Abstract
Background
The fungi in the gastrointestinal tract, the gut mycobiota, are now recognised as a significant part of the gut microbiota, and they may be important to human health. In contrast to the adult gut mycobiota, the establishment of the early gut mycobiota has never been described, and there is little knowledge about the fungal transfer from mother to offspring.
Methods
In a prospective cohort, we followed 298 pairs of healthy mothers and offspring from 36 weeks of gestation until 2 years of age (1516 samples) and explored the gut mycobiota in maternal and offspring samples. Half of the pregnant mothers were randomised into drinking probiotic milk during and after pregnancy. The probiotic bacteria included Lactobacillus rhamnosus GG (LGG), Bifidobacterium animalis subsp. lactis Bb-12 and Lactobacillus acidophilus La-5. We quantified the fungal abundance of all the samples using qPCR of the fungal internal transcribed spacer (ITS)1 segment, and we sequenced the 18S rRNA gene ITS1 region of 90 high-quantity samples using the MiSeq platform (Illumina).
Results
The gut mycobiota was detected in most of the mothers and the majority of the offspring. The offspring showed increased odds of having detectable faecal fungal DNA if the mother had detectable fungal DNA as well (OR = 1.54, p = 0.04). The fungal alpha diversity in the offspring gut increased from its lowest at 10 days after birth, which was the earliest sampling point. The fungal diversity and fungal species showed a succession towards the maternal mycobiota as the child aged, with Debaryomyces hansenii being the most abundant species during breast-feeding and Saccharomyces cerevisiae as the most abundant after weaning. Probiotic consumption increased the gut mycobiota abundance in pregnant mothers (p = 0.01).
Conclusion
This study provides the first insight into the early fungal establishment and the succession of fungal species in the gut mycobiota. The results support the idea that the fungal host phenotype is transferred from mother to offspring.
Trial registration
Clinicaltrials.gov NCT00159523
Electronic supplementary material
The online version of this article (doi:10.1186/s40168-017-0319-x) contains supplementary material, which is available to authorized users.
Keywords: Gut microbiota, Mycobiota, Fungi, Newborn, Infant, Infant health, Probiotics
Background
The fungi that populate the gastrointestinal tract (gut mycobiota) have recently been recognised as a substantial part of the gut microbiota and can be important for human health [1]. The adult gut mycobiota, which probably comprise approximately 13% of the gut microbial volume, consists of a species selection from approximately 140 different fungal genera [2, 3], with the most abundant ones being Candida, Saccharomyces and Cladosporium spp. [1].
Gut microbiota has been extensively studied over the last two decades. The Human Microbiome Project, or HMP [4], and the Metagenomics of the Human Intestinal Tract (MetaHIT) [5] have contributed greatly to our knowledge of the human microbial community structure, although no comprehensive and uniformly processed database can represent the human gut microbiome [6]. Fungal communities are far less studied. However, a positive association between the archaeon Methanobrevibacter and Candida with relative abundance differences in Prevotella might exist. Similarly, the relative abundance differences in Bacteriodes are associated with the archaeon Nitrososphaera, and they are negatively correlated with gut fungi [7].
The human gut mycobiota confers several physiological effects to the human body. These fungi consume nutrients and may facilitate nutrient extraction and assist in digestion through enzyme and vitamin production [7, 8]. The gut mycobiota is also essential as a form of antigen exposures to train the immune system and its responses. Through activating the fungus-specific pathogen-recognition receptors (PRRs) and adjacent mechanisms, defences against harmful pathogens and likewise a tolerance towards helpful commensals are formed [1, 9, 10].
However, for some humans, fungi can have unfavourable impacts, and the term fungal dysbiosis describes a state of unbalanced mycobiota associated with disease [11]. This phenomenon is most extensively studied in immunocompromised patients who regularly contract opportunistic commensal fungal infections [1] and in patients with obesity and inflammatory bowel disease (IBD) [12–15]. Obesity and metabolic disorders have been associated with the increased presence and abundance of Saccharomycetes spp., Dipodascaceae spp. and Tremellomycetes spp. [12]. Obesity and metabolic syndrome are pro-inflammatory states, and Tremellomycetes spp. are associated with higher inflammation levels. Accordingly, a lower abundance of the ascomycotic Eurotiomycetes spp. and particularly less of the zygomycotic Mucor spp. might actually protect against an unhealthy metabolic profile [12, 13]. By contrast, IBD patients host lower concentrations of Saccharomyces cerevisiae and more Candida albicans than healthy subjects do. In this disorder, an increased Basidiomycota-Ascomycota ratio is also observed, along with increased fungal diversity and richness [14, 15]. Interestingly, many IBD and obesity patients also produce anti-S. cerevisiae antibodies [1, 16, 17], although they host different abundance levels of these gut fungi. Taken together, these findings imply that the gut mycobiota could be aetiologically important in human diseases.
An understanding of the role of the gut mycobiota is emerging in relation to physiological as well as pathophysiological processes, but there is little knowledge of how the mycobiome is shaped from early life. High abundances of the genera Penicillium, Aspergillus and Candida (species-non-specific) were found in 10 Italian children who were each sampled once from 0 to 2 years of age [18]. Additionally, some common species have been studied. C. albicans and Malassezia spp. are partly transferred vertically from mothers to their offspring [19–21], supporting the theory that fungi colonise the neonatal gut through the birth canal. Culturing has also shown that the Candida spp. prevalence in neonates is 23%, and it more than doubles to 50% within 4 months [20]. The paradigm of the sterile intrauterine environment is now shifting, and several studies confirm the prenatal presence of commensal bacterial taxa in the placenta and amniotic fluid and the possible transmission of these bacteria to the foetus long before birth [22–26]. Corresponding knowledge on fungi is scarce. Early life microbiomes can also be affected by maternal exposure during pregnancy, ranging from high-fat diets to probiotics [27, 28]. Generally, we know that the mycobiome may be shaped by bacteria-fungus interactions [29, 30], as well as by the diet, by probiotics and by antibiotic administration (as shown in mice) [7, 31–33]. However, to our knowledge, the settling of early mycobiota with respect to the quantity, diversity and association with the maternal mycobiome has not been described before nor has the probiotic impact on the mycobiota been investigated.
In this prospective cohort, we describe the gut mycobiota in 298 pairs of healthy pregnant women and offspring from birth to 2 years of age. We report major shifts in the fungal abundance and diversity within these populations, and it supports the idea of a succession of mycobiotic hosting from mother to child and over the first 2 years of life.
Methods
Material
We selected 298 mother-offspring pairs with at least one pair of faecal samples from mothers and offspring who participated in the Probiotics in the Prevention of Allergy among Children in Trondheim study (ProPACT) (Table 1). The ProPACT study is a population-based, randomised, placebo-controlled and double-blinded trial on probiotics from Trondheim, Norway, and it has been described in detail elsewhere [34, 35]. Briefly, the pregnant women who attended the regular Norwegian Antenatal Care Programme were asked to participate by completing questionnaires on their health and risk factors and by collecting faecal samples from themselves and their offspring. The health questionnaire details, including antibiotic administration, were collected at 36 weeks of gestation and 6 weeks, 1 year and 2 years after birth. Although we do not have details on the antibiotic administration in the offspring before 10 days of life, no offspring that were delivered by caesarean section received antibiotics during labour, but one developed septicaemia afterwards and was treated accordingly. The probiotic milk administration was double-blinded and randomly provided to one half of the pregnant population from 36 weeks of gestation until 3 months after birth. The probiotic milk contained 5 × 1010 colony-forming units (CFUs) of Lactobacillus rhamnosus GG (LGG), 5 × 1010 CFUs of Bifidobacterium animalis subsp. lactis Bb-12 and 5 × 1010 CFUs of Lactobacillus acidophilus La-5 per day. The remaining half received placebos in the form of heat-treated fermented skimmed milk with no probiotic bacteria.
Table 1.
Maternal and offspring characteristics
| Maternal age at delivery (years (SD)) | 29.6 (± 3.9) |
| Caesarean sections | 12.8% |
| Probiotic users | 49.4% |
| Antibiotic therapy during pregnancy | 7.2% |
| Male offspring | 46.4% |
| Gestational age (weeks (SD)) | 40.4 (± 1.5) |
| Birth weight (kg (SD)) | 3.6 (± 0.4) |
| Birth length (cm (SD)) | 50.7 (± 3.3) |
| Breast-fed at 3 months | 97.1% |
| Formula-fed at 3 months | 6.5% |
| Proportion of children receiving antibiotic treatment within | |
| - 6 weeks | 6.3% |
| - 1 year | 17.2% |
| - 2 years | 44.4% |
A total of 1516 faecal samples were collected (Table 2). Maternal samples were collected at 35–38 gestational weeks of pregnancy and 3 months postpartum. Offspring faeces were obtained at 10 days, 3 months, 1 year and 2 years, and they were sampled from the diapers. Faecal samples were stored in a Cary-Blair transport medium, immediately frozen to − 18 °C at home, and collected and held in a frozen state until their permanent storage at − 80 °C before further analyses.
Table 2.
DNA quantification and 18 rRNA gene ITS1 region sequencing of faecal samples
| Pregnant | Postpartum | 10 days | 3 months | 1 year | 2 years | Total | |
|---|---|---|---|---|---|---|---|
| All faecal samples (count) | 248 | 253 | 274 | 246 | 247 | 248 | 1516 |
| Detected fungal ITS1 | 221 (89%) | 220 (87%) | 153 (54%) | 148 (60%) | 163 (66%) | 189 (76%) | 1094 (72%) |
| Sequenced samples | 47 (19%) | 27 (11%) | 28 (10%) | 4 (2%) | 7 (3%) | 12 (5%) | 125 (8%) |
| Passed rarefaction and taxonomic classification | 28 (11%) | 25 (10%) | 15 (6%) | 4 (2%) | 7 (3%) | 11 (4%) | 90 (6%) |
Quantification
We used a protocol for bacterial DNA extraction that involved mechanical and chemical cell lysis. The stool samples were homogenised by bead beating with acid-washed glass beads (Sigma). We isolated the DNA with an LGC mag nucleic extraction maxi kit (LGC Genomics, Middlesex, UK) together with a KingFisher FLEX magnetic particle processor (ThermoScientific, Waltham, MA) according to the manufacturer’s recommendations, including a negative control as contamination control. Fungal internal transcribed spacer 1 (ITS1) amplicons were constructed using the primer pairs ITS1F (CTTGGTCATTTAGAGGAAGTAA) and ITS2 (GCTGCGTTCTTCATCGATGC), according to Tang et al. [36]. The fungal ITS quantities in 1516 samples were assessed with a LightCycler qPCR (Roche) of 50 cycles, using thermocycles comprising 95 °C in 15 min, then (95 °C in 30 s, 56 °C in 30 s, 72 °C for 45 s) × 50. For each qPCR plate, we included positive and negative controls (S. cerevisiae and sterile water, respectively). The qPCR cycle threshold (CT) value cut-off for fungal detection was set to either within the value of the negative control or to 45 cycles, because DNA quantification beyond 45 cycles can produce misleading results.
Sequencing of the 18S rRNA gene ITS1 region
Since many of the samples had low ITS DNA quantities, we chose to sequence the 18S ribosomal RNA (rRNA) gene ITS1 region of only those samples with sufficiently high ITS DNA quantities. We used a CT value of less than 35 cycles as the cut-off for sequencing. For the sequencing preparation, we measured the ITS DNA concentrations of the 125 selected ITS DNA samples (in addition to four positive and four negative controls) with FLx 800 cse (Cambrex), and they were normalised with a Biomek 3000 (Beckman Coulter) and prepared for amplicon sequencing using Illumina MiSeq v3 600-cycle chemistry, according to the producer’s instructions. Four positive and four negative controls were also included to the library. The library was quantified by using a Droplet Digital PCR (ddPCR, BioRad) and then diluted to a concentration recommended for sequencing. We then performed gene paired-read sequencing of the 18S rRNA gene ITS1 region on a MiSeq platform (Illumina). Resulting sequencing reads were first filtered out based on the quality score (minimum average q-score 25) and the barcode (no mismatches in the barcode were allowed). Remaining sequences were then pair-end joined and further filtered through UPARSE algorithm (max expected error (maxEE) value set to 0.25). The sequencing of the 18S rRNA gene ITS1 region produced a total of 3,722,830 reads. The median number of reads per sample was 16,355 reads and the mean was 27,991 reads, ranging from 3 to 119,463 reads per sample. We then used 6000 reads per sample as a cut-off for the rarefaction to ensure even representation of each sample in the dataset. The final dataset comprised 100 samples with more than 6000 sequences per sample and a total of 214 operational taxonomic units (OTUs). Ten of the samples were later discarded due to incorrect inclusion criteria, resulting in the inclusion of 90 samples in the analysis (Table 2).
We used the QIIME (Quantitative Insights into Microbial Ecology) pipeline for quality filtering and diversity estimation, whereas the UPARSE algorithm was used for OTU clustering [37]. In applying the rarefaction cut-off at 6000 reads per sample, we ensured minimal losses in the number of samples whilst maintaining the diversity (Additional file 1: Table S1 and Additional file 2: Figure S1).
The alpha diversity refers to the fungal diversity within each sample, and it was calculated by using Simpson’s reciprocal index, which describes how many OTUs prevail in each sample [38]. The beta diversity expresses the difference between the samples in terms of the number and abundance of OTUs within an age group, and it was calculated with the Bray-Curtis dissimilarity index.
Since there is no well-established quality annotation database designed for mycobiotic taxonomy assignment at present, and because fungi are often subject to misclassification, we used a conservative concordance system for the taxonomic annotation. We compared the OTU sequences with the four databases as follows: GenBank (NCBI, ≥ 97% identity and E value < 10−50); the Warcup Fungal ITS and UNITE Fungal ITS (User-friendly Nordic ITS Ectomycorrhiza Database with a bootstrapping threshold of 80%) through the Ribosomal Database Project (RDP) Classifier (https://rdp.cme.msu.edu/classifier/classifier.jsp); and the Targeted Host-Associated Fungi ITS Database (THF) [36], which was especially curated for the gut mycobiome (see Additional file 3 and Additional file 4). A concordance of at least three of these databases at the lowest taxonomic level or two databases with a justifiable was determined as sufficient qualification for the final assignment of each OTU. We followed the recent taxonomic reclassification for the fungi by manually curating the classification of OTU representative sequences with Index Fungorum as the reference [39].
Statistical methods
A standard curve was made to convert the qPCR CT values into fungal ITS copy concentrations in the faecal samples. The averages of three dilutions of the positive control for each qPCR plate of known fungal concentrations of S. cerevisiae were used for the calculation (see Additional file 5). The fungal ITS copy concentrations were logarithmically expressed to obtain a normal distribution. The fungal DNA data from the offspring samples were analysed using a linear mixed model for the fungal DNA concentration and a mixed logistic regression for the presence of fungal DNA. The models included a random intercept for mother-offspring pair, and age, maternal fungal DNA concentration/presence, the mode of delivery and maternal probiotics use were used as covariates. The effect of antibiotic use was studied in a separate model because we lacked information on the antibiotic use within 10 days of life. The interaction terms were investigated and included in the final analyses if significant. For independent data, multiple linear regression analyses were performed to test the associations between the fungal DNA abundance and clinical characteristics. The diversities and OTU abundances between the groups were analysed with non-parametric Mann-Whitney U tests and Kruskal-Wallis tests. By this, we disregard the potential within-subjects correlations; nevertheless, a non-parametric test for repeated measurements, i.e. Friedman’s test was not applicable due to the low numbers of observations in some groups. We defined the statistical significance as p < 0.05 and corrected the non-parametric analyses for multiple testing by controlling the false discovery rate through the Benjamini-Hochberg procedure. The statistical analyses were conducted using MATLAB 2016a (The MathWorks, Inc.), STATA 14 (StataCorp) and SPSS Statistics version 23.0 (IBM).
Results
Fungal DNA concentration and diversity
In total, 88% of the mothers and 56–76% of the offspring had detectable gut fungi (Fig. 1). The total fungal abundance was quantified by the amount of fungal ITS DNA copies in the sample. The samples from the pregnant women had the highest fungal DNA concentrations of all the groups (3.85 log(copies/mL) and 95% CI 3.62–4.07), which were significantly higher than those of the postpartum mothers (3.05 and 2.82–3.26, p < 0.001) (Fig. 2, Additional file 1: Table S2). Among the offspring samples, there was a tendency to uncover the highest fungal DNA concentration at 10 days (2.81 and 2.55–3.08), which then fell to the lowest levels at 1 year (2.19 and 1.94–2.45) before an increase at 2 years.
Fig. 1.
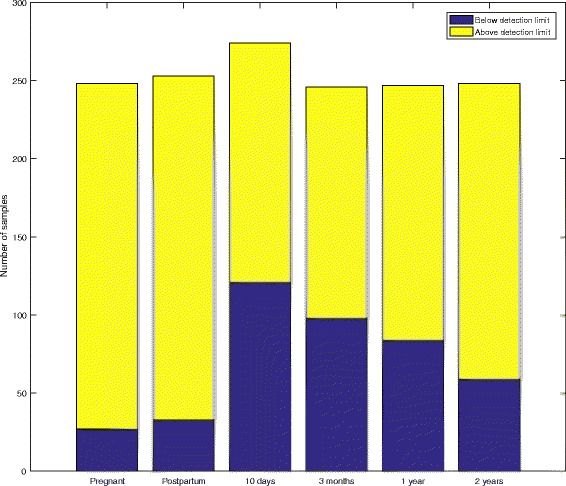
Detection of fungal ITS DNA. The counts of samples with detected and non-detected fungal ITS DNA for each age group. The detection limit was set to a higher fungal ITS concentration than the negative control or within a CT value of 45 cycles
Fig. 2.
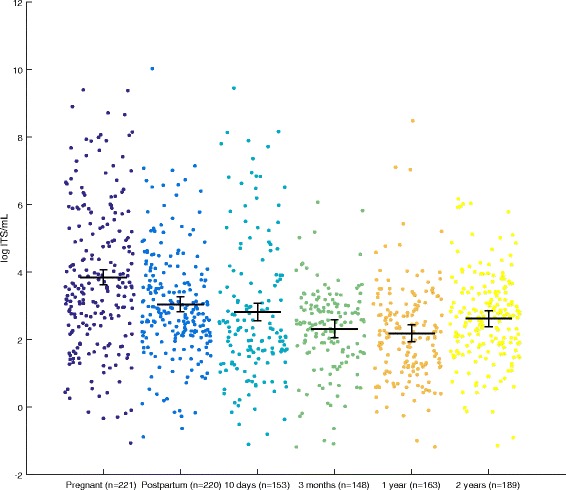
Fungal ITS DNA concentration in maternal and offspring faecal samples. A scatter plot of the fungal ITS DNA concentrations (log ITS copies per mL, mean and 95% CI). The concentration of the ITS copies quantifies the amounts of fungi in the samples
Among all the groups, the alpha diversity was lowest in the 10-day samples (1.21, p < 0.05, Simpson’s reciprocal index) (Fig. 3a) and tended to be higher in the pregnant women (2.16, ns). This finding indicates that only 1–3 fungal species prevailed in mothers as well as offspring. The alpha diversity showed a consistent tendency to increase from birth to 2 years, but it did not reach significance due to the low number of samples in each group. A similar distribution was observed for the mean number of OTUs in each age group (Fig. 3b). In contrast to the alpha diversity, the beta diversity was highest at 10 days after birth (0.97, median Bray-Curtis dissimilarity index) and showed a spread between 0.6 and 1.0 in the other maternal and offspring samples (Fig. 3c). In summary, the mothers tended to have both higher alpha and beta diversity in pregnancy than postpartum. In the offspring, the alpha diversity seemed to increase steadily from birth, whereas the beta diversity was highest in 10-day-old offspring.
Fig. 3.
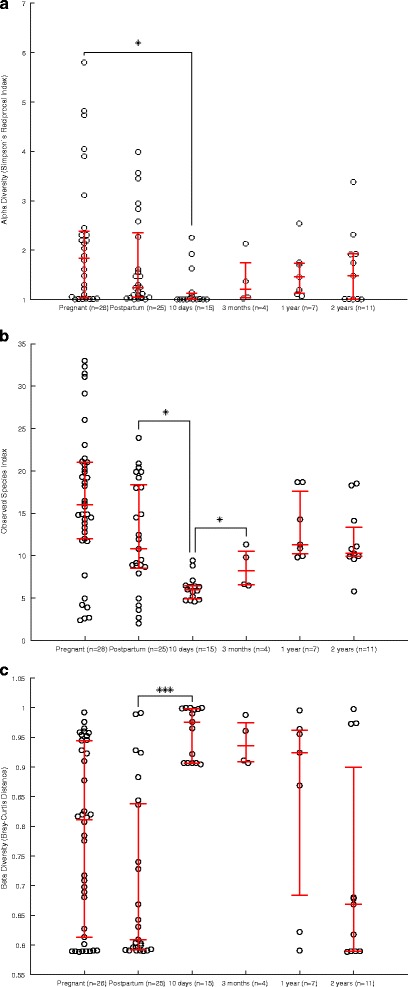
Alpha and beta diversity for the faecal samples. A scatter plot of the diversities; red whiskers designate the median and interquartile ranges. a Alpha diversity as Simpson’s reciprocal index. The Simpson reciprocal index describes how many OTUs prevail in each sample [36]. b The observed species index describes the sample richness, i.e. how many OTUs are detected in each sample. c The beta diversity as Bray-Curtis Distance describes the between-sample diversity from 0 to 1
The PCoA plot at 6000 reads distinguished the 10-day–3-month-old offspring (breast-fed and/or formula-fed) from the other samples. Conversely, the samples from the 1–2-year-old offspring (fed a diet more similar to their mothers) converged towards the maternal pattern (Additional file 6: Figure S2).
OTU taxonomic classification
In applying our stringent annotation method, 140 out of 245 OTUs were annotated at least up to fungal phylum, and 101 OTUs were classified by genus (Additional file 4).
Twelve OTUs differed significantly in their abundance between age groups, whilst they made up > 1% of the total relative abundance (Additional file 7: Figure S3). S. cerevisiae was most abundant in mothers and in offspring from 1 year of age onwards, whereas it was detected in very low quantities in offspring at 10 days and 3 months after birth. Debaryomyces hansenii exhibited its greatest abundance in offspring at 10 days and 3 months (Fig. 4a–c). The 10-day, 1-year and 2-year samples were richer in Rhodotorula mucilaginosa, whereas the 3-month samples showed a greater presence of Candida parapsilosis and a Cladosporium sp.
Fig. 4.
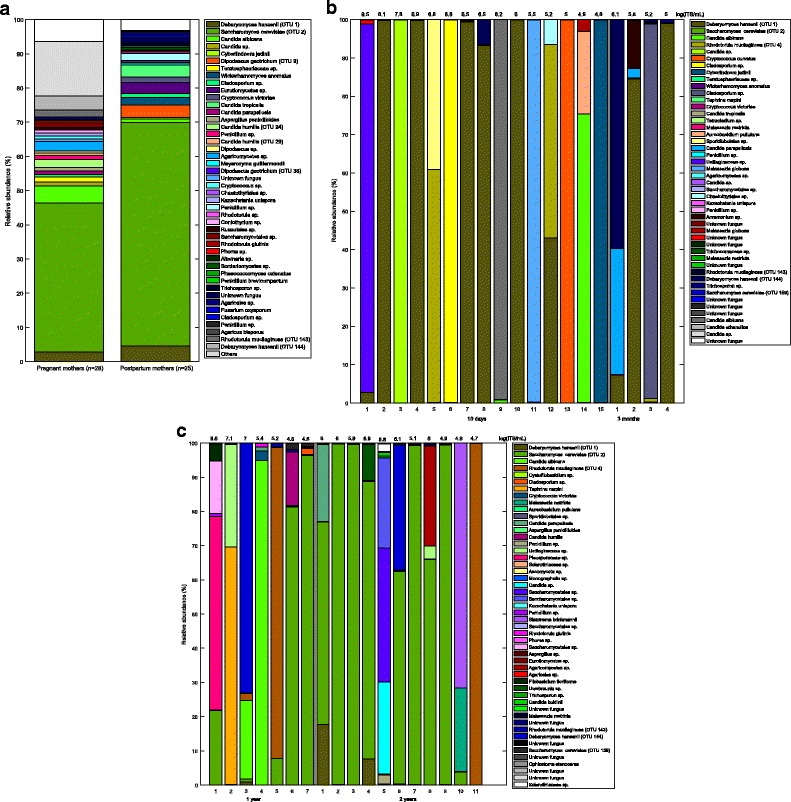
a–c OTU abundances for all groups. Bar charts of the relatively most abundant OTUs in a mothers, b offspring from 10 days to 3 months and c offspring for 1–2 years. Each coloured box represents an OTU. The individual fungal ITS DNA concentration is on top of each bar
Ascomycota spp. comprised 86.4% of the fungi in all the age groups, with no significant difference between the age groups (p = 0.74). In total, 88.6% of the fungal community consisted of yeast species with no significant difference between the age groups (p = 0.60).
Transfer of fungi from the mothers to the offspring
The odds of detecting fungal DNA in the offspring samples increased if the mothers also had detectable fungal DNA (odds ratio (OR) = 1.54 (95% CI 1.01–2.34, p = 0.04)) compared to mothers with no detectable fungi upon mixed logistic regression (Additional file 1: Table S3). Investigating the interactions, this effect was strongest 10 days after birth (OR = 3.7 (1.24–11.0), p = 0.019). In particular, we observed no effects of mode of delivery nor did we see any effects of maternal probiotic use or offspring antibiotic use. The intraclass coefficient (ICC) was < 0.01, which indicates that the repeated measurements were largely unrelated.
We found no significant associations between the offspring fungal DNA concentrations and the maternal fungal DNA concentrations, probiotics, mode of delivery or the offspring antibiotic use (Additional file 1: Table S4). The ICC was < 0.01, which shows that the repeated measurements were largely unrelated.
By sequencing the 18S rRNA gene ITS1 regions of merely high-quantity samples, 5 mother-offspring pairs remained. In these pairs, there were 11 overlapping species, with D. hansenii and S. cerevisiae being the most frequently overlapping ones (Fig. 5). These two species were also retained between pregnant and postpartum mother pairs (data not shown). Several other species were also present in the mother-offspring overlap, and these mostly belonged to the Saccharomycetaceae family, including Candida spp., in addition to R. mucilaginosa, Malassezia spp. and Cladosporium spp.
Fig. 5.
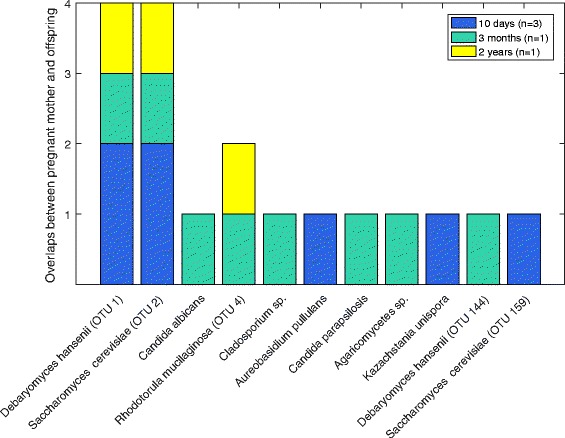
Overlapping OTUs between pregnant woman and their offspring. The counts of overlapping OTUs in five mother-offspring pairs from the sequencing of the 18S rRNA gene ITS1 regions
Probiotics and fungal DNA concentrations in the mothers and the offspring
The pregnant mothers who were randomised to receive probiotics had significantly increased fungal DNA concentrations compared to the controls (p < 0.01, Additional file 1: Table S5). Adjusting for a history of antibiotic treatment did not change the effect estimator. One S. cerevisiae strain (OTU 159) tended to be underrepresented in the probiotic-receiving pregnant women (p = 0.07); however, the distributions of the other S. cerevisiae strain (OTU 2) remained the same (Additional file 8: Figure S4).
Discussion
In this study, we showed that the gut fungi are detectable in most mothers and the majority of their offspring already at the age of 10 days. S. cerevisiae was the most abundant fungal species in mothers and 1- to 2-year-old offspring, whereas D. hansenii prevailed during the first months of life. Furthermore, there was an increased risk of fungal DNA presence in offspring if the mother had detectable fungi. We also found that the maternal fungal DNA concentrations increased when the pregnant mothers drank probiotics.
Almost 90% of the mothers and 60–80% of the offspring had detectable gut fungi, which highlights fungi as an inherent part of the gut microbiota. These proportions cohere with the findings of an Italian cross-sectional fungal cultivation study on children and adults [18].
Many of the fungal species that we detected in the offspring have previously been detected in the adult gut (see Additional file 5). Strikingly, D. hansenii, an ubiquitous Saccharomycetaceae species that is often used in the food industry as a cheese yeast [40], dominates the offspring gut mycobiota during the breast-feeding period. D. hansenii can stem from breast milk because this fluid was the only dietary component at 3 months, and similar yeasts (C. albicans) have been found in breast milk before [41]. D. hansenii has also been found on the facial skin of children [42]. D. hansenii has previously been cultured from human faeces and has been shown to grow well in milky environments like cheese and possibly breast milk, since some strains are known to grow at 37 °C [20, 40]. All these findings suggest that D. hansenii could be an autochthonous species of the early mycobiota.
We found S. cerevisiae in the newborn offspring, which has not been shown before. Intriguingly, S. cerevisiae first surges and then becomes a dominant species at 1 year of age, after the introduction of food containing S. cerevisiae (e.g. bread) into the diet. This finding suggests both a birth-related and a dietary means of colonisation. S. cerevisiae is present in most diets and has been found and cultured from faecal samples. However, it is also capable of causing opportunistic infections [20, 43], implying that it is an autochthonous species. We observed that S. cerevisiae was substantially more abundant in offspring and adult gut mycobiota than previously described [1, 9], and there was a relatively low occurrence of Candida spp.; this variation may be due to different regional diets, host genetics or fungal detection methods. Some studies have shown that by using culture-dependent techniques, one would obtain relatively more Candida spp. than when using culture-independent techniques [2, 18], which could partly explain the rather limited amounts of Candida spp. in our study.
Some of the identified fungi should be regarded as transients that do not colonise the gut. The OTUs Agaricomycetes/Agaricales sp. are likely from edible and non-colonising fungi, e.g. the button mushroom Agaricus bisporus, and similarly, Ustilaginaceae spp. are well-known plant pathogens. These fungi are generally of lower quantities and are often known not to live in anaerobic and body temperature environments; they are thus most likely transients from food. Not all OTUs were annotated to the species level because the fungal databases still are under development. However, our strict classification improved the chance of an accurate classification.
We found that maternal fungal hosting makes the offspring more inclined to host fungi. This effect was strongest at 10 days after birth. The increased chance of fungal hosting suggests that these mother-offspring pairs share physiological fungal hosting abilities. Because fungi are ubiquitous in the environment, they may originate from the mother during birth, the mother’s breast milk, parental skin or anywhere else in the hospital or home environment with which the offspring come in contact. However, we did not observe any OTU abundance difference regarding vaginal or caesarean delivery. Thus, we find indications for the transfer of fungal hosting between mothers and offspring that appear to be independent of the mode of delivery.
The overlapping OTUs in the mother and offspring guts were mostly Saccharomycetaceae spp. This fungal family seems to have adapted well to the human gut environment and may be the species that are fittest to survive the transfer into the newborn fungal host environment. In addition to the gut mycobiota, Saccharomycetaceae spp. are also the most abundant species in healthy human mouth mycobiota [44].
The fungal abundance varied by age, which supports the idea that physiological fungal succession occurs in the early gut mycobiota. We have shown that the gut mycobiota is establishing already at 10 days after birth, albeit at a lower abundance and diversity than what is detected in their mothers’ guts. At 10 days, the gut fungi have just started to fight for their positions in a seemingly first-come first-serve model; the reduction in fungal abundance is probably determined by feeding, gut immunity and their interactions. The decrease at 3 months may be due to a previously described temporarily high abundances of Bifidobacterium spp. and Lactobacillus spp. that exhibit fungal antagonism [30, 45]. Upon approaching 2 years of age, the gut mycobiota consists of fungi specific for the adult mycobiota, as observed in our study.
Interestingly, pregnant mothers that received probiotics showed a higher abundance of gut fungi. This finding could indicate that the probiotic bacteria used in our study promote the symbiotic growth of gut fungi, like other lactic acid bacteria that are known to grow mutually with yeasts [46].
Due to a strict ITS quantity cut-off, a smaller proportion of the samples was sequenced. It cannot be excluded that the lower-abundance samples have different compositions. Nevertheless, in this unselected study population of mothers and offspring, it is reasonable to surmise that this selection could reflect the gut mycobiota of a healthy gut. The study design gave us limited control of the faecal sampling, but all the mothers were well informed about how to collect and quickly freeze the samples to avoid contamination and improve preservation. In the future, a lysis protocol optimised for fungal DNA extraction would be preferable, but this approach would require the fungal extraction analysis of a representative selection of human-associated fungi that is not yet available.
Conclusion
Our findings provide the first insight into the gut mycobiota that is established in offspring and into the transfer of fungal hosting from mother to child. This study covers a large, unselected population cohort of mothers and offspring, and it broadens the field of gut mycobiota as a new research area. The ways in which the early mycobiota can affect a child’s normal physiology with respect to growth, immunity and metabolism remain to be elucidated.
Additional files
Rarefaction table of sequenced samples. Table S2. ITS DNA concentration for all age groups. Table S3. Detectable fungal DNA in offspring according to maternal characteristics and offspring age. Table S4. Fungal DNA concentration in offspring according to maternal presence and offspring age. Table S5. Association between fungal DNA concentration in pregnant mothers and maternal use of antibiotics and probiotics. (XLSX 16 kb)
Number of samples vs. rarefaction cut-off. To compare the samples, a rarefaction is performed to obtain the same number of sequences in each sample. By increasing the rarefaction cut-off, the number of observed species increases with the sacrifice in the number of included samples. Using 6000 sequences as the rarefaction cut-off is a reasonable trade-off. (PDF 6 kb)
Concordance of fungal databases and final assignment. (XLSX 101 kb)
Table of fungal species. (XLSX 18 kb)
Concentration and standard curve calculations. (DOCX 32 kb)
Principal coordinates analysis (PCoA). A PCoA plot at 6000 reads per sample. (PDF 8 kb)
Significantly different OTUs between groups. A significantly different abundance of OTUs between groups in terms of relative abundance, as tested by Kruskal-Wallis test. Each bar represents an OTU, for which the relative abundances of all the groups are added. Only species > 1% of the relative abundance in at least one age group are included in the analysis. (PDF 5 kb)
OTU abundances for probiotics in pregnant mothers. The OTU abundances in pregnant mothers with and without probiotics. Each coloured box represents an OTU. (PDF 5 kb)
Acknowledgements
Great thanks to all the mothers and children who participated in the study, to Inga Leena Angell for the laboratory aid, to the ObeCe Research Centre at St. Olav’s University Hospital and to the Medical Student Research Programme at NTNU.
Funding
This study was funded by the Research Council of Norway and internal funds from NTNU – Norwegian University of Science and Technology. The probiotics in the ProPACT study were provided by the TINE BA.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to the Norwegian Authorities’ legislation on the sharing of personal yet non-identifiable data. These data are stored at the Norway University of Life Sciences in Ås, Norway, and they are available upon reasonable request.
Abbreviations
- CFU
Colony-forming unit
- CI
Confidence intervals
- CT
Cycle threshold
- ddPCR
Droplet Digital Polymerase Chain Reaction
- IBD
Inflammatory bowel disease
- ICC
Intraclass coefficient
- ITS
Internal transcribed spacer
- OR
Odds ratio
- OTU
Operational taxonomic unit
- ProPACT
Probiotics in the Prevention of Allergy among Children in Trondheim Study
- PRR
Pathogen-recognition receptor
- QIIME
Quantitative Insights into Microbial Ecology
- qPCR
(Real-time) quantitative polymerase chain reaction
- SD
Standard deviation
- Sp. and spp.
Species (singular) and species (plural)
Authors’ contributions
All the authors contributed to the research question. TØ conducted the sampling. KS performed the laboratory work and the statistical analyses, and KS wrote the manuscript draft. All the authors revised and approved the final article.
Ethics approval and consent to participate
This study was approved by the Regional Committee for Medical and Health Research Ethics for Central Norway (ref: 120-2000 and 2014/1796/REK midt) and the Norwegian Data Inspectorate (ref: 2003/953-3 KBE/-). At least one of each child’s parent was informed and signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s40168-017-0319-x) contains supplementary material, which is available to authorized users.
Contributor Information
Kasper Schei, Phone: +47 90262522, Email: kasperschei@gmail.com.
Ekaterina Avershina, Email: ekaterina.avershina@nmbu.no.
Torbjørn Øien, Email: torbjorn.oien@ntnu.no.
Knut Rudi, Email: knut.rudi@nmbu.no.
Turid Follestad, Email: turid.follestad@ntnu.no.
Saideh Salamati, Email: saideh.salamati@stolav.no.
Rønnaug Astri Ødegård, Email: ronnaug.odegard@ntnu.no.
References
- 1.Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouba N, Drancourt M. Digestive tract mycobiota: a source of infection. Medecine et maladies infectieuses. 2015;45:9–16. doi: 10.1016/j.medmal.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Hamad I, Raoult D, Bittar F. Repertory of eukaryotes (eukaryome) in the human gastrointestinal tract: taxonomy and detection methods. Parasite Immunol. 2016;38:12–36. doi: 10.1111/pim.12284. [DOI] [PubMed] [Google Scholar]
- 4.Huse SM, Ye Y, Zhou Y, Fodor AA. A core human microbiome as viewed through 16S rRNA sequence clusters. PLoS One. 2012;7:e34242. doi: 10.1371/journal.pone.0034242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 2015;517:165–169. doi: 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome medicine. 2013;5:63. doi: 10.1186/gm467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzetto L, De Filippo C, Cavalieri D. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease. Eur J Immunol. 2014;44:3166–3181. doi: 10.1002/eji.201344403. [DOI] [PubMed] [Google Scholar]
- 11.Walker AW, Lawley TD. Therapeutic modulation of intestinal dysbiosis. Pharmacol Res. 2013;69:75–86. doi: 10.1016/j.phrs.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Mar Rodriguez M, Perez D, Javier Chaves F, Esteve E, Marin-Garcia P, Xifra G, et al. Obesity changes the human gut mycobiome. Sci Rep. 2015;5:14600. doi: 10.1038/srep14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wensveen FM, Valentić S, Šestan M, Turk Wensveen T, Polić B. The “big bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45:2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 14.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039-48. [DOI] [PMC free article] [PubMed]
- 15.Ott SJ, Kuhbacher T, Musfeldt M, Rosenstiel P, Hellmig S, Rehman A, et al. Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand J Gastroenterol. 2008;43:831–841. doi: 10.1080/00365520801935434. [DOI] [PubMed] [Google Scholar]
- 16.Salamati S, Martins C, Kulseng B. Baker’s yeast (Saccharomyces cerevisiae) antigen in obese and normal weight subjects. Clinical obesity. 2015;5:42–47. doi: 10.1111/cob.12079. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldi M, Perricone R, Blank M, Perricone C, Shoenfeld Y. Anti-Saccharomyces cerevisiae autoantibodies in autoimmune diseases: from bread baking to autoimmunity. Clin Rev Allergy Immunol. 2013;45:152–161. doi: 10.1007/s12016-012-8344-9. [DOI] [PubMed] [Google Scholar]
- 18.Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, et al. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front Microbiol. 2016;7:1227. doi: 10.3389/fmicb.2016.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231–235. doi: 10.1097/INF.0b013e31815bb69d. [DOI] [PubMed] [Google Scholar]
- 20.Suhr MJ, Hallen-Adams HE. The human gut mycobiome: pitfalls and potentials—a mycologist’s perspective. Mycologia. 2015;107:1057–1073. doi: 10.3852/15-147. [DOI] [PubMed] [Google Scholar]
- 21.Ward TL, Knights D, Gale CA. Infant fungal communities: current knowledge and research opportunities. BMC Med. 2017;15:30. doi: 10.1186/s12916-017-0802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stinson LF, Payne MS, Keelan JA. Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017;43:352–369. doi: 10.1080/1040841X.2016.1211088. [DOI] [PubMed] [Google Scholar]
- 24.Prince AL, Chu DM, Seferovic MD, Antony KM, Ma J, Aagaard KM. The perinatal microbiome and pregnancy: moving beyond the vaginal microbiome. Cold Spring Harbor Perspect Med. 2015;5 [DOI] [PMC free article] [PubMed]
- 25.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soderborg TK, Borengasser SJ, Barbour LA, Friedman JE. Microbial transmission from mothers with obesity or diabetes to infants: an innovative opportunity to interrupt a vicious cycle. Diabetologia. 2016;59:895–906. doi: 10.1007/s00125-016-3880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason KL, Erb Downward JR, Mason KD, Falkowski NR, Eaton KA, Kao JY, et al. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect Immun. 2012;80:3371–3380. doi: 10.1128/IAI.00449-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21:334–341. doi: 10.1016/j.tim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dollive S, Chen YY, Grunberg S, Bittinger K, Hoffmann C, Vandivier L, et al. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS One. 2013;8:e71806. doi: 10.1371/journal.pone.0071806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsubara VH, Bandara HM, Mayer MP, Samaranayake LP. Probiotics as antifungals in mucosal candidiasis. Clin Infect Dis. 2016;62:1143–1153. doi: 10.1093/cid/ciw038. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Bansal A, Chakrabarti A, Singhi S. Evaluation of efficacy of probiotics in prevention of candida colonization in a PICU—a randomized controlled trial. Crit Care Med. 2013;41:565–572. doi: 10.1097/CCM.0b013e31826a409c. [DOI] [PubMed] [Google Scholar]
- 34.Dotterud CK, Storro O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol. 2010;163:616–623. doi: 10.1111/j.1365-2133.2010.09889.x. [DOI] [PubMed] [Google Scholar]
- 35.Dotterud CK, Avershina E, Sekelja M, Simpson MR, Rudi K, Storro O, et al. Does maternal perinatal probiotic supplementation alter the intestinal microbiota of mother and child? J Pediatr Gastroenterol Nutr. 2015;61:200–207. doi: 10.1097/MPG.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 36.Tang J, Iliev ID, Brown J, Underhill DM, Funari VA. Mycobiome: approaches to analysis of intestinal fungi. J Immunol Methods. 2015;421:112–121. doi: 10.1016/j.jim.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Meth. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 38.Magurran AE. Measuring biological diversity. Maldan: Blackwell Pub; 2004. [Google Scholar]
- 39.Hibbett DS, Taylor JW. Fungal systematics: is a new age of enlightenment at hand? Nat Rev Microbiol. 2013;11:129–133. doi: 10.1038/nrmicro2963. [DOI] [PubMed] [Google Scholar]
- 40.Breuer U, Harms H. Debaryomyces hansenii—an extremophilic yeast with biotechnological potential. Yeast (Chichester, England) 2006;23:415–437. doi: 10.1002/yea.1374. [DOI] [PubMed] [Google Scholar]
- 41.Mutschlechner W, Karall D, Hartmann C, Streiter B, Baumgartner-Sigl S, Orth-Höller D, et al. Mammary candidiasis: molecular-based detection of Candida species in human milk samples. Eur J Clin Microbiol Infect Dis. 2016:1–5. [DOI] [PubMed]
- 42.Arzumanyan VG, Magarshak OO, Semenov BF. Yeast fungi in patients with allergic diseases: species variety and sensitivity to antifungal drugs. Bull Exp Biol Med. 2000;129:601–604. doi: 10.1007/BF02434889. [DOI] [PubMed] [Google Scholar]
- 43.Vaughan-Martini A, Martini A. Chapter 61 - Saccharomyces Meyen ex Reess (1870) A2 - Kurtzman, Cletus P. In: Fell JW, Boekhout T, editors. The yeasts. Fifth. London: Elsevier; 2011. pp. 733–746. [Google Scholar]
- 44.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Furukawa S, Watanabe T, Toyama H, Morinaga Y. Significance of microbial symbiotic coexistence in traditional fermentation. J Biosci Bioeng. 2013;116:533–539. doi: 10.1016/j.jbiosc.2013.05.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefaction table of sequenced samples. Table S2. ITS DNA concentration for all age groups. Table S3. Detectable fungal DNA in offspring according to maternal characteristics and offspring age. Table S4. Fungal DNA concentration in offspring according to maternal presence and offspring age. Table S5. Association between fungal DNA concentration in pregnant mothers and maternal use of antibiotics and probiotics. (XLSX 16 kb)
Number of samples vs. rarefaction cut-off. To compare the samples, a rarefaction is performed to obtain the same number of sequences in each sample. By increasing the rarefaction cut-off, the number of observed species increases with the sacrifice in the number of included samples. Using 6000 sequences as the rarefaction cut-off is a reasonable trade-off. (PDF 6 kb)
Concordance of fungal databases and final assignment. (XLSX 101 kb)
Table of fungal species. (XLSX 18 kb)
Concentration and standard curve calculations. (DOCX 32 kb)
Principal coordinates analysis (PCoA). A PCoA plot at 6000 reads per sample. (PDF 8 kb)
Significantly different OTUs between groups. A significantly different abundance of OTUs between groups in terms of relative abundance, as tested by Kruskal-Wallis test. Each bar represents an OTU, for which the relative abundances of all the groups are added. Only species > 1% of the relative abundance in at least one age group are included in the analysis. (PDF 5 kb)
OTU abundances for probiotics in pregnant mothers. The OTU abundances in pregnant mothers with and without probiotics. Each coloured box represents an OTU. (PDF 5 kb)
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to the Norwegian Authorities’ legislation on the sharing of personal yet non-identifiable data. These data are stored at the Norway University of Life Sciences in Ås, Norway, and they are available upon reasonable request.


