Abstract
Background
Hepatitis B virus (HBV) infection is a major cause of chronic liver disease worldwide. In immunocompromised patients, the chronicity rates of HBV infection are higher, but the rates of hepatitis Be antigen (HBeAg) and HBsAg loss and seroconversion to anti-HBe and anti-HBs are lower than those in immunocompetent subjects. This study aimed to evaluate articles on the prevalence of HBsAg in people living with human immunodeficiency virus (HIV) /AIDS (PLWHA) in Latin America and the Caribbean (LAC).
Methods
We searched the PubMed, Latin American and Caribbean Health Sciences, and Embase databases for studies up to November 2016 on infection with HIV and HBV in LAC without period or language restrictions. We did not include case reports, case series, review articles, comments, or studies with a sample size smaller than 100. We also evaluated the quality of the articles using a list of criteria totaling 21 items.
Results
Of the 28 selected articles (n = 18,457) published from 1999 to 2016, 18 studies (64.3%) were from Brazil, 3 (10.7%) were from Argentina, 2 (7.1%) were from Chile, 2 (7.1%) were from Cuba, 1 (3.6%) was from Colombia, 1 (3.6%) was from Venezuela, and 1 (3.6%) was from Jamaica. The mean score for the assessment of the study quality was 11.6 (range: 8–16). The estimated pooled prevalence of HBsAg among PLWHA in the selected studies was 7.0% (95% CI 7.0–7.0%). The pooled prevalence of HBsAg was 8.0% (95% CI 8.0–9.0%) in the studies published from 1999 to 2006 and 6.0% (95% CI 5.0–6.0%) in the studies published during the later timeframe.
Conclusions
The results of this review indicate the need to increase the investment in preventive measures against hepatitis B, particularly when the impact of adequate vaccination in this population is considered. Future studies with larger sample sizes are needed in LAC to determine the true prevalence of hepatitis B throughout the region and to clarify and address the risk factors associated with the acquisition of infection.
Electronic supplementary material
The online version of this article (10.1186/s12879-017-2695-z) contains supplementary material, which is available to authorized users.
Keywords: Hepatitis B, Hepatitis B virus, HIV, Human immunodeficiency virus, Latin America, The Caribbean, Prevalence, Systematic review, Review
Background
Hepatitis B virus (HBV) infection is a major cause of chronic liver disease worldwide, and approximately 350 million people are chronically infected. In the absence of antiviral therapy, 15% to 40% of patients positive for the hepatitis B surface antigen (HBsAg) develop progressive liver disease, cirrhosis, hepatocellular carcinoma, and terminal liver failure [1]. Because HBV is primarily transmitted via the parenteral and sexual routes and during the perinatal period similar to human immunodeficiency virus (HIV), coinfection with HIV and HBV is common [2].
HIV infection appears to have a negative impact on the natural history of HBV infection. In immunocompromised patients, the chronicity rates of HBV infection are higher, but the rates of hepatitis Be antigen (HBeAg) and HBsAg loss and seroconversion to anti-HBe and anti-HBs are lower than those in immunocompetent subjects [3–5].
Patients with HIV infection have lower serum alanine aminotransferase and albumin levels and higher serum HBV DNA levels than patients with HBV monoinfection. Additionally, the prevalence of cirrhosis is higher in HIV-positive patients [6]. Coinfection with HIV and HBV is associated with a higher rate of HBV reactivation and an increased incidence of cirrhosis and death from cirrhosis in cases with low CD4 counts. Coinfection is also associated with a higher incidence of hepatocellular carcinoma [7, 8].
Other studies have suggested that HBV is associated with a rapidly progressive course of HIV infection [9]. A retrospective analysis indicated that the risk of death in 64 individuals coinfected with HIV and HBV was approximately two-fold higher than that in individuals with HIV monoinfection [10].
The estimated prevalence of hepatitis B among people living with HIV/AIDS (PLWHA) is 5–20%. Therefore, approximately 2 to 4 million people living with HIV have chronic hepatitis B coinfection [2, 11]. Although several studies have evaluated the prevalence of hepatitis B in PLWHA in Latin America and the Caribbean (LAC), the results are conflicting among the evaluated regions and even within the same geographic area. This study aimed to evaluate the studies on the prevalence of HBsAg in LAC countries or territories.
Methods
We conducted a systematic review of published articles on the prevalence of HBV infection in PLWHA in LAC countries or territories. Our review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement published in 2009 [12] (Additional file 1).
Search strategies
We searched Medline via the PubMed, Latin American and Caribbean Health Sciences (Literatura Latino-Americana e do Caribe em Ciências da Saúde–Lilacs) via BVS, and Embase databases for studies up to November 2016 on infection with HIV and HBV in LAC without period and language restrictions. In Medline, we used the terms [(HIV OR Acquired Immunodeficiency Syndrome Virus OR AIDS OR HTLV OR Human Immunodeficiency Virus OR Human TCell) AND (HBV OR Hepatitis B OR Hepatitis B Virus) AND (name of each country or territory from LAC)]. In Lilacs, we used the terms [(HIV OR AIDS OR HUMAN IMMUNODEFICIENCY VIRUS) [Words] AND (HBV OR HEPATITIS B) [Words]]. In Embase, we searched the terms [(HIV OR AIDS OR HUMAN IMMUNODEFICIENCY VIRUS) AND (HBV OR HEPATITIS B)]. We manually searched the references of the selected studies and reviewed articles on the subjects to identify other relevant studies. Disagreements on the identification of relevant studies were discussed until a consensus was reached.
The studies eligible for reading of the titles and abstracts were selected independently by two researchers (E.A. and M.N.), and a list of potentially relevant studies was generated. Articles for inclusion in the review were selected after reading the full text (A.A.B. and W.M.B.).
The articles included were those that contained data on HBV infection in PLWHA, a serological diagnosis of HIV and HBV, and estimates of the prevalence of HBsAg in HIV-infected individuals.
Study selection
We included original articles that reported the prevalence of HBV in PLWHA in LAC with a sample size ≥100. We did not include case reports, case series, review articles, comments, studies whose participants did not live in LAC, or studies that contained the same case series presented in other publications. Regarding the latter studies, the article with the most complete data was included in the study. We excluded self-reported HIV and/or HBV infections, data obtained from mandatory reporting of HIV and/or HBV (e.g., databases of the local Ministry of Health), and studies whose participant selection method was unclear.
We used the following definitions: (1) HIV infection: the presence of anti-HIV antibodies measured by enzyme immunoassay; (2) HBV infection: the presence of HBsAg; and (3) Latin America and the Caribbean: the countries and territories of Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, French Guiana, Guyana, Paraguay, Peru, Suriname, Uruguay, Venezuela, Belize, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Aruba, Antigua and Barbuda, Aruba, Bahamas, Barbados, Bonaire, British Virgin Islands, Cayman Islands, Cuba, Curaçao, Dominica, Dominican Republic, Grenada, Guadalupe, Haiti, Jamaica, Martinique, Montserrat, Puerto Rico, Saba, Saint Barthelemy, Saint Kitts and Nevis, Saint Lucia, Saint Martin, Saint Vincent and The Grenadines, Sint Eustatius, Sint Maarten, Trinidad and Tobago, Turks and Caicos Islands, and the United States Virgin Islands.
Data extraction
Data were collected independently by two investigators (A.A.B. and W.M.B.), and disagreements were resolved via discussions and a consensus. The following data were collected from the articles: author name(s), year of publication, country (state in the case of Brazilian studies), period of data collection, type of study, sample size, mean age, gender of the participants, and number of HBsAg-positive individuals.
Some studies did not report all of the variables necessary for the calculation of prevalence. In these cases, the missing variables were calculated using other reported values (e.g., the numerator was calculated from the values of the denominator and the prevalence).
Quality assessment of the studies
Based on the criteria proposed by Boyle [13], Fowkes and Fulton [14], Loney [15], and Prins [16], we created a list of criteria related to the adequacy of the sample (11 items: adequate design, prospective collection, definition of the target population, probability sampling, sample size calculation, inclusion and exclusion criteria, specification of the collection period, specification of the age range, adequate participant selection, acceptable sample loss, and sample representativeness), data collection (4 items: standardization of collection, clear definition of the outcome, definition of the outcome, and description of the outcome measurement), and data analysis and presentation (6 items: description of statistical analysis, total number of participants, number of events (outcomes), prevalence by sex and age, prevalence with confidence interval, and satisfactory confidence interval), for a total of 21 items. The items were scored as positive or negative, and the importance of each item was not weighted. Higher scores (positive items) corresponded to higher-quality studies for our review. This assessment was made independently by two researchers (E.A. and M.N.). Disagreements in the evaluation of the quality of the studies were discussed, and a consensus was reached.
Statistical analysis
As a function of the expected heterogeneity among the selected studies, all meta-analyses were performed using random-effects models based on the DerSimonian and Laird method, which took into account variation among studies. Heterogeneity was assessed using Cochran’s Q statistic (expressed as χ2 and p values) and the I2 statistic, which described the percentage of variation among studies explained by heterogeneity rather than by chance. I2 values higher than 25%, 50%, and 75% are considered evidence of low, moderate, and high heterogeneity among studies, respectively. Low I2 values indicate that the variability among estimates is compatible with random variation. Additionally, we investigated potential sources of heterogeneity using a meta-regression analysis. The following factors were assessed using both uni- and multivariate models: publication year (comparison between studies published from 1999 to 2006 and those published from 2007 to 2016), geographical area (comparison between studies conducted in Brazil and elsewhere), study design (whether the data collection was cross-sectional or non-cross-sectional), sample size (continuous variable), and quality score (whether the quality score was ≤10 or >10). We also performed an analysis considering two groups of studies: those published during the period from 1999 to 2006 and those published during the period from 2007 to 2016. In the studies published within the last ten years (2006 to 2016), we also analyzed the results stratified by sex and age group (<40 years and ≥40 years). All analyses were performed using Stata version 13 (Stat Corp LP, TX, USA) with the commands metan (for the random-effects meta-analysis) and metareg (for the meta-regression).
Results
We identified 1304 publications in Medline, Lilacs, and Embase, and no additional articles were found by the manual search (Fig. 1). After excluding duplicates (80), 1224 articles were selected for reading of the titles and abstracts.
Fig. 1.
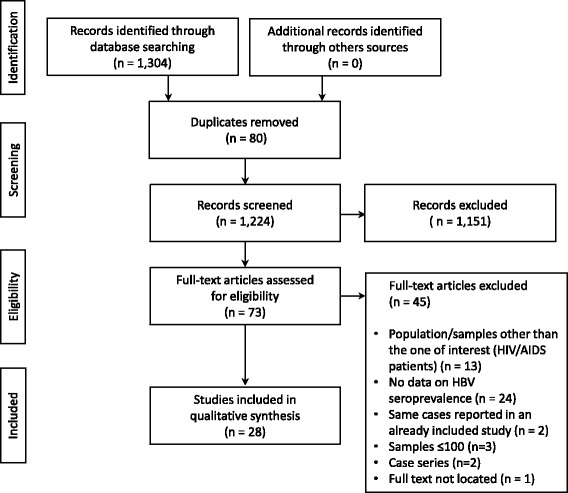
Flowchart of the identification, inclusion, and exclusion of the studies
After reading, 1151 articles were excluded, and 73 articles were selected for reading of the full text. Of these, we selected 28 articles (n = 18,457) published from 1999 to 2016 for data extraction.
Of the 28 publications on the prevalence of infection with HBV in PLWHA, 18 studies (64.3%) were from Brazil, 3 (10.7%) were from Argentina, 2 (7.1%) were from Chile, 2 (7.1%) were from Cuba, 1 (3.6%) was from Colombia, 1 (3.6%) was from Venezuela, and 1 (3.6%) was from Jamaica (Table 1). The sample sizes ranged from 129 to 2994 participants (mean: 659; median: 471). The mean score of the study quality assessment was 11.6 (range: 8–16). No study was scored ≤7, 25 studies (89.3%) had scores between 8 and 14, and 3 (10.7%) studies had scores between 15 and 21 (maximum score: 21). The most common reasons precluding higher scores were the methods used for sample selection and the presentation of the results (the details of the quality assessment of the studies are shown in the Additional files 2 and 3). The characteristics of the studies and the sample populations are shown in Table 1.
Table 1.
Prevalence studies of HBsAg in people living with HIV/AIDS
| Author | Year | Geographical region | Collection period | Study design | Total | Mean age | Gender (% M) | Outcome | HBsAg + (%) | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Oliveira et al. [23] | 2016 | Brazil | 2009–2010 | Cross-sectional | 505 | 37.6 | 60.2 | 25 | 4.9 | 15 |
| Brandao et al. [24] | 2015 | Brazil | 2011 | Cross-sectional | 495 | 40 | 73.9 | 19 | 3.8 | 14 |
| Vieira et al. [25] | 2015 | Brazil | 2008–2009 | Cross-sectional | 297 | NA | 49.8 | 8 | 2.6 | 10 |
| Martins et al. [26] | 2014 | Brazil | 2012–2013 | Cross-sectional | 300 | 44.6 | 59.7 | 7 | 2.3 | 14 |
| Bautista-Amorocho et al. [27] | 2014 | Colombia | 2009–2010 | Cross-sectional | 275 | 37.4 | 65.1 | 9 | 3.3 | 13 |
| Jasper et al. [28] | 2014 | Venezuela | 2002–2011 | Retrospective | 418 | NA | 64.1 | 13 | 3.1 | 10 |
| Freitas et al. [29] | 2014 | Brazil | 2009–2011 | Cross-sectional | 848 | 41.6 | 57 | 21 | 2.5 | 13 |
| Oliveira et al. [30] | 2014 | Brazil | 2006–2008 | Cross-sectional | 768 | NA | 65.4 | 46 | 6 | 11 |
| Otto-Knapp et al. [31] | 2013 | Chile | 2001–2007 | Retrospective | 1907 | 37.2 | 84.8 | 161 | 8.5 | 12 |
| Tornatore et al. [32] | 2012 | Brazil | 2006–2008 | Cross-sectional | 130 | 26.2 | 0 | 3 | 2.3 | 10 |
| Benzaken et al. [33] | 2012 | Brazil | 2009 | Cross-sectional | 598 | 29 | 47.7 | 35 | 5.9 | 16 |
| Laufer et al. [34] | 2010 | Argentina | 2004–2005 | Cross-sectional | 593 | 38 | 65.6 | 20 | 3.3 | 11 |
| Perez et al. [35] | 2009 | Chile | 1990–2007 | Retrospective | 311 | 40.9 | 90.9 | 19 | 6.1 | 9 |
| Sampaio et al. [36] | 2009 | Brazil | 2004 | Cross-sectional | 429 | 39.3 | 60.1 | 44 | 10.3 | 13 |
| Zago et al. [37] | 2007 | Brazil | 1993–2004 | Retrospective | 851 | 35 | 52.1 | 32 | 3.8 | 13 |
| Quarleri et al. [38] | 2007 | Argentina | 2004–2005 | Cross-sectional | 593 | 39 | 66 | 22 | 3.7 | 11 |
| Braga et al. [39] | 2006 | Brazil | 1998–2003 | Retrospective | 704 | NA | 65.1 | 45 | 6.4 | 12 |
| Tovo et al. [40] | 2006 | Brazil | NA | Retrospective | 343 | 34.4 | 62.4 | 14 | 4.6 | 10 |
| Grinsztejn et al. [41] | 2006 | Brazil | 1996–2004 | Cross-sectional | 458 | 35.4 | 0 | 8 | 1.9 | 9 |
| Pereira et al. [42] | 2006 | Brazil | 2004 | Cross-sectional | 1000 | 37.2 | 53 | 37 | 3.7 | 13 |
| Corredor et al. [43] | 2005 | Cuba | 2000–2004 | Retrospective | 2994 | NA | NA | 309 | 10.3 | 8 |
| Monteiro et al. [44] | 2004 | Brazil | 1999–2004 | Cross-sectional | 406 | 34.2 | NA | 32 | 7.9 | 15 |
| Souza et al. [45] | 2004 | Brazil | 1992–1995 | Retrospective | 401 | NA | 64.8 | 34 | 8.5 | 13 |
| Pavan et al. [46] | 2003 | Brazil | 1992–1995 | Retrospective | 232 | 30.8 | 69.4 | 12 | 5.3 | 10 |
| Smikle et al. [47] | 2003 | Jamaica | 2001–2002 | Retrospective | 129 | NA | 38 | 19 | 15 | 10 |
| Mendes-Correa et al. [48] | 2000 | Brazil | 1996 | Retrospective | 1693 | NA | 68.2 | 96 | 5.7 | 10 |
| Rodriguez et al. [49] | 2000 | Cuba | NA | Cross-sectional | 295 | 30 | 72.5 | 15 | 5.1 | 10 |
| Fainboim et al. [50] | 1999 | Argentina | 1994–1995 | Cross-sectional | 484 | 29 | 74.2 | 70 | 14.5 | 10 |
The estimated prevalence of HBsAg in the 28 selected studies in LAC ranged from 2.0% (95% CI 1.0–5.0%) to 15.0% (95% CI 9.0–24.0%); the pooled prevalence was 7.0% (95% CI 7.0–7.0%). The heterogeneity found was substantial among the estimates (I2 = 88.4%, p = 0.00) (Fig. 2).
Fig. 2.
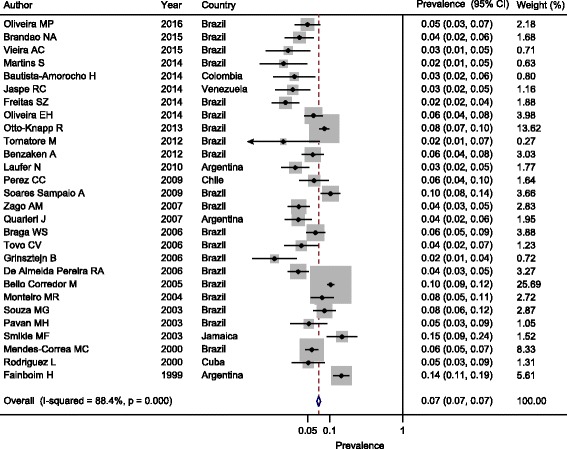
Estimated pooled prevalence of HBsAg in the LAC region
A possible source of heterogeneity may have been the period in which the selected studies were published (from 1999 to 2006 or 2007–2016) (meta-regression coefficient: −0.410846; p = 0.033) (Table 2). None of the other factors investigated was significantly associated with heterogeneity.
Table 2.
Univariate meta-regression analysis of the prevalence of HBV infection among individuals living with HIV/AIDS
| Meta-regression coefficient | 95% CI | P | |
|---|---|---|---|
| Publication year (1999–2006 vs. 2007–2016) | −0.410846 | −0.789861 to −0.032406 | 0.033 |
| Country (Brazil vs. other) | −0.3252341 | −0.7287418 to 0.0782735 | 0.114 |
| Data collection (cross-sectional vs. other) | −0.3525707 | −0.7476732 to 0.0425319 | 0.080 |
| Sample size | −0.0002151 | −0.0000918 to 0.000522 | 0.170 |
| Quality score | −0.0212113 | −0.1197399 to 0.0773112 | 0.673 |
Figure 3 shows that the estimated pooled prevalence of HBsAg was 8.0% (95% CI 8.0–9.0%) in the 12 studies published from 1999 to 2006 and 6.0% (95% CI 5.0–6.0%) in the remaining selected studies published from 2007 to 2016 (Fig. 4). Considering the second study period (2007 to 2016), the estimated prevalence of HBsAg for males in seven selected studies ranged from 3.0% (95% CI 1.0–8.0%) to 10.0% (95% CI 8–11%); the pooled prevalence was 8.0% (95% CI 7.0–9.0%) (Additional file 4). For females (8 studies), the estimated prevalence of HBsAg ranged from 1.0% (95% CI 0.0–5.0%) to 4.0% (95% CI 3.0–8.0%); the pooled prevalence was 2.0% (95% CI 2.0–3.0%) (Additional file 5). For the participants aged ≥40 years (3 studies), the pooled prevalence was 5.0% (95% CI 4.0–6.0%) (Additional file 6), whereas in participants younger than 40, the pooled prevalence was 3.0% (95% CI 2.0–4.0%) (Additional file 7).
Fig. 3.
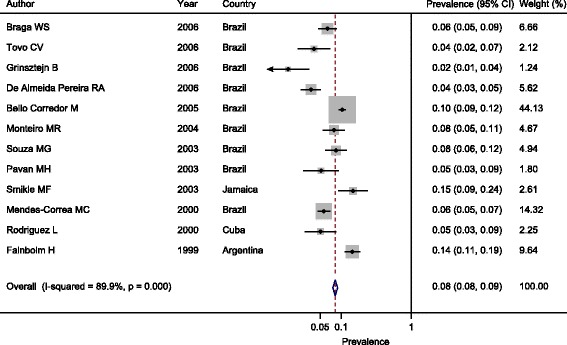
Estimated pooled prevalence of HBsAg in the 12 studies published during the period from 1999 to 2006
Fig. 4.
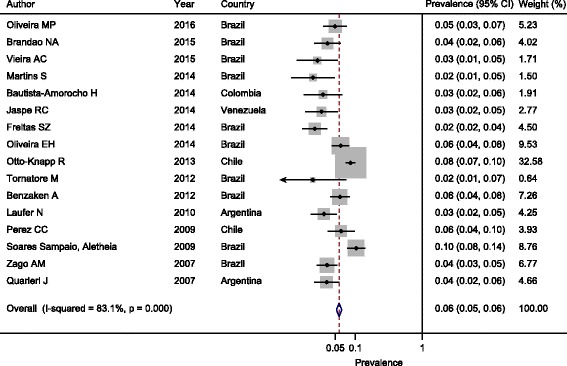
Estimated pooled prevalence of HBsAg in the 16 studies published during the period from 1999 to 2006
During the period from 1999 to 2006, 3 articles were published that analyzed the factors associated with HBV exposure. The factors most frequently associated with HBV infection were increasing age and homosexuality. During the later period (2007 to 2016), six studies performed the same analysis. The factors most frequently associated with HBV were the male sex, increasing age, intravenous drug use, and a history of sexually transmitted diseases.
Discussion
HBV infection is one of the leading causes of morbidity and mortality among people living with HIV/AIDS and other populations with immune deficiencies. In our review of the results of the available studies, we attempted to present a comprehensive overview of the literature on the subject and gain new insights into the distribution of HBsAg among PLWHA.
We systematically reviewed the studies conducted in LAC and found 28 studies from 7 countries (n = 18,457). Our review indicated that the pooled prevalence of hepatitis B in PLWHA in LAC was approximately 7.0%. In studies published during the last 10 years, the estimated pooled prevalence of HBsAg among PLWHA was 6.0%. During the latter period, the prevalence of HBsAg in male participants and participants aged ≥40 years was higher than the prevalence in women and in participants younger than 40 years of age.
We did not find any review study on the prevalence of hepatitis B among PLWHA in LAC. In areas of low endemicity, such as the United States and Europe, HBV and HIV are usually acquired in adulthood by sexual and percutaneous transmission [17]. In these regions, the prevalence of HBV coinfection among individuals with HIV varies from 5% to 10% depending on the route of infection. Konopnicki et al. [2] reported data on the prevalence of HBsAg in the EuroSIDA study. This prospective observational cohort study evaluated patients with HIV-1 from 72 centers located in Europe and, more recently, in Argentina and Israel. The authors found that 498 of 5728 patients (8.7%) were positive for HBsAg. The highest prevalence rates for HBsAg were found in Argentina (17.8%), northern and central Europe (9.1%), southern Europe (8.9%), and eastern Europe (5.9%). This result suggests that the prevalence of chronic hepatitis B in this group is 100-fold higher than the prevalence in the general population [2]. A study of patients with HIV infection from 11 geographic regions in the United States [11] indicated that the HBsAg prevalence was 7.6%, and it was higher in male homosexuals than in heterosexuals; moreover, the prevalence of HBV was 2.3% in participants who used antiretroviral drugs containing lamivudine and 7.8% among participants who did not use lamivudine. In countries with intermediate and high endemicity, the main transmission routes were perinatal and during childhood. Generally, HBV infection precedes HIV infection by decades [17]. In the endemic area of Sub-Saharan Africa, HBsAg is found in up to 36% of the HIV-infected population [18]. The authors of one systematic review investigated the prevalence of hepatitis B and C in Sub-Saharan Africa and found rates ranging from 1.1% to 35.7%. The highest rates were found in western African regions (Nigeria, Ghana, Ivory Coast, Gambia, and Burkina Faso). In a study conducted in patients from the TREAT Asia HIV Observational Database (TAHOD), which involved a multicenter cohort of patients with HIV in the Asia-Pacific region, the authors reported that 10.4% (591/5656) of the participants were HBsAg-positive [19]. A study conducted in China evaluated the prevalence of hepatitis B in 1958 HIV-infected patients [20]. Of the 1958 patients, 186 (9.5%) were HBsAg-positive. The rates varied widely depending on the studied region of the country; the participants from the eastern region had the highest prevalence (14.5%), whereas those from the central region had the lowest prevalence (5.0%).
Both tropical and central Latin America exhibited a significant decrease in the prevalence of HBsAg between 1990 and 2005 [21]. Tropical Latin America (Brazil and Paraguay) was reclassified from an intermediate endemicity region to a low endemicity region. Similarly, the prevalence in central Latin America (Colombia, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, and Venezuela) decreased during this period, and a shift to low endemicity was observed for most age groups in 2005. In other regions, such as Andean Latin America (Bolivia, Ecuador, and Peru) and South Latin America (Argentina, Chile, and Uruguay), the endemicity levels remained intermediate even though the prevalence decreased with age.
The heterogeneity of the data on the prevalence of HBsAg in PLWHA also significantly limited the interpretation of the results. However, the analysis shown in Table 3 (prevalence of HBsAg in the general population in selected countries based on data by Schweitzer et al. [22]) indicated that the actual prevalence of HBsAg in PLWHA was highly likely to be greater than the prevalence in the general population. The reported prevalence in studies published during the last ten years (Fig. 4) suggests that the prevalence of HBsAg among PLWHA is on average 6.7 times (range: 1.5 to 15.9) higher than that in the general population. Additionally, the overall prevalence of HBsAg in Brazil, Colombia, and Venezuela decreased when comparing the data from 1990 and 2005 [21]. The countries shifted from an intermediate endemicity region (2–8%) to a low endemicity region (<2%), likely due to the implementation of vaccination against hepatitis B in children younger than 1 year of age in 1998. However, the prevalence of HBsAg in PLWHA appeared to remain between 2% and 8%.
Table 3.
Prevalence of HBsAg in the general population in the selected countries according to Schweitzer et al. [22]
| Prevalence of HBsAg | ||
|---|---|---|
| Country | % | 95% CI |
| Argentina | 0.77% | 0.77–0.78 |
| Brazil | 0.65% | 0.65–0.66 |
| Chile | 0.68% | 0.34–1.35 |
| Colombia | 2.29% | 1.86–2.82 |
| Cuba | 1.30% | 0.62–2.70 |
| Jamaica | 3.76% | 2.65–5.29 |
| Venezuela | 0.48% | 0.44–0.52 |
The available data from the last 10 years showed that male individuals aged ≥40 years were the most affected by HBV infection. Age is a known risk factor associated with HBV, likely due to increasing exposure over time. We observed that the male sex was also a risk factor, likely due to the more frequent exposure to risk factors involved in HBV transmission, especially sexual contact.
The results of this review indicate that the prevention of HBV infection in PLWHA and HIV infection in the population with chronic hepatitis B has not been effective in LAC. Of the known strategies to prevent HBV infection, the most efficient is universal vaccination. In addition to immunization against hepatitis B, active recognition of chronic hepatitis B carriers is necessary. This measure is necessary to allow this population to receive specific treatment and to prevent the progression to well-known complications, which are more severe and rapidly progressive in patients with immunodeficiency, and the spread of infection, which is transmitted primarily via the sexual and vertical routes.
This study has limitations. Studies on the prevalence of HBsAg are unavailable in various regions. Population-based studies are rare in all LAC regions, including areas in which the prevalence of HBsAg in the general population is at the intermediate level of endemicity, such as Belize (4.71%), the Dominican Republic (4.09%), Jamaica (3.76%), and Suriname (3.9%), and those with high levels of endemicity, such as Haiti (13.55%) [22]. Thus, more studies are necessary to elucidate the burden of coinfection with HIV and HBV in LAC. However, our results indicate the need to increase the investment in preventive measures against hepatitis B, particularly when the impact of adequate vaccination in this population is considered.
Conclusions
The estimated pooled prevalence of HBsAg among PLWHA in the selected studies was 7.0% (95% CI 7.0–7.0%), and the prevalence was 6.0% in the studies published within the last 10 years. The heterogeneity found was substantial among the estimates (I2 = 88.4%, p = 0.00). A possible source of heterogeneity was the period in which the selected studies were published (from 1999 to 2006 or 2007–2016) (meta-regression coefficient: −0.410846; p = 0.033).
Additional files
Prisma 2009 Checklist. (PDF 85 kb)
Instrument for the assessment of the quality of the studies. Describes the items considered for the assessment of the quality of the studies. (PDF 64 kb)
Assessment of the quality of the studies. Contains data corresponding to the quality of the studies that describe their (quality) total score relative to the following items: sampling process, procedures used for data collection, and data analysis and description. (PDF 68 kb)
Estimated pooled prevalence of HBsAg in males during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 276 kb)
Estimated pooled prevalence of HBsAg in women during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 362 kb)
Estimated pooled prevalence of HBsAg in individuals aged 40 years and over during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 309 kb)
Estimated pooled prevalence of HBsAg in individuals under 40 years of age during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 259 kb)
Acknowledgements
Not applicable.
Funding
The authors declare that they did not receive any funding for this research.
Availability of data and materials
All data analyzed during this study are included in this published article (and its supplementary information files) and are available from the included studies, which are fully referenced.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- HBV
Hepatitis B virus
- HIV
Human immunodeficiency virus
- LAC
Latin America and the Caribbean
- PLHA
People living with HIV/acquired immune deficiency syndrome
Authors’ contributions
FMT was responsible for the study design, data analysis, interpretation, and drafting of the manuscript. EA and MN performed the searches in the databases and selected articles based on their titles and abstracts, and MN assessed the quality of the studies. WMB and AAB selected the studies included in the review, extracted data from the selected articles based on a full-text analysis, contributed to the data analysis and interpretation and were responsible for the critical revision of the manuscript content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
No ethical approval was sought, because approval was deemed unnecessary for this systematic review.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-017-2695-z) contains supplementary material, which is available to authorized users.
Contributor Information
Fatima Mitiko Tengan, Email: fatima.tengan@uol.com.br.
Edson Abdala, Email: eabdala@uol.com.br.
Marisa Nascimento, Email: marisa.nascimento@hc.fm.usp.br.
Wanderley Marques Bernardo, Email: wmbernardo@usp.br.
Antonio Alci Barone, Email: aabarone@uol.com.br.
References
- 1.Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1–21. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 3.Bodsworth NJ, Cooper DA, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis. 1991;163:1138–1140. doi: 10.1093/infdis/163.5.1138. [DOI] [PubMed] [Google Scholar]
- 4.Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol. 2006;44:S65–S70. doi: 10.1016/j.jhep.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138–S145. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 6.Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–1310. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 7.Di Martino V, Thevenot T, Colin JF, Boyer N, Martinot M, Degos F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology. 2002;123:1812–1822. doi: 10.1053/gast.2002.37061. [DOI] [PubMed] [Google Scholar]
- 8.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the multicenter cohort study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/S0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 9.Eskild A, Magnus P, Petersen G, Sohlberg C, Jensen F, Kittelsen P, et al. Hepatitis B antibodies in HIV-infected homosexual men are associated with more rapid progression to AIDS. AIDS. 1992;6:571–574. doi: 10.1097/00002030-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Chun HM, Roediger MP, Hullsiek KH, Thio CL, Agan BK, Bradley WP, et al. Hepatitis B virus coinfection negatively impacts HIV outcomes in HIV seroconverters. J Infect Dis. 2012;205:185–193. doi: 10.1093/infdis/jir720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571–577. doi: 10.1086/377135. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health. 1998;1:37–39. doi: 10.1136/ebmh.1.2.37. [DOI] [Google Scholar]
- 14.Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ. 1991;302:1136–1140. doi: 10.1136/bmj.302.6785.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–176. [PubMed] [Google Scholar]
- 16.Prins J, Blanker MH, Bohnen AM, Thomas S, Bosch JL. Prevalence of erectile dysfunction: a systematic review of population-based studies. Int J Impot Res. 2002;14:422–432. doi: 10.1038/sj.ijir.3900905. [DOI] [PubMed] [Google Scholar]
- 17.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Matthews PC, Geretti AM, Goulder PJ, Klenerman P. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in sub-Saharan Africa. J Clin Virol. 2014;61:20–33. doi: 10.1016/j.jcv.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Wong WW, Law MG, Kiertiburanakul S, Yunihastuti E, Merati TP, et al. Hepatitis B and C co-infection in HIV patients from the TREAT Asia HIV observational database: analysis of risk factors and survival. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Han Y, Qiu Z, Li Y, Li Y, Song X, et al. Prevalence of hepatitis B and C viruses in HIV-positive patients in China: a cross-sectional study. J Int AIDS Soc. 2016;19:20659. doi: 10.7448/IAS.19.1.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira MP, Lemes PS, Matos MA, Del-Rios NH, Carneiro MA, Silva ÁM, et al. Overt and occult hepatitis B virus infection among treatment-naive HIV-infected patients in Brazil. J Med Virol. 2016;88:1222–1229. doi: 10.1002/jmv.24462. [DOI] [PubMed] [Google Scholar]
- 24.Brandão NA, Pfrimer IA, Martelli CM, Turchi MD. Prevalence of hepatitis B and C infection and associated factors in people living with HIV in Midwestern Brazil. Braz J Infect Dis. 2015;19:426–430. doi: 10.1016/j.bjid.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira AC, Tizzot MRPA, Santos VLP, Bovo F, Reason IM. Epidemiological analysis of serological markers of hepatitis B in HIV+ patients from Curitiba and metropolitan region. J Bras Patol Med Lab. 2015;51:17–21. [Google Scholar]
- 26.Martins S, Livramento Ad, Andrigueti M, Kretzer IF, Machado MJ, Spada C, et al. The prevalence of hepatitis B virus infection markers and socio-demographic risk factors in HIV-infected patients in Southern Brazil. Rev Soc Bras Med Trop. 2014;47:552–558 doi:10.1590/0037-8682-0109-2014. [DOI] [PubMed]
- 27.Bautista-Amorocho H, Castellanos-Domínguez YZ, Rodríguez-Villamizar LA, Velandia-Cruz SA, Becerra-Peña JA, Farfán-García AE. Epidemiology, risk factors and genotypes of HBV in HIV-infected patients in the northeast region of Colombia: high prevalence of occult hepatitis B and F3 subgenotype dominance. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaspe RC, Sulbarán YF, Loureiro CL, Martínez N, Devesa M, Rodríguez Y, et al. Genetic diversity of hepatitis B virus and hepatitis C virus in human immunodeficiency virus type 1-co-infected patients from Venezuela. J Med Microbiol. 2014;63:1099–1104. doi: 10.1099/jmm.0.067496-0. [DOI] [PubMed] [Google Scholar]
- 29.Freitas SZ, Soares CC, Tanaka TS, Lindenberg AS, Teles SA, Torres MS, et al. Prevalence, risk factors and genotypes of hepatitis B infection among HIV-infected patients in the state of MS, central Brazil. Braz J Infect Dis. 2014;18:473–480. doi: 10.1016/j.bjid.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira EH, Lima Verde RM, Pinheiro LM, Benchimol MG, Aragão AL, Lemos JA, et al. HBV infection in HIV-infected subjects in the state of Piaui, Northeast Brazil. Arch Virol. 2014;159:1193–1197. doi: 10.1007/s00705-013-1921-2. [DOI] [PubMed] [Google Scholar]
- 31.Otto-Knapp R, Cortes CP, Saavedra F, Wolff M, Weitzel T. Hepatitis B prevalence and influence on HIV treatment outcome and mortality in the Chilean AIDS cohort. Int J Infect Dis. 2013;17:e919–e924. doi: 10.1016/j.ijid.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Tornatore M, Gonçalves CV, Bianchi MS, Germano FN, Garcés AX, Soares MA, et al. Co-infections associated with human immunodeficiency virus type 1 in pregnant women from southern Brazil: high rate of intraepithelial cervical lesions. Mem Inst Oswaldo Cruz. 2012;107:205–210. doi: 10.1590/S0074-02762012000200009. [DOI] [PubMed] [Google Scholar]
- 33.Benzaken A, Sabidó M, Galban E, Rodrigues Dutra DL, Leturiondo AL, Mayaud P. HIV and sexually transmitted infections at the borderlands: situational analysis of sexual health in the Brazilian Amazon. Sex Transm Infect. 2012;88:294–300. doi: 10.1136/sextrans-2011-050309. [DOI] [PubMed] [Google Scholar]
- 34.Laufer N, Quarleri J, Bouzas MB, Juncos G, Cabrini M, Moretti F, et al. Hepatitis B virus, hepatitis C virus and HIV coinfection among people living with HIV/AIDS in Buenos Aires, Argentina. Sex Transm Dis. 2010;37:342–343. doi: 10.1097/OLQ.0b013e3181d73c0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez CC, Cerón AI, Fuentes LG, Zañartu SC, Balcells MME, Ajenjo HC, et al. Hepatitis B, C, Treponema pallidum and toxoplasma gondii co-infections in HIV infected patients. Rev Med Chil. 2009;137:641–648. [PubMed] [Google Scholar]
- 36.Sampaio AS, de Alencar LCA, Moura P, Correia J, Barreto S, Castelo A. Prevalencia de la co-infección con hepatitis B y C em pacientes HIV positivos y factores de riesgo associados. Actualizadiones en SIDA 2009;17:12–17.
- 37.Zago AM, Machado TF, Cazarim FL, Miranda AE. Prevalence and risk factors for chronic hepatitis B in HIV patients attended at a sexually-transmitted disease clinic in Vitoria, Brazil. Braz J Infect Dis. 2007;11:475–478. doi: 10.1590/S1413-86702007000500007. [DOI] [PubMed] [Google Scholar]
- 38.Quarleri J, Moretti F, Bouzas MB, Laufer N, Carrillo MG, Giuliano SF, et al. Hepatitis B virus genotype distribution and its lamivudine-resistant mutants in HIV-coinfected patients with chronic and occult hepatitis B. AIDS Res Hum Retrovir. 2007;23:525–531. doi: 10.1089/aid.2006.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braga WS, da Costa Castilho MDC, dos Santos IC, Moura MA, Segurado AC. Low prevalence of hepatitis B virus, hepatitis D virus and hepatitis C virus among patients with human immunodeficiency virus or acquired immunodeficiency syndrome in the Brazilian Amazon Basin. Rev Soc Bras Med Trop. 2006;39:519–522. doi: 10.1590/S0037-86822006000600001. [DOI] [PubMed] [Google Scholar]
- 40.Tovo CV, Dos Santos DE, de Mattos AZ, de Almeida PR, de Mattos AA, Santos BR. Ambulatorial prevalence of hepatitis B and C markers in patients with human immunodeficiency virus infection in a general hospital. Arq Gastroenterol. 2006;43:73–76. doi: 10.1590/S0004-28032006000200002. [DOI] [PubMed] [Google Scholar]
- 41.Grinsztejn B, Bastos FI, Veloso VG, Friedman RK, Pilotto JH, Schechter M, et al. Assessing sexually transmitted infections in a cohort of women living with HIV/AIDS, in Rio de Janeiro, Brazil. Int J STD AIDS. 2006;17:473–478. doi: 10.1258/095646206777689071. [DOI] [PubMed] [Google Scholar]
- 42.de Almeida Pereira RADA, Mussi AD, de Azevedo e Silva VC, Souto FJ. Hepatitis B virus infection in HIV-positive population in Brazil: results of a survey in the state of Mato Grosso and a comparative analysis with other regions of Brazil. BMC Infect Dis. 2006;6:34 doi:10.1186/1471-2334-6-34. [DOI] [PMC free article] [PubMed]
- 43.Bello Corredor M, Rodríguez Lay Lde L, Gutiérrez Moreno A, Sariego Frómeta S, Montalvo Villalba MC, Sánchez Sol A. [Detection of hepatitis B and hepatitis C markers in HIV positive patients, 2000–2004]. Rev Cuba Med Trop 2005;57:212–213. [PubMed]
- 44.Monteiro MR, do Nascimento MM, Passos AD, Figueiredo JF. [Soroepidemiological survey of hepatitis B virus among HIV/AIDS patients in Belém, Pará--Brasil]. Rev Soc Bras Med Trop 2004;37:27–32 doi:10.1590/S0037-86822004000700004. [DOI] [PubMed]
- 45.Souza MG, Passos AD, Machado AA, Figueiredo JF, Esmeraldino LE. HIV and hepatitis B virus co-infection: prevalence and risk factors. Rev Soc Bras Med Trop. 2004;37:391–395. doi: 10.1590/S0037-86822004000500004. [DOI] [PubMed] [Google Scholar]
- 46.Pavan MHP, Aoki FH, Monteiro DT, Gonçales NSL, Escanhoela CA, Gonçales Júnior FL. Viral hepatitis in patients infected with human immunodeficiency virus. Braz J Infect Dis. 2003;7:253–261. doi: 10.1590/S1413-86702003000400005. [DOI] [PubMed] [Google Scholar]
- 47.Smikle MF, Heslop O, Vickers I, Dowe G, Deer D, Sue-Ho R, et al. A serosurvey of hepatitis B virus, hepatitis C virus, human T lymphotropic virus type-1 and syphilis in HIV-1-infected patients in Jamaica. West Indian Med J. 2003;52:14–17. [PubMed] [Google Scholar]
- 48.Mendes-Corrêa MC, Barone AA, Cavalheiro N, Tengan FM, Guastini C. Prevalence of hepatitis B and C in the sera of patients with HIV infection in Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2000;42:81–85. doi: 10.1590/S0036-46652000000200004. [DOI] [PubMed] [Google Scholar]
- 49.Rodríguez L, Collado-Mesa F, Aragón U, Díaz B, Rivero J. Hepatitis B virus exposure in human immunodeficiency virus seropositive Cuban patients. Mem Inst Oswaldo Cruz. 2000;95:243–245. doi: 10.1590/S0074-02762000000200019. [DOI] [PubMed] [Google Scholar]
- 50.Fainboim H, González J, Fassio E, Martínez A, Otegui L, Eposto M, et al. Prevalence of hepatitis viruses in an anti-human immunodeficiency virus-positive population from Argentina. A multicentre study. J Viral Hepat. 1999;6:53–57. doi: 10.1046/j.1365-2893.1999.t01-1-6120135.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prisma 2009 Checklist. (PDF 85 kb)
Instrument for the assessment of the quality of the studies. Describes the items considered for the assessment of the quality of the studies. (PDF 64 kb)
Assessment of the quality of the studies. Contains data corresponding to the quality of the studies that describe their (quality) total score relative to the following items: sampling process, procedures used for data collection, and data analysis and description. (PDF 68 kb)
Estimated pooled prevalence of HBsAg in males during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 276 kb)
Estimated pooled prevalence of HBsAg in women during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 362 kb)
Estimated pooled prevalence of HBsAg in individuals aged 40 years and over during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 309 kb)
Estimated pooled prevalence of HBsAg in individuals under 40 years of age during the period from 2007 to 2016 in Latin America and the Caribbean. (PDF 259 kb)
Data Availability Statement
All data analyzed during this study are included in this published article (and its supplementary information files) and are available from the included studies, which are fully referenced.


