Abstract
Background and Aims
Radially symmetrical, five-winged fossil fruits from the highly diverse early Eocene Laguna del Hunco flora of Chubut Province, Patagonia, Argentina, are named, described and illustrated. The main goals are to assess the affinities of the fossils and to place them in an evolutionary, palaeoecological and biogeographic context.
Methods
Specimens of fossil fruits were collected from the Tufolitas Laguna del Hunco. They were prepared, photographed and compared with similar extant and fossil fruits using published literature. Their structure was also evaluated by comparing them with that of modern Ceratopetalum (Cunoniaceae) fruits through examination of herbarium specimens.
Key Results
The Laguna del Hunco fossil fruits share the diagnostic features that characterize modern and fossil Ceratopetalum (symmetry, number of fruit wings, presence of a conspicuous floral nectary and overall venation pattern). The pattern of the minor wing (sepal) veins observed in the Patagonian fossil fruits is different from that of modern and previously described fossil Ceratopetalum fruits; therefore, a new fossil species is recognized. An apomorphy (absence of petals) suggests that the fossils belong within crown-group Ceratopetalum.
Conclusions
The Patagonian fossil fruits are the oldest known record for Ceratopetalum. Because the affinities, provenance and age of the fossils are so well established, this new Ceratopetalum fossil species is an excellent candidate for use as a calibration point in divergence dating studies of the family Cunoniaceae. It represents the only record of Ceratopetalum outside Australasia, and further corroborates the biogeographic connection between the Laguna del Hunco flora and ancient and modern floras of the Australasian region.
Keywords: Argentina, calibration, Ceratopetalum, Cunoniaceae, Eocene, fossil fruit, Laguna del Hunco, Patagonia, Schizomerieae
INTRODUCTION
The genus Ceratopetalum includes eight species found in eastern Australia, New Guinea, New Britain and several other small islands in the same region (Fig. 1; Hoogland, 1960; Fortune Hopkins and Hoogland, 2002; Rozefelds and Barnes, 2002). It is a member of the Cunoniaceae, a primarily southern hemispheric family that comprises about 27 genera, some of which are placed into six formally recognized tribes, with seven additional genera currently not included in a tribe (Bradford and Barnes, 2001; Bradford et al., 2004; Sweeney et al., 2004). Plants of this family are woody, with opposite or whorled leaves; they typically have bisexual flowers, often with 4–5 sepals and petals, although some members are apetalous (Fortune Hopkins and Hoogland, 2002; Bradford et al., 2004). Fruits are highly variable and encompass dry dehiscent (follicles, dehiscent capsules) and indehiscent (indehiscent capsules, samaroid fruits) types, and fleshy types (drupes and berries) (Dickison, 1984; Bradford and Barnes, 2001). Morphological evolution of reproductive structures within the family, and especially of fruit types, is complex, with inflorescence, flower and fruit structure being prone to homoplasy and providing few completely unambiguous synapomorphies for the genera and tribes (Bradford and Barnes, 2001).
Fig. 1.
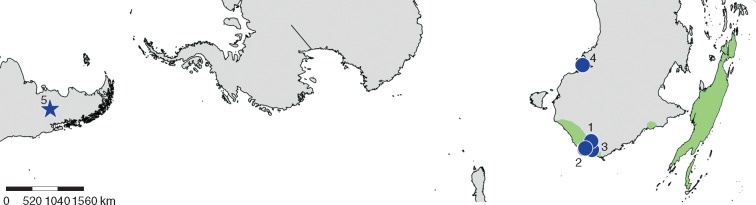
Distribution of extant Ceratopetalum (green) and Ceratopetalum fossils (blue circles, Laguna del Hunco flora marked with a blue star). Numbers adjacent to the circles on the map match the numbers in Table 1. The range of extant Ceratopetalum is after Hoogland (1960, fig. 1), Fortune Hopkins and Hoogland (2002, fig. 15) and Barnes et al. (2001, fig. 1); Australian fossil localities are from Barnes et al. (2001, fig. 2). The base map was produced using SimpleMappr (www.simplemappr.net).
Ceratopetalum belongs within the monophyletic tribe Schizomerieae, which also includes the genera Anodopetalum, Platylophus and Schizomeria (Bradford and Barnes, 2001; Bradford et al., 2004). Anodopetalum and Platylophus are monotypic (Bradford et al., 2004); Anodopetalum is endemic to Tasmania (Barker and Brown, 1994), while Platylophus is restricted to south-western Cape South Africa (Dyer, 1975; Bond and Goldblatt, 1984). Schizomeria (approx. 10 species), like Ceratopetalum, is found in eastern Australia, New Guinea and other islands in the same region (Fig. 1; Hoogland, 1960; Rozefelds and Barnes, 2002; Fortune Hopkins and Hoogland, 2002). Bradford and Barnes (2001) found that the monophyly of Schizomerieae is supported by the following morphological synapomorphies: petals with incised margins; an annular floral nectary; and a heterogeneous pollen tectum. Within Schizomerieae, each genus can be distinguished in part on the basis of its fruit type. Fruits of Anodopetalum are interpreted as berries (Dickison, 1984) or septicidal capsules (Barnes and Rozefelds, 2000); Schizomeria produces drupes (Fortune Hopkins and Hoogland, 2002); and Platylophus produces inflated indehiscent capsules (Bradford and Barnes, 2001; Bradford et al., 2004). Ceratopetalum has a highly distinctive indehiscent fruit with 4–6 woody wings derived from the enlarged sepals (Hoogland, 1960; Bradford and Barnes, 2001; Rozefelds and Barnes, 2002; Bradford et al., 2004). Several studies have identified this fruit type and/or the semi-inferior ovary position of Ceratopetalum as unique apomorphies defining the genus amongst the genera of Schizomerieae (Hufford and Dickison, 1992; Bradford and Barnes, 2001; Rozefelds and Barnes, 2002); other genera of Schizomerieae have superior ovaries (Rozefelds and Barnes, 2002).
Cunoniaceae have a fossil record extending into the Cretaceous. Most cunoniaceous fossils are known from sediments of the Southern Hemisphere, although two taxa with ambiguous affinities for the family were reported from Cretaceous deposits of the Northern Hemisphere. The older of these, Tropidogyne pikei, was described by Chambers et al. (2010) based on a single flower preserved in amber from the earliest Cenomanian (Late Cretaceous) of Burma (Myanmar) and compared with Ceratopetalum (for revised age, see Shi et al. 2012). Given the age of this fossil, it seems unlikely that it represents a crown-group member of Cunoniaceae and, indeed, Chambers et al. (2010) suggested that the two genera might not be closely related. The younger Cretaceous taxon, Platydiscus peltatus, was described by Schönenberger et al. (2001) based on charcoalified flowers from the Santonian to Campanian (Late Cretaceous) of Sweden. Schönenberger et al. (2001) placed the species within Cunoniaceae but also noted a resemblance between the floral structure of Platydiscus and that of the unrelated rosid families Anisophyllaceae (Cucurbitales) and Cunoniaceae (Oxalidales) [see also Matthews et al. (2001) and Matthews and Endress (2002) on Platydiscus; see The Angiosperm Phylogeny Group IV (2016), for the current classification].
Confirmed fossil records of the Cunoniaceae are known only from Australia, Antarctica and South America. According to a review by Barnes et al. (2001), 11 modern genera of Cunoniaceae and one fossil genus (Weinmanniaphyllum) are reported from the fossil record of Australia beginning in the late Paleocene, where they are represented by remains of leaves, cuticles, wood and reproductive structures. Cunoniaceous pollen, although it cannot be attributed with precision to modern genera, is also known (Barnes et al., 2001). Outside of Australia, the fossil record of the family is generally recognized on the basis of wood and pollen. Several species based on wood specimens have been described from the Cenomanian to early Campanian of King George Island (Isla 25 de Mayo) and Livingston Island, South Shetland Islands, Antarctica (Torres, 1985; Poole et al., 2000, 2001). Additional reports come from the Paleogene to Miocene of Chilean and Argentinean Patagonia (Petriella, 1972; Brea et al., 2004, 2012, 2015; Terada et al., 2006). Fossil pollen grains with affinities for Cunoniaceae are known from the Late Cretaceous to late Paleocene of the Antarctic Peninsula (Cranwell, 1959; Askin, 1992) and the late Paleocene to mid-Miocene of Patagonia (Petriella and Archangelsky, 1975; Romero and Castro, 1986; Barreda et al., 2007). In the Quaternary, pollen is known from deposits in Argentina and Chile (e.g. Heusser, 1974; Arbazúa et al., 2004), as well as other countries in South America, such as Colombia (e.g. Schreve-Brinkman, 1978; Hooghiemstra, 1989). The fossil record of Ceratopetalum was heretofore restricted to Australia, where it is based solely on fossil fruits of Eocene to Miocene age (Table 1; Fig. 1).
Table 1.
Fossil record of Ceratopetalum
| Species | Age | Locality/formation | State/country | Reference |
|---|---|---|---|---|
| C. priscum Holmes & Holmes (1) | Middle Miocene | Quarry H and A/Chalk Mountain | New South Wales, Australia | Holmes and Holmes (1992); Barnes and Hill, (1999) |
| C. westermannii R.W. Barnes & R. S. Hill (2) | Early Miocene | Elands | New South Wales/Australia | Barnes and Hill (1999) |
| C. wilkinsonii (Ett.) Holmes & Holmes (3) | Late Eocene–early Oligocene | Old Rose Valley Lead | New South Wales/Australia | Holmes and Holmes (1992); Barnes and Hill, (1999) |
| C. maslinensis R.W.Barnes & R.S.Hill (4) | Middle Eocene | Maslin Bay/North Maslin Sand | South Australia/Australia | Barnes and Hill (1999) |
| C. edgardoromeroi Gandolfo & Hermsen (5) | Early Eocene | LH4, LH6 and LH25/Tufolitas Laguna del Hunco | Chubut, Argentina | This study |
Additional reports considered invalid by Barnes, Hill and Bradford (2001) have been omitted.
Numbers following species names correspond to the locality numbers in Fig. 1.
In this contribution, we report radially symmetrical, five-winged fossil fruits with unique characters supporting their inclusion within the genus Ceratopetalum. These fossils come from the early Eocene Tufolitas Laguna del Hunco flora, Chubut Province, Patagonia, Argentina (Fig. 1). They are significant because they represent the first record of Cunoniaceae based on reproductive macrofossils from South America, the oldest unambiguous record for cunoniaceous reproductive macrofossils worldwide and the first known occurrence of the genus Ceratopetalum (extant or fossil) outside of Australasia.
MATERIALS AND METHODS
The Tufolitas Laguna del Hunco belongs to the Middle Río Chubut Volcanic–Pyroclastic Complex (Aragón and Mazzoni, 1997; Aragón et al., 2004). Sediments of the Tufolitas Laguna del Hunco outcrop in north-western Chubut Province, Patagonia, Argentina (Fig. 1); they represent tuffaceous lacustrine deposits that have yielded one of the most species-rich Eocene macrofloras yet discovered (Wilf et al., 2003, 2005). The fossil fruits were recovered from three (LH4, LH6 and LH25) of a total of 27 quarried localities (see Wilf et al., 2003 for coordinates). The age for all the localities is Ypresian or early Eocene [approx. 52 million years ago (Ma)] as calculated by radiometric and palaeomagnetic methods (see Wilf et al., 2003, 2005; and Wilf, 2012, for additional discussion of the age). The fossils were trimmed in the field, and additional preparation was performed by staff of the Museo Paleontológico Egidio Feruglio (MEF; Trelew, Chubut Province, Argentina).
Specimens described herein are housed in the palaeobotanical collection of the MEF (specimen number prefix MPEF-Pb).
Extant Ceratopetalum material used for comparison to the fossil specimens comes from herbarium sheets that are held at the L. H. Bailey Hortorium (BH), Plant Biology Section, School of Integrative Plant Science, Cornell University, Ithaca, NY, USA and the National Herbarium of Victoria, Royal Botanic Gardens Melbourne (MEL), Victoria, Australia. Fossils were photographed using a Nikon D70 Digital SLR camera at BH and MEF. Images of extant Ceratopetalum were taken at MEL using an Epson Expression 10000 XL, Model J181A scanner mounted in a HerbScan framework, and at BH with a Nikon D800e camera. Plates were prepared using Adobe Photoshop CS4 Extended ver. 11.0 (1990–2008 Adobe Systems Inc.), and line drawings of the venation patterns of the sepals of the fossil and extant Ceratopetalum and schematic diagrams of venation patterns were produced with Adobe Illustrator Creative Cloud 2015.0.0 Release (1987–2015 Adobe Systems Inc.).
RESULTS
Family. Cunoniaceae R. Br. 1814
Tribe. Schizomerieae J. C. Bradford & R. W. Barnes 2001
Genus. Ceratopetalum Sm. 1793
Species. Ceratopetalum edgardoromeroi Gandolfo & Hermsen, sp. nov.
cf. Cunoniaceae, sp. no. TY129 (LH4-169 = MPEF-Pb 5087), Wilf et al. (2005), appendix table 2.
Table 2.
Floral characters of Ceratopetalum species
| No. of wings | Wing shape | Wing apex | Wing base | Length (mm) | Width (mm) | Petals | Nectary disc | ‘Ovary’ diameter (mm) | Stamens on fruit | |
|---|---|---|---|---|---|---|---|---|---|---|
| gum | 4–6 | Narrowly to broadly obovate | Acute | Not constricted | 9·8–16·1 | 2·7–6·7 | Present | Present | 3·4–4·8 | Present |
| ape | 4–6 | Obovate to ovate | Acute | Constricted | 6·3–8·9 | 2·1–4·3 | Absent | Present | 3–4·4 | Present |
| cor | 4–6 | Obovate | Acute | Slightly constricted | At least 7 | ? | Absent | Present | ? | ? |
| hyl | 4 | Narrowly obovate to lanceolate | Acute to obtuse | Slightly constricted | 6·6–11·2 | 2·2–3 | Absent | Present | 3·6–4 | Present |
| suc | 4–5 | Elliptical to obovate | Acute | Slightly constricted | 8·3–12·6 | 2·4–4·1 | Absent | Present | 3·6–4·2 | Present |
| vir | 4–6 | Obovate to lanceolate | Acute | Constricted | 11·5–13·5 | 3·4–4·9 | Absent | Present | 6·1–7·3 | Present |
| tet | 4 | Ovate to obovate | Acute | Slightly constricted or not | 8·8–17 | 3·8–5·1 | Absent | Present | 6–8 | Present |
| iug | 4 | Narrowly to broadly obovate | Acute | Not constricted | 15 | 4·8–5 | Absent | Present | ? | Present |
| mac | 4–5 | Obovate to lanceolate | Acute | Constricted | 10–13 | 3·2–4·5 | Absent | Present | 5·4–5·9 | Present |
| *pri | 5 | Narrow oblong | Obtuse | Not constricted | 7–10 | 3–4 | Present | Present | 3–5 | Absent |
| *wes | 5 | Narrow obovate | Rounded | Not constricted | 6–9 | 1·8–2·2 | Absent | Absent? | 1–1·2 | Present |
| *mas | 5–6 | Narrow oblong | Acute to obtuse | Not constricted | 5–5·5 | ∼1·5–2 | Absent | Absent? | ∼2·1 | Absent |
| *wil | 5 | Ovate to elliptical | Obtuse | Not constricted | ∼10 | 4–5·5 | Present | Present | 6·5 | Absent |
| *edg | 5 | Narrow obovate | Rounded | Constricted | 10 | 2–4 | Absent | Present | 4–5 | Absent |
gum, C. gummiferum; ape, C. apetalum; cor, C. corymbosum; hyl, C. hylandii; suc, C. succirubrum; vir, C. virchowii; tet, C. tetrapterum; iug, C. iugumensis; mac, C. macrophyllum; *pri, C. priscum; *wes, C. westermannii; *mas, C. maslinensis; *wil, C. wilkinsonii; edg, *C. edgardoromeroi.
Denotes fossils.
Data for extant species are gathered from Hoogland (1960), Fortune Hopkins and Hoogland (2002), and Rozefelds and Barnes (2002); data for fossil species from Holmes and Holmes (1992), Barnes and Hill (1999) and this contribution.
All measurements are given in mm.
Unknown dicot sp., sp. no. TY145 (LH6-1017 = MPEF-Pb 5085), Wilf et al. (2005), appendix table 2.
Specific diagnosis. Fruit with five wings (sepals) surrounding a circular central region. Each wing obovate, apex rounded and base constricted, vascularized by three primary veins; midvein prominent and two lateral veins weaker; primary veins branching in the distal two-thirds of each wing, branches sometimes anastomosing to form a partially closed reticulum. Petals absent. Annular nectary present, prominent.
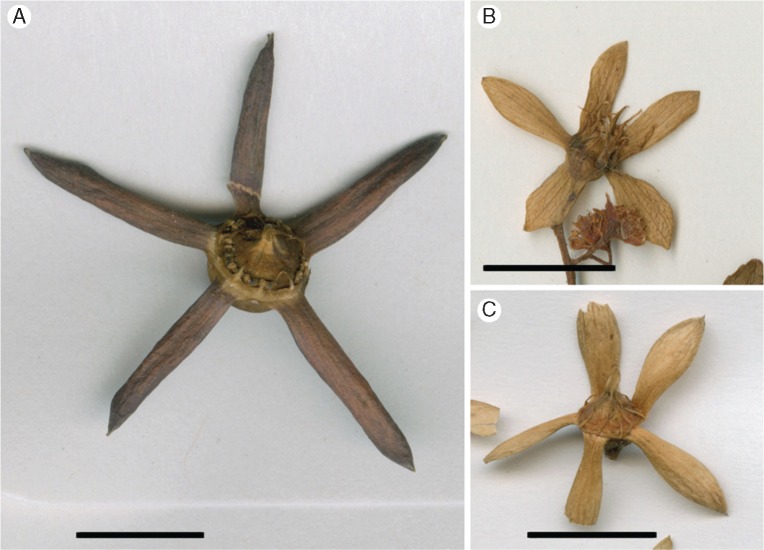
Holotype designated here. MPEF-Pb 5085a, b (LH6), Fig. 2A, B. Repository, Museo Paleontológico Egidio Feruglio (MEF).
Fig. 2.
Ceratopetalum edgardoromeroi Gandolfo and Hermsen, sp. nov. (A) Overall view of the holotype showing the five wings and the circular central region with nectary and semi-inferior ovary. MPEF-Pb 5085a. Scale bar = 10 mm. (B) Detail of the central region showing the nectary and radiating veins (arrows show examples). MPEF-Pb 5085a. Scale bar = 2 mm. (C) Overall view of the specimen showing three complete or nearly complete wings and two wing bases, prominent nectary, central styles marking the position of the ovary and inter-wing veins (IWV). MPEF-Pb 5086b. Scale bar = 5 mm. (D) Detail of fruit wing showing three primary veins emerging from the central region and branching distally to form a reticulum of minor veins. MPEF-Pb 5086a. Scale bar = 5 mm. (E) Detail of the circular central region showing ends of radiating veins (RV), inter-wing veins (IWV), intersections of the inter-wing and radiating veins (I) and well-preserved encircling vein (EV) crossing beneath a wing. Styles (ST) are visible in the centre of the nectary. MPEF-Pb 5086b. Scale bar = 5 mm.
Type locality, age and stratigraphy. Laguna del Hunco paleoflora locality LH6, Ypresian (early Eocene) Tufolitas Laguna del Hunco, Chubut Province, Argentina.
Additional material examined. MPEF-Pb 5084 (LH4), MPEF-Pb 5087 (LH4) and MPEF-Pb 5086a, b (LH25).
Etymology
The species epithet ‘edgardoromeroi’ is erected in honor of Edgardo J. Romero, a prestigious Argentinean palaeobotanist, for his many contributions to our understanding of the angiosperm palaeofloras of Patagonia, Argentina.
Species description
The fruits are radially symmetrical, apparently indehiscent and lacking petals. They are 2·3–2·6 cm in diameter. Each fruit has a calyx of five persistent wings (sepals) attached to a circular central region (Fig. 2A, C). Each wing is narrowly obovate with a rounded apex, a slightly constricted base and an entire margin (Fig. 2A, C); wing dimensions are approx. 1 cm long and 0·2–0·4 cm wide. Each wing is vascularized by three primary veins, a prominent midvein and two less prominent lateral veins (Fig. 2A, C, D). The midvein radiates from within the central region of the fruit and proceeds in a slightly sinuous pattern to the tip of the wing, giving off lateral branches (Fig. 2A, C, D). Each lateral vein is continuous basally, with the nearest lateral vein in the adjacent wing, forming an inter-wing vein that follows the edge of the central region (Fig. 2C, E). Each lateral vein also branches distally, becoming indistinct in its course as it does so (Fig. 2C, D). The branches of the primary veins anastomose occasionally, yielding a reticulum in the distal two-thirds of the wing (Fig. 2C, D).
The circular central region of the fruit is 0·5–0·6 cm in diameter and has two distinct zones, a thick outer ring and an inner circular zone (Fig. 2A–C, E). The thick outer ring is interpreted as representing an annular nectary and is 0·1–0·2 cm wide; the inner circular zone is interpreted as representing the upper part of the ovary and is 0·4–0·5 cm in diameter (Fig. 2A–C, E). Thick veins radiating from the central region of the fruit are visible on and at the edge of the nectary (Fig. 2A–C, E). Some of these radiating veins are the veins that continue into the wings to become the wing midveins (Fig. 2A–C, E); alternating with these are radiating veins that fuse with each inter-wing vein at its midpoint (Fig. 2B, E). Since one radiating vein that proceeds into each wing alternates with one radiating vein that fuses with an inter-wing vein (Fig. 2B, E), the fruit is interpreted as having had ten radiating veins.
A prominent vein crosses the base of a wing near the outer edge of the nectary on one specimen; this vein intersects the wing midvein at a right angle and merges with the inter-wing veins on either side (Fig. 2C, E). Given the symmetry of the fruit and the remnants of what appear to be additional, similar veins near the outer edge of the nectary, the fruit may have had a thick vein proceeding across the base of each wing and fusing with the interwing veins on either side, thus forming a continuous series of veins encircling the periphery of the nectary.
The nectary region shows remnants of tissue, perhaps including vascular tissue forming thin veins, although no particular venation pattern was observed beyond that described above (Fig. 2A–C, E). One specimen shows at least two styles, although four structures may be present (Fig. 2C, E), indicating that there were either two unlobed styles, two bilobed styles or four styles; thus, carpel number is inferred to be two or four. Based on the prominence of the annular nectary relative to the ovary (Fig. 2A–C, E), the ovary is interpreted as at least semi-inferior.
DISCUSSION
Assignment to Ceratopetalum and recognition of new species
The floral and fruit morphology of extant Ceratopetalum have been described in detail by, for example, Hoogland (1960), Dickison (1984), Barnes and Hill (1999), Matthews et al. (2001), Fortune Hopkins and Hoogland (2002) and Rozefelds and Barnes (2002). Extant Ceratopetalum species produce fruits with a radially symmetrical, persistent calyx of 4–6 wings (enlarged sepals) attached to a central receptacle (Fig. 3A–C). Stamens are persistent on the fruits and are found both alternating with and opposite the wings (Fig. 3A–C). The ovary is semi-inferior, indehiscent and composed of two or sometimes three carpels; a conspicuous annular nectary surrounds the upper portion of the ovary in the flowers (see, for example, fig. 5 in Rozefelds and Barnes, 2002), although its appearance is less pronounced in the fruit (Fig. 3A, C).
Fig. 3.
Extant Ceratopetalum fruits. (A) C. macrophyllum. MEL2277402. (B) C. gummiferum. MEL648233. (C) C. apetalum. MEL2048597. Scale bar = 1 cm.
Fig. 5.
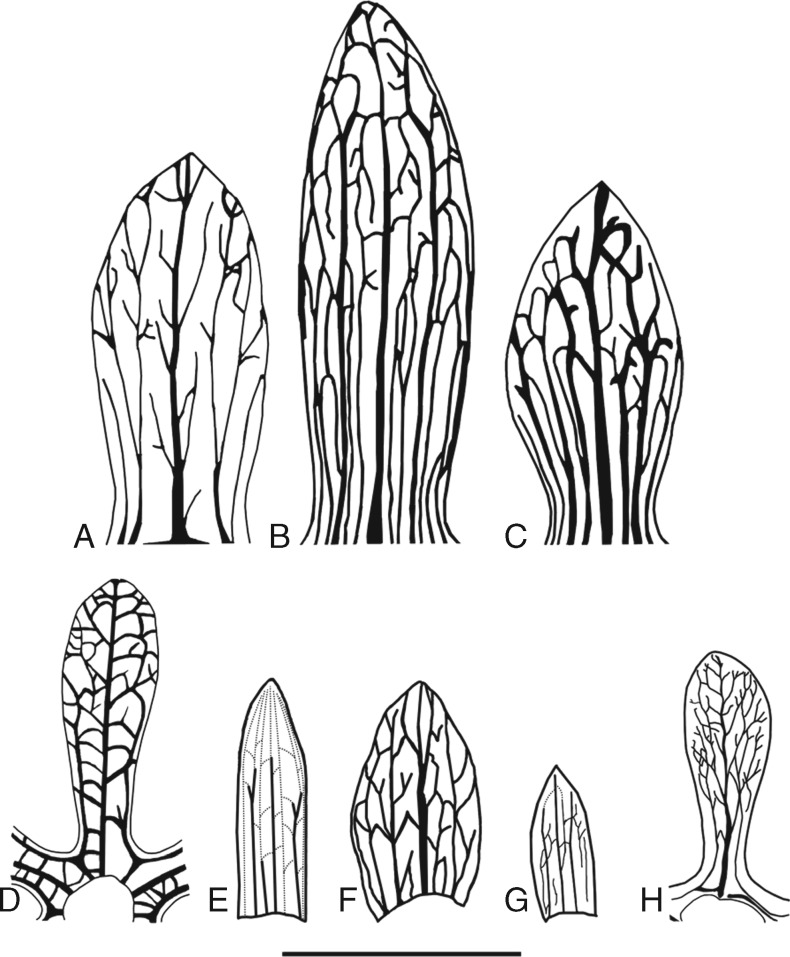
Line drawing of wing morphology and venation pattern of selected extant and all fossil species of Ceratopetalum. (A–C) Extant species. (A) C. gummiferum. (B) C. succirubrum. (C) C. virchowii. (D–H) Fossil species. (D) C. westermannii. (E) C. priscum. (F) C. wilkinsonii. (G) C. maslinensis. (H) C. edgardoromeroi. See Tables 1 and 2 for descriptions; (A–D) are redrawn from Barnes and Hill (1999). Scale bar = 1 cm.
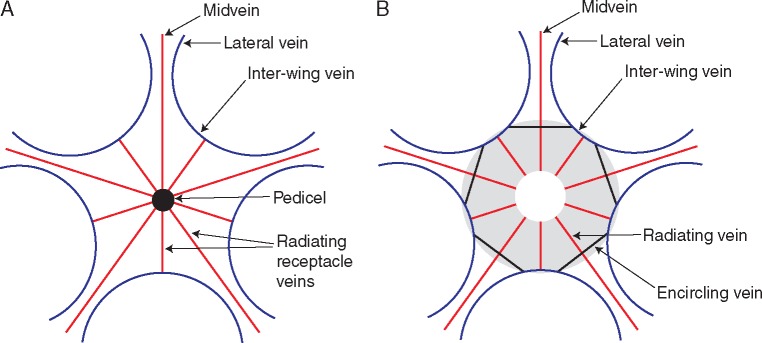
According to Barnes and Hill (1999), extant Ceratopetalum flowers and fruits share a similar venation pattern, as reconstructed here (Fig. 4A) based on descriptions by Dickison (1975), Barnes and Hill (1999) and Matthews et al. (2001), as well as fig. 3 of Barnes and Hill (1999): each sepal/fruit wing is fed by three primary veins that emerge from the receptacle (i.e. extra-ovarian floral tissue fused to the ovary wall), a midvein with one lateral vein on either side; additional, less prominent lateral wing veins may also be present in the wing bases of some species. The lateral primary veins from adjacent wings are continuous basally, forming inter-wing veins. Prominent veins radiating from the receptacle to form the wing midveins alternate with veins that fuse with the inter-wing veins near the edge of the receptacle. The number of veins radiating from the receptacle of the flower is thus twice the number of fruit wings, or equal to the number of stamens.
Fig. 4.
Schematic diagrams showing fruit venation patterns of Ceratopetalum. (A) Extant Ceratopetalum. (B) C. edgardoromeroi. The shaded area represents the region interpreted as the nectary in the fossil fruits. The central white circle represents the region of the ovary. While it is presumed that the radiating veins would have passed beneath the ovary to the fruit pedicel as in extant Ceratopetalum (Barnes and Hill, 1999), this has been left intentionally ambiguous in the drawing since it was not observed. Encircling veins are here reconstructed as crossing beneath each wing.
The three-veined sepals and inter-sepal veins formed by the fusion of lateral veins from adjacent sepals are characteristics shared across flowers of nearly all genera of Cunoniaceae that have been examined (Dickison, 1975; Barnes and Hill, 1999; Matthews et al., 2001; Bradford et al., 2004). Therefore, flower or fruit venation pattern alone is not necessarily diagnostic for the genus, but must be considered in conjunction with other features.
Ceratopetalum edgardoromeroi fruits are largely consistent with extant Ceratopetalum in their morphology and venation pattern. Ceratopetalum edgardoromeroi is a five-winged fruit with wings attached to a circular region interpreted here as consisting of a central ovary surrounded by a prominent annular nectary (Fig. 2A–C, E). Due to the prominence of the nectary in relation to the relatively small region interpreted as representing the gynoecium (Fig. 2A–C, E), the Patagonian fossils are interpreted as probably having had a semi-inferior ovary, although the position of the ovary cannot be definitively confirmed due to the orientation in which the fruits are preserved. Based on the presence of 2–4 structures interpreted as styles on one specimen (Fig. 2C, E), C. edgardoromeroi is interpreted to be bicarpellate or tetracarpellate; although bicarpellate flowers are a synapomorphy for a large clade of Cunoniaceae including tribe Schizomerieae, flowers with more than two carpels have originated separately several times, including within Schizomerieae (Bradford and Barnes, 2001). Ceratopetalum edgardoromeroi fruits do not have stamens preserved, which conflicts with the fruits of modern Ceratopetalum species. Lack of persistent stamens is, however, consistent with other fossil fruits assigned to Ceratopetalum, except for C. westermannii (Table 2).
The venation pattern of the fruits, preserved in two specimens (Fig. 2A–E), is similar to that described for extant species (cf. Fig. 4A and B). Wings have three primary veins, and lateral primary veins from adjacent wings fuse to form inter-wing veins (Figs 2A–C, E and4B). Veins radiate from the circular region at the centre of the fruit, continuing into the wings or ending at the inter-wing veins (Figs 2A–C and4B). In one specimen, these veins appear to widen at the periphery of the nectary, perhaps representing the attachment points of the stamens (Fig. 2A, B). Although the radiating veins are observed in or at the margins of the nectary in C. edgardoromeroi, they are assumed to be passing beneath the nectary proper, presumably through tissue beneath or surrounding the ovary (i.e. the receptacle). Large veins crossing the bases of the wings and partially or wholly encircling the periphery of the nectary in C. edgardoromeroi (Figs 2C, E and 4B) are one feature not noted as characterizing the extant genus. These veins may be associated with the nectary or, more probably, the rim of the receptacle or even the base of the calyx. The nectary also shows the remnants of thick tissue or perhaps minor veins (Fig. 2A–C, E); Matthews et al. (2001) noted phloem in the nectary of extant C. gummiferum flowers.
Within Cunoniaceae, the only other taxon that produces fruit with five wings formed by a persistent calyx is Pullea (tribe Codieae); this taxon, like Ceratopetalum, also has a semi-inferior ovary (Fortune Hopkins and Hoogland, 2002), which is relatively uncommon amongst genera of Cunoniaceae (Bradford and Barnes, 2001). The similarities in fruit morphology between Ceratopetalum and Pullea were interpreted as likely to be the result of convergent evolution (Bradford and Barnes, 2001). Pullea is native to Fiji, Australia, New Guinea and other small islands north of Australia as far north as Morotai (Hoogland, 1979; Fortune Hopkins and Hoogland, 2002). According to Fortune Hopkins and Hoogland (2002), Pullea can be distinguished from Ceratopetalum by its imbricate sepal aestivation, a feature that can apparently be inferred even from the fruiting stage; in contrast, Ceratopetalum flowers have valvate sepal aestivation (Rozefelds and Barnes, 2002). Additionally, Pullea fruits have papery wings and relatively long, persistent styles (Fortune Hopkins and Hoogland, 2002).
Extant and fossil Ceratopetalum species can be distinguished by certain fruit characteristics, including a combination of wing (sepal) number, wing morphology, wing venation pattern and presence or absence of petals and stamens on mature fruits (Table 2).Ceratopetalum edgardoromeroi can be distinguished from other known species by a combination of wing number (five), wing shape (obovate with rounded apex and constricted base), absence of petals and stamens, and wing venation. The partially closed reticulum formed by the minor veins in particular seems to be unique for this species among fossil and extant Ceratopetalum (Fig. 5A–H).
Character evolution and age of Ceratopetalum
Ceratopetalum edgardoromeroi is typical of fossil Ceratopetalum fruits in having five wings (sepals) and in lacking stamens (Table 2). Based on a morphological phylogenetic analysis of Schizomerieae focusing on intrageneric relationships of Ceratopetalum, Rozefelds and Barnes (2002) found that tetramerous flowers are derived within the genus, and that they had originated twice: once in C. iugumensis and once in a clade including C. hylandii, C. succirubrum and C. tetrapterum. Thus, all fossils appear to have the plesiomorphic condition for perianth merosity. All extant Ceratopetalum species (except C. corymbosum, in which this character has not been documented) retain whole stamens or remnants of stamens on their fruits (Fig. 3A–C; Rozefelds and Barnes, 2002), whereas all fossil species, except C. westermannii (early Miocene), lack them (Holmes and Holmes, 1992; Barnes and Hill, 1999; this study). This would suggest either that the stamens were routinely lost during preservation of Ceratopetalum fossils, or that persistence of the stamens on the fruits is a novel structural feature that appeared some time during the early Miocene.
Only three species of Ceratopetalum have a corolla: the extant C. gummiferum (Fig. 3B) and the Australian fossil fruits C. priscum and C. wilkinsonii (Holmes and Holmes, 1992; Barnes and Hill, 1999; Rozefelds and Barnes, 2002). If the absence of petals in the fossil fruits is interpreted as a true absence of petals as in most extant species of Ceratopetalum, the remaining species lack petals (Table 2). Petals with incised margins are a synapomorphy for the tribe Schizomerieae as a whole (Bradford and Barnes, 2001), whereas the absence of petals is thought to be apomorphic within Ceratopetalum (Rozefelds and Barnes, 2002). The lack of petals in C. edgardoromeroi, now the oldest documented occurrence of fossils assignable to Ceratopetalum, would thus indicate that the loss of petals within the genus occurred before the early Eocene. Previously, the loss of petals was considered to have dated minimally to the middle Eocene, as evidenced by lack of petals in C. maslinensis (Rozefelds and Barnes, 2002). Lack of petals in C. maslinensis was also previously considered to indicate that Ceratopetalum itself had originated prior to the middle Eocene (Rozefelds and Barnes, 2002). Since C. edgardoromeroi likewise lacks petals, suggesting that it nests within crown-group Ceratopetalum, the age of the genus must now be considered to date minimally to the early Eocene.
Criteria for choosing fossils to use in divergence dating analyses have been suggested by Gandolfo et al. (2008) and Parham et al. (2012). The age of the C. edgardoromeroi specimens is well constrained, the source locality of the fossils is well documented, the identity of the fossils is well founded on the basis of their overall structure and an apomorphy (lack of petals) convincingly places them within crown-group Ceratopetalum; thus, C. edgardoromeroi would make a suitable calibration point for divergence dating analyses. The LH4 locality occurs below a stratigraphic layer most recently dated as 52·22 ± 0·29 Ma (Wilf et al., 2003, 2005; Wilf, 2012), making the numerical minimum age currently associated with C. edgardoromeroi 51·93 Ma. Notably, in addition to being the oldest fossil record of Ceratopetalum, C. edgardoromeroi is the oldest fossil record for tribe Schizomerieae.
Palaeoecology and palaeobiogeography
Extant Ceratopetalum species have a disjunct distribution confined to Australasia (Fig. 1). In Australia, Ceratopetalum is found in sub-tropical to warm temperate rain forests of eastern New South Wales and south-eastern Queensland, as well as north-eastern Queensland (Baur, 1957, 1979; Hoogland, 1960; Floyd, 1990; Rozefelds and Barnes, 2002). Only one species grows outside Australia, occurring in New Britain, New Guinea and small islands nearby (Fortune Hopkins and Hoogland, 2002; Rozefelds and Barnes, 2002), where it has been documented from sub-tropical rain forests (Takeuchi, 1999a, b, 2002; Paul, 2011). Ceratopetalum shows a biogeographic pattern typical of the Laguna del Hunco flora, since the genus is extinct in South American today but is a component of the Paleogene to recent rain forest vegetation of Australasia (see, for example, Wilf et al., 2009, 2014; Kooyman et al., 2014; Merkhofer et al., 2015).
The palaeocommunity in which C. edgardoromeroi grew was composed of pteridophytes, including Dicksonia, Sticherus, Todea and Pteridaceae (Carvalho et al., 2010, 2013); conifers, such as Agathis, Dacrycarpus and Papuacedrus (Wilf et al., 2009, 2014; Wilf, 2012); the ginkgophyte Ginkgoites (Villar de Seoane et al., 2015); and angiosperms, including magnoliids (Atherospermataceae and Monimiaceae: Knight and Wilf 2013), eudicots (other Cunoniaceae, Akania, Eucalyptus, Gymnostoma, Juglandaceae and several Proteaceae: Romero and Hickey, 1976; Gandolfo et al., 1988, 2011; Zamaloa et al., 2006; González et al., 2007; Gandolfo and Hermsen, 2012; Hermsen et al., 2012; Hermsen and Gandolfo, 2016), and the monocot Ripogonum (Carpenter et al., 2014). This palaeocommunity is similar in composition to modern rain forest communities of Australia and New Guinea where extant Ceratopetalum occurs. Ceratopetalum is dominant in some rain forest alliances in Australia (see Baur, 1957, 1979; Burges and Johnston, 1953; Floyd, 1990; Kooyman et al., 2014), while it is typically an occasional component of plant communities in Papua New Guinea (Takeuchi, 1999a, 2002; Paul, 2011). The few specimens (four specimens in total) of C. edgardoromeroi found in the Laguna del Hunco flora suggest that it was not a prominent component of the Laguna del Hunco palaeocommunity, but rather relatively rare.
Conclusions
In their paper on the evolution of angiosperm fruits, Collinson and van Bergen (2004) separated winged fruits into several categories based on wing position. Since the Patagonian fossils have multiple wings, they belong to Collinson and van Bergen’s (2004: p. 377) category ‘Multiple wings (‘helicopters/propellers’)’, which includes fossil fruits with at least three (and typically more) wings oriented in a more or less horizontal position. Within this category, nine genera have a Paleogene fossil record (Table 3): Asterocarpinus (Betulaceae), Ceratopetalum (Cunoniaceae), Chaneya (probably within Simaroubaceae), Cruciptera (Juglandaceae), Tetrapterys (Malpighiaceae), Trilobium (Anacardiaceae?), and Calycites, Ozakia and Raskya (affinity uncertain). With the exception of Ceratopetalum, all genera representing multiwinged helicopter-type fruits are known from Northern Hemisphere palaeofloras of Europe, eastern Asia and/or western North America (Table 3). This report of Ceratopetalum is the first documented occurrence of a multiwinged helicopter-type fruit genus showing an intercontinental connection between Southern Hemisphere continents, and the first Paleogene report of this fruit type from South America. The helicopter-type Laguna del Hunco fruits described in this study clearly represent a new record of fossil Ceratopetalum: they have five wings, an annular floral nectary, a semi-inferior ovary and a floral/fruit venation pattern consistent with Cunoniaceae and Ceratopetalum (Fig. 4A, B).
Table 3.
Paleogene multiwinged, helicopter-type fruits (see Collinson and van Bergen, 2004)
| Genus | Family | Continent | Age | Publication(s) |
|---|---|---|---|---|
| Asterocarpinus | Betulaceae | North America | Late Eocene–early Oligocene | Manchester and Crane (1987); Meyer and Manchester (1997) |
| Calycites | Unknown | Europe | Paleocene | Crane (1988) |
| North America | Paleocene–middle Eocene | Crane (1988); Wehr (1995) | ||
| Ceratopetalum | Cunoniaceae | South America | Eearly Eocene | This study |
| Australia | Middle Eocene–middle Miocene | Holmes and Holmes (1992); Barnes and Hill (1999) | ||
| Chaneya | Simaroubaceae? | North America | Middle Eocene–? late Oligocene | Wang and Manchester (2000) |
| Asia | Late Eocene–middle Miocene | Wang and Manchester (2000) | ||
| Cruciptera | Juglandaceae | Europe | Middle Eocene | Manchester (1991, 1999); Manchester et al. (1994) |
| North America | Middle Eocene–Oligocene | Manchester (1991, 1999, 2000); Wehr (1995); Meyer and Manchester (1997) | ||
| Ozakia | Unknown | North America | ?Oligocene–Miocene | Manchester and Uemura (2014) |
| Asia | Late Miocene | Manchester and Uemura (2014) | ||
| Raskya | Unknown | Europe | Late Eocene–early Oligocene | Manchester and Hably (1997); Thiebaut (1999) |
| Tetrapterys | Malpighiaceae | Europe | Early Oligocene | Hably and Manchester (2000) |
| Trilobium | Anacardiaceae? | Europe | Middle Eocene–Oligocene | Wilde and Frankenhäuser (2010) |
The lack of petals on C. edgardoromeroi suggests that they belong within the crown-group for the genus. The fruits are assigned to a new species, C. edgardoromeroi, primarily on the basis of the venation pattern of their wings, which differs from that described for other fossil and modern Ceratopetalum fruits (Fig. 5A–H). Due to their well-documented geographic and stratigraphic provenance, as well as the features establishing their position within the genus Ceratopetalum, the fossils provide a robust minimum age for the genus of 51·93 Ma. Ceratopetalum edgardoromeroi fits comfortably into the biogeographic pattern already established for the Laguna del Hunco flora, as the genus Ceratopetalum has previously been described only from Australasia.
ACKNOWLEDGEMENTS
We thank G. Jordan, R. Carpenter and P. Wilf for discussions related to these fossils; the curators of BH and MEL, K. C. Nixon and D. Cantrill, respectively, for access to their collections; H. Barnes (MEL, Australia) for photographs of extant Ceratopetalum fruits; J. Svitko (Cornell University) for photographs of extant material; J. Gallego (MEF) for photographs of fossil material; M. Caffa, L. Canessa, B. Cariglino, M. Carvalho, N. R. Cúneo, I. Escapa, J. Gallego, R. Horwitt, A. Iglesias, K. R. Johnson, P. Puerta, L. Reiner, E. Ruigomez, J. Svitko, P. Wilf and S. Wing for assistance in the field, lab and collections; and the Nahueltripay family for generously providing land access. This work was supported by the National Science Foundation grants [DEB-0345750, DEB-0918932, DEB-0919071, DEB-1556136, DEB-1556666] and a Fulbright Fellowship to M.A.G.
LITERATURE CITED
- Aragón E, Mazzoni MM.. 1997. Geología y estratigrafía del complejo volcánico piroclástico del río Chubut medio (Eoceno), Chubut, Argentina. Revista de la Asociación Geológica Argentina 52: 243–256. [Google Scholar]
- Aragón E, Aguilera YE, Consoli VC, Cavarozzi CE, Ribot A.. 2004. Las Andesitas Estrechura del Complejo Volcánico Piroclástico del río Chubut medio (Paleoceno-Eoceno medio). Revista de la Asociación Geológica Argentina 59: 619–633. [Google Scholar]
- Arbazúa AM, Villagrán C, Moreno PI.. 2004. Deglacial and postglacial climate history in east-central Isla Grande de Chiloé, southern Chile (43°S). Quaternary Research 62: 49–59. [Google Scholar]
- Askin RA. 1992. Late Cretaceous–Early Tertiary Antarctic outcrop evidence for past vegetation and climates. Antarctic Research Series 59: 61–73. [Google Scholar]
- Barker PCJ, Brown MJ.. 1994. Anodopetalum biglandulosum: growth form and abundance in Tasmanian rainforest. Australian Journal of Ecology 19: 435–443. [Google Scholar]
- Barnes RW, Hill RS.. 1999. Ceratopetalum fruits from Australian Cainozoic sediments and their significance for petal evolution in the genus. Australian Systematic Botany 12: 635–645. [Google Scholar]
- Barnes RW, Rozefelds AC.. 2000. Comparative morphology of Anodopetalum (Cunoniaceae). Australian Systematic Botany 13: 267–282. [Google Scholar]
- Barnes RW, Hill RS, Bradford JC.. 2001. The history of Cunoniaceae in Australia from macrofossil evidence. Australian Journal of Botany 49: 301–320. [Google Scholar]
- Barreda V, Anzótegui LM, Prieto AR, et al. 2007. Diversificación y cambios de las angiospermas durante el Neógeno de Argentina. Asociación Paleontológica Argentina, Publicación Especial 11: 173–191. [Google Scholar]
- Baur GN. 1957. Nature and distribution of rain-forests in New South Wales. Australian Journal of Botany 5: 190–233. [Google Scholar]
- Baur GN. 1979. Forest types in New South Wales. Forestry Commission of New South Wales Research Note 17: 1–81. [Google Scholar]
- Bond P, Goldblatt P.. 1984. Plants of the Cape Flora, a descriptive catalogue. Journal of South African Botany Supplement 13: 1–445. [Google Scholar]
- Bradford JC, Barnes RW.. 2001. Phylogenetics and classification of Cunoniaceae (Oxalidales) using chloroplast DNA sequences and morphology. Systematic Botany 26: 354–385. [Google Scholar]
- Bradford JC, Fortune-Hopkins HC, Barnes RW.. 2004. Cunoniaceae In:K Kubitzki, ed. The families and genera of vascular plants, VI Flowering plants, Dicotyledons. New York: Springer-Verlag, 91–111. [Google Scholar]
- Brea M, Zucol AF, Raigemborn MS, Matheos S.. 2004. Leños fósiles del Paleoceno Superior (Grupo Río Chico), Provincia del Chubut, Argentina. Ameghiniana 41 (Suppl): 7R–8R (abstract). [Google Scholar]
- Brea M, Zucol AF, Iglesias A.. 2012. Fossil plant studies from late Early Miocene of the Santa Cruz Formation: paleoecology and paleoclimatology at the passive margin of Patagonia, Argentina In: SF Vizcaíno, RF Kay, MS Bargo, eds. Early Miocene paleobiology in Patagonia: high-latitude paleocommunities of the Santa Cruz Formation. Cambridge: Cambridge University Press, 104–128. [Google Scholar]
- Brea M, Artabe AE, Franzese JR, et al. 2015. Reconstruction of a fossil forest reveals details of the palaeocology, palaeoenvironments and climatic conditions in the late Oligocene of South America. Palaeogeography, Palaeoclimatology, Palaeoecology 418: 19–42. [Google Scholar]
- Burges A, Johnston RD.. 1953. The structure of a New South Wales subtropical rain forest. Journal of Ecology 41: 72–83. [Google Scholar]
- Carpenter RJ, Wilf P, Conran JG, Cúneo NR.. 2014. A Paleogene trans-Antarctic distribution for Ripogonum (Ripogonaceae: Liliales)? Palaeontologia Electronica 17: 39A; 9p; palaeo-electronica.org/content/2014/921-early-eocene-ripogonum. [Google Scholar]
- Carvalho MJ, Wilf P, Gandolfo MA, Cúneo NR, Johnson KR.. 2010. Ferns from the Laguna del Hunco paleoflora (51.9 Ma), Patagonia, Argentina, reveal biogeographic links to rainforest Gondwana. Geological Society of America Abstracts with Programs 42: 373. [Google Scholar]
- Carvalho MJ, Wilf P, Hermsen EJ, Gandolfo MA, Cúneo NR, Johnson KR.. 2013. First record of Todea (Osmundaceae) in South American, from the early Eocene paleorainforests of Laguna del Hunco (Patagonia, Argentina). American Journal of Botany 100: 1831–1848. [DOI] [PubMed] [Google Scholar]
- Chambers KL, Poinar G Jr, Buckley R.. 2010. Tropidogyne, a new genus of Early Cretaceous eudicots (Angiospermae) from Burmese Amber. Novon 20: 23–29. [Google Scholar]
- Collinson ME, van Bergen PF.. 2004. Evolution of angiosperm fruit and seed dispersal biology and ecophysiology: morphological, anatomical and chemical evidence from fossils In: AR Hemsley, I Poole, eds. The evolution of plant physiology: from whole plants to ecosystems. London: Elsevier, 343–377. [Google Scholar]
- Crane PR. 1988. Abelia-like frutis from the Palaeogene of Scotland and North America. Tertiary Research 9: 21–30. [Google Scholar]
- Cranwell LM. 1959. Fossil pollen from Seymour Island, Antarctica. Nature 184: 1782–1785. [Google Scholar]
- Dickison WC. 1975. Studies on floral anatomy of the Cunoniaceae. American Journal of Botany 62: 433–447. [Google Scholar]
- Dickison WC. 1984. Fruits and seeds of the Cunoniaceae. Journal of the Arnold Arboretum 65: 149–190. [Google Scholar]
- Dyer RA. 1975. The genera of southern African flowering plants, Volume 1 Dicotyledons. Department of the Agricultural Technical Services, Pretoria, South Africa. [Google Scholar]
- Floyd AG. 1990. Australian rainforests in New South Wales, Vols 1 and 2. Chipping Norton: Surrey Beatty & Sons Pty Limited and National Parks and Wildlife Service of New South Wales. [Google Scholar]
- Fortune Hopkins HC, Hoogland RD.. 2002. Cunoniaceae. Flora Malesiana, Series 1 16: 53–165. [Google Scholar]
- Gandolfo MA, Hermsen EJ.. 2012. The emerging Patagonian fossil record of Cunoniaceae and its biogeographical significance. Japanese Journal of Palynology (Special Issue, Abstracts: IPC/IOPC 2012) 58: 66–67. [Google Scholar]
- Gandolfo MA, Dibbern MC, Romero EJ.. 1988. Akania patagonica n. sp. and additional material on Akania americana Romero & Hickey (Akaniaceae), from Paleocene sediments of Patagonia. Bulletin of the Torrey Botanical Club 115: 83–88. [Google Scholar]
- Gandolfo MA, Nixon KC, Crepet WL.. 2008. Selection of fossils for calibration of molecular dating models. Annals of the Missouri Botanical Garden 95: 34–42. [Google Scholar]
- Gandolfo MA, Hermsen EJ, Zamaloa MC, et al. 2011. Oldest known Eucalyptus macrofossils are from South America. PLoS One 6: e21084. doi:10.1371/journal.pone.0021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González CC, Gandolfo MA, Zamaloa MC, Cúneo NR, Wilf P, Johnson KR.. 2007. Revision of the Proteaceae macrofossil records from Patagonia, Argentina. Botanical Review 73: 235–266. [Google Scholar]
- Hably L, Manchester SR.. 2000. Fruits of Tetrapterys (Malpighiaceae) from the Oligocene of Hungary and Slovenia. Review of Palaeobotany and Palynology 111: 93–101. [DOI] [PubMed] [Google Scholar]
- Hermsen EJ, Gandolfo MA.. 2016. Fruits of Juglandaceae from the Eocene of South America. Systematic Botany 41: 316–328. [Google Scholar]
- Hermsen EJ, Gandolfo MA, Zamaloa MC.. 2012. The fossil record of Eucalyptus in Patagonia. American Journal of Botany 99: 1356–1374. [DOI] [PubMed] [Google Scholar]
- Heusser CJ. 1974. Vegetation and climate of the Southern Chilean lake district during and since the last interglaciation. Quaternary Research 4: 290–315. [Google Scholar]
- Holmes WBK, Holmes FM.. 1992. Fossils flowers of Ceratopetalum Sm. (Family Cunoniaceae) from the Tertiary of Eastern Australia. Proceedings of the Linnean Society of New South Wales 113: 265–270. [Google Scholar]
- Hooghiemstra H. 1989. Quaternary and Upper-Pliocene glaciations and forest development in the tropical Andes: evidence from a long high-resolution pollen record from the sedimentary basin of Bogotá, Colombia. Palaeogeography, Palaeoclimatology, Palaeoecology 72: 11–26. [Google Scholar]
- Hoogland RD. 1960. Studies in the Cunoniaceae. I. The genera Ceratopetalum, Gillbeea, Aistopetalum, and Calycomis. Australian Journal of Botany 8: 318–341. [Google Scholar]
- Hoogland RD. 1979. Studies in the Cunoniaceae. II. The genera Caldcluvia, Pullea, Acsmithia, and Spiraeanthemum. Blumea 25: 481–505. [Google Scholar]
- Hufford L, Dickison WC.. 1992. A phylogenetic analysis of Cunoniaceae. Systematic Botany 17: 181–200. [Google Scholar]
- Knight CL, Wilf P.. 2013. Rare leaf fossils of Monimiaceae and Atherospermataceae (Laurales) from Eocene Patagonian rainforests and their biogeographic significance. Palaeontologia Electronica 16: 26A; 39p; palaeo-electornica/content/2013/546-eocene-laurales-from-patagonia. [Google Scholar]
- Kooyman RM, Wilf P, Barreda VD, et al. 2014. Paleo-Antarctic rainforest into the modern Old World tropics: the rich past and threatened future of the ‘southern wet forest survivors’. American Journal of Botany 101: 2121–2135. [DOI] [PubMed] [Google Scholar]
- Manchester SR. 1991. Cruciptera, a new juglandaceous winged fruit from the Eocene and Oligocene of western North America. Systematic Botany 16: 715–725. [Google Scholar]
- Manchester SR. 1999. Biogeographical relationships of North American Tertiary floras. Annals of the Missouri Botanical Garden 86: 472–522. [Google Scholar]
- Manchester SR. 2000. Late Eocene fossil plants of the John Day Formation, Wheeler County, Oregon. Oregon Geology 62: 51–63. [Google Scholar]
- Manchester SR, Crane PR.. 1987. A new genus of Betulaceae from the Oligocene of western North America. Botanical Gazette 148: 263–273. [Google Scholar]
- Manchester SR, Hably L.. 1997. Revision of ‘Abelia’ fruits from the Paleogene of Hungary, Czech Republic and England. Review of Palaeobotany and Palynology 96: 231–240. [Google Scholar]
- Manchester SR, Uemura K.. 2014. Ozakia, a new genus of winged fruit shared between the Miocene of Japan and western North America. Journal of Plant Research 127: 187–192. [DOI] [PubMed] [Google Scholar]
- Manchester SR, Collinson ME, Goth K.. 1994. Fruits of the Juglandaceae from the Eocene of Messel, Germany, and implications for early Tertiary phytogeographic exchange between Europe and western North America. International Journal of Plant Sciences 155: 388–394. [Google Scholar]
- Matthews ML, Endress PK.. 2002. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae). Botanical Journal of the Linnean Society 140: 321–381. [Google Scholar]
- Matthews ML, Endress PK, Schönenberger J, Friis EM.. 2001. A comparison of floral structures of Anisophylleaceae and Cunoniaceae and the problem of their systematic position. Annals of Botany 88: 439–455. [Google Scholar]
- Merkhofer L, Wilf P, Haas MT, et al. 2015. Resolving Australian analogs for an Eocene Patagonian paleorainforest using leaf size and floristics. American Journal of Botany 102: 1160–1173. [DOI] [PubMed] [Google Scholar]
- Meyer HW, Manchester SR.. 1997. The Oligocene Bridge Creek flora of the John Day Formation, Oregon. University of California Publications in Geological Sciences 141: 1–195, plates 1–75. [Google Scholar]
- Parham JF, Donoghue PC, Bell CJ, et al. 2012. Best practices for justifying fossil calibrations. Systematic Biology 61: 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul OK. 2011. Tree species composition of Crater Mountain Wildlife Management Area, EHP-Papua New Guinea. Journal of Agricultural Science 1: 25–30. [Google Scholar]
- Petriella B. 1972. Estudio de maderas petrificadas del Terciario inferior del area central de Chubut (Cerro Bororó). Revista del Museo de La Plata Sección Paleontología 6: 159–249. [Google Scholar]
- Petriella B, Archangelsky S.. 1975. Vegetación y ambiente en el Paleoceno de Chubut. Primer Congreso de Argentino de Paleontología y Bioestratigrafía, Actas 2: 257–270. [Google Scholar]
- Poole I, Cantrill DJ, Hayes P, Francis J.. 2000. The fossil record of Cunoniaceae: new evidence from Late Cretaceous wood of Antarctica? Review of Palaeobotany and Palynology 111: 127–144. [DOI] [PubMed] [Google Scholar]
- Poole I, Hunt RJ, Cantrill DJ.. 2001. A fossil wood flora from King George Island: ecological implications for an Antarctic Eocene Vegetation. Annals of Botany 88: 33–54. [Google Scholar]
- Romero EJ, Castro MT.. 1986. Material fúngico y granos de polen de angiospermas de la Formación Río Turbio (Eoceno), provincia de Santa Cruz, República Argentina. Ameghiniana 23: 101–118. [Google Scholar]
- Romero EJ, Hickey LJ.. 1976. A fossil leaf of Akaniaceae from Paleocene beds in Argentina. Bulletin of the Torrey Botanical Club 103: 126–131. [Google Scholar]
- Rozefelds AC, Barnes RW.. 2002. The systematic and biogeographical relationships of Ceratopetalum (Cunoniaceae) in Australia and New Guinea. International Journal of Plant Sciences 163: 651–673. [Google Scholar]
- Schönenberger J, Friis EM, Matthews ML, Endress PK.. 2001. Cunoniaceae in the Cretaceous of Europe: evidence from fossil flowers. Annals of Botany 88: 423–437. [Google Scholar]
- Schreve-Brinkman EJ. 1978. A palynological study of the Upper Quaternary sequence on the El Abra Corridor and Rock Shelters (Colombia). Palaeogeography, Palaeoclimatology, Palaeoecology 25: 1–109. [Google Scholar]
- Shi G, Grimaldi DA, Harlow GE, et al. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research 37: 155–163. [Google Scholar]
- Sweeney PW, Bradford JC, Lowry PP II. 2004. Phylogenetic position of the New Caledonian endemic genus Hooglandia (Cunoniaceae) as determined by maximum parsimony analysis of chloroplast DNA. Annals of the Missouri Botanical Garden 91: 266–274. [Google Scholar]
- Takeuchi W. 1999a. Botanical results from the 1995 Bismarck–Ramu expedition in Papua New Guinea. Sida 18: 751–782. [Google Scholar]
- Takeuchi W. 1999b. New plants from Crater Mt. Papua New Guinea, and an annotated checklist of the species. Sida 18: 941–986. [Google Scholar]
- Takeuchi W. 2002. Botanical summary of a lowland ultrabasic flora in Papua New Guinea. Sida 20: 1491–1559 [Google Scholar]
- Terada K, Askawa TO, Nishida H.. 2006. Fossil woods from Arroyo Cardenio, Chile Chico Province, Aisen (XI) Region, Chile In: H Nishida, ed. Post-Cretaceous floristic changes in southern Patagonia, Chile. Tokyo, Japan: Chuo University, 57–65. [Google Scholar]
- The Angiosperm Phylogeny Group IV. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- Thiebaut M. 1999. A new locality of Raskya vetusta (Ettingshausen) Manchester & Hably from France. Revue Paléobiologie Genève 18: 509–515. [Google Scholar]
- Torres T. 1985. Plantas fósiles de la Antártica. Boletín Antártico Chileno 5: 17–31. [Google Scholar]
- Villar de Seoane L, Cúneo NR, Escapa IH, Wilf P, Gandolfo MA.. 2015. Ginkgoites patagonica (Berry) comb. nov. from the Eocene of Patagonia, last ginkgoalean record in South America. International Journal of Plant Sciences 176: 346–363. [Google Scholar]
- Wang Y, Manchester SR.. 2000. Chaneya, a new genus of winged fruit from the Tertiary of North America and Eastern Asia. International Journal of Plant Sciences 161: 167–178. [DOI] [PubMed] [Google Scholar]
- Wehr WC. 1995. Early Tertiary flowers, fruits, and seeds of Washington Stare and adjacent areas. Washington Geology 23: 3–16. [Google Scholar]
- Wilde V, Frankenhäuser H.. 2010. A new species of four-winged fruits (Trilobium maii sp. nov.) from the middle Eocene of Eckfeld (Eifel, Germany). Review of Palaeobotany and Palynology 159: 143–151. [Google Scholar]
- Wilf P. 2012. Rainforest conifers of Eocene Patagonia: attached cones and foliage of the extant Southeast Asian and Australasian genus Dacrycarpus (Podocaepaceae). American Journal of Botany 99: 562–584. [DOI] [PubMed] [Google Scholar]
- Wilf P, Cúneo NR, Johnson KR, Hicks JF, Wing SL, Obradovich JD.. 2003. High plant diversity in Eocene South America: evidence from Patagonia. Science 300: 122–125. [DOI] [PubMed] [Google Scholar]
- Wilf P, Johnson KR, Cúneo NR, Smith ME, Singer BS, Gandolfo MA.. 2005. Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. American Naturalist 165: 634–650. [DOI] [PubMed] [Google Scholar]
- Wilf P, Little SA, Iglesias A. et al. 2009. Papuacedrus (Cupressaceae) in Eocene Patagonia: a new fossil link to Australasian rainforests. American Journal of Botany 96: 2031–2047. [DOI] [PubMed] [Google Scholar]
- Wilf P, Escapa IH, Cúneo NR, Kooyman RM, Johnson KR, Iglesias A.. 2014. First South American Agathis (Araucariaceae), Eocene of Patagonia. American Journal of Botany 101: 156–179. [DOI] [PubMed] [Google Scholar]
- Zamaloa MC, Gandolfo MA, González CC, Romero EJ, Cúneo NR, Wilf P.. 2006. Casuarinaceae from the Eocene of Patagonia, Argentina. International Journal of Plant Sciences 167: 1279–1289. [Google Scholar]