Abstract

As the speed of chromatographic enantioseparations advances to the point where the enantiomers of most chiral compounds can be resolved in less than a minute, some in less than a second, we pose the question of how this field is likely to develop over the next few years. Are we approaching a fundamental speed limit, or will further technological advances continue to deliver faster and faster separations? Are faster separations even needed for chemical research, and if so, how will they help? We herein examine this trend, investigating the barriers that currently limit speed and offering some insights into the continued evolution of fast chromatographic enantioseparations.
Short abstract
The enantiomers of most chiral compounds can now be chromatographically resolved in less than a minute, some in less than a second. How will this field develop over the next few years?
Introduction
For several decades, chromatography using chiral stationary phases (CSPs) has been the preferred technique for measuring enantiopurity in the chemical sciences. Prior to the first CSP commercialization in 1981, the use of polarimetry prevailed, a technique fraught with problems of high material demand, unpredictable solvent effects, nonlinear concentration response, and fundamental incompatibility with the study of impure compounds. Chiral chromatography addresses all of these shortcomings, but was only widely embraced by the larger organic chemistry and bioscience community during the 1990s, when the availability of generally useful CSPs and the experience of using them became widespread. Today, the enantiomers of almost any chiral molecule can be chromatographically resolved in minutes, and the technique has become a mainstay of research in organic synthesis, pharmaceutical analysis, drug metabolism, agrochemistry, food science, bioanalysis, and a variety of other fields.
Recent developments in column and instrumentation design and new experimental strategies are leading to dramatic increases in the speed of chromatographic enantioseparation. Where analysis times on the order of 20–40 min were commonplace just a few years ago, recent improvements now allow most enantiomer pairs to be chromatographically resolved within 1 min, with many being separable in just a few seconds. These fast separations are allowing analytical measurements to keep pace with the high throughput experimentation approaches that are increasingly used in the discovery and development of new medicines. We explore the factors contributing to this remarkable revolution in analytical speed, asking the questions of whether further improvements are likely and in what ways such improvements will be of value to chemical researchers.
Fundamentals of Chromatographic Enantioseparation and Band Broadening
A variety of physical, instrumental, chemical, and experimental factors have contributed to recent increases in the speed of chromatographic enantioseparations. Some background explanation will help to put these advances into perspective. Chromatographic enantioseparation is possible when a mixture of injected analyte enantiomers interacts with a CSP under flow conditions to form transient diastereomeric adsorbates of differing free energy, with energy differences as small as 0.025 kcal/mol being sufficient for chromatographic resolution. Adsorption and desorption of enantiomers by the CSP is fast relative to movement through the column, with the enantiomer that spends proportionally more time adsorbed onto the CSP making slower progress through the column (Figure 1a). The retention of an analyte is described by k, enantioselectivity by α, peak efficiency by N, and resolution by Rs (Figure 1b). In addition to experiencing chromatographic retention, an analyte sample injected onto the head of a column as a thin band can become broadened and dispersed as it traverses the column under flowing conditions. The degree of band broadening depends upon a number of factors including differences in path length resulting from inefficient packing with nonideal particles, diffusion of the sample in the longitudinal direction during the course of the chromatographic experiment, resistance to mass transfer caused by the need for analytes to diffuse into and out of the chromatographic particles, slow desorption kinetics, and mixing during transit of the sample through the connecting tubing between the injector and the column and from the column to the detector (Figure 1c).
Figure 1.
Fundamentals of chromatographic enantioseparation. (a) Chromatographic resolution of enantiomers is made possible by the formation of transient diastereomeric adsorbates of differing free energy between the enantiomers of the analyte and a chiral stationary phase consisting of a single enantiomer of a chiral selector immobilized on an inert chromatographic support. (b) Key terms and equations. (c) Controlling chromatographic band broadening is critically important for fast chromatographic enantioseparations.
Evolution of Chromatographic Particle Design
Recent improvements in the speed of chromatographic enantioseparation stem from innovations in chromatographic instrumentation to reduce extracolumn volume and, more importantly, from the development of new columns with greatly reduced band broadening. A rapid evolution of chromatographic particle technology over the past few years has enabled the development of these more efficient columns.1 For many years, porous silica gel (SiO2) has been the preferred material for chromatographic stationary phases, with the presence of strongly adsorbed or covalently bonded selector molecules serving to modulate chromatographic selectivity. Early technology employed silica gel produced by fracturing bulk material, with sieving to afford discrete particle size ranges. While the resulting irregular silica gel is still used for flash chromatography applications, the irregular nature of this material leads to nonuniform packing within the column at the microscopic level, which in turn leads to nonuniform flow paths through the column, resulting in band broadening. Furthermore, columns packed with irregular silica particles tend to crush, settle, and compress when repeatedly subjected to high pressure operating conditions, resulting in the generation of column voids and leading to further band broadening. Since the 1980s, most HPLC columns have been packed with spherical silica particles produced by emulsion polymerization technology. By adjusting mixing rates and the presence of various solvents and porogens, both the particle size and the pore size within the particles can be systematically altered, with subsequent sorting by mechanical winnowing or other processes being used to obtain highly monodisperse spherical particles. Columns packed with these particles afford improved peak efficiency (i.e., sharper peaks) relative to irregular particles, while also being more resistant to crushing under high pressure operating conditions.2,3
Columns packed with smaller diameter porous silica spheres generally afford better peak efficiency than larger diameter particles, as the requirement of analytes to diffuse into and out of adsorbent particles can lead to considerable band broadening. The standard silica particles used in HPLC have decreased from the 30–100 μm irregular particles used in the 1970s to 10 or 5 μm spherical particles used in the 1980s and 1990s, to the 3 μm particles of the 2000s and the sub 2 μm particles that are the current industry standard (Figure 2). In addition to improving peak efficiency, the adoption of smaller particles has led to undesirable increases in column back pressure, which has necessitated a series of instrument improvements to enable operation at higher pressure. Core–shell particles, a recently introduced concept in chromatography particle technology, contain a solid core surrounded by a porous exterior.4 With these particles, the mean path length that an analyte must travel when diffusing into and out of a particle is much less than with a fully porous particle of the same diameter. Columns packed with these particles exhibit excellent chromatographic efficiency (peak shape) and, equally important, afford greatly reduced backpressure relative to a comparable column packed with the same size fully porous particles. The outstanding resolution afforded by highly efficient columns packed with modern particles allows surplus resolution to be exchanged for increased speed.
Figure 2.

Evolution of silica chromatography particle technology.
Trading Excess Resolution for Speed
The quality of a chromatographic separation is expressed in terms of resolution (Rs), with Rs = 0 denoting no separation whatsoever between the peaks of interest, and Rs = 1.5 denoting a “touching band” separation, in which the two chromatographic peaks are just barely fully resolved. Modern high efficiency columns often afford enantioseparations with Rs values of 5, 10, or even greater. In such cases, excess resolution can be traded for speed by shortening the column or increasing the flow rate, modifier concentration, or temperature. Since resolution varies as the square root of column length, a simple formula permits the rapid estimation of the minimum analysis time available via column shortening.5 This relationship clearly shows that the speed of many chromatographic enantioseparations can be drastically improved by the use of shorter columns operating near their pressure limits. Recent results from Merck,6,7 University of Rome,8−10 and University of Texas at Arlington11,12 have shown some impressively fast enantioseparations, with resolution of both enantiomers being complete in just a few seconds using short columns from 0.5 to 5 cm in length. The interplay of particle size, flow rate, and backpressure on chromatographic resolution is illustrated in Figure 3.
Figure 3.
How the interplay of particle size, column length, flow rate, and extracolumn volume influences resolution (Rs) in the chromatographic separation of enantiomers. Early era chromatogram peaks were minutes wide (a), but as smaller particle technology became available, excess resolution could be traded for speed (b–h). With short and/or narrow columns, minimizing extracolumn volume becomes an important concern (i). Chromatograms prepared using the open source software package, HPLC Simulator (hplcsimulator.org).13
The Practice of Ultrafast Enantioseparation
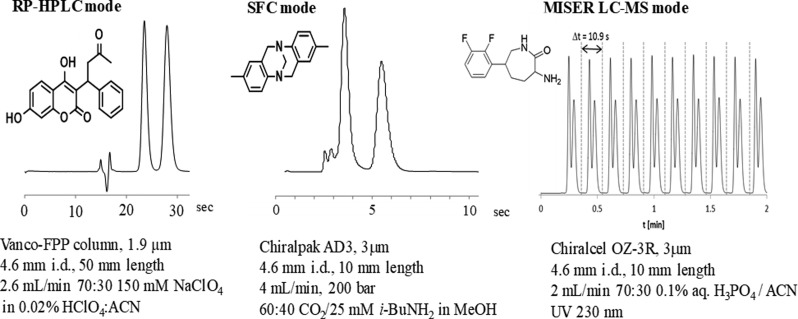
Prompted by disbelief expressed by routine chiral chromatography users upon seeing these new ultrafast resolution results, we investigated the general applicability of the approach to ensure that truly fast separations were not limited to a few special case examples.14 Using a test set of 50 racemates, including a number of difficult to resolve pharmaceutical intermediates, we found that 43 could be resolved within 1 min, and 25 within 30 s. In addition to the use of short columns at high flow rates, it is important to note that many of these separations employ supercritical fluid chromatography (SFC), which is well-known for offering speed advantages relative to conventional liquid chromatography, owing to the decreased viscosity and enhanced diffusivity of the condensed CO2 based eluent. Ultrafast enantioseparations reduce analysis time, especially when used in the MISER (multiple injections in a single experimental run) mode, where interinjection computer delays relating to initiating runs and file saving are eliminated.15 In addition, ultrafast enantioseparations can be used as a fast second dimension for analysis in two-dimensional chromatography studies7 and for high frequency online monitoring of kinetic reaction profiles.16 Several representative ultrafast enantioseparations are shown in Figure 4.
Figure 4.
Representative samples of ultrafast chromatographic enantioseparations.14,16
Method development for ultrafast enantioseparations can be surprisingly straightforward. Application of a simple algorithm to a conventional chromatogram displaying excess resolution affords an estimate of the fastest separation time that can be achievable through reduction in column length.5 Somewhat surprisingly, this easily obtained “fastest time for enantioseparation” provides a good prediction of the speed that can be obtained by changes in mobile phase, flow rate, temperature, and other parameters. In general, method development involves a change from gradient to isocratic elution conditions, with selection of an eluent composition that affords adequate resolution of the first eluted enantiomer from unretained components and injection disturbances. Short columns from 1 to 5 cm in length are typically used so as to minimize chromatographic void time (the time required to elute an unretained component from the column). Choice of lower viscosity eluents is an important factor in achieving optimal speeds, with selection of a fast flow rate that produces a backpressure near the operating limit for the column and instrument being preferred. As each chromatographic run is quite short, an iterative approach to method development can typically afford an optimized ultrafast chromatographic method in 15–30 min.
Ultrafast chromatographic separations require some special considerations and experimental techniques. The extracolumn volume of a typical HPLC instrument coming from connecting tubing, solvent mixers, and flow cells, while perfectly suitable for conventional analysis, may lead to serious band broadening problems when shorter 5 mm or 1 cm length columns are used. Consequently, downsizing tubing volumes, optimizing connector fittings, and scaling down injection volumes can help achieve improved performance.17 Researchers at University of Texas at Arlington have undertaken systematic studies of other limiting factors, most notably the need to adjust detector sampling rates such that the observed signal accurately reflects what is passing through the detector.18 With a sampling rate of only 10 Hz, eluted peaks that are only a fraction of a second wide will be represented by only a few data points. Thus, requirements for ultrafast chromatography are pushing many detectors and instruments to their current limits.
It should also be noted that packing short columns with high efficiency (N/m) can be quite challenging. While column vendors have many years of experience in packing 25, 15, 10, or even 5 cm long columns with near-ideal efficiency, the challenges with 1 cm, 5 mm, or even shorter columns can be significant, leading to inferior performance and variable outcomes. Increased experience will undoubtedly lead to improvements over time, but the current standard of stainless steel column housings, frits, and end fittings may well give way to microfluidic chip-based column technologies or other formats that afford improved ability to minimize extracolumn volumes and nonideal stationary phase distribution.
The slowness of many current autosamplers can create an additional concern. While autosamplers with a 30 s to 1 min cycle time for sample injection were perfectly suitable just a few years ago, they now create a bottleneck for ultrafast chromatographic analysis. A dual needle autosampler with an injection cycle time of 10 s has been recently introduced by Agilent, greatly improving the speed of high throughput MISER analysis19 and kinetic profiling.16 Nevertheless, even faster autosampler technology is needed to realize the potential of the most recent developments in ultrafast chromatography.
Is There a Moore’s Law for Ultrafast Enantioseparation?
With the speed of chromatographic enantioseparations doubling every few years, it is natural to consider the ultimate limits for ultrafast chromatographic enantioseparation. Are we approaching a fundamental speed limit, or will further technological advances continue to deliver faster and faster separations? Continued evolution in column and instrument design will likely allow several additional doublings in the next decades before reaching ultimate limits. We herein consider several key factors affecting speed, including the following.
Column Length and Injection Volume
Currently, ultrafast chromatography columns with 5 mm length are challenging to prepare, and accessing even shorter columns of 1 mm or less will require alternative approaches. While chromatography today is still dominated by stainless steel column housings, frits, and tubing connectors, microfluidic chip columns made of glass, silicon, or other materials offer an attractive option for creating short columns with tightly controlled extracolumn volume. The possibility of incorporating on-chip sample injectors and detectors can help to reduce band broadening to an absolute minimum. Along with this decrease in column length, a corresponding decrease in diameter is also likely, as preserving a uniform elution profile across a column with the aspect ratio of a pancake can be challenging. It should be pointed out that this miniaturization of column size has a number of ancillary advantages, not the least of which is a dramatic decrease in the amount of costly stationary phase material required to carry out an analysis, with the amount within a conventional column being sufficient for packing dozens or even hundreds of microcolumns.20 Interestingly, as the overall volume of the column diminishes, the volume of the sample injection must decrease as well, with larger injections leading to broader peaks and poorer resolution. Thus, adoption of nanoliter injection technology for ultrafast chromatography can be expected.
Particle Size, Operating Pressure, and Column Design
As we have seen, advances in the development of smaller chromatographic particles and the advent of core–shell particle technology has been a leading factor in recent increases in the speed of chromatographic separations. Columns packed with sub-micrometer particles have been explored by Jorgensen and others using chromatography at extreme pressures,21 often with corresponding innovations in column and frit design. The cost and complexity of instruments operating at these extreme pressures will likely place a practical limit on the widespread utilization of such approaches. However, other formats beyond the close packing of spherical particles within stainless steel tubes may allow for even greater performance with more conventional instrumentation. As we have seen, much of the advantage of small particle chromatography stems from reduced band broadening for analytes diffusing into and out of smaller stationary phase particles. Alternative approaches for creating a thin adsorptive layer on the walls of a channel22 or an engineered fluidic surface23 may afford improved chromatographic performance. Such structures could perhaps be accessed via traditional coating and bonding of polymers, or by stepwise synthesis or controlled growth of selector materials from a functionalized surface to afford selective adsorbents with a short path length for diffusion capable of high quality separations with minimal band broadening.
Stationary Phases
The development of improved CSPs has not been a major driver of increased separation speeds in recent years, with the optimization of physical parameters contributing to band broadening playing a much greater role. Improvements in the performance and generality of chiral selectors will undoubtedly occur and can be expected to be important for particular separations. Although high selectivity (α) would seem to be a key need for fast separations, excessive selectivity can actually be deleterious to speed. Importantly, some retention of the first eluted enantiomer relative to the void volume is important for accurate quantitation, and while some selectivity is required for enantioseparation (α > 1), an infinitely high selectivity factor means that the second enantiomer will never elute from the column. Therefore, for a given analyte, an intermediate selectivity value will afford the fastest enantioseparation. As the science of controlling physical band broadening in chromatographic separation becomes more firmly established, we can expect that renewed emphasis on the development of chromatographic media and methods that afford optimal selectivity for fast analysis will become increasingly important.
Chiral selectors bonded or adsorbed onto porous silica particles have been the principal approach used in chromatography over the past half-century, but there is reason to believe that other formats may afford comparable or even superior performance. Although results to date are somewhat modest, the controlled growth of chiral metal organic framework (MOF) or covalent organic framework (COF) materials within chromatographic columns could enable the desired formation of selective adsorbents with a short path length for diffusion and with preferred properties for minimal band broadening.24
Equipment Limitations
Current needs for decreased sample injection volume and increased sampling rate of detectors will likely be overcome with relatively minor modifications to existing equipment, although the data coming from such dense sampling will require additional data handling modifications. Current limitations on autosampler speed will likely present a more formidable obstacle. The industry standard can now achieve a 10 s sampling interval using a dual needle autosampler, but much faster injection cycle times on the order of 1 s are needed. Engineering a solution to this problem may be difficult and will likely involve a departure from traditional robotic arm injector approaches. One possible solution could involve an approach in which 4, 8, 12, or more sample wells are simultaneously sampled by a parallel injector syringe array, which then simultaneously injects into parallel ports within a fluidic device such that the spacing between samples arriving at the column head is appropriate for the time scale of the ultrafast separation. Such improvements would afford high speed chromatographic separations with analysis times comparable to those of plate reader spectroscopic devices.
Are Faster Separations Needed for Chemical Research, and If So, How Will They Help?
Faster enantioseparations are critically important in some areas of research, such as high throughput screening of enantioselective catalysts, enzymatic reactions, or crystallization-based resolutions. As high throughput experimentation has become firmly established in pharmaceutical discovery and development, workflows that incorporate 96, 384, or even 1536 reactions in a single investigation are on the rise, leading to significant bottlenecks in research operations when conventional analytical approaches are used. For example, analysis of a single 1536 well microplate using a 2 min separation method will require more than 2 days of around the clock analysis time. Fast analytical methods enable short experimental cycle times, allowing experimental results to be obtained within a single day.25 Ultrafast chromatographic screening is often used as a triage approach in high throughput experimentation, enabling rapid identification of the most promising experiments, which are then studied in greater detail using more conventional analytical assays.
Ultrafast chromatography is now becoming competitive with sensor and spectroscopy approaches for high throughput analysis of enantiopurity that employ optical plate readers, mass spectrometers, and other high speed analytical tools. Importantly, such approaches, while offering very fast analysis speed, are often plagued by sample interference issues that are readily addressed using chromatographic separation approaches.26 This is especially true for the analysis of enantiopurity, where suitable spectroscopic methods for high throughput enantiopurity determination that are both generally useful and interference-free are currently lacking.
Researchers engaged in conventionally paced research may find that existing instrumentation and columns afford satisfactory analytical support. When only a few measurements are made each day, it makes little difference if analysis time is 5 min or 5 s. Furthermore, traditional chromatography can even offer reasonable analytical support for limited scale high throughput experimentation when sample analysis is carried out overnight by automated instrumentation. It is only when analysis becomes a significant bottleneck in research operations, or when real time rather than next day results become a priority, that the adoption of ultrafast chromatographic analysis becomes truly compelling.
Conclusions
The recent trend toward faster chromatographic enantioseparations will continue over the next few years, likely leading to a general ability to chromatographically analyze the enantiomers of most compounds within 1 s. Continued evolution of chromatography instrumentation and column technology can be expected, with microflow instrumentation and chip-based columns gradually replacing existing technologies. Needed technological innovations include the development of high speed or multiparallel injector technology to take full advantage of these increases in chromatographic speed, as well as high speed detector sampling, near column injector and detection technologies, and the development of new column and chromatographic stationary phase technologies. While these increases in the speed of chromatographic enantioseparation will initially enable researchers in high throughput experimentation, other researchers will also reap benefits through the real-time analysis of experimental results and the use of densely packed profiling of reaction kinetics. Finally, while the discussion in this article has focused on the separation of two component mixtures of enantiomers, many of these principles can be extended to ultrafast separations of more complex multicomponent mixtures of stereoisomers or achiral analytes.
The author declares no competing financial interest.
References
- Gritti F.; Guiochon G. The current revolution in column technology: How it began, where is it going?. J. Chromatogr. A 2012, 1228, 2–19. 10.1016/j.chroma.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Fountain K. J.; Neue U. D.; Grumbach E. S.; Diehl D. M. Effects of extra-column band spreading, liquid chromatography system operating pressure, and column temperature on the performance of sub-2-um porous particles. J. Chromatogr. A 2009, 1216, 5979–5988. 10.1016/j.chroma.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Gritti F.; Guiochon G. Perspectives on the evolution of the column efficiency in liquid chromatography. Anal. Chem. 2013, 85, 3017–3035. 10.1021/ac3033307. [DOI] [PubMed] [Google Scholar]
- González-Ruiz V.; Olives A. I.; Martín M. A. Core-shell particles lead the way to renewing high-performance liquid chromatography. TrAC, Trends Anal. Chem. 2015, 64, 17–28. 10.1016/j.trac.2014.08.008. [DOI] [Google Scholar]
- Welch C. J.; Regalado E. L. Estimating optimal time for fast chromatographic separations. J. Sep. Sci. 2014, 37, 2552–2558. 10.1002/jssc.201400508. [DOI] [PubMed] [Google Scholar]
- Regalado E. L.; Welch C. J. Pushing the speed limit in enantioselective supercritical fluid chromatography. J. Sep. Sci. 2015, 38, 2826–2832. 10.1002/jssc.201500270. [DOI] [PubMed] [Google Scholar]
- Barhate C. L.; Regalado E. L.; Lee J.; Contrella N. D.; Jo J.; Makarov A. A.; Armstrong D. W.; Welch C. J. Ultrafast chiral chromatography as the second dimension in two-dimensional liquid chromatography experiments. Anal. Chem. 2017, 89, 3545–3553. 10.1021/acs.analchem.6b04834. [DOI] [PubMed] [Google Scholar]
- Ismail O. H.; Ciogli A.; Villani C.; DeMartino M.; Pierini M.; Cavazzini A.; Bell D. S.; Gasparrini F. Ultrafast high efficiency enantioseparations by means of a teicoplanin-based chiral stationary phase made on sub 2 μm totally porous silica particles of narrow size distribution. J. Chromatogr. A 2016, 1427, 55–68. 10.1016/j.chroma.2015.11.071. [DOI] [PubMed] [Google Scholar]
- Ismail O. H.; Pasti L.; Ciogli A.; Villani C.; Kocergin J.; Anderson S.; Gasparrini F.; Cavazzini A.; Catani M. Pirkle-type chiral stationary phase on core-shell and fully porous particles: Are superficially porous particles always the better choice toward ultrafast high-performance enantioseparations?. J. Chromatogr. A 2016, 1466, 96–104. 10.1016/j.chroma.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Kotoni D.; Ciogli A.; Molinaro C.; D’Acquarica I.; Kocergin J.; Szczerba T.; Ritchie H.; Villani C.; Gasparrini F. Introducing enantioselective ultrahigh-pressure liquid chromatography (eUHPLC): Theoretical inspections and ultrafast separations on a new sub-2-μm Whelk-O1 stationary phase. Anal. Chem. 2012, 84, 6805–6813. 10.1021/ac301335b. [DOI] [PubMed] [Google Scholar]
- Patel D. C.; Breitbach Z. S.; Wahab M. F.; Barhate C. L.; Armstrong D. W. Gone in seconds; praxis, performance and peculiarities of ultrafast chiral liquid chromatography with superficially porous particles. Anal. Chem. 2015, 87, 9137–9148. 10.1021/acs.analchem.5b00715. [DOI] [PubMed] [Google Scholar]
- Wahab M. F.; Wimalasinghe R. M.; Wang Y.; Barhate C. L.; Patel D. C.; Armstrong D. W. Salient sub-second separations. Anal. Chem. 2016, 88, 8821–8826. 10.1021/acs.analchem.6b02260. [DOI] [PubMed] [Google Scholar]
- Carr P. W.; Wang X.; Stoll D. R. Effect of Pressure, Particle Size, and Time on Optimizing Performance in Liquid Chromatography. Anal. Chem. 2009, 81, 5342–5353. 10.1021/ac9001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhate C.; Joyce L. A.; Makarov A. A.; Zawatzky K.; Bernardoni F.; Schafer W. A.; Armstrong D. W.; Welch C. J.; Regalado E. L. Ultrafast chiral separation for high throughput enantiopurity analysis. Chem. Commun. 2017, 53, 509–512. 10.1039/C6CC08512A. [DOI] [PubMed] [Google Scholar]
- Welch C. J.; Gong X.; Schafer W.; Pratt W.; Brkovic T.; Pirzada Z.; Cuff J. F.; Kosjek B. MISER Chromatography (Multiple Injections in a Single Experimental Run): The Chromatogram is the Graph. Tetrahedron: Asymmetry 2010, 21, 1674–1681. 10.1016/j.tetasy.2010.05.029. [DOI] [Google Scholar]
- Zawatzky K.; Grosser S. T.; Welch C. J. Facile Kinetic Profiling of Chemical Reactions using MISER Chromatographic Analysis. Tetrahedron 2017, 73, 5048–5053. 10.1016/j.tet.2017.05.048. [DOI] [Google Scholar]
- Vanderlinden K.; Broeckhoven K.; Vanderheyden Y.; Desmet G. Effect of pre- and post-column band broadening on the performance of high-speed chromatography columns under isocratic and gradient conditions. J. Chromatogr. A 2016, 1442, 73–82. 10.1016/j.chroma.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Wahab M. F.; Dasgupta P. K.; Kadjo A. F.; Armstrong D. W. Sampling Frequency, Response Times and Embedded Signal Filtration in Fast, High Efficiency Liquid Chromatography: A Tutorial. Anal. Chim. Acta 2016, 907, 31–44. 10.1016/j.aca.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Zawatzky K.; Barhate C. L.; Regalado E. L.; Mann B. F.; Marshall N.; Moore J. C.; Welch C. J. Overcoming Speed Limits in High Throughput Chromatographic Analysis. J. Chromatogr. A 2017, 1499, 211–216. 10.1016/j.chroma.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Welch C. J.; Hyun M. H.; Kubota T.; Schafer W.; Bernardoni F.; Choi H. J.; Wu N.; Gong X.; Lipshutz B. Microscale HPLC Enables a New Paradigm for Commercialization of Complex Chiral Stationary Phases. Chirality 2008, 20, 815–819. 10.1002/chir.20548. [DOI] [PubMed] [Google Scholar]
- Jorgenson J. W. Capillary liquid chromatography at ultrahigh pressures. Annu. Rev. Anal. Chem. 2010, 3, 129–50. 10.1146/annurev.anchem.1.031207.113014. [DOI] [PubMed] [Google Scholar]
- Hara T.; Futagami S.; Eeltink S.; De Malsche W.; Baron G.; Desmet G. Very High Efficiency Porous Silica Layer Open-Tubular Capillary Columns Produced via in-Column Sol–Gel Processing. Anal. Chem. 2016, 88, 10158–10166. 10.1021/acs.analchem.6b02713. [DOI] [PubMed] [Google Scholar]
- Regnier F. E.; He B.; Lin S.; Busse J. Chromatography and electrophoresis on chips: critical elements of future integrated, microfluidic analytical systems for life science. Trends Biotechnol. 1999, 17, 101–106. 10.1016/S0167-7799(98)01294-3. [DOI] [PubMed] [Google Scholar]
- Kuang X.; Ma Y.; Su H.; Zhang J.; Dong Y. B.; Tang B. High-Performance Liquid Chromatographic Enantioseparation of Racemic Drugs Based on Homochiral Metal–Organic Framework. Anal. Chem. 2014, 86, 1277–1281. 10.1021/ac403674p. [DOI] [PubMed] [Google Scholar]
- Buitrago Santanilla A.; Regalado E. L.; Pereira T.; Shevlin S.; Bateman K.; Campeau L. C.; Schneeweis J.; Berritt S.; Shi Z.C.; Nantermet P.; Liu Y.; Helmy R.; Welch C. J.; Vachal P.; Davies I. W.; Cernak T.; Dreher S. D. Nanomole Scale High-Throughput Chemistry for the Synthesis of Complex Molecules. Science 2015, 347, 49–53. 10.1126/science.1259203. [DOI] [PubMed] [Google Scholar]
- Schafer W.; Bu X.; Gong X.; Joyce L.; Welch C. J.. High Throughput Analysis for High Throughput Experimentation in Organic Chemistry. In Enabling Technologies for Organic Synthesis; Welch C., Ed.; Comprehensive Organic Synthesis, 2nd ed.; Knochel P., Molander G. A., Eds.; Elsevier: 2014; Vol. 9. [Google Scholar]