urine samples were measured by American International Biotechnology, LLC (AI BioTech, Richmond, VA), via validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) using the procedure described by Li et al. (34). Dilutional linearity was demonstrated throughout the range of plasma and urine concentrations in the samples tested with a percent relative error of less than 11%.
The lower and upper limits of quantitation for fosfomycin in human plasma samples were 0.556 and 9.270 μg/ml, respectively. A total of 1,289 unique samples were analyzed in 15 analytical runs, which all met acceptance criteria for standard curve and quality control (QC) samples. The accuracy of the method was determined by comparing the mean measured concentrations with theoretical concentrations of each analyte in the QC samples. The overall mean absolute percent deviation from theoretical values was 2.69%. The intra-assay precision was determined from quality control samples at the low, medium, and high end of the calibration curve and demonstrated percent relative standard deviations (%RSD) ranging from 2.79 to 4.97%. Interassay precision and accuracy determined from standard curves prepared independently demonstrated %RSD ranging from 3.50 to 7.53%.
The lower and upper limits of quantitation for fosfomycin in human urine samples were 0.741 and 9.270 μg/ml, respectively. A total of 602 unique samples were analyzed in 10 runs which met acceptance criteria for standard curve and QC samples. The overall mean absolute percent deviation from theoretical values was 2.78%. The intra-assay precision was determined from quality control samples at the low, medium, and high end of the calibration curve and demonstrated %RSD ranging from 2.24 to 4.65%. Interassay precision and accuracy determined from standard curves prepared independently demonstrated %RSD ranging from 3.76 to 6.60%.
Pharmacokinetic analysis.
Noncompartmental analyses (WinNonlin, version 7; Pharsight Corporation, Cary, NC) were used to generate pharmacokinetic parameters of each subject for fosfomycin in plasma. Reported parameters following i.v. administration of fosfomycin included peak plasma concentration (Cmax), time of maximum concentration (Tmax), volume of distribution (V), clearance (CL), and elimination half-life (t1/2). Reported parameters following oral administration of fosfomycin tromethamine included fraction bioavailable (F%), peak plasma concentration (Cmax), time of maximum concentration (Tmax), volume of distribution (V/F), clearance (CL/F), and elimination half-life (t1/2). The AUC was calculated with the linear trapezoidal method. Reported parameters for fosfomycin in human urine following i.v. or oral administration included amount of drug excreted during the urine collection interval (Ae), cumulative amount excreted from time zero (Cum Ae), fraction of dose excreted during the collection interval (fe), cumulative fraction of dose excreted from time zero (Cum fe), and renal clearance (CLR).
Laboratory and safety assessment.
Safety was monitored by clinical laboratory tests, physical examination, 12-lead ECGs, vital signs, and monitoring of adverse events. Safety evaluations were conducted at screening, admission to the study center, and on day 3 of all three study drug administration regimens. The investigators assessed subjects for the occurrence of adverse events throughout the study along with their severity, as assessed via Common Terminology Criteria for Adverse Events (CTCAE) (35), and their relationship with study drug. A safety monitoring committee of independent evaluators was also appointed to monitor subjects' safety.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under contract number HHSN272200800024C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
E.W. has no conflict of interest to disclose. K.A.R. serves as a scientific advisor for Zavante Therapeutics, Inc. E.J.E.-G. is an employee of Zavante Therapeutics, Inc.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00775-17.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States. Available from http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf Accessed 26 January 2015.
- 2.Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. 2016. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents 47:269–285. doi: 10.1016/j.ijantimicag.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. 2016. Fosfomycin. Clin Microbiol Rev 29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falagas ME, Vouloumanou EK, Togias AG, Karadima M, Kapaskelis AM, Rafailidis PI, Athanasiou S. 2010. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 65:1862–1877. doi: 10.1093/jac/dkq237. [DOI] [PubMed] [Google Scholar]
- 5.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis 46:1069–1077. doi: 10.1086/527442. [DOI] [PubMed] [Google Scholar]
- 7.Rudenko N, Dorofeyev A. 2005. Prevention of recurrent lower urinary tract infections by long-term administration of fosfomycin trometamol. Double blind, randomized, parallel group, placebo controlled study. Arzneimittelforschung 55:420–427. [DOI] [PubMed] [Google Scholar]
- 8.Pullukcu H, Tasbakan M, Sipahi OR, Yamazhan T, Aydemir S, Ulusoy S. 2007. Fosfomycin in the treatment of extended spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infections. Int J Antimicrob Agents 29:62–65. doi: 10.1016/j.ijantimicag.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Qiao LD, Zheng B, Chen S, Yang Y, Zhang K, Guo HF, Yang B, Niu YJ, Wang Y, Shi BK, Yang WM, Zhao XK, Gao XF, Chen M. 2013. Evaluation of three-dose fosfomycin tromethamine in the treatment of patients with urinary tract infections: an uncontrolled, open-label, multicentre study. BMJ Open 3:e004157. doi: 10.1136/bmjopen-2013-004157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florent A, Chichmanian RM, Cua E, Pulcini C. 2011. Adverse events associated with intravenous fosfomycin. Int J Antimicrob Agents 37:82–83. doi: 10.1016/j.ijantimicag.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Meissner A, Haag R, Rahmanzadeh R. 1989. Adjuvant fosfomycin medication in chronic osteomyelitis. Infection 17:146–151. doi: 10.1007/BF01644014. [DOI] [PubMed] [Google Scholar]
- 12.Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. 2010. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin Microbiol Infect 16:184–186. doi: 10.1111/j.1469-0691.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- 13.Mirakhur A, Gallagher MJ, Ledson MJ, Hart CA, Walshaw MJ. 2003. Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J Cyst Fibros 2:19–24. doi: 10.1016/S1569-1993(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 14.Nissen LR, Jacobsen J, Ravn TJ, Wahlgreen C, Auning-Hansen H. 1986. Fosfomycin-ampicillin versus gentamicin-ampicillin in the treatment of critically ill patients with pneumonia. Infection 14:246–249. doi: 10.1007/BF01644272. [DOI] [PubMed] [Google Scholar]
- 15.Portier H, Kazmierczak A, Lucht F, Tremeaux JC, Chavanet P, Duez JM. 1985. Cefotaxime in combination with other antibiotics for the treatment of severe methicillin-resistant staphylococcal infections. Infection 13(Suppl 1):S123–S128. doi: 10.1007/BF01644232. [DOI] [PubMed] [Google Scholar]
- 16.Ueda T, Masaoka T, Shibata H, Nagai K, Kanamaru A, Horiuchi A, Hasegawa H, Kitani T, Taniguchi N, Yonezawa T, Tsubakio T, Kawagoe H, Shinohara Y. 1983. Clinical evaluation of high dose intravenous injection of fosfomycin on the severe infections associated with the treatment of haematological disorders. Jpn J Antibiot 36:311–315. [PubMed] [Google Scholar]
- 17.Forest Pharmaceuticals, Inc. 2007. Monurol (fosfomycin tromethamine) package insert. Forest Pharmaceuticals, Inc., St. Louis, MO: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf Accessed 11 May 2015. [Google Scholar]
- 18.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. 2009. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 34:506–515. doi: 10.1016/j.ijantimicag.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Goto M, Sugiyama M, Nakajima S, Yamashina H. 1981. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob Agents Chemother 20:393–397. doi: 10.1128/AAC.20.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frossard M, Joukhadar C, Erovic BM, Dittrich P, Mrass PE, Van Houte M, Burgmann H, Georgopoulos A, Muller M. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob Agents Chemother 44:2728–2732. doi: 10.1128/AAC.44.10.2728-2732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borsa F, Leroy A, Fillastre JP, Godin M, Moulin B. 1988. Comparative pharmacokinetics of tromethamine fosfomycin and calcium fosfomycin in young and elderly adults. Antimicrob Agents Chemother 32:938–941. doi: 10.1128/AAC.32.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergan TTS, Albini E. 1993. Pharmacokinetic profile of fosfomycin trometamol. Chemotherapy (Basel) 29:297–301. doi: 10.1159/000239140. [DOI] [PubMed] [Google Scholar]
- 23.Bergan T. 1990. Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection 18:65–69. doi: 10.1007/BF01641417. [DOI] [PubMed] [Google Scholar]
- 24.Segre G, Bianchi E, Cataldi A, Zannini G. 1987. Pharmacokinetic profile of fosfomycin trometamol (Monuril). Eur Urol 13:56–63. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes NJ, Gardiner BJ, Neely MN, Grayson ML, Ellis AG, Lawrentschuk N, Frauman AG, Maxwell KM, Zembower TR, Scheetz MH. 2015. Optimal timing of oral fosfomycin administration for pre-prostate biopsy prophylaxis. J Antimicrob Chemother 70:2068–2073. doi: 10.1093/jac/dkv067. [DOI] [PubMed] [Google Scholar]
- 26.Gardiner BJ, Mahony AA, Ellis AG, Lawrentschuk N, Bolton DM, Zeglinski PT, Frauman AG, Grayson ML. 2014. Is fosfomycin a potential treatment alternative for multidrug-resistant gram-negative prostatitis? Clin Infect Dis 58:e101–. doi: 10.1093/cid/cit704. [DOI] [PubMed] [Google Scholar]
- 27.Yanez JA, Remsberg CM, Sayre CL, Forrest ML, Davies NM. 2011. Flip-flop pharmacokinetics–delivering a reversal of disposition: challenges and opportunities during drug development. Ther Deliv 2:643–672. doi: 10.4155/tde.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrison KL, Sahin S, Benet LZ. 2015. Few drugs display flip-flop pharmacokinetics and these are primarily associated with classes 3 and 4 of the BDDCS. J Pharm Sci 104:3229–3235. doi: 10.1002/jps.24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishizawa T, Sadahiro S, Hosoi K, Tamai I, Terasaki T, Tsuji A. 1992. Mechanisms of intestinal absorption of the antibiotic, fosfomycin, in brush-border membrane vesicles in rabbits and humans. J Pharmacobiodyn 15:481–489. doi: 10.1248/bpb1978.15.481. [DOI] [PubMed] [Google Scholar]
- 30.Grif K, Dierich MP, Pfaller K, Miglioli PA, Allerberger F. 2001. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J Antimicrob Chemother 48:209–217. doi: 10.1093/jac/48.2.209. [DOI] [PubMed] [Google Scholar]
- 31.Docobo-Perez F, Drusano GL, Johnson A, Goodwin J, Whalley S, Ramos-Martin V, Ballestero-Tellez M, Rodriguez-Martinez JM, Conejo MC, van Guilder M, Rodriguez-Bano J, Pascual A, Hope WW. 2015. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 59:5602–5610. doi: 10.1128/AAC.00752-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusanya OO, Bhavnani SM, Forrest A, Ambrose PG. 2015. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 59:7170–7177. doi: 10.1128/AAC.04955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepak AJ, Zhao M, VanScoy B, Taylor DS, Ellis-Grosse E, Ambrose PG, Andes DR. 2017. In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (fosfomycin for injection) in the neutropenic murine thigh infection model against E. coli, K. pneumoniae, and P. aeruginosa. Antimicrob Agents Chemother doi: 10.1128/aac.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Chen X, Dai X, Chen H, Zhong D. 2007. Rapid and selective liquid chromatographic/tandem mass spectrometric method for the determination of fosfomycin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 856:171–177. doi: 10.1016/j.jchromb.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 35.Anonymous. 2015. Common terminology criteria for adverse events (CTCAE), version 4.0. U.S. Department of Health and Human Services. National Institutes of Health, National Cancer Institute, Washington, DC; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Accessed 1 June 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


