ABSTRACT
Mycobacterium abscessus is a highly pathogenic drug-resistant rapidly growing mycobacterium. In this study, we evaluated the in vitro, intracellular, and in vivo activities of LCB01-0371, a novel and safe oxazolidinone derivative, for the treatment of M. abscessus infection and compared its resistance to that of other oxazolidinone drugs. LCB01-0371 was effective against several M. abscessus strains in vitro and in a macrophage model of infection. In the murine model, a similar efficacy to linezolid was achieved, especially in the lungs. We induced laboratory-generated resistance to LCB01-0371; sequencing analysis revealed mutations in rplC of T424C and G419A and a nucleotide insertion at the 503 position. Furthermore, LCB01-0371 inhibited the growth of amikacin-, cefoxitin-, and clarithromycin-resistant strains. Collectively, our data indicate that LCB01-0371 might represent a promising new class of oxazolidinones with improved safety, which may replace linezolid for the treatment of M. abscessus.
KEYWORDS: drug resistance, LCB01-0371, Mycobacterium abscessus, oxazolidinone
INTRODUCTION
Mycobacterium abscessus complex is made up of pathogenic rapidly growing mycobacteria (RGM) that are ubiquitous in soil and water (1). This species is distantly related to slow-growing mycobacterial (SGM) species, such as M. tuberculosis and M. leprae, which cause tuberculosis and leprosy, respectively, in humans. Originally, M. abscessus was considered to belong to the M. chelonae group, but in 1992, M. abscessus was reclassified as an individual species (1). M. abscessus causes a variety of infections with a high fatality rate (2, 3). Over the past 10 years, this species has been highlighted as an important human pathogen responsible for a wide spectrum of soft tissue infections, disseminated infections, and severe chronic pulmonary infections, mostly in immunosuppressed and cystic fibrosis (CF) patients (4). In South Korea and the United States, M. abscessus is the second most common cause of nontuberculous mycobacterium (NTM) lung disease, behind the M. avium complex (MAC); its prevalence is also on the rise in eastern Asian countries, including South Korea and Japan (5, 6).
M. abscessus is the most pathogenic and chemotherapy-resistant RGM (7); this species is resistant to most of the antibiotics, including antituberculosis drugs currently on the market. This is a major problem for public health care. Currently, the treatment of an M. abscessus infection of the skin or soft tissue includes a combination of an oral macrolide (e.g., clarithromycin or azithromycin) and another parenteral drug (e.g., amikacin, cefoxitin, or imipenem) for up to 6 months. However, for pulmonary infections, no antibiotic class has been shown to produce long-term sputum smear conversion (8). Therefore, the treatment of pulmonary infection is very difficult, and no appropriate drug therapy regimen has been established.
With regard to drug resistance, M. abscessus possesses a number of mechanisms that contribute to its intrinsic and acquired resistances. For example, M. abscessus contains a naturally thick and waxy mycobacterial cell envelope that acts as a physical barrier to protect it against toxic drugs. This internal protection does not allow the toxic agent to reach its target; consequently, M. abscessus can survive in the presence of many antibiotics. Furthermore, the M. abscessus genome encodes MmpL proteins and ABC-type multidrug transporters that are involved in drug efflux. This intrinsic transporter system pumps drugs out of the cell and physiologically protects the bacteria against toxic molecules (9). Moreover, M. abscessus has the ability to modify or inactivate antibiotics using enzymes. For example, M. abscessus harbors enzymes that modify aminoglycoside drugs through the relocation of acetyl or phosphate residues in crucial positions within the antibiotic, which renders them inactive. The expressed enzymes, such as rifampin ADP-ribosyltransferase, as well as a monooxygenase, may be involved in its resistance to rifampin (7). Recently, researchers also have observed that M. abscessus expresses the inducible erythromycin ribosome methyltransferase (erm) gene after treatment with a macrolide, which results in a poor response to clarithromycin and erythromycin (10). M. abscessus has already acquired antibiotic resistance via the spontaneous mutation of critical targets of antibiotics, rather than through horizontal transmission. Mutations in the peptidyltransferase-binding region of the 23S rRNA gene (rrl) and the 16S rRNA gene (rrs) have been reported to confer acquired resistance to aminoglycosides and macrolides in M. abscessus (7, 11).
Recently, linezolid was the first oxazolidinone antibacterial agent to be applied to the treatment of NTMs, including M. abscessus (12, 13). Linezolid has broad-spectrum activity against most Gram-positive bacteria, including streptococci, vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA). It is also effective in the treatment of chronic and extensively drug-resistant tuberculosis. However, the efficacy of linezolid against NTMs varies among different derivatives, and the clinical use of linezolid in patients with NTMs can result in adverse events (e.g., peripheral neuropathy and cytopenias) in more than one-third of patients, regardless of concomitant vitamin B6 use, although it can be tolerated for 6 months or longer at a once-daily 600-mg dose in a multidrug regimen (14). In addition, linezolid therapy involves the potential risks of decreased platelets, red blood cells, and white blood cell count (myelosuppression) (15). Thus, well-defined indications and the appropriate monitoring of treatment are required during the treatment course. Oxazolidinone agents with greater safety are therefore strongly desirable for the treatment of M. abscessus.
LCB01-0371, a novel oxazolidinone with a cyclic amidrazone, was synthesized by LegoChem BioSciences, Inc. (Daejeon, Republic of Korea). Recently, the phase 1 clinical trial for safety, tolerability, and pharmacokinetics of LCB01-0371 was completed (16). In the present study, we have discussed the results of in vitro and in vivo studies of the effects of LCB01-0371 against M. abscessus. LCB01-0371 was found to effectively inhibit M. abscessus growth, not only in vitro, but also in mouse lungs in vivo compared with linezolid. Furthermore, LCB01-0371 killed all strains of bacteria, regardless of their resistance to amikacin, cefoxitin, or clarithromycin. We also identified a single nucleotide polymorphism in the rplC gene as the molecular target responsible for LCB01-0371 resistance in M. abscessus. Given the in vivo observed in vivo efficacy, we consider LCB01-0371 to be a strong potential candidate for the treatment of M. abscessus lung disease with improved safety.
RESULTS AND DISCUSSION
MIC values and intracellular activity of LCB01-0371.
LCB01-0371 is metabolically stable compound synthesized by LegoChem BioSciences, Inc. (Daejeon, Republic of Korea) (Fig. 1). The in vitro pharmacokinetic profile demonstrates no cytochrome P450 (CYP) inhibition, no cytotoxicity, and a reasonable plasma protein binding profile (human, 37%; rat, 57%; mouse, 69%). In comparison with linezolid, an increased percentage of reticulocytes in the blood of rats and a trend toward higher platelet counts were observed in subjects that received multiple doses of LCB01-0371 in a phase 1b trial (Fig. S1 and S2 in the supplemental material) (17). Therefore, we decided to evaluate the use of LCB01-0371 in the treatment of M. abscessus infections as a safer compound of the oxazolidinone class than linezolid (Fig. S1B).
FIG 1.
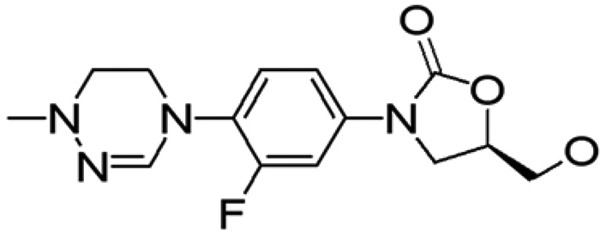
Chemical structure of LCB01-0371.
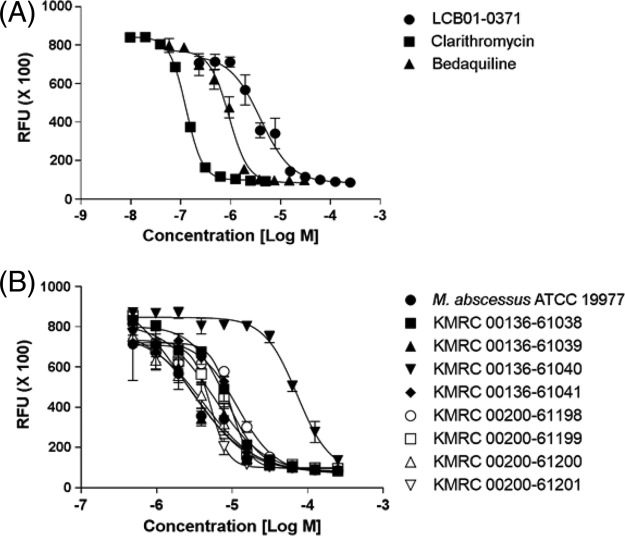
To investigate whether LCB01-0371 affected the survival of M. abscessus, we tested the susceptibility of M. abscessus ATCC 19977 to LCB01-0371. As shown in Fig. 2A and Table 1, the survival of M. abscessus was greatly decreased in the presence of LCB01-0371 (MIC50, 1.2 μg/ml). LCB01-0371 showed in vitro activity comparable to that with other reference compounds, such as clarithromycin and bedaquiline, which have different mechanisms of action. In our study, the in vitro MICs of clarithromycin and bedaquiline against M. abscessus were 0.1 and 0.5 μg/ml, respectively (Table 1). Clarithromycin was the drug of choice for M. abscessus infections until the identification of inducible clarithromycin-resistant strains that express the erythromycin ribosome methyltransferase gene erm(41). Although the M. abscessus strain is susceptible to clarithromycin after 3 days of in vitro incubation, the strain will become clarithromycin resistant upon the extension of the incubation time to 2 weeks because of the induction of methyltransferase synthesis. The treatment of this M. abscessus infection then becomes almost impossible if there are no alternative drugs (18). Bedaquiline was included as a reference compound in this study, as it targets ATP synthase with a range of effects specifically restricted to mycobacteria; it received conditional approval from the U.S. Food and Drug Administration in December 2012 for use in patients with multidrug-resistant tuberculosis. In our experiment, bedaquiline showed effective in vitro activity against M. abscessus (Fig. 2A), although it was almost inactive in the nude mouse model (19, 20).
FIG 2.
In vitro activity of LCB01-0371. (A) Activity of LCB01-0371 against Mycobacterium abscessus in culture broth medium. Bedaquiline and clarithromycin were used as positive controls. (B) Activity of LCB01-0371 against M. abscessus clinical isolates. Dose-response curves were plotted from REMA data for M. abscessus strains treated with LCB01-0371. Data are expressed as the mean ± standard deviation (SD) of triplicates for each concentration. RFU, relative fluorescence units.
TABLE 1.
MIC of antimicrobial agents to M. abscessus strains
| Strain | MIC50 (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| LCB01-0371 | Bedaquiline | Linezolid | Sutezolid | Amikacin | Clarithromycin | Cefoxitin | |
| M. abscessus ATCC 19977 | 1.2 | 0.5 | 2.1 | 1.8 | 7.4 | 0.1 | 14.0 |
| M. abscessus clinical isolates | |||||||
| KMRC 00136-61038 | 2.5 | ||||||
| KMRC 00136-61039 | 0.7 | ||||||
| KMRC 00136-61040 | 22.3 | ||||||
| KMRC 00136-61041 | 2.9 | ||||||
| KMRC 00200-61198 | 3.5 | ||||||
| KMRC 00200-61199 | 1.7 | ||||||
| KMRC 00200-61200 | 1.2 | ||||||
| KMRC 00200-61201 | 1.6 | ||||||
| Drug-resistant mutant | |||||||
| LCB01-0371R | 28.5 | 0.5 | 18.4 | 29.4 | 0.1 | ||
| AMK-R | 1.3 | >50 | |||||
| CFX-R | 1.6 | >40 | |||||
| CLA-R | 1.5 | >70 | |||||
We have evaluated the antibacterial activity of LCB01-0371 against eight clinical strains of M. abscessus, including rough (R)- and smooth (S)-colony morphotypes. LCB01-0371 was equally effective against eight M. abscessus clinical isolates from different sources selected from the Korean Mycobacterial Resource Center (KMRC) (Fig. 2B). Different morphotypes, such as R and S colonies, can be determined based on their glycopeptidolipid (GPL) content; both morphotypes have been identified in human airways. Although human data are scarce, the R morphotype tends to be a much more virulent pathogen, which is involved in the chronic colonization of CF airways. In addition, the R morphotype is more proinflammatory than the S morphotype. Intravenous (i.v.) M. abscessus infection models in C57BL/6 mice showed much higher mortality and levels of induced tumor necrosis factor alpha (TNF-α) in the R morphotype than in the S morphotype (21). As shown in Fig. 2B, LCB01-0371 was active against both morphotypes in our study, which suggested a much greater clinical impact. Although LCB01-0371 exhibited slightly higher activity against a particular clinical isolate (KMRC 00136-61040), these results demonstrated that LCB01-0371 was effective in vitro against both the reference strain and the clinical R- and S-colony morphotype strains.
The intracellular antimicrobial activity of LCB01-0371 against M. abscessus was assessed after 8 h of replication inside murine bone marrow-derived macrophage (mBMDM) macrophages. As shown in Fig. 3, LCB01-0371 dramatically decreased the number of intracellular mycobacteria present at 2 days after infection at concentrations of 0.1, 1, and 10 μg/ml. LCB01-0371 treatment led to a 79% reduction in mycobacteria, which was comparable to that elicited by clarithromycin (96%). Hence, we concluded that LCB01-0371 was active against intracellular M. abscessus. This result demonstrated that LCB01-0371 was an effective compound for the in vitro and intracellular inhibition of M. abscessus.
FIG 3.
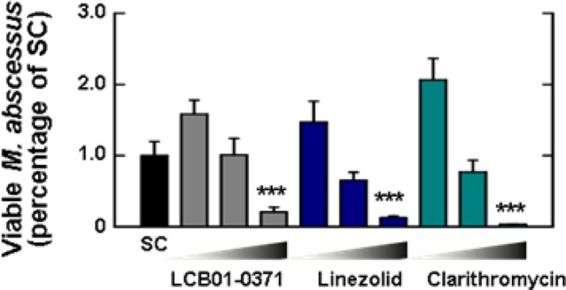
Intracellular activity of LCB01-0371. The activity of LCB01-0371 on intracellular Mycobacterium abscessus was tested in bone marrow-derived macrophages (BMDMs). Cells were infected at a multiplicity of infection (MOI) of 3 with M. abscessus ATCC 19977 and treated with LCB01-0371 at 0.1, 1, and 10 μg/ml, linezolid at 0.1, 1, and 10 μg/ml, and clarithromycin at 0.1, 1, and 10 μg/ml. The experiment was performed in triplicate, and results are shown as mean ± standard error of the mean (SEM). SC, solvent control. ***, P < 0.001.
Effect of LCB01-0371 in the murine model.
The effects of LCB01-0371 therapy in mice intranasally infected with M. abscessus are shown in Fig. 4. Owing to the preclinical efficacy of 100 mg/kg of body weight linezolid in mouse models of infection, the similar in vivo pharmacokinetic profiles of linezolid and LCB01-0371, and the absence of adverse effects at a dose of 1,200 mg twice daily (BID) in a follow-up phase 2 clinical study, LCB01-0371 was administered at 100 mg/kg in the mouse in vivo efficacy study (Tables S1 and S2) (22, 23). When LCB01-0371 was administered at 100 mg/kg daily (by gavage), the CFU counts tended to be decreased in the lungs of mice at 7 days after infection. On 7H10 agar medium supplemented with 10% oleic acid, albumin, dextrose, and catalase (OADC), LCB01-0371 showed a statistically significant effect compared with the saline control. Mice treated with 100 mg/kg LCB01-0371 resulted in the reduction of CFU in lungs to 3.7 log10, which was very similar to the values obtained for linezolid (Fig. 4A). This result demonstrated that the in vivo activity of LCB01-0371 against M. abscessus in the mouse model was comparable to that of linezolid, an antibiotic that is used for the treatment of M. abscessus infection. Thus, we suggested that LCB01-0371 could replace linezolid for the treatment of lung infections, owing to its improved safety profile, such as reduced myelosuppression in a human study (phase 1b trial) (Fig. S1 and S2) and low toxicity (Table S3). Clarithromycin (200 mg/kg) resulted in a somewhat increased CFU level compared with LCB01-0371 and linezolid.
FIG 4.
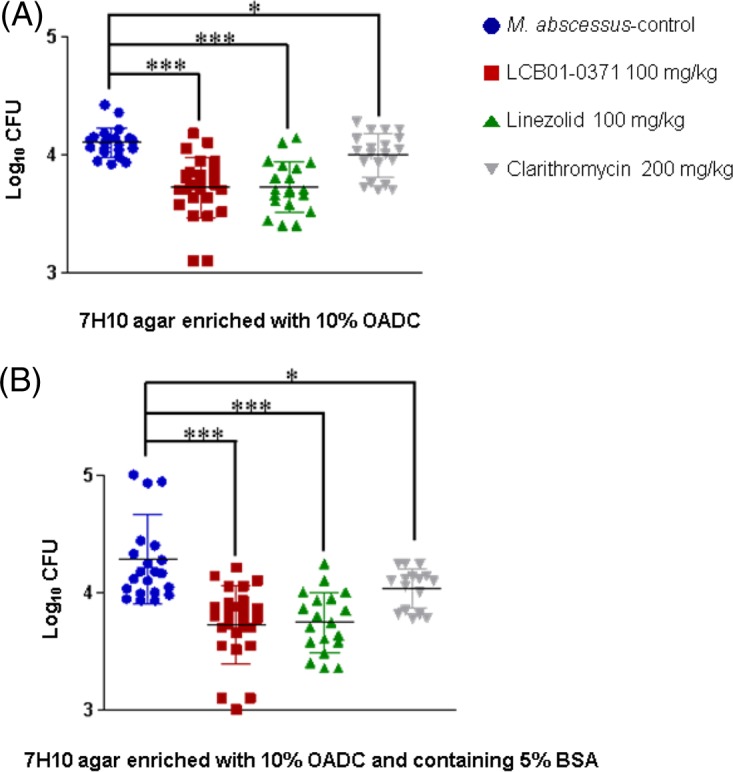
In vivo efficacy of LCB01-0371, linezolid, and clarithromycin against Mycobacterium abscessus infection in two different media. C57BL/6 WT female mice (n = 8) were infected intranasally with 1 × 107 CFU M. abscessus. After 2 days of infection, mice were treated for 4 consecutive days with 100 mg/kg LCB01-0371 and linezolid. Mice were sacrificed at 7 days after M. abscessus infection. Clarithromycin (200 mg/kg) and saline were used as positive and negative controls, respectively. The lungs were harvested, and bacterial load was determined using 7H10 agar enriched with 10% OADC (A) and 7H10 agar enriched with 10% OADC supplemented with 5% bovine serum albumin (B). Error bars represent SEM from two independent experiments (*, P < 0.05; ***, P < 0.001).
Furthermore, we evaluated the drug carryover effect by using 5% bovine serum albumin (BSA)-containing 7H11 agar medium at physiological concentrations. The drug carryover effect is defined as an overestimation of the in vivo efficacy of a compound owing to high drug-protein binding interactions (24). To minimize the carryover phenomenon, which produces inaccurate in vitro inhibition results as a result of the presence of high drug concentrations in tissue-originated samples, we used a medium with high protein concentration (Middlebrook 7H10 agar containing 5% BSA). However, as shown in Fig. 4B, there was no significant difference in CFU number for LCB01-0371 in 5% BSA-containing 7H10 agar medium compared with normal 7H10 medium supplemented with 10% OADC, which suggested that protein binding may not influence LCB01-0371 efficacy in vivo. Interestingly, all untreated groups showed significant spontaneous clearance of bacilli at approximately 3 log10 CFU per lung from the initial inocula at 7 days postinfection in comparison with treated mice. Based on this phenomenon, we speculated that the activities of LCB01-0371, linezolid, and clarithromycin were tested in an optimistic model that was different from the human infection mdoel, which was characterized by chronicity. As gamma interferon (IFN-γ) is critically important in the host defense against M. abscessus infection, an IFN-γ knockout (GKO) mouse model should provide a much suitable murine model for the M. abscessus in vivo efficacy study (25).
Finally, the highest dose test was conducted with LCB01-0371 in the presence or absence of 5% BSA. After infection with M. abscessus via the intravenous (i.v.) route, single doses (QD) of LCB01-0371, linezolid, and clarithromycin at 1,000 mg/kg of body weight were administered (by gavage) to C57BL/6 wild-type female mice. Bacterial loads in the lung, liver, and spleen of infected mice were evaluated at 7 days after infection. The linezolid treatment of mice was much more potent than that of LCB01-0371 in the spleen and liver (Fig. S3A and B). However, LCB01-0371 showed a slightly higher potential for clearance of M. abscessus than that of linezolid in the lungs. Thus, we concluded that for high doses, LCB01-0371 was more effective than linezolid in the lungs but less effective in the spleen and liver.
Selection of spontaneous LCB01-0371-resistant mutants of M. abscessus and their molecular target.
Through experiments on solid media, we were able to select spontaneous mutants that were resistant to LCB01-0371. M. abscessus was plated at 25, 50, and 100 times the MIC50 values for LCB01-0371. At 100 times the MIC50, spontaneously resistant mutants to LCB01-0371 were isolated and named LCB01-0371R1, LCB01-0371R2, LCB01-0371R3, LCB01-0371R4, and LCB01-0371R5. The frequency of the isolation of spontaneously resistant mutants against LCB01-0371 was 4.9 × 10−8. To evaluate the specific mechanism of resistance of these mutants to LCB-0371, the susceptibility was determined using current drugs of choice with different mechanisms of action, such as clarithromycin and bedaquiline. In detail, clarithromycin is a protein synthesis inhibitor that binds to the 23S rRNA of the 50S subunit of M. abscessus and inhibits the translation of peptides. A region of the rrl gene which encodes the peptidyltransferase domain of the 23S rRNA is associated with clarithromycin point mutations at positions A2058 and A2059. In addition, the erm gene is also involved in inducible resistance to clarithromycin (10). Bedaquiline is an ATP synthesis inhibitor that inhibits AtpE (mycobacterial ATP synthase), an essential enzyme in mycobacterial energy metabolism. Similar to M. tuberculosis, M. abscessus is also susceptible to bedaquiline (MIC, 0.25 μg/ml), and the induced bedaquiline-resistant mutants were reported to contain two types of single nucleotide polymorphism (SNP) mutations in atpE (Asp28→Ala and Ala63→Pro). As seen in Fig. 5A to C and Table 1, only LCB01-0371R showed strong resistance against LCB01-0371 and was sensitive to clarithromycin and bedaquiline, with an MIC 24 times higher (28.5 μg/ml) than that of the wild type. Conversely, the MIC of the wild type and LCB01-0371R remained unchanged after clarithromycin and bedaquiline treatment.
FIG 5.
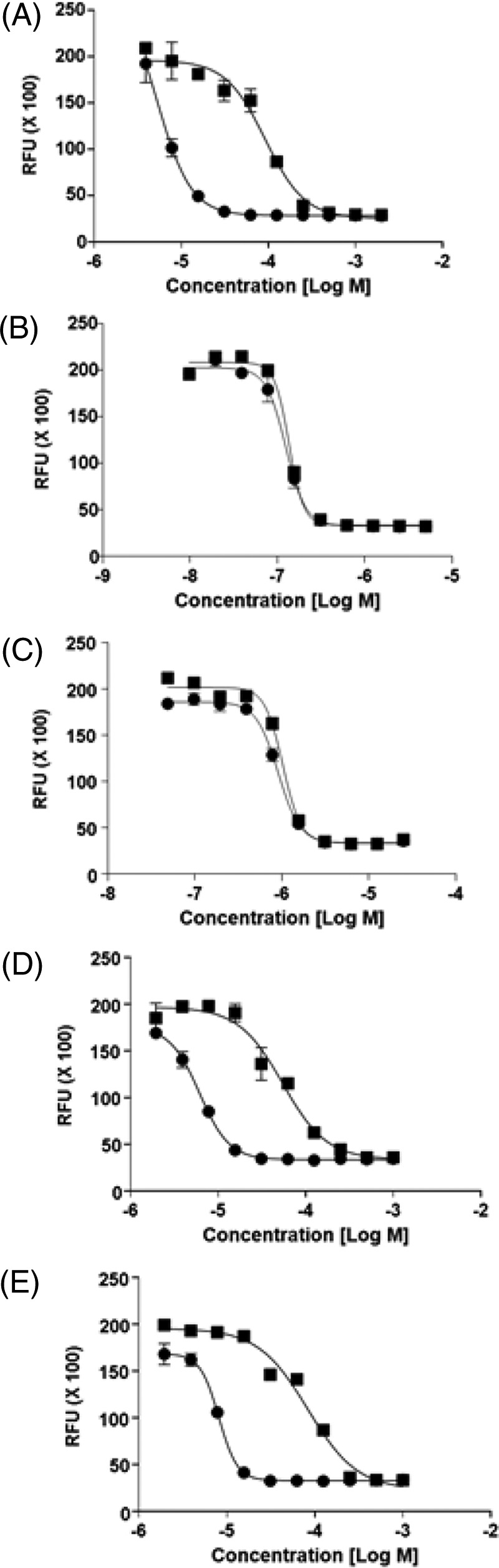
Evaluation of LCB01-0371 resistance. (A) Dose-response curve of LCB01-0371 against Mycobacterium abscessus ATCC 19977 (closed circle) and laboratory-generated LCB01-0371-resistant mutant (closed square). LCB01-0371-specific resistance was evaluated by treatment with drugs with different mechanisms of action, e.g., clarithromycin (B) and bedaquiline (C). Cross-resistance was observed in other oxazolidinone agents, such as linezolid (D) and sutezolid (E). Data are shown with± SD of triplicates.
Furthermore, we investigated whether LCB01-0371 had the same molecular target as the other compounds of the oxazolidinone class, such as linezolid and sutezolid (PNU-100480). Linezolid binds to the 23S subunit of the bacterial ribosome to prevent protein synthesis; in vitro testing indicated that M. abscessus was susceptible to linezolid (26). As seen in Fig. 5D and E and Table 1, three different oxazolidinones (including LCB01-0371) showed similar MIC shifts against LCB01-0371R. These resistant clones displayed a significant increase in MIC50 to LCB01-0371, as well as cross-resistance with linezolid and sutezolid. These data suggest that LCB01-0371R shares a molecular target with other members of the oxazolidinone class. Thus, we concluded that LCB01-0371R is a genuine oxazolidinone-specific resistant mutant.
With regard to molecular targeting, the known target genes rrl (encoding 23S rRNA) and rplC (encoding 50S ribosomal protein L3) have been implicated in the acquisition of resistance to oxazolidinones. For example, resistance to one of the oxazolidinones, linezolid, was found to result from mutations at positions G2061T, G2576T, C2848A, A2810T, G2270C, G2270T, and G2746A in the M. tuberculosis rrl gene. However, recently, another molecular target of the oxazolidinone class was reported in M. tuberculosis. Linezolid, sutezolid, and AZD5847 were found to be resistant to the T460C (Cys154Arg in L3 protein) mutation in the rplC gene in M. tuberculosis (27–29). Therefore, we further analyzed the molecular targets of LCB01-0371 by sequencing the rplC and rrl genes derived from M. abscessus LCB01-0371R strains. As shown in Table 2, five LCB01-0371R strains had mutations at various positions in the rplC gene. First, LCB01-0371R1 exhibited a mutation at the position T424C (Cys142Arg). Based on the alignment of the sequence with that of rplC of M. tuberculosis, we identified this T424C mutation at the same position as those induced by linezolid and sutezolid on the rplC gene of M. tuberculosis. Thus, these results demonstrated that M. abscessus had a resistance mechanism against LCB01-0371 similar to that found in M. tuberculosis against oxazolidinone class compounds. Furthermore, another rplC mutation was identified at position G419A (Gly140Asp), and a cytosine nucleotide insertion between positions 502 and 503 in the rplC gene was also found in three LCB01-0371R strains. It is noteworthy that these new mutations and nucleotide insertions were involved in the reduction in susceptibility to LCB01-0371 in the rplC gene, although this resistance mechanism needs further study. Future studies of the docking of LCB01-0371 to the M. abscessus rplC protein will provide an understanding of how new mutations and nucleotide insertions at different positions contribute to LCB01-0371 resistance. Further isolated clinical strains that harbor this mutation in rplC will provide more evidence to support our result. In this study, we could not identify the rrl mutation in the LCB-0371R-resistant isolates.
TABLE 2.
Characteristics of five laboratory-generated LCB01-0371-resistant M. abscessus strains
| M. abscessus strain | rrl mutation(s) detected | rplC mutation(s) detecteda | Nucleotide changeb | Amino acid change |
|---|---|---|---|---|
| ATCC 19977 | None | None | ||
| LCB01-0371R1 | None | GGC CGC GCC ACC CCG | T424C | Cys142Arg |
| LCB01-0371R2 | None | ATC GAT GGC TGC GCC | G419A | Gly140Asp |
| LCB01-0371R3 | None | CAG AAC CCT GGT GGT | Ins 503C | |
| LCB01-0371R4 | None | CAG AAC CCT GGT GGT | Ins 503C | |
| LCB01-0371R5 | None | CAG AAC CCT GGT GGT | Ins 503C |
Underlined bases indicate those that changed due to a mutation.
Ins, insertion.
Next, we tested whether LCB01-0371 was effective for inhibition of the growth of drug-resistant strains that were laboratory generated at high concentrations of amikacin, cefoxitin, and clarithromycin, which are currently used in anti-M. abscessus regimens. All laboratory-generated resistant mutants showed high drug resistance to each antibiotic, as shown in Fig. S4A to C. However, the laboratory-generated amikacin-, cefoxitin-, and clarithromycin-resistant mutants (AMK-R, CFX-R, and CLA-R, respectively) were fully inhibited by LCB01-0371 (Fig. 6 and S5). All the AMK-R, CFX-R, and CLA-R variants tested were as sensitive to LCB01-0371 as the wild type was, with same MIC range and without differences between the strains or resistance to other drugs (Table 1). These results confirmed that LCB01-0371 was active against amikacin-, cefoxitin-, and clarithromycin-sensitive and -resistant strains. Thus, LCB01-0371 should be considered an active drug for the treatment of amikacin-, cefoxitin-, and clarithromycin-resistant M. abscessus.
FIG 6.
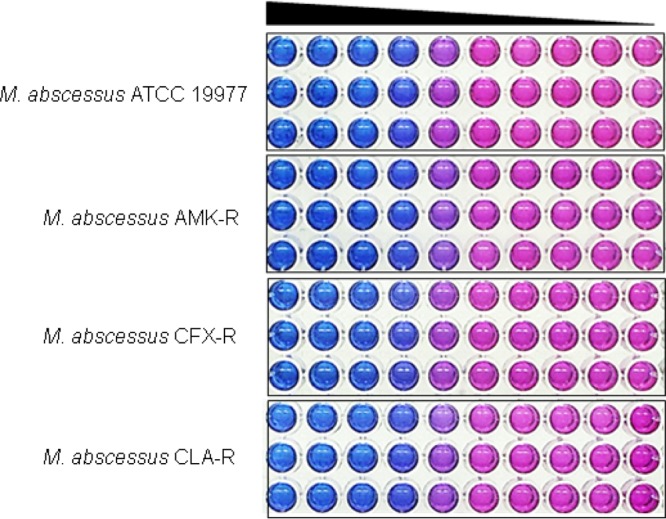
Determination of sensitivity to LCB01-0371. Mycobacterium abscessus ATCC 19977 and amikacin-, cefoxitin-, and clarithromycin-resistant mutants (AMK-R, CFX-R, and CLA-R, respectively) were tested for their ability to grow in 7H9 medium in the presence of LCB01-0371. No differences in LCB01-0371 sensitivity were observed in the different strains. Resazurin reports bacterial viability via color change from blue to pink. Each experiment was performed in triplicate.
In the present study, we described the in vitro, intracellular, and in vivo activity profiles of the novel oxazolidinone LCB01-0371. Previously, LCB01-0371 was suggested to have good in vivo activity against Gram-positive pathogens, such as Staphylococcus aureus, Enterococcus faecalis, Streptococcus pneumoniae, and Haemophilus influenzae. Its activity was more potent than linezolid against both Gram-positive and Gram-negative bacteria (16), but Mycobacterium fits neither of these categories completely. Mycobacteria are structurally Gram-positive bacteria, because they have a poor true outer membrane that contains a thick peptidoglycan layer. However, they also share properties with Gram-negative organisms, as they do not retain Gram staining (30). Therefore, it is unknown whether the novel oxazolidinone LCB01-0371 can be applied to mycobacterial infections. Therefore, we focused on M. abscessus, a new antibiotic nightmare. M. abscessus possesses intrinsic and acquired resistance to commonly used antibiotics, which limits the available chemicals for treatment (7). Currently, the recommended regimen for M. abscessus infection includes the combination of clarithromycin with amikacin and one other injectable drug, such as cefoxitin or imipenem. However, this combination therapy is controversial, because its efficacy varies among patients (7). Recently, new studies have reported the use of an oxazolidinone, such as linezolid, for treatment. Linezolid has been suggested as a potential candidate for M. abscessus treatment, owing to its efficacy against clinical isolates of M. abscessus in vitro (MIC range, 0.5 to 128 μg/ml), with comparable in vivo efficacy to clarithromycin for the treatment of M. abscessus infection in the Drosophila melanogaster model (5). However, linezolid is recognized to lead to significant adverse effects. Specifically, patients who have taken linezolid for the treatment of atypical mycobacterial infections experienced neuropathy, anemia, headaches, nausea, taste alteration, itching, insomnia, and bulimia. Moreover, expanded therapy with linezolid involved higher rates of adverse events, such as myelosuppression (15). Therefore, linezolid was not recommended for long-term treatment. In this context, it is necessary to develop new oxazolidinones with better safety profiles. The absorption, distribution, metabolism, excretion, and toxicity (ADMET) test of LCB01-0371 showed high aqueous solubility and good absorption, distribution, metabolism, excretion, toxicity, and pharmacokinetic profiles. In addition, a phase 1 clinical trial of LCB01-0371 was recently completed to determine the safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy male subjects in a randomized, double-blind, placebo-controlled, single-dose, dose escalation study (31). Therefore, all the efficacies shown in this study are significant in the consideration of a replacement for linezolid in the treatment of M. abscessus infections. In our study, we concluded that LCB01-0371 was highly effective against wild-type and clinical isolates of M. abscessus in vitro, intracellularly, and in vivo, with activity comparable to that of linezolid. Thus, LCB01-0371 is a promising candidate, with an improved safety profile, which can be further developed for the treatment of M. abscessus infections.
MATERIALS AND METHODS
Bacterial strains and culture.
M. abscessus ATCC 19977 was used in this study. All clinical strains were obtained from the Korea Mycobacterium Resource Center (KMRC). M. abscessus AMK-R, CFX-R, and CLA-R were laboratory-generated amikacin-, cefoxitin-, and clarithromycin-resistant mutant strains, respectively. M. abscessus strains were grown at 37°C in Middlebrook 7H9 broth supplemented with 10% albumin-dextrose-catalase (ADC) or on Middlebrook 7H10 plates supplemented with 10% oleic acid-ADC (OADC). Escherichia coli DH5α was grown in LB broth and agar. LCB01-0371 was provided by LegoChem BioSciences, Inc. (Daejeon, Republic of Korea). Linezolid, clarithromycin, and sutezolid were purchased from Sigma-Aldrich. Bedaquiline was purchased from Santa Cruz Biotech, solubilized in accordance with the manufacturer's instructions, divided into aliquots, and stored at −20°C.
MIC determination using REMA.
The MICs of all the antibiotics used in this study were determined using the resazurin microtiter assay (REMA) plate method for a range of drug concentrations. Briefly, 100 μl of 7H9 broth supplemented with 10% ADC was added to every well of a 96-well microtiter plate, except for the peripheral wells, which were filled with 200 μl of sterilized water to prevent evaporation during incubation. Two-fold serial dilutions of antibiotics were added directly to the wells. The plates were sealed and incubated at 37°C for 3 days. Thirty microliters of 0.025% resazurin solution (Sigma Chem. Co.) was added to each well, and the plates were reincubated overnight. Bacterial growth was indicated by a color change from blue to pink; the MIC was defined as the minimum compound concentration that prevented the color change in the resazurin solution. Fluorescence was measured by excitation at 530 nm and emission at 590 nm using a Synergy H1 multimode reader (BioTek) in bottom-reading mode. The MIC was calculated using Prism 6 for Windows software (GraphPad Software, Inc., La Jolla, CA).
Generation and characterization of spontaneous resistant mutants.
The selection of spontaneously resistant mutants to LCB01-0371 was performed as follows. LCB01-0371-resistant mutants were selected on 7H10 agar plates containing 25, 50, and 100 times the MIC values for LCB01-0371. The resistance phenotype was confirmed by testing for a shift in MIC50 values. To characterize the LCB01-0371-resistant mutants, single colonies were selected for rrl and rplC amplification and sequencing. DNA from the five resistant mutants was extracted from liquid cultures grown in Middlebrook 7H9-ADC. After centrifugation, the pellet was resuspended in 1,000 μl of distilled water and incubated at 95°C for 1 h before the harvest of genomic DNA for PCR amplification. PCRs were performed by using primers for rrl (forward, 5′-GGC AAA ATA CCC CCG TAA CT-3′; reverse, 5′-ACG GTC CGA GGT TAG AGG TT-3′) and rplC (forward, 5′-AAA CCA TGG CAA GAA AAG GA-3′; reverse, 5′-GAG CCT TGA CTG CGA TCT TC-3′). Laboratory-generated amikacin-, cefoxitin-, and clarithromycin-resistant mutants were selected by plating the CFU of M. abscessus ATCC 19977 on Middlebrook 7H10 agar supplemented with 10% OADC, with amikacin (160 μg/ml), cefoxitin (160 μg/ml), and clarithromycin (20 μg/ml), respectively. Twenty colonies were selected, and their resistances to amikacin, cefoxitin, and clarithromycin were determined using the REMA method, as described above.
Mice and culture of BMDMs.
C57BL/6 mice were purchased from Samtaco Bio (Gyeonggi-do, South Korea). Primary bone marrow-derived macrophages (BMDMs) were isolated in 6-week-old mice and differentiated for 5 days in medium containing macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA). The culture medium was Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Lonza), 50 U/ml penicillin, and 50 μg/ml streptomycin. All animal-related procedures were approved by the Animal Care and Use Committee of Chungnam National University.
M. abscessus infection in vivo and CFU assays.
M. abscessus infection was diluted with phosphate-buffered saline (PBS) to a final volume of 10 or 100 μl per mouse. Wild-type (WT) mice were intranasally or intravenously injected with M. abscessus (1 × 107 CFU/mouse). After 2 days, the mice were orally administered LCB-0371, clarithromycin, and linezolid for 4 days, consecutively. At 7 days after M. abscessus infection, the mice were killed, and their spleens, livers, and lungs were homogenized in PBS. Serial dilutions of the homogenates were plated on 7H10 medium supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC). For the in vitro infection procedure, BMDMs were plated at a concentration of 2 × 105 cells/well and infected for 4 h with M. abscessus. The cells were washed with PBS to remove extracellular bacteria and treated with LCB-0371, clarithromycin, and linezolid in medium for 2 days. Thereafter, the intracellular bacteria were harvested, and the lysates were diluted 10-fold in PBS. Each sample was plated on 7H10 agar plates and incubated at 37°C in a 0.5% CO2 incubator for 7 days.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant HI15C0395), and the National Research Foundation of Korea (grant 2016R1D1A1A02937214) from research funds of Chungnam National University and funds of the research promotion program, Gyeongsang National University, 2016. Jinsun Jeong was supported by the BK21plus program through the National Research Foundation (NRF), funded by the Ministry of Education of Korea.
English language editing of the article was conducted by Editage, a division of Cactus Communications.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02752-16.
REFERENCES
- 1.Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. 2015. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 21:1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanguinetti M, Ardito F, Fiscarelli E, La Sorda M, D'Argenio P, Ricciotti G, Fadda G. 2001. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J Clin Microbiol 39:816–819. doi: 10.1128/JCM.39.2.816-819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernut A, Le Moigne V, Lesne T, Lutfalla G, Herrmann JL, Kremer L. 2014. In vivo assessment of drug efficacy against Mycobacterium abscessus using the embryonic zebrafish test system. Antimicrob Agents Chemother 58:4054–4063. doi: 10.1128/AAC.00142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obregón-Henao A, Arnett KA, Henao-Tamayo M, Massoudi L, Creissen E, Andries K, Lenaerts AJ, Ordway DJ. 2015. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob Agents Chemother 59:6904–6912. doi: 10.1128/AAC.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh CT, Moon C, Park OK, Kwon SH, Jang J. 2014. Novel drug combination for Mycobacterium abscessus disease therapy identified in a Drosophila infection model. J Antimicrob Chemother 69:1599–1607. doi: 10.1093/jac/dku024. [DOI] [PubMed] [Google Scholar]
- 6.Koh W-J, Stout JE, Yew W-W. 2014. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. Int J Tuberc Lung Dis 18:1141–1148. doi: 10.5588/ijtld.14.0134. [DOI] [PubMed] [Google Scholar]
- 7.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 8.Cremades R, Santos A, Rodríguez JC, Garcia-Pachón E, Ruiz M, Royo G. 2009. Mycobacterium abscessus from respiratory isolates: activities of drug combinations. J Infect Chemother 15:46–48. doi: 10.1007/s10156-008-0651-Y. [DOI] [PubMed] [Google Scholar]
- 9.Sassi M, Drancourt M. 2014. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics 15:359. doi: 10.1186/1471-2164-15-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SH, Yoo HK, Kim SH, Koh WJ, Kim CK, Park YK, Kim HJ. 2014. The drug resistance profile of Mycobacterium abscessus group strains from Korea. Ann Lab Med 34:31–37. doi: 10.3343/alm.2014.34.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo RF, Curry C, Taylor N, Budvytiene I, Banaei N. 2015. Rapid detection of acquired and inducible clarithromycin resistance in Mycobacterium abscessus group by a simple real-time PCR assay. J Clin Microbiol 53:2337–2339. doi: 10.1128/JCM.00132-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novosad SA, Beekmann SE, Polgreen PM, Mackey K, Winthrop KL, M. abscessus Study Team. 2016. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis 22:511–514. doi: 10.3201/eid2203.150828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RA, Rom WN, Addrizzo-Harris DJ. 2010. The use of linezolid and nebulized amikacin in a case of Mycobacterium chelonae/Mycobacterium abscessus pulmonary disease. Chest J 138:86A. doi: 10.1378/chest.10760. [DOI] [Google Scholar]
- 14.Deng J, Su LX, Liang ZX, Liang LL, Yan P, Jia YH, Zhang XG, Feng D, Xie LX. 2013. Effects of vitamin B6 therapy for sepsis patients with linezolid-associated cytopenias: a retrospective study. Curr Ther Res Clin Exp 74:26–32. doi: 10.1016/j.curtheres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Y, Chai D, Falagas ME, Vouloumanou EK, Wang R, Guo D, Bai N, Liang B, Liu Y. 2012. Immediate hematological toxicity of linezolid in healthy volunteers with different body weight: a phase I clinical trial. J Antibiot (Tokyo) 65:175–178. doi: 10.1038/ja.2011.142. [DOI] [PubMed] [Google Scholar]
- 16.Jeong JW, Jung SJ, Lee HH, Kim YZ, Park TK, Cho YL, Chae SE, Baek SY, Woo SH, Lee HS, Kwak JH. 2010. In vitro and in vivo activities of LCB01-0371, a new oxazolidinone. Antimicrob Agents Chemother 54:5359–5362. doi: 10.1128/AAC.00723-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anti-Infective Drugs Advisory Committee. 2014. NDA 205435, NDA 205436. Tedizolid phosphate for the treatment of acute bacterial skin and skin structure infections. Anti-Infective Drugs Advisory Committee meeting, March 31, 2014 U.S. Food and Drug Administration, Silver Spring, MD https://wayback.archive-it.org/7993/20170405204503/https:/www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM390790.pdf. [Google Scholar]
- 18.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. 2014. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:574–576. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerat I, Cambau E, Roth Dit Bettoni R, Gaillard J-L, Jarlier V, Truffot C, Veziris N. 2014. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis 209:905–912. doi: 10.1093/infdis/jit614. [DOI] [PubMed] [Google Scholar]
- 21.Caverly LJ, Caceres SM, Fratelli C, Happoldt C, Kidwell KM, Malcolm KC, Nick JA, Nichols DP. 2015. Mycobacterium abscessus morphotype comparison in a murine model. PLoS One 10:e0117657. doi: 10.1371/journal.pone.0117657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marra A, Lamb L, Medina I, George D, Gibson G, Hardink J, Rugg J, Van Deusen J, O'Donnell JP. 2012. Effect of linezolid on the 50% lethal dose and 50% protective dose in treatment of infections by Gram-negative pathogens in naive and immunosuppressed mice and on the efficacy of ciprofloxacin in an acute murine model of septicemia. Antimicrob Agents Chemother 56:4671–4675. doi: 10.1128/AAC.00276-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams KN, Stover CK, Zhu T, Tasneen R, Tyagi S, Grosset JH, Nuermberger E. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother 53:1314–1319. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lounis N, Gevers T, Van Den Berg J, Verhaeghe T, Van Heeswijk R, Andries K. 2008. Prevention of drug carryover effects in studies assessing antimycobacterial efficacy of TMC207. J Clin Microbiol 46:2212–2215. doi: 10.1128/JCM.00177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordway D, Henao-Tamayo M, Smith E, Shanley C, Harton M, Troudt J, Bai X, Basaraba RJ, Orme IM, Chan ED. 2008. Animal model of Mycobacterium abscessus lung infection. J Leukoc Biol 83:1502–1511. doi: 10.1189/jlb.1007696. [DOI] [PubMed] [Google Scholar]
- 26.Wallace RJ, Brown-Elliott BA, Ward SC, Crist CJ, Mann LB, Wilson RW. 2001. Activities of linezolid against rapidly growing mycobacteria. Antimicrob Agents Chemother 45:764–767. doi: 10.1128/AAC.45.3.764-767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Chen J, Cui P, Shi W, Shi X, Niu H, Chan D, Yew WW, Zhang W, Zhang Y. 2016. Mycobacterium tuberculosis mutations associated with reduced susceptibility to linezolid. Antimicrob Agents Chemother 60:2542–2544. doi: 10.1128/AAC.02941-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balasubramanian V, Solapure S, Iyer H, Ghosh A, Sharma S, Kaur P, Deepthi R, Subbulakshmi V, Ramya V, Ramachandran V, Balganesh M, Wright L, Melnick D, Butler SL, Sambandamurthy VK. 2014. Bactericidal activity and mechanism of action of azd5847, a novel oxazolidinone for treatment of tuberculosis. Antimicrob Agents Chemother 58:495–502. doi: 10.1128/AAC.01903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makafe GG, Cao Y, Tan Y, Julius M, Liu Z, Wang C, Njire MM, Cai X, Liu T, Wang B, Pang W, Tan S, Zhang B, Yew WW, Lamichhane G, Guo J, Zhang T. 2016. Role of the Cys154Arg substitution in ribosomal protein L3 in oxazolidinone resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 60:3202–3206. doi: 10.1128/AAC.00152-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hett EC, Rubin EJ. 2008. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 72:126–156. doi: 10.1128/MMBR.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pucci MJ, Bush K. 2013. Investigational antimicrobial agents of 2013. Clin Microbiol Rev 26:792–821. doi: 10.1128/CMR.00033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.