ABSTRACT
OXA-244 is a single-point-mutant derivative of OXA-48 displaying reduced carbapenemase activity. Here, we report the microbiological features of seven OXA-244-producing Escherichia coli isolates. Only one isolate grew on ChromID Carba Smart medium (bioMérieux), but six of the seven isolates grew on ChromID extended-spectrum-β-lactamase (ESBL) medium (bioMérieux), as they coproduced an ESBL and/or a plasmid-encoded cephalosporinase. The production of a carbapenemase was detected in 57.1%, 71.4%, 71.4%, and 100% of the E. coli isolates using the Carba NP test, the Rapidec Carba NP test (bioMérieux), a matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) hydrolysis assay (Bruker), and the OXA-48 K-SeT assay (Coris BioConcept), respectively. Our results indicate that OXA-244-producing E. coli isolates are difficult to detect, which may lead to their silent spread.
KEYWORDS: OXA-244, detection, screening, tests, carbapenemase activity, OXA-48-like
TEXT
The emergence of carbapenemase-producing Enterobacteriaceae (CPE) is becoming a major clinical issue (1). In this context, expert committees have set up guidelines to prevent the spread of CPEs (2). Thus, it is recommended to screen individuals at risk of being colonized, especially patients who were hospitalized previously in countries with high CPE prevalence, in order to isolate colonized patients as soon as possible, to implement contact precautions, and to strongly recommend cohorting with dedicated nursing staff. Screening procedures involving plating of rectal swabs on screening medium to detect all carbapenem-resistant isolates, with high sensitivity and sufficient specificity to rule out CPE carriage, should be performed as soon as possible (2, 3).
OXA-244, a single-point-mutant derivative of OXA-48 with reduced carbapenemase activity, was initially observed in a Spanish Klebsiella pneumoniae isolate (4). Subsequently, it was fortuitously found in a CTX-M-producing Escherichia coli isolate in Germany (5), in four Enterobacter aerogenes isolates in Russia (6), in E. coli VAL from France (7), and in an E. coli isolate from Southeast Asia that coproduced CTX-M-14 (8). While the blaOXA-244 gene was chromosomally encoded in the E. coli VAL isolate, it was plasmid located in K. pneumoniae and E. aerogenes (4, 6, 7).
The aim of this study was to evaluate different screening approaches and confirmatory tests useful for detecting OXA-244-producing E. coli (OXA-244-Ec) isolates. In addition, we investigated the genetic relatedness of seven OXA-244-Ec isolates from different geographical origins that were received at the French National Reference Center (F-NRC) for CPEs.
In August 2015, a 44-year-old Egyptian man was admitted to the Bicêtre Hospital (Le Kremlin-Bicêtre, France) for an episode of erysipelas of his right leg, which was treated with intravenous amoxicillin. After 2 weeks, the patient was discharged with a favorable outcome. Because this patient (as a repatriated patient) was considered to be at risk for multidrug-resistant (MDR) bacterial carriage according to French CPE guidelines, rectal swabs were plated on ChromID Carba Smart medium (bioMérieux, La Balme-les-Grottes, France), a selective chromogenic biplate for screening for CPE, and ChromID ESBL medium (bioMérieux), a chromogenic plate for screening for extended-spectrum-β-lactamase (ESBL)-producing Enterobacteriaceae. The ChromID Carba Smart medium remained sterile, while E. coli 85H4 grew on the ChromID ESBL plate (Table 1). Antimicrobial susceptibilities, as determined by the disk diffusion technique on Mueller-Hinton agar (Bio-Rad, Marnes-La-Coquette, France) and interpreted according to the EUCAST breakpoints, as updated in 2016 (http://www.eucast.org/clinical_breakpoints/), revealed that the E. coli 85H4 isolate was highly resistant to temocillin (absence of an inhibition zone) and displayed reduced susceptibility to ertapenem (zone diameter of 19 mm), thus requiring confirmatory testing for carbapenemase production, as recommended by EUCAST (9). The results of the Rapidec Carba NP test (bioMérieux) were positive for E. coli 85H4, although no colony grew on the ChromID Carba Smart plate (10). In-house PCR sequencing, as described previously (11), revealed the presence of a gene coding for OXA-244, a R214G OXA-48 variant (Table 1) (4). Since OXA-244-Ec 85H4 also produced an ESBL, the ChromID ESBL medium was used to screen 34 contact patients for the Egyptian patient; for 4 patients, positive E. coli cultures on ChromID ESBL medium were obtained. Antibiotic susceptibility testing and in-house PCR testing of five independent colonies revealed that none carried the blaOXA-244 gene (data not shown).
TABLE 1.
Clinical and phenotypic characteristics of the OXA-244-Ec isolates
| Isolate | STa | Cloneb | Approximate plasmid size (kb)c | Year of isolation | Source of isolation | Origin | MIC (mg/liter) and susceptibilityd |
Inhibition zone (mm) for MOXe | Test resultsf |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMP | MEM | ETP | TEM | OXA-48 K-SeT | Carba NP | Rapidec Carba NP | MALDI-TOF MS | ChromID ESBL | ChromID Carba Smartg | ||||||||
| 86J1 | ST-361 | 1 | 160, 110, 70 | 2015 | Rectal | Egypt | 0.5 (S) | 0.5 (S) | 2 (R) | >1,024 | 7 | + | + | + | + | + | +/− |
| 62D3 | ST-1722 | 2 | Abs | 2014 | Urine | Unknown | 0.38 (S) | 0.38 (S) | 1 (I) | 128 | 21 | + | + | + | + | + | −/− |
| 69E6 | ST-38 | 3 | Abs | 2014 | Rectal | Unknown | 0.25 (S) | 0.38 (S) | 3 (R) | 128 | 20 | + | +/− | + | + | + | −/− |
| 78B5 | ST-38 | 3 | Abs | 2015 | Rectal | Unknown | 0.38 (S) | 0.5 (S) | 3 (R) | 256 | 21 | + | + | + | + | + | −/− |
| VAL (4 isolates) | ST-38 | 3 | 120, 60, 10 | 2013 | Urine | France | 0.5 (S) | 0.75 (S) | 2 (R) | 96 | 21 | + | − | +/− | − | − | −/− |
| 73G4 | ST-3541 | 4 | 115 | 2015 | Unknown | Egypt | 0.25 (S) | 0.19 (S) | 0.75 (I) | 128 | 20 | + | + | + | + | + | −/− |
| 85H4 | ST-3541 | 4 | 115 | 2015 | Rectal | Egypt | 0.38 (S) | 0.25 (S) | 2 (R) | 384 | 20 | + | +/− | +/− | − | + | −/− |
Overview of STs identified by the MLST 1.8 server (19).
Rep-PCR analysis was performed using the DiversiLab technique.
Abs, absent.
Susceptible (S), intermediate (I), and resistant (R) interpretations were according to the 2016 EUCAST guidelines (http://www.eucast.org/clinical_breakpoints/). IMP, imipenem; MEM, meropenem; ETP, ertapenem; TEM, temocillin.
MOX, moxalactam.
+, positive test or culture result; −, negative test or culture result; +/−, equivocal test result.
Bacterial growth was checked on both sides of the biplate (ChromID OXA-48/ChromID Carba).
Phenotypic characterization of OXA-244-Ec.
The ability to reliably detect OXA-244-Ec using ChromID Carba Smart and ChromID ESBL plates and to confirm the presence of a carbapenemase was further investigated with E. coli VAL (7) and five other OXA-244-Ec isolates referred to the F-NRC for CPEs. The susceptibilities to different antibiotics of the seven OXA-244-Ec isolates are shown in Table 2. For all OXA-244-Ec isolates, 100 μl of a 0.5 McFarland solution was plated on ChromID ESBL and ChromID Carba Smart media. Only one of the seven isolates did not grow on the ChromID ESBL medium (Table 1); that isolate was susceptible to cephalosporins, which explained the absence of growth on the ChromID ESBL medium (Table 2). In contrast, only one isolate grew on the OXA-48 side of the ChromID Carba Smart plate (Table 1) and none grew on the Carba side; that strain displayed the highest MICs for temocillin (>1,024 mg/liter) and for moxalactam, a β-lactam classically used for testing impermeability problems (Table 1) (12). All of the OXA-244-Ec isolates exhibited only slightly decreased susceptibility to carbapenems, which explained the absence of growth on the Carba side of the ChromID Carba Smart plate (Table 1). The biochemical confirmation tests used for carbapenemase detection were positive for 57.1% (4/7 isolates), 71.4% (5/7 isolates), 71.4% (5/7 isolates), and 100% (7/7 isolates) of the isolates using the Carba NP test (13), the Rapidec Carba NP test (bioMérieux) (10), the MBT STAR-BL test, a commercial matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based assay (Maldi-Biotyper; Bruker, Illkirch, France), and a lateral flow immunoassay (LFIA) called the OXA-48 K-SeT assay (Coris BioConcept, Gembloux, Belgium) (14) respectively (Table 1).
TABLE 2.
Resistance genes and phenotypic susceptibility of the OXA-244-Ec isolates
| Isolate | Acquired resistance genesa |
Observed phenotypeb |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Lac | AMG | CST | FOS | C | FQ | SUL | TR | TET | AMX | AMC | CTX | AMGc | CST | FOS | C | FQ | SXT | TET | |
| 86J1 | blaOXA-244, blaTEM-1b, blaCMY-42 | aph3-1b, aph6-1d, aadA1 | None | None | None | None | sul1, sul2 | dfrA1 | tetB | R | R | R | S | S | S | S | R | R | R |
| 62D3 | blaOXA-244, blaCMY-2 | None | None | None | None | None | None | None | None | R | R | R | S | S | S | S | S | S | S |
| 69E6 | blaOXA-244, blaTEM-1b, blaCTX-M-14b | aadA1 | None | None | catA1 | None | None | dfrA1 | None | R | R | R | S | S | S | R | S | R | S |
| 78B5 | blaOXA-244, blaTEM-1b, blaCTX-M-14b | aadA1 | None | None | catA1 | None | None | dfrA1 | None | R | R | R | S | S | S | R | S | R | S |
| VAL (4 isolates) | blaOXA-244, blaTEM-1b | aph3-1b, aph6-1d, aadA1 | None | None | catA1 | None | sul2 | dfrA1, dfrA14 | tetB, tetD | R | R | S | S | S | S | R | S | R | R |
| 73G4 | blaOXA-244, blaTEM-1b, blaCTX-M-27 | aph3-1b, aph6-1d, aph3-1a | None | None | None | None | sul2 | dfrA14 | tetB | R | R | R | S | S | S | S | S | R | R |
| 85H4 | blaOXA-244, blaTEM-1b, blaCTX-M-27 | aph3-1b, aph6-1d, aph3-1a | None | None | None | None | sul2 | dfrA14 | tetB | R | R | R | S | S | S | S | S | R | R |
Overview of resistance genes detected in the isolates by ResFinder (17). β-Lac, β-lactam; AMG, aminoglycoside; CST, colistin; FOS, fosfomycin; C, chloramphenicol; FQ, fluoroquinolone; SUL, sulfonamide; TR, trimethoprim; TET, tetracycline.
Antimicrobial susceptibilities were determined by the disc diffusion technique and interpreted according to the EUCAST breakpoints (http://www.eucast.org/clinical_breakpoints/). S, susceptible; R, resistant; AMX, amoxicillin; AMC, amoxicillin-clavulanic acid; CTX, cefotaxime; SXT, sulfamethoxazole-trimethoprim.
The aminoglycoside tested were amikacin, gentamicin, tobramycin, and netilmicin.
Molecular detection of resistance genes.
Using the commercially available Xpert Carba-R v2 assay, as recommended by the manufacturer (Cepheid, Toulouse, France) (15, 16), blaOXA-48-like genes were detected in all seven OXA-244-Ec isolates. Whole-genome sequencing (WGS) was performed to determine the resistome of these OXA-244-Ec isolates using the ResFinder server (http://cge.cbs.dtu.dk/services/ResFinder-2.1) (17) (Table 2). A good correlation between the genetic profile and the phenotypic resistance profile for routinely tested β-lactams, colistin, fosfomycin, phenicol, sulfonamide-trimethoprim, and tetracycline antibiotics was found (Table 2). For aminoglycosides, only netilmicin, amikacin, tobramycin, and gentamicin were tested. However, different aminoglycoside resistance genes (e.g., aph3-1a, aph3-1b, aph6-1d, and aadA1), which confer resistance to other aminoglycosides that were not tested because they were not clinically relevant, were found in some of the isolates. Of note, isolate OXA-244-Ec 86J1 was resistant to fluoroquinolones due to substitutions in the quinolone resistance determinant region, as revealed by analysis with the RAST server (rast.nmpdr.org) (18) and comparison with that of K. pneumoniae ATCC 13883 (GenBank accession no. DQ673325), i.e., in codons 83 (Ser83-Leu) and 87 (Asp87-Asn) for GyrA and in codons 80 (Ser80-Ile) and 84 (Glu84-Gly) for ParC, which are known to confer fluoroquinolone resistance.
Among the seven isolates, only E. coli VAL had no other β-lactam resistance gene besides the blaOXA-244 gene; consequently, that strain was susceptible to cephalosporins (7). For the remaining six isolates, an ESBL gene (blaCTX-M-14 or blaCTX-M-27) or a plasmid-encoded cephalosporinase gene (blaCMY-42 or blaCMY-2) was always associated with the blaOXA-244 gene (Table 2).
Genetic relatedness of OXA-244-Ec isolates.
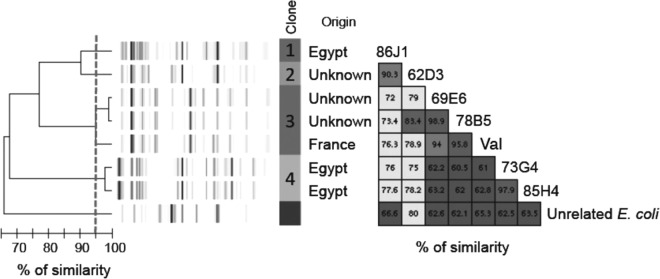
The seven OXA-244-Ec isolates corresponded to four different clones, as revealed by repetitive element sequence-based PCR (rep-PCR) using the DiversiLab system (bioMérieux), following the manufacturer's recommendations. Two distinct clones were identified among the Egyptian isolates (Fig. 1 and Table 1). Multilocus sequence typing (MLST) results deduced from WGS data using the MLST 1.8 server (https://cge.cbs.dtu.dk/services/MLST) (19) confirmed the rep-PCR results, as each rep-PCR pattern corresponded to a different sequence type (ST), i.e., ST-38, ST-361, ST-1722, or ST-3541 (Table 1).
FIG 1.
Rep-PCR analysis using the DiversiLab technique, showing a dendrogram and computer-generated image of rep-PCR banding patterns of OXA-244-Ec isolates and an E. coli isolate of an unrelated strain. As recommended by the manufacturer, a cutoff value of 95% similarity defined a cluster.
Genetic environment and support of blaOXA-244 genes.
For three strains (78B5, 62D3, and 69E6), no plasmids could be detected after electrophoresis of Kieser-extracted DNA (11) (see Fig. S1 in the supplemental material). Electroporation of the extracted plasmids, as described previously (11), yielded E. coli TOP10 transformants for only three strains (86J1, 85H4, and 73G4). However, only ESBL/plasmid-encoded blaAMP-C genes were found in those transformants. Thus, all of these findings suggest a chromosomal location for the blaOXA-244 gene. PCR mapping of the blaOXA-244 gene flanking sequences showed that all were bracketed by two IS1R copies, forming an IS1R-made composite transposon named Tn51098 (Fig. S2A). In all isolates, although they belonged to different rep-PCR patterns or STs, Tn51098 was inserted into a gene encoding an intrinsic endonuclease from E. coli, as described previously (7) (Fig. S2B). Dissemination of E. coli isolates harboring a chromosomally located blaOXA-48-like gene has recently been linked to one ST, namely, ST38 (20, 21). In our study, however, four different STs that have integrated the blaOXA-244 carbapenemase gene into the chromosome were found, indicating that diffusion could be more related to the mobility of blaOXA-244-carrying IS1R-made composite transposons (Tn51098) than to clonal expansion.
Conclusions.
Detection of CPEs remains a challenge for clinical microbiology laboratories (22), especially with OXA-244-Ec isolates, since they do not grow on ChromID Carba Smart plates, one of the most used types of medium for the screening of CPEs (7, 23–25). However, as most OXA-244-Ec isolates also produced an ESBL, they could grow on ChromID ESBL medium. In the absence of expanded-spectrum hydrolyzing enzymes (such as in E. coli VAL), detection of OXA-244-Ec strains would rely only on molecular tests directly with rectal swabs (15) and on LFIA tests, such as OXA-48 K-SeT, with cultured bacteria (14). In France, OXA-244-Ec strains are still rare, i.e., 0%, 0.3% (two isolates), 0.2% (two isolates), 0.6% (six isolates), and 0.7% (eight isolates) of all OXA-48-like enzymes in 2012, 2013, 2014, 2015, and 2016, respectively, but whether this indicates a real low prevalence or is the result of underdetection is not known.
Accession number(s).
The E. coli genome sequences of isolates 86J1, 62D3, 69E6, 78B5, 35J9, 73G4, and 85H4, used in this study, were deposited in GenBank under the accession numbers MKGU00000000, MKGY00000000, MKGZ00000000, MKGT00000000, MKGX00000000, MKGV00000000, and MKGW00000000, respectively.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by grants from Assistance Publique-Hôpitaux de Paris, Santé Publique France, and the French Ministry of Education and Research through the Université Paris Sud. T.N., N.F., and L.D. are members of the Laboratory of Excellence Lermit, supported by a grant from the Agence Nationale pour la Recherche (grant ANR-10-LABX-33).
L.D. holds an international patent for the Carba NP test that was filed on behalf of INSERM Transfert and subsequently licensed to bioMérieux.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00818-17.
REFERENCES
- 1.Doi Y, Paterson DL. 2015. Carbapenemase-producing Enterobacteriaceae. Semin Respir Crit Care Med 36:74–84. doi: 10.1055/s-0035-1544208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundmann H, Livermore DM, Giske CG, Canton R, Rossolini GM, Campos J, Vatopoulos A, Gniadkowski M, Toth A, Pfeifer Y, Jarlier V, Carmeli Y. 2010. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill 15(46):pii=19711 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19711. [DOI] [PubMed] [Google Scholar]
- 3.Viau R, Frank KM, Jacobs MR, Wilson B, Kaye K, Donskey CJ, Perez F, Endimiani A, Bonomo RA. 2016. Intestinal carriage of carbapenemase-producing organisms: current status of surveillance. Methods Clin Microbiol Rev 29:1–27. doi: 10.1128/CMR.00108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oteo J, Hernández JM, Espasa M, Fleites A, Sáez D, Bautista V, Pérez-Vázquez M, Fernández-García MD, Delgado-Iribarren A, Sánchez-Romero I, García-Picazo L, Miguel MD, Solís S, Aznar E, Trujillo G, Mediavilla C, Fontanals D, Rojo S, Vindel A, Campos J. 2013. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J Antimicrob Chemother 68:317–321. doi: 10.1093/jac/dks383. [DOI] [PubMed] [Google Scholar]
- 5.Valenza G, Nickel S, Pfeifer Y, Eller C, Krupa E, Lehner-Reindl V, Höller C. 2014. Extended-spectrum-β-lactamase-producing Escherichia coli as intestinal colonizers in the German community. Antimicrob Agents Chemother 58:1228–1230. doi: 10.1128/AAC.01993-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fursova NK, Astashkin EI, Knyazeva AI, Kartsev NN, Leonova ES, Ershova ON, Alexandrova IA, Kurdyumova NV, Sazikina SY, Volozhantsev NV, Svetoch EA, Dyatlov IA. 2015. The spread of blaOXA-48 and blaOXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Ann Clin Microbiol Antimicrob 14:46. doi: 10.1186/s12941-015-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potron A, Poirel L, Dortet L, Nordmann P. 2016. Characterisation of OXA-244, a chromosomally-encoded OXA-48-like β-lactamase from Escherichia coli. Int J Antimicrob Agents 47:102–103. doi: 10.1016/j.ijantimicag.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 8.van Hattem JM, Arcilla MS, Bootsma MC, van Genderen PJ, Goorhuis A, Grobusch MP, Molhoek N, Oude Lashof AM, Schultsz C, Stobberingh EE, Verbrugh HA, de Jong MD, Melles DC, Penders J. 2016. Prolonged carriage and potential onward transmission of carbapenemase-producing Enterobacteriaceae in Dutch travelers. Future Microbiol 11:857–864. doi: 10.2217/fmb.16.18. [DOI] [PubMed] [Google Scholar]
- 9.Giske CG, Martinez-Martinez L, Cantón R, Stefani S, Skov R, Glupczynski Y, Nordmann P, Wootton M, Miriagou V, Simonsen GS, Zemlickova H, Cohen-Stuart J, Gniadkowski M. 2013. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. EUCAST, Basel, Switzerland: http://www.eucast.org/resistance_mechanisms/. [Google Scholar]
- 10.Dortet L, Agathine A, Naas T, Cuzon G, Poirel L, Nordmann P. 2015. Evaluation of the RAPIDEC® CARBA NP, the Rapid CARB Screen® and the Carba NP test for biochemical detection of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 70:3014–3022. doi: 10.1093/jac/dkv213. [DOI] [PubMed] [Google Scholar]
- 11.Cuzon G, Naas T, Truong H, Villegas M-V, Wisell KT, Carmeli Y, Gales AC, Navon-Venezia S, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase blaKPC-2. Gene Emerg Infect Dis 16:1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsu Y, Murakami K, Nishikawa T. 1981. Penetration of moxalactam into its target proteins in Escherichia coli K-12: comparison of a highly moxalactam resistant mutant with its parent strain. Antimicrob Agents Chemother 20:613–619. doi: 10.1128/AAC.20.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dortet L, Bréchard L, Poirel L, Nordmann P. 2014. Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP test. J Med Microbiol 63:772–776. doi: 10.1099/jmm.0.071340-0. [DOI] [PubMed] [Google Scholar]
- 14.Dortet L, Jousset A, Sainte-Rose V, Cuzon G, Naas T. 2016. Prospective evaluation of the OXA-48 K-SeT assay, an immunochromatographic test for the rapid detection of OXA-48-type carbapenemases. J Antimicrob Chemother 71:1834–1840. doi: 10.1093/jac/dkw058. [DOI] [PubMed] [Google Scholar]
- 15.Dortet L, Fusaro M, Naas T. 2016. Improvement of the Xpert Carba-R kit for the detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:3832–3837. doi: 10.1128/AAC.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyos-Mallecot Y, Ouzani S, Dortet L, Fortineau N, Naas T. 2017. Performance of the Xpert® Carba-R v2 in the daily workflow of a hygiene unit in a country with a low prevalence of carbapenemase-producing Enterobacteriaceae. Int J Antimicrob Agents 49:774–777. doi: 10.1016/j.ijantimicag.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Pontén T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turton JF, Doumith M, Hopkins KL, Perry C, Meunier D, Woodford N. 2016. Clonal expansion of Escherichia coli ST38 carrying a chromosomally integrated OXA-48 carbapenemase gene. J Med Microbiol 65:538–546. doi: 10.1099/jmm.0.000248. [DOI] [PubMed] [Google Scholar]
- 21.Potron A, Poirel L, Rondinaud E, Nordmann P. 2013. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro Surveill 18(31):pii=20549 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20549. [DOI] [PubMed] [Google Scholar]
- 22.Hrabák J, Chudáčkova E, Papagiannitsis CC. 2014. Detection of carbapenemases in Enterobacteriaceae: a challenge for diagnostic microbiological laboratories. Clin Microbiol Infect 20:839–853. doi: 10.1111/1469-0691.12678. [DOI] [PubMed] [Google Scholar]
- 23.Girlich D, Anglade C, Zambardi G, Nordmann P. 2013. Comparative evaluation of a novel chromogenic medium (chromID OXA-48) for detection of OXA-48 producing Enterobacteriaceae. Diagn Microbiol Infect Dis 77:296–300. doi: 10.1016/j.diagmicrobio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Oueslati S, Nordmann P, Poirel L. 2015. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother 70:1059–1063. [DOI] [PubMed] [Google Scholar]
- 25.Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, Nordmann P. 2013. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents 41:325–329. doi: 10.1016/j.ijantimicag.2012.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.