ABSTRACT
Vulvovaginal candidiasis (VVC) is a global health problem affecting ∼75% of women at least once in their lifetime. Here we examined the epidemiology of VVC in a patient cohort to identify the causative organisms associated with VVC. Biofilm-forming capacity and antifungal sensitivity profiles were also assessed. We report a shifting prevalence of Candida species with heterogeneous biofilm-forming capacity, which is associated with altered antifungal drug sensitivity.
KEYWORDS: Candida, biofilm, vulvovaginal candidiasis, fluconazole
TEXT
Fungal infections play a surprisingly substantial, yet unrecognized, health burden on the global population (1). Vulvovaginal candidiasis (VVC) is one example of such infections; it is estimated to be the most common fungal infection in a number of countries worldwide (2–4). Approximately 138 million women worldwide complain of >4 episodes of VVC per year due to treatment failure, clinically defined as recurrent VVC (RVVC) (5–7). These unresolved infections not only have a high impact on the quality of life of these women, but can also lead to further health complications (8). Candida albicans is historically reported as the predominant organism isolated from VVC, accounting for over 90% of infections (9, 10). However, evidence of a dynamic shift in yeast epidemiology has been demonstrated through an increasing prevalence of non-C. albicans species (NCAS), which accounts for 11 to 80% of infections, depending on geographical location (8). Nevertheless, C. albicans, a well-characterized biofilm-forming organism, remains a prominent pathogen in this disease. Resistance to antifungal therapy as a result of biofilm formation is a likely contributor to failed treatment. While it is widely accepted that biofilms contribute to the pathogenesis of bacterial vaginosis (BV) (11, 12), their role in VVC remains contested despite overwhelming evidence to suggest otherwise (13–15).
An anonymized series of high vaginal swabs (HVS) (n = 300) were obtained throughout April 2016 from women visiting their general practitioner (GP) and referral clinics in the NHS Greater Glasgow and Clyde area for at least the second time (16). These women were symptomatic at the time of sampling, with the causative organism identified using matrix-assisted laser desorption–ionization-time of flight mass spectrometry (MALDI-TOF MS), with Escherichia coli used pre- and post-yeast sampling to ensure accuracy of testing.
A total of 71% (n = 212) were identified as C. albicans, followed by 15% (n = 47) as Candida glabrata, 6% (n = 17) as Candida dubliniensis, and 3% (n = 10) as Candida parapsilosis (Fig. 1). The remaining 5% of isolates included Candida tropicalis, Candida lusitaniae, and Candida guilliermondii. These data are in line with recent epidemiological patterns showing a shift in NCAS prevalence within VVC (8). However, a caveat of our study is the limitation of a single geographical location, which may influence the species distribution. Future studies should include various institutes worldwide in order to fully assess the shift in VVC epidemiology.
FIG 1.
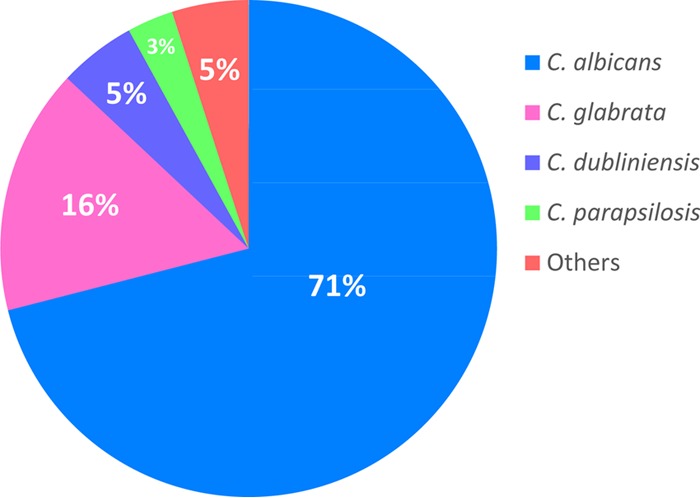
Distribution of organisms isolated from VVC patients. A total of 300 VVC isolates were identified using MALDI-TOF MS, with yeast species proportionally represented in the figure.
To determine the biofilm-forming capability of these isolates, all VVC strains (n = 300) were standardized to 1 × 106 cells/ml in RPMI 1640 and grown as biofilms in 96-well plates for 24 h. Biofilms were washed with phosphate-buffered saline (PBS) and biomass was assessed using the crystal violet (CV) assay (17). Here we have shown that vaginal isolates were able to form differential biofilms, regardless of species (Fig. 2). C. albicans displayed the greatest heterogeneity with regard to biofilm biomass, with isolates ranging from OD570 (optical density at 570 nm) of 0.008 to 1.478, with a mean of 0.416. The second most prevalent species, C. glabrata, had significantly lower biomass than C. albicans (P < 0.05) and C. dubliniensis (P < 0.01), with a mean OD570 of 0.271. This apparent biofilm heterogeneity may affect the management of VVC infections, as these sessile communities are known to be notoriously recalcitrant to antifungal therapy and biofilm heterogeneity has been shown to correlate with success of in vitro antifungal therapy (17).
FIG 2.
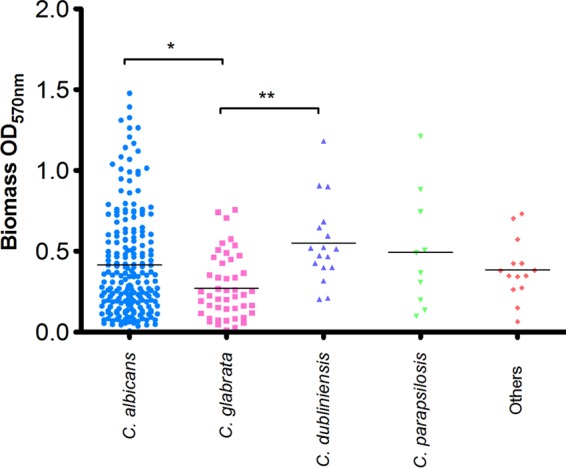
VVC isolates display varied biofilm formation. A total of 300 VVC isolates were screened for biofilm formation using a biomass stain, as described in Materials and Methods. Each isolate was tested in quadruplicate, with the mean represented by a horizontal black bar. Statistical analysis was carried out using a one-way ANOVA. *, P < 0.05; **, P < 0.01.
Planktonic and biofilm antifungal susceptibility testing was carried out as described previously to determine the MICs (18). Briefly, cells were standardized in RPMI 1640 before being treated with fluconazole (FLZ) (Sigma, Dorset, UK) for 24 h, at a range of concentrations (0.0625 to 32 mg/liter). Planktonic MIC (pMIC) was determined as the lowest concentration able to completely inhibit growth on visual inspection. Sessile MIC (sMIC) analysis were performed on 24 h preformed biofilms, with sMIC recorded at 50% inhibition using an XTT (2,3-bis[2-methoxy-4-nitro-5-sulfo-phenyl]-2H-tetrazolium-5-caboxanilide) metabolic reduction assay (19). Here we have shown that FLZ, the first line antifungal used to treat VVC, was ineffective against most isolates, with planktonic MICs ranging from < 0.0625 to >32 mg/liter (Table 1). Specifically, the pMIC50 for FLZ was 4 mg/liter for C. albicans, C. glabrata, and C. dubliniensis, although for biofilms pMIC50 was >32 mg/liter. When planktonic cells were stratified based on identification as C. albicans and NCAS it was shown that 41% and 26% of the isolates, respectively, were insensitive to FLZ at >32 mg/liter, whereas for sessile cells this rose to 51% and 56% of the isolates, respectively. Interestingly, similar susceptibility profiles were observed for C. albicans and C. glabrata, despite C. glabrata being known to be a low biofilm former (20). This reduced sensitivity in C. glabrata can be associated with its intrinsic resistance to fluconazole due to the overexpression of multidrug transporters (21).
TABLE 1.
Susceptibility profile of vaginal Candida isolates to fluconazole
| Profile parameter | Fluconazole MIC (mg/liter) fora: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
C. albicans (n = 212) |
C. glabrata (n = 47) |
C. dubliniensis (n = 17) |
C. parapsilosis (n = 10) |
Other Candida spp. (n = 14) |
||||||
| pMIC | sMIC | pMIC | sMIC | pMIC | sMIC | pMIC | sMIC | pMIC | sMIC | |
| Range | 0.0625–>32 | 0.125–>32 | <0.0625–>32 | 0.5–>32 | 0.125–>32 | 0.125–>32 | 1–>32 | 1–>32 | 0.0625–>32 | 1–>32 |
| MIC50 | 4 | >32 | 4 | >32 | 4 | >32 | 1 | 4 | 1 | >32 |
| MIC90 | >32 | >32 | >32 | >32 | >32 | >32 | 16 | >32 | >32 | >32 |
n = 300. Abbreviations: pMIC, planktonic MIC; sMIC, sessile MIC.
VVC is not a reportable disease, making epidemiological studies difficult. However, this study provides a snapshot of the species identified within a VVC population, demonstrating that NCAS are responsible for an increasing number of these infections. This corresponds with previous studies reporting an ongoing dynamic shift in yeast epidemiology (22, 23), which is potentially driven by inappropriate use of over-the-counter azoles (10). Irrespectively, C. albicans remained the most dominant species in this study, which raises the question of why a high number of isolates displayed reduced susceptibility to FLZ. We demonstrated the ability of these clinical isolates to form heterogeneous biofilms. The presence of these communities in VVC may explain why C. albicans infections remain unresponsive to FLZ therapy, an antifungal highly ineffective against C. albicans biofilms (24). We cannot discount the potential for heteroresistance phenotypes within these populations (25). The contribution of biofilms to VVC pathogenesis remains poorly understood, though many researchers are beginning to consider them to be important determinants of disease (13, 14), further emphasizing the need for research in this field. Collectively, the data from this investigation highlight the necessity for careful consideration of the causative organism in VVC, the biofilm phenotype, and its accentuated antifungal sensitivity profiles, all of which may improve antifungal treatment in this area.
REFERENCES
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Klimko N, Kozlova Y, Khostelidi S, Shadrivova O, Borzova Y, Burygina E, Vasilieva N, Denning DW. 2015. The burden of serious fungal diseases in Russia. Mycoses 58(Suppl):S58–S62. doi: 10.1111/myc.12388. [DOI] [PubMed] [Google Scholar]
- 3.Corzo-Leon DE, Armstrong-James D, Denning DW. 2015. Burden of serious fungal infections in Mexico. Mycoses 58(Suppl):S34–S44. doi: 10.1111/myc.12395. [DOI] [PubMed] [Google Scholar]
- 4.Giacomazzi J, Baethgen L, Carneiro LC, Millington MA, Denning DW, Colombo AL, Pasqualotto AC, in association with the Lp. 2016. The burden of serious human fungal infections in Brazil. Mycoses 59:145–150. doi: 10.1111/myc.12427. [DOI] [PubMed] [Google Scholar]
- 5.De Bernardis F, Arancia S, Sandini S, Graziani S, Norelli S. 2015. Studies of immune responses in Candida vaginitis. Pathogens 4:697–707. doi: 10.3390/pathogens4040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurley R, De Louvois J. 1979. Candida vaginitis. Postgrad Med J 55:645–647. doi: 10.1136/pgmj.55.647.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobel JD. 2016. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 214:15–21. doi: 10.1016/j.ajog.2015.06.067. [DOI] [PubMed] [Google Scholar]
- 8.Goncalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. 2016. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 42:905–927. doi: 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- 9.Linhares LM, Witkin SS, Miranda SD, Fonseca AM, Pinotti JA, Ledger WJ. 2001. Differentiation between women with vulvovaginal symptoms who are positive or negative for Candida species by culture. Infect Dis Obstet Gynecol 9:221–225. doi: 10.1155/S1064744901000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobel JD. 2007. Vulvovaginal candidosis. Lancet 369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 11.Jung HS, Ehlers MM, Lombaard H, Redelinghuys MJ, Kock MM. 2017. Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit Rev Microbiol. doi: 10.1080/1040841X.2017.1291579. [DOI] [PubMed] [Google Scholar]
- 12.Hardy L, Cerca N, Jespers V, Vaneechoutte M, Crucitti T. 2017. Bacterial biofilms in the vagina. Res Microbiol. doi: 10.1016/j.resmic.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL Jr, Noverr MC. 2010. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156:3635–3644. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muzny CA, Schwebke JR. 2015. Biofilms: An Underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin Infect Dis 61:601–606. doi: 10.1093/cid/civ353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobel JD. 2015. Editorial commentary: vaginal biofilm: much ado about nothing, or a new therapeutic challenge? Clin Infect Dis 61:607–608. doi: 10.1093/cid/civ358. [DOI] [PubMed] [Google Scholar]
- 16.NHS G. 2011. STI diagnostics redesign. http://www.sandyford.org/professionals/clinical-guidance/gumstis/.
- 17.Sherry L, Rajendran R, Lappin DF, Borghi E, Perdoni F, Falleni M, Tosi D, Smith K, Williams C, Jones B, Nile CJ, Ramage G. 2014. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol 14:182. doi: 10.1186/1471-2180-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherry L, Millhouse E, Lappin DF, Murray C, Culshaw S, Nile CJ, Ramage G. 2013. Investigating the biological properties of carbohydrate derived fulvic acid (CHD-FA) as a potential novel therapy for the management of oral biofilm infections. BMC Oral Health 13:47. doi: 10.1186/1472-6831-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R. 2017. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis 23:328–331. doi: 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whaley SG, Rogers PD. 2016. Azole resistance in Candida glabrata. Curr Infect Dis Rep 18:41. doi: 10.1007/s11908-016-0554-5. [DOI] [PubMed] [Google Scholar]
- 22.Brandolt TM, Klafke GB, Goncalves CV, Bitencourt LR, Martinez AM, Mendes JF, Meireles MC, Xavier MO. 2017. Prevalence of Candida spp. in cervical-vaginal samples and the in vitro susceptibility of isolates. Braz J Microbiol 48:145–150. doi: 10.1016/j.bjm.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cetin M, Ocak S, Gungoren A, Hakverdi AU. 2007. Distribution of Candida species in women with vulvovaginal symptoms and their association with different ages and contraceptive methods. Scand J Infect Dis 39:584–588. doi: 10.1080/00365540601148491. [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Wang H, Zhu L. 2016. Quercetin assists fluconazole to inhibit biofilm formations of fluconazole-resistant Candida albicans in in vitro and in vivo antifungal managements of vulvovaginal candidiasis. Cell Physiol Biochem 40:727–742. doi: 10.1159/000453134. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Ami R, Zimmerman O, Finn T, Amit S, Novikov A, Wertheimer N, Lurie-Weinberger M, Berman J. 2016. Heteroresistance to fluconazole is a continuously distributed phenotype among Candida glabrata clinical strains associated with in vivo persistence. mBio 7:e00655-16. doi: 10.1128/mBio.00655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


