Abstract
The liver, the largest organ with multiple synthesis and secretion functions in mammals, consists of hepatocytes and Kupffer, stem, endothelial, stellate and other parenchymal cells. Because of early and extensive contact with the external environment, hepatic ischaemia reperfusion (IR) may result in mitochondrial dysfunction, autophagy and apoptosis of cells and tissues under various pathological conditions. Because the liver requires a high oxygen supply to maintain normal detoxification and synthesis functions, it is extremely susceptible to ischaemia and subsequent reperfusion with blood. Consequently, hepatic IR leads to acute or chronic liver failure and significantly increases the total rate of morbidity and mortality through multiple regulatory mechanisms. An increasing number of studies indicate that mitochondrial structure and function are impaired after hepatic IR, but that the health of liver tissues or liver grafts can be effectively rescued by attenuation of mitochondrial dysfunction. In this review, we mainly focus on the subsequent therapeutic interventions related to the conservation of mitochondrial function involved in mitigating hepatic IR injury and the potential mechanisms of protection. Because mitochondria are abundant in liver tissue, clarification of the regulatory mechanisms between mitochondrial dysfunction and hepatic IR should shed light on clinical therapies for alleviating hepatic IR‐induced injury.
Keywords: hepatic, ischaemia reperfusion, liver transplantation, pre‐condition, mitochondria
Introduction
The liver, the largest organ with multiple synthesis and secretion functions in mammals, consists of hepatocytes and Kupffer, stem, endothelial, stellate and other parenchymal cells. Because of the early and extensive contact with the external environment, hepatic ischaemia reperfusion (IR) may result in mitochondrial dysfunction, autophagy and apoptosis of cells and tissues under various pathological conditions. Because the liver requires a high oxygen supply to maintain normal detoxification and synthesis functions, it is extremely susceptible to ischaemia and subsequent reperfusion with blood 1. Hepatic IR, a frequent clinical outcome of pathological conditions, may result in mitochondrial dysfunction, a wave of parenchymal cell death and an abundance of cytotoxic inflammatory responses 2. Consequently, hepatic IR leads to acute or chronic liver failure and significantly increases the total rate of morbidity and mortality through multiple regulatory mechanisms.
Under different pathological conditions, hepatic IR injury can be classified into different types according to the environmental temperature. Warm IR injury has been observed in low‐flow situations 3, 4; in contrast, cold IR injury has been observed solely in liver transplantation (LT) in which the excised graft was subjected to hypothermic preservation before warm reperfusion 5. In clinical conditions, warm IR occurs more frequently than cold hepatic IR and leads to initial deficiencies and incompetence of liver allografts. Furthermore, most of the animal models that have been used in experimental studies are hepatic partial hepatectomy models that imitate warm hepatic IR to induce hepatocellular damage and apoptosis. Before LT, the liver graft is preserved in cold buffer, and reperfusion induces apoptosis in sinusoidal endothelial cells because of the characteristic lack of organized basal membrane5. LT is the ultimate choice for end‐stage liver disease because it induces hepatic injury and apoptosis of hepatocytes and other cells, and hepatic IR leads to liver graft dysfunction.
Mitochondria play a role as the primary target in an early stage of hepatic IR (Fig. 1), and the increased level of reactive oxygen species (ROS) promotes the release of various inflammatory signals and adversely affects the metabolic processes in liver tissues 6. During hepatic ischaemia, aerobic energy metabolism is shifted to an anaerobic state, accompanied with a decrease in respiratory enzymes and mitochondrial antioxidant enzymes for adenosine triphosphate (ATP) production, but the accumulation of lipid peroxidation and Ca2+ content is initiated after hepatic IR 7. The attenuation of mitochondrial permeability transition (MPT), phospholipid cardiolipin, the respiratory control index, ADP/O, and state 3 respiration have also been observed during the pathological changes 8, 9. Although the synthesis of pH‐dependent enzymes is activated and the pH values are restored to primary levels after reperfusion, the impairments continue to extensively and aggressively damage the liver 10. Additionally, inflammatory cells and various cytokines are released as a defence against the adverse effects 11, and the activities of nicotinamide adenine dinucleotide NAD (NADH) cytochrome c reductase and succinate cytochrome c reductase are not consistently decreased after reperfusion. Consequently, the severity of hepatic IR injury cannot be predicted by solely the expression levels of mitochondrial respiratory enzymes 12. Moreover, the protein expression levels of 234 genes have been found to be significantly altered after hepatic IR, according to a proteomic analysis of liver mitochondria 13. Although all these changes contribute to the microcirculation failure and cellular destruction during hepatic IR, it has been demonstrated that the initial injury can be inhibited by the attenuation of mitochondrial dysfunction 14. Heme oxygenase‐1 (HO‐1) and heat shock protein (HSP)‐70 are upregulated to conserve the cytoskeleton integrity and protect against hepatic IR‐induced injury 15. The expression of HO‐1 is regulated through the inhibition of tumour necrosis factor (TNF)/TNF receptor 1, specifically by the modulation of the death‐inducing signalling complex formation and mitochondrial TNF receptor 1 translocation during hepatic IR 16. Additionally, the p38‐mitogen‐activated protein kinase (MAPK) as well as the extracellular signal‐regulated kinases 1/2 (ERK 1/2) signalling pathways eliminate hepatic IR injury 17.
Figure 1.
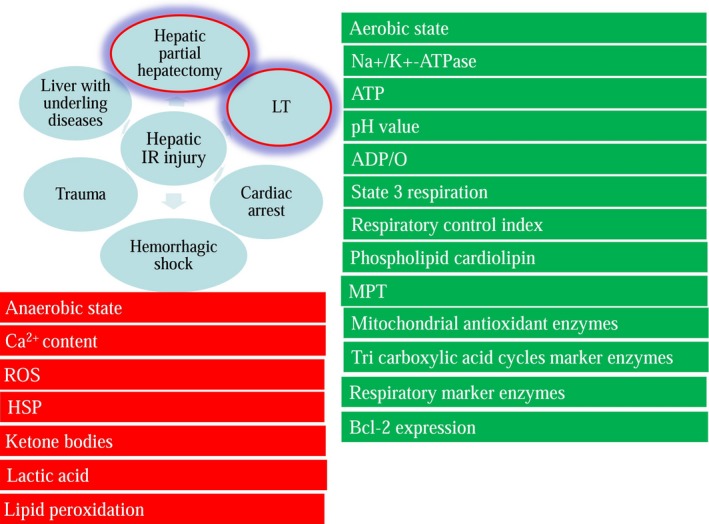
Hepatic IR can be classified into warm IR and LT‐induced IR; the parameters in the red boxes are upregulated, and the parameters in green boxes are down‐regulated during the hepatic ischaemic process.
Hepatic IR injury induces apoptosis and necrosis of cells and leads to irreversible damage if this process is not intervened timely. Apoptosis is an orderly form of cell death for clearance of unneeded cells to keep natural homeostasis during development, growth or ageing; in contrast, necrosis is marked by rapid loss of plasma membrane integrity and stimulated inflammation 18. In general, apoptosis rate can be determined by specific markers including Bax, caspase, PARP‐1,TNF, TUNEL, etc 19, 20, 21, but it now appears that these markers will not specifically distinguish between apoptosis and necrosis 22. In hepatic IR model, hepatic necrosis rate is closely correlated with alanine aminotransferase (ALT) levels, and this phenomenon is more apparent after 24 hr of reperfusion 23. In addition, hepatic IR model shows reduced expression levels of mitochondrial enzymes accompanied by increasing number of necrotic and apoptotic cells 24. However, apoptosis was also termed as the early mechanism while necrosis was termed as the principal mechanism of cell death in hepatic IR models 25, 26. In view of this controversy, necroapoptosis was proposed to emphasize both necrosis and apoptosis exist in hepatic IR models 27. Although necroptosis is determined as programmed necrosis via receptor‐interacting protein 1 pathway, it is not always present in the post‐ischaemic liver but correlated to caspase activation 28. To eliminate adverse outcomes, an increasing amount of pre‐treatments (Fig. 2) including ischaemic pre‐conditioning (IPC), pre‐treatments involving exposure to non‐physiological oxygen levels, pharmaceutical pre‐conditioning, and gene targetting approaches that can significantly reduce injury after hepatic IR are under investigation. In this review, we mainly focus on the subsequent therapeutic interventions related to the conservation of mitochondrial function involved in mitigating hepatic IR injury and the potential mechanisms for the protection. Because mitochondria are abundant in liver tissue, elucidation of the regulatory mechanisms between mitochondrial dysfunction and hepatic IR‐induced injury should shed light on clinical therapies for alleviating hepatic injury during various pathological injuries.
Figure 2.
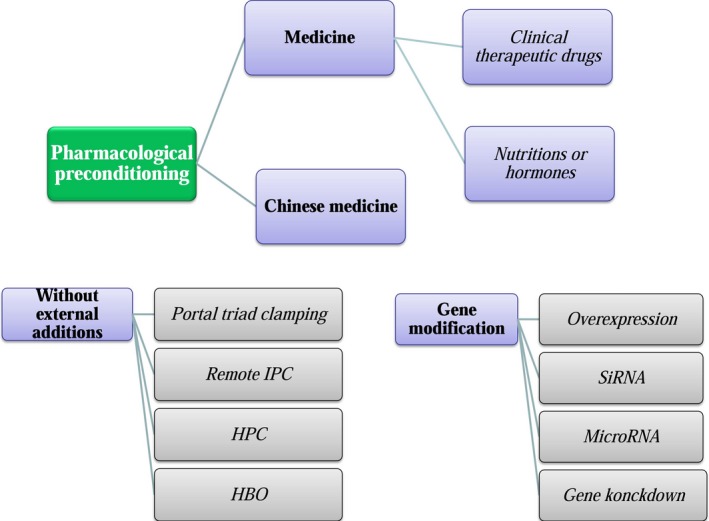
Pre‐conditions are categorized into three categories, and pharmacological pre‐conditions that have been investigated in recent years are marked in green.
IPC for attenuating hepatic IR injury
Multiple clinical and animal studies have demonstrated that IPC improves the outcomes of hepatic IR by influencing the survival rates of hepatocytes and non‐parenchymal cells 29. IPC is executed as a short period of portal triad clamping and a subsequent longer period of reperfusion, and then, the tissues or organs are incubated during a relatively long period of ischaemia 30. During the brief ischaemic exposure before hepatic IR, an enzyme with antioxidant properties, namely HO‐1, is initially activated and consequently maintains normal function 31. After clamping of the hepatic blood vessels, there is a mild increase in peroxides, which stimulates cellular adaptation 32 and mitochondrial preservation 33. This adaptive prerequisite condition notably conserves hepatic ATP synthase activity, ATP level and tolerance to MPT 34. In multiple clinical tests, it has concluded that IPC results in a decreased hospital length of stay and decreased transfusion rates 35. Although IPC seems to have a protective effect against hepatic IR injury, it may exert noxious effects on small liver remnants 36. Recently, Ye et al. 37 have demonstrated that IPC exerts no apparent effects on decreasing the level of serum transaminases and rates of morbidity and mortality. Because there is inevitable heterogeneity among patients, IPC varies with ischaemia time and respiration time, and an IPC protocol has various influences on hepatic IR. Additionally, compared with topical hypothermia, IPC cannot decrease inflammatory cytokines, inducible nitric oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate (NADPH)‐quinone oxidoreductase‐1 as efficiently as topical hypothermia, and the combination of the two pre‐treatments confers no additional advantage 38.
Because IPC through portal triad clamping is complicated to execute, remote IPC through other parts of the body can be easily controlled. Lai et al. 39 have demonstrated that remote IPC on the limbs results in favourable changes in mitochondrial oxygenation, serum bicarbonate and hepatic venous nitric oxide (NO), NO protects against MPT‐dependent apoptosis through the cGMP‐dependent kinase signalling pathway 40. One study has executed IPC in the right hind limbs of rats with six cycles of pre‐condition clamping and reperfusion and has found that the attenuated liver necrosis is mediated by the up‐regulation of interleukin(IL)‐6 41. In addition to remote IPC on limbs, intestinal IPC has also eliminated hepatic IR‐induced injury by decreasing inflammatory cytokines and enhancing Bcl‐2 expression 42. However, investigators also found that there was no additional protective effect of IPC in patients undergoing liver resection under continuous or intermittent vascular occlusion 43. Consequently, the detailed protocols for clinical usage of IPC should be adjusted according to the detailed conditions.
Pre‐treatments with un‐physiological oxygen content before hepatic IR
Other techniques in addition to clamping have been used to obtain an ischaemic environment; hypoxic pre‐conditioning (HPC) has been executed by exposing the liver to an altitude chamber for several days, and the release of protective protein, namely HO‐1, is significantly induced with a diminished elevation of serum ALT levels after hepatic IR 39. Hypoxic pre‐conditioning on isolated liver grafts also up‐regulates Bcl‐2 expression and significantly decreases the apoptosis rate after hepatic IR 44. One study has found that mice receiving breathing oxygen at a low concentration before hepatic IR, compared with control mice, released lower levels of apoptotic cytokines and myeloperoxidase 45.
Hyperbaric oxygen (HBO) therapy at several absolute atmospheres can improve the prognosis of severe diseases including acute brain injury, and acute lung injury. 46, 47. The protective effects of liver injury in HBO pre‐conditioning remain controversial because this pre‐treatment does not increase hepatocellular energy metabolism or repair mitochondrial oedema after hepatic IR 48, 49. Intriguingly, Losada et al. 50 have reported that HBO pre‐conditioning protects the liver from mitochondrial dysfunction and decreases the levels of hepatic injury markers during the ischaemia and reperfusion processes. Although HBO pre‐conditioning has been proven to effectively eliminate ALT and to improve the expression of HO‐1, it cannot decrease the levels of aspartate aminotransferase (AST) and malondialdehyde (MDA) 51, 52. Notably, the time of HBO exposure is also critical for determining whether the effects in hepatic IR models are beneficial or deleterious 53. Yu et al. 54 have shown that pre‐conditioning by one‐dose HBO but not three‐dose HBO effectively inhibits subsequent hepatic IR injury in rats. The pre‐condition of oxygen concentration should be further compared to optimize the protection of liver mitochondrial function without additional pharmacokinetics.
Pharmacological pre‐conditioning before hepatic IR
Various experiments have demonstrated that pharmacological pre‐conditioning may protect liver tissue against IR injury and maintain the mitochondrial function or may alter the signalling pathways attenuating the functional impairments. Pharmacological treatments for preventing hepatic IR injury typically have damaging side‐effects in patients, and it is crucial to develop new drugs that are safe and usable for clinical tests or applications. Moreover, because of the lack of adequate clinical trials, pharmacological pre‐conditioning for reducing hepatic IR‐induced injury remains a controversial issue.
Clinical therapeutic drugs
Many therapeutic drugs have been tested and demonstrated to have protective effects in mitochondrial function after hepatic IR injury (Table 1).
Table 1.
Clinical therapeutic drugs and their potential mechanisms for hepatic warm IR
| Piperazine | Potential mechanisms | Model | References |
|---|---|---|---|
| Cyclosporine | Preventing MPT and decreasing cytochrome c release | Mouse | 58 |
| Minocycline/ doxycycline | Inhibiting mitochondrial Ca2+ uptake and eliminating the Ca2+‐induced MPT | In vitro rat hepatocytes | 55 |
| Carbamazepine | Preventing calcium overload and calpain activation | Mouse | 56 |
| 17β‐estradiol | Decreasing the apoptosis rate of hepatocytes by up‐regulating the ratio of Bcl‐2/Bax, decreasing cytochrome c release, and decreasing activities of caspase‐related genes, consequently improving the 7‐day survival rate | Rat | 85 |
| Gadolinium chloride | Inhibiting the release of serum aminotransferases and TNF‐α, decreasing mitochondrial MDA and suppressing the release of caspase‐3 | Rat | 62 |
| Thrombomodulin | Protecting against hepatectomy‐induced macrophage/monocyte infiltration and improving the proliferation rate of hepatocytes | Rat | 65 |
| Amlodipine | Prohibiting the uptake of mitochondrial Ca2+ and inhibiting the Ca2+‐induced MPT | Rat | 74 |
| Edaravone | Suppressing the IR‐induced disorganization of mitochondrial structures | Rat | 138 |
| Levosimendan | Enhancing the hepatic microcirculation and decreasing histological damage, serum aminotransferase level, DNA damage and liver redox homeostasis | Rat | 75 76 |
| Diazoxide | Decreasing liver mitochondrial dysfunction, but the MDA content and MPO activity were not affected | Rat | 77 |
| Vinpocetine | Inhibiting the release of IL‐1β and IL‐6 while enhancing the expression of GSH | Rat | 68 |
| CV159 | Decreasing the release of HMGB‐1 and iNOS but elevating the level of eNOS | Rat | 59 |
| Eritoran | Preventing inflammatory cellular responses by inhibiting HMGB1‐mediated inflammatory signalling | Mouse | 60 |
| Remifentanil | Improving MMP and inhibiting mitochondrial swelling and synthesis of superoxide dismutase, simultaneously decreasing high levels of IR‐induced TNF‐α and NF‐κB‐p65 | Rat | 70 |
| Propofol | Preserving the respiratory activity and normal energy metabolism, thus limiting free radical production and PTP opening promoting the phosphorylation of mitochondrial GSK‐3β at Ser9, and consequently restraining the opening of MPT and MMP collapse | Rat | 71 72 |
| Flurbiprofen | Preserving respiratory activity and normal energy metabolism, thus limiting free radical production and PTP opening, promoting the phosphorylation of mitochondrial GSK‐3β at Ser9, and consequently restraining the opening of MPT and MMP collapse | Mouse | 139 |
Some drugs that directly interfere with mitochondrial metabolism are widely used in current therapies. Pre‐treatment with minocycline and doxycycline decreases the uptake of mitochondrial Ca2+ and eliminates the Ca2+‐mediated MPT 55; additionally, carbamazepine prevents calcium overload and calpain activation, which are induced by IR 56. CF102, as an effective A3 adenosine receptor agonist, promotes the proliferation of hepatocytes and rescues damaged liver function by down‐regulating the nuclear factor kappa B (NF‐κB) signalling pathway 57. N‐methyl‐4‐isoleucine cyclosporine improves liver regeneration after massive hepatectomy by preventing MPT and decreasing cytochrome c release 58. The high‐mobility group box protein B1 (HMGB1) is positively associated with significant damage and metabolic imbalance in the liver, and a calcium channel blocker named CV159, which significantly decreases the release of HMGB‐1 and iNOS but elevates the level of eNOS, can protect against hepatic IR‐induced injury 59. Eritoran, an inhibitor of toll‐like receptor (TLR)‐4, also prevents inflammatory cellular responses by inhibiting HMGB1‐mediated inflammatory signalling 60.
Recent studies have demonstrated that hepatic IR injury is closely related to acute inflammatory responses and the release of Kupffer cells, monocytes and neutrophils. IL‐18 binding protein significantly decreases the IR‐induced liver injury and necroapoptosis of Kupffer cells, whereas blocking IL‐18 inhibited the release of NF‐κB, c‐Jun, myeloperoxidase and IL‐32 and concurrently up‐regulates inflammatory neutrophils and lymphocytes 61. Gadolinium chloride, a drug inhibiting Kupffer cells, inhibits the levels of serum aminotransferases and TNF‐α, decreases mitochondrial MDA and suppresses the release of caspase‐3 after hepatic IR 62. Tripeptide glutathione (GSH), which has substantial antioxidant properties, is present in hepatocytes at high concentrations 63, and GSH detoxifies ROS generated by Kupffer cells with or without peroxidase 64. Thrombomodulin protects against hepatectomy‐induced macrophage/monocyte infiltration and significantly improves the proliferation rate of hepatocytes 65. Although cilostazol up‐regulates the expression of HO‐1, mitochondrial biogenesis and mtDNA content, the protective effects are neutralized by the inhibition of HO‐1 or nuclear factor E2‐related factor 2(Nrf2) 66. To the best of our knowledge, many antineoplastic drugs result in strong inflammatory responses in vivo and inhibit tumour cells from proliferating and progressing, thus leading to multiple organ failure. Interestingly, these drugs have also been found to have antitoxic effects on hepatic IR‐induced injury: infliximab significantly inhibits inferior impairments in vivo models, owing to its selective antioxidant and anti‐TNF‐α effects 67. Vinpocetine inhibits the release of IL‐1β and IL‐6 but enhances the expression of GSH in warm hepatic IR rat models 68. As a strong immunosuppressive agent, FK506 decreases apoptosis in sinusoidal endothelial cells and inhibits the activation of inflammation, consequently maintaining normal microcirculation in hepatic IR animals 69.
Anaesthetics or analgesics are used to eliminate the perception of pain in patients, but these drugs have also been found to decrease hepatic IR injury. Remifentanil pre‐conditioning improves mitochondrial membrane potential (MMP) and inhibits mitochondrial swelling and synthesis of superoxide dismutase while simultaneously decreasing IR‐induced high levels of TNF‐α and NF‐κB‐p65 in liver tissues 70. By inhibiting the expression of hypoxia inducible factor (HIF)‐1α, propofol preserves the respiratory activity and normal energy metabolism, thus limiting free radical production and permeability transition pore (PTP) opening 71. Furthermore, propofol promotes the phosphorylation of mitochondrial glycogen synthase kinase (GSK)‐3β at Ser9, thus consequently restraining the opening of MPT and MMP collapse 72. Sevoflurane pre‐treatment decreases the release of heparan sulphate and syndecan‐1 and attenuates the release of serum aminopherases in a time‐dependent manner, thus decreasing the shedding of endothelial glycocalyx and cell death rate 73.
Intriguingly, an increasing number of antihypertensive drugs, including angiotensin‐converting enzyme inhibitors, beta blockers, diuretics and calcium channel blockers, for eliminating the impairment from hepatic IR injury are under investigation. For example, amlodipine blocks the uptake of mitochondrial Ca2+ and inhibits Ca2+‐induced MPT 74. Pre‐treatment with levosimendan, a calcium sensitizer, significantly enhances hepatic microcirculation and decreases histological damage, the serum aminotransferase level, DNA damage and liver redox homeostasis 75. Additionally, levosimendan suppresses the release of Bax, caspase‐9, AKT and endothelial nitric oxide synthase (eNOS) in hepatic IR models; oxidative damage induced by hepatic IR injury have also been found to be attenuated in a dose‐dependent manner 76. Although diazoxide significantly decreases liver mitochondrial dysfunction after hepatic IR, the MDA content and myeloperoxidase (MPO) activity are not affected in the liver 77. A similar drug, trimetazidine, effectively rescue hepatic IR injury in rat model, but repeated administration of this drug confers more protection by significantly enhancing signalling pathways including phosphorylated adenosine monophosphate‐activated protein kinase (AMPK) and eNOS 78.
Drugs affecting nutrition or hormone levels
In addition to the powerful drugs described above, drugs affecting nutrition or hormone levels can also affect the maintenance of mitochondrial function and hepatocellular structure. Intravenous glycine administration results in the maintenance of cellular energy production and decreases cytokine levels and hepatocellular injury by increasing the fluxion of portal blood and hepatic microcirculation, thus preserving the plasma membrane and cytochrome oxidase activity after warm hepatic IR 79, 80. Additionally, pharmaceuticals including desferal and menadione effectively inhibit mitochondrial calcein quenching and mitochondrial ROS and MPT, thus inhibiting hepatic IR injury 19, 81. Protein kinase A, a specific peptide inhibitor, effectively abolishes cytosol‐induced inhibition of MPT and hepatic IR injury 82. Owing to its high water‐solubility and stability, a novel selenocysteine containing the 7‐mer peptide attenuates hepatic IR‐induced injury by suppressing free radicals and up‐regulating Bcl2/Bax expression 83. Polyethylene glycol‐conjugated cysteine‐modified lysine dendrimers eliminate ROS production and prolong circulation time in hepatic IR injury 84. The basal levels of hormones determine the level of damage resistance in vivo, and it is important to maintain or enhance some hormones in vivo to improve the outcomes in hepatic IR models. 17β‐estradiol has been found to decrease the apoptosis rate in hepatocytes by up‐regulating the ratio of Bcl‐2/Bax, to decrease cytochrome c release, and to decrease the activities of caspase‐related genes, thereby improving the 7‐day survival rate after hepatic IR injury 85. Pre‐treatment with flavonoids, which inhibit the TLR4 pathway but activate the Sirt1/Nrf2 pathway, down‐regulate IL‐1β, IL‐6 and TNF‐α but improve oxidative stress in hepatic IR rats 86. Because of its less side‐effect, this kind of drugs may serve as a novel and useful route to repair hepatic IR‐induced injury.
Chinese medicine
Intriguingly, multiple Chinese medicines have emerged as treatments for hepatic IR injury, although some Chinese medicines have previously been found to be toxic and can lead to hepatic dysfunction. Paeoniflorin attenuates hepatic IR injury by acting as a strong immunosuppressor 87. Trans‐resveratrol negatively alters the production of related cytokines and reverses the TLR4/NF‐κB signalling pathway in liver tissues undergoing hepatic IR 88.
Tetrandrine, a traditional Chinese medicine with calcium channel blocking capacity exerts a protective effect on hepatic IR models by reducing oxidative stress and maintaining superoxide dismutase activity 89. Astaxanthin significantly decreases hepatic production of xanthine oxidase and protein carbonyl after IR, whereas parenchymal cell damage, mitochondrial swelling, and impairment of rough endoplasmic reticulum are reversed to some extent 90. Recently, catechins, which are extracted from green tea, have been found to be protective by maintaining manganese superoxide dismutase (MnSOD) 91. Additionally, sulforaphane exerts antioxidant effects in a rat hepatic IR model by activating the Nrf2 signal, ameliorating oxidative stress, and preserving ATPase activity 92. Cryptotanshinone attenuates serum aminotransferase levels and hepatic IR injury by inhibiting the c‐Jun N‐terminal kinase (JNK) and MAPK signalling pathways 93. Eupatilin, a pharmacologically active flavone derived from the Artemisia species, promotes the accumulation of HSP and Bcl‐2, attenuates the release of iNOS and cleaves caspase‐3 after hepatic IR 94. All these Chinese medicines demonstrate protective effects via multiple signalling pathways including calcium resistance, immunosuppression and interference in energy metabolism.
Genetic modification strategies for warm hepatic IR injury
In addition to external pre‐treatments involving ischaemic environments or pharmacokinetics, recent studies have proposed gene targetting strategies to decrease hepatic IR injury. The loss of Plexin C1 decreases injuries induced by hepatic IR, as evidenced by lower levels of LDH, aspartate and ALT and fewer neutrophils in ischaemic hepatic tissue 95. The inhibition of gene transcription exerts anti‐inflammatory response and anti‐apoptosis effects on these models. For example, genetic ablation of CCL2 decreases the levels of neutrophil recruitment 96, and knockdown of TNF‐α‐induced‐protein 3 inhibits Bax expression and mitochondrial apoptosis 97. Although no differences in the cell death rate have been found in JNK2‐knockout mice, these mice exhibit less ALT release and less necrosis, a higher survival rate and overall better Kaplan‐Meier survival 98. Sphingosine kinase‐2 inhibition decreases the levels of NO synthase, NFκB‐p65 and TNF‐α; thus, mitochondrial depolarization, MPT and neutrophil infiltration are down‐regulated in vivo and in vitro hepatic IR models; intriguingly, the inhibition significantly increases the survival rates of mice after hepatic IR 99.
Most gene targetting investigations have focused on the inhibitory effects of genes on hepatic IR injury, some genes play critical roles in maintaining normal liver functions. Prdx6‐knockout mice exhibit more mitochondrial dysfunction and hepatocellular injury, and the generation of mitochondrial hydrogen peroxide is increased 13. Furthermore, overexpression of Nrf2 decreases the release of inflammatory cytokines and 8‐isoprostanes, thus protecting the liver against hepatic IR injury 100. A plasmid with a receptor activator for NF‐κB‐Fc has been found to exert prominent effects, protecting against hepatocellular apoptosis in hepatic IR mice by inhibiting NF‐κB nuclear translocation, JNK phosphorylation and HIF‐1α expression 101. Since TLR is primarily recognized as a vector in immunoreactions 102, galactose‐conjugated liposome nanoparticle TLR4 siRNA delivery have been found to efficiently attenuate neutrophil and lipid peroxidase accumulation and to suppress IL‐1 and TNF‐α expression as well as hepatic IR injury in a mouse model 103. In the same manner, the single‐stranded and non‐coding small RNA microRNA‐370 targets 3′ un‐translated regions of TGF‐β receptor II and induces hepatic IR injury, and inhibition of microRNA‐370 decreases the levels of serum aminotransferase and pro‐inflammatory cytokines 104.
Pre‐conditions for LT to eliminate cold hepatic IR injury
According to the complex processes in LT including partial or whole liver procurements, conservation in destined buffer, organ rewarming and final transplantation, isolated liver tissues cumulatively acquire injuries because of the gradual exposure to non‐physiological and harmful conditions. Unfortunately, some drugs exert side‐effects on the LT procedure and result in serious damage in liver grafts. For example, colloid hydroxyethyl starch prevents interstitial oedema when added into UW preservation solution but lead to stasis of blood and incomplete wash out of donor organs before transplantation 105, 106. On the other hand, intravenous administration of pan‐caspase inhibitors to the recipient abolished the previously observed protective effects 107. Although most of the administration during LT provided promising results, a multicentre studies are still necessary for confirming these results.
Pre‐conditions for excised liver grafts
In addition to these impairments in LT, the conservation of function in an excised liver graft is more important than that that observed in warm IR, and the optimization of protocols can be extremely useful for improving the survival rates and prognosis.
Before the extraction of liver grafts, IPC partly alleviates operation‐induced liver injury and reduces the IL‐6 level, thereby inhibiting the generation of multiple inflammatory cytokines 108; thus, the survival time of the liver graft is prolonged 109. Additionally, the 90‐min hypoxic environment before LT notably increases the expression level of HIF‐1α and protects against hepatic IR injury by promoting glucose metabolism 110. Addition of ulinastatin and simvastatin inhibits the release of inflammatory cytokines and apoptotic genes in a dose‐dependent manner, thus allowing liver grafts to withstand cold IR 111, 112. Bortezomib efficiently protects liver grafts against cold IR injury even at low doses and is thus considered a powerful protective agent for the maintenance of liver function 113. Even in reduced‐size LT models, bortezomib has been found to decrease oxidative stress, ER stress, mitochondrial dysfunction and hepatic IR injury; the expression levels of some well‐known IR protective proteins including NO synthase, HO‐1 and HSP70 are upregulated, and liver regeneration is accelerated 114. Addition of activated protein C (APC) to the preservation solution exerts cytoprotective effects by decreasing portal pressure, inflammatory cytokine release and hepatocellular apoptosis 115. Furthermore, the combination of Institute Georges Lopez 1 with trimetazidine in a preservation solution eliminates LT‐induced injury by up‐regulating sirtuin 1, AMPK signalling and inhibiting mTOR signalling 116. As mentioned above, some protocols of genetic modification effectively improve abnormal liver function in warm IR models and also sustain hepatocellular function in LT models. Injection of a recombinant adenovirus with Bcl‐2 into the portal vein of a rat donor liver and subsequent refrigeration of the liver graft at 4°C for 4 hr has been found to significantly increase hepatic Bcl‐2 expression and decrease the LDH level 117.
Pre‐treatments for liver recipients
After transplantation with excised liver grafts, portal blood is perfused to initiate hepatic function recovery. Although serum pH values normalize slowly after hepatic IR, the heamodynamics stabilize quickly in LT models 118. Losartan administered to donors and recipients before LT has been found to result in the maintenance of liver function by inhibition of multiple damaging signalling pathways 119. The dichloroacetate diisopropylamine pre‐treatment in liver recipients is associated with a preservation of hepatocellular mitochondria and promotes the recovery of donor liver function after LT by down‐regulating the release of cytochrome c 120. The carbonic anhydrase inhibitor acetazolamide protects against cold hepatic IR injury by decreasing the MAPK signal and up‐regulating eNOS 121. Dexmedetomidine alleviates hepatic IR injury by suppressing intercellular adhesion molecule 1 and improving post‐operative liver function 122. Additionally, Chinese medicines have also been shown to conserve liver function in LT models. For example, Sprague‐Dawley rats that administered astragaloside IV before surgery have higher survival rates as a result of down‐regulation of TNF‐α levels and NF‐κB expression 123.
The JNK pathway is upregulated after LT, and knockout of JNK2 decreases lipid peroxidation, mitochondrial cytochrome c release, mitochondrial depolarization and hepatocellular necrosis 124. Adenoviruses encoding human IL‐10 or beta‐galactosidase significantly prolong the survival time of LT grafts by maintaining hepatocellular integrity and liver function 125. Furthermore, injection of adenoviruses encoding human IL‐10 conserves hepatic integrity by suppressing the NFκB signalling pathway and increasing the expression levels of HO‐1 and Bcl‐2 126. The potential regulated mechanism is attributed to diminished activities of cytochrome c and caspase‐3 and simultaneous up‐regulation of HO‐1 and Bcl‐2 expression 125.
Under the development of operative technology, an increasing number of patients with end‐stage‐liver disease have received LT to prolong the survival time, and most of the grafts for clinical use are steatotic or cadaveric. Additionally, few detailed investigations of the molecular signalling pathways in human LT models exist to allow the study of regulatory mechanisms.
Hepatic IR exerts various effects according to liver health
Although pre‐treatments in vivo and in vitro significantly maintain mitochondrial function and liver function, liver tissue responds to hepatic IR differently and exhibits extremely different reactions of ROS generation according to the health of liver 127. Steatotic and aged liver models are much more sensitive to external stimulations 128, 129. Fatty livers exhibit decreased MMP and delayed repolarization 113, and these models are particularly vulnerable to hepatic IR 130. Additionally, the unhealthy state of steatotic livers increases the level of large lipid droplets and ROS generation but decreases ATP‐dependent energy metabolism 129. There is an abundance of cholesterol and depolarization in the hepatic mitochondria of steatotic livers; thus, the beneficial mitochondrial GSH level is significantly decreased 131.
Mitochondrial function in fatty livers significantly decreases after 5 hrs of cold preservation, because the energy metabolism of steatotic livers is impaired, and the primary dysfunction is increased compared with that in lean livers132. Chu et al. 133 have demonstrated that steatotic livers exhibit significant mitochondrial dysfunction by altering the activity of mitochondrial complex I, and are more susceptible to prolonged cold ischaemia in LT models. Consequently, steatotic livers are excluded for partial liver transplantation according to the baseline health of the liver 134. Because steatotic and aged liver organs are easily impaired in function, the regulatory pathways help to improve the physiologic and morphologic outcomes of hepatic tissues.
Although steatotic livers are highly sensitive to injury, some pre‐conditional protocols are also effective in improving mitochondrial function and liver function after hepatic IR injury. Although IPC exerts no effect on mitochondrial function, it significantly normalizes the function of fatty livers 135. The protective effect of suppressing mitochondrial MPT in pre‐conditional protocols is more significant in young rats than in old rats after hepatic IR injury 128. Metformin pre‐conditioning attenuates the hepatic IR‐induced necro‐inflammatory reaction and mitochondrial dysfunction, decreases the levels of serum aminotransferases, and eliminates lipoperoxidation in high‐fat diet‐fed rats 136. Three inhibitors of autophagy including 3‐methyladenine, bafilomycin A1 and exendin 4 protect against hepatic IR injury by decreasing the expression levels of autophagy‐associated proteins and maintaining mitochondrial function in obese mice 130. Tauroursodeoxycholic acid decreases the hepatic inflammatory response after partial hepatectomy by inactivating mitochondrial anion channels, decreasing the release of cytochrome c and activating the level of caspase‐9 signalling; consequently, the apoptosis and necrosis rates of hepatocytes are decreased and liver regeneration is promoted in steatotic livers 3. Although APC has been found to be un‐protective in an early stage of hepatic IR in a steatotic mouse model, it has been proven to be an effective protective agent in a late stage through the adenosine monophosphate kinase signalling pathway 137. It is important to investigate protocols to eliminate lipid droplets and reverse the fat deposition in liver tissue, thereby allowing effectively the maintenance of liver function after warm or cold IR and increasing the survival rate in various pathological conditions.
Conclusions
Pathological states can be categorized into various categories including liver with underlying diseases, hepatic partial hepatectomy, trauma, haemorrhagic shock, cardiac arrest, LT state. Mitochondrial dysfunction is an important cellular event contributing to hepatic IR, and the restoration of mitochondrial function sustains cell and tissue survival in vivo and in vitro models. All ischaemia and reperfusion processes trigger a series of inflammatory responses including the activation of tissue macrophages and recruitment of neutrophils. Although mitochondrial dysfunction may be immediately initiated, and ROS formation causes higher rates of cell damage, the regulation of mitochondrial function maintains the balance of energy metabolism and normal hepatic function. Because of the mechanisms between warm hepatic IR and cold hepatic IR are not the same, treatments should be investigated according to its own features for potential clinical therapies.
More strategies are under investigation and are being mechanistically tested to improve the proliferation rate or decrease the death rate of hepatocytes or other non‐parenchymal cells in a hepatic IR injury state. As our review demonstrated, various mechanisms participate in the pathological process of hepatic IR, the mechanisms vary according to different experimental conditions including in vitro or in vivo condition, type of ischaemia, period of ischaemia, graft subclinical situation, etc. Therapeutic interventions including IPC, pre‐treatments with non‐physiological oxygen content, pharmaceutical pre‐conditioning and genetic modifications may be promising strategies to improve the antioxidant capacity in hepatic IR models in vitro and in vivo. Since gene modification shows vector toxicity and low transfection efficiencies, and leads to adequate mutants in warm or cold hepatic IR models. In addition, siRNA and micro RNA targetting specific genes to elaborate release of inflammatory and cell death factors is an advanced approach for hepatic IR. Because of complex procedures including partial or whole liver procurements, conservation in destined buffer, organ rewarming and final transplantation in LT models, the isolated liver tissues cumulatively acquire irreversible injuries. Pathological processes should be inhibited by effective routes which exert less side‐effect on human bodies. LT is an emergency procedure and leaves very little time to pre‐treat the donor with genetic approaches, thus it may be impossible to exert gene modification on clinical patients to improve the outcome of hepatic IR. Furthermore, some drugs mentioned above have possible side‐effects and frequently limit their use in human LT procedure. Moreover, elimination of LT‐induced injury should be focused on the donor organ's health, patient's own health and the precise surgical procedures. Although some procedures in our review may be unrealistic for clinical usage, these procedures and their regulative pathways help to clarifying the underling mechanisms for hepatic IR. Then, clinical therapies can be effectively developed for improving long‐term survival rate during various pathological processes.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81471794), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (no. 81121002) and the National Science and Technology Major Project (no. 2012ZX10002004).
References
- 1. Yoon SY, Kim CY, Han HJ, et al Protective effect of ischemic postconditioning against hepatic ischemic reperfusion injury in rat liver. Ann Surg Treat Res. 2015; 88: 241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhai Y, Petrowsky H, Hong JC, et al Ischaemia‐reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013; 10: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ben Mosbah I, Alfany‐Fernandez I, Martel C, et al Endoplasmic reticulum stress inhibition protects steatotic and non‐steatotic livers in partial hepatectomy under ischemia‐reperfusion. Cell Death Dis. 2010; 1: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogaki S, Taguchi K, Watanabe H, et al Carbon monoxide‐bound red blood cell resuscitation ameliorates hepatic injury induced by massive hemorrhage and red blood cell resuscitation via hepatic cytochrome P450 protection in hemorrhagic shock rats. J Pharm Sci. 2014; 103: 2199–206. [DOI] [PubMed] [Google Scholar]
- 5. Peralta C, Jimenez‐Castro MB, Gracia‐Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013; 59: 1094–106. [DOI] [PubMed] [Google Scholar]
- 6. Spencer NY, Zhou W, Li Q, et al Hepatocytes produce TNF‐alpha following hypoxia‐reoxygenation and liver ischemia‐reperfusion in a NADPH oxidase‐ and c‐Src‐dependent manner. Am J Physiol Gastrointest Liver Physiol. 2013; 305: G84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pronobesh C, Dagagi AV, Pallab C, et al Protective role of the calcium channel blocker amlodipine against mitochondrial injury in ischemia and reperfusion injury of rat liver. Acta Pharm. 2008; 58: 421–8. [DOI] [PubMed] [Google Scholar]
- 8. Martens JC, Keilhoff G, Halangk W, et al Lipidomic analysis of molecular cardiolipin species in livers exposed to ischemia/reperfusion. Mol Cell Biochem. 2015; 400: 253–63. [DOI] [PubMed] [Google Scholar]
- 9. Okatani Y, Wakatsuki A, Reiter RJ, et al Protective effect of melatonin against mitochondrial injury induced by ischemia and reperfusion of rat liver. Eur J Pharmacol. 2003; 469: 145–52. [DOI] [PubMed] [Google Scholar]
- 10. Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013; 19: 1683–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ponticelli C. Ischaemia‐reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014; 29: 1134–40. [DOI] [PubMed] [Google Scholar]
- 12. Lin NC, Liu CS, Chang CJ, et al Changes in mitochondrial respiratory enzyme activity after ischemia‐reperfusion injury in living‐donor liver transplantation. Transplant Proc. 2010; 42: 721–4. [DOI] [PubMed] [Google Scholar]
- 13. Eismann T, Huber N, Shin T, et al Peroxiredoxin‐6 protects against mitochondrial dysfunction and liver injury during ischemia‐reperfusion in mice. Am J Physiol Gastrointest Liver Physiol. 2009; 296: G266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang SJ, Li W, An W. Adenoviral gene transfer of hepatic stimulator substance confers resistance against hepatic ischemia‐reperfusion injury by improving mitochondrial function. Hum Gene Ther. 2013; 24: 443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaouali MA, Bejaoui M, Calvo M, et al Polyethylene glycol rinse solution: an effective way to prevent ischemia‐reperfusion injury. World J Gastroenterol. 2014; 20: 16203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaeschke H, Woolbright BL. Role of heme oxygenase 1 in TNF/TNF receptor‐mediated apoptosis after hepatic ischemia/reperfusion in rats. Shock 39: 380–388, 2013. Shock. 2013; 40: 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Shen J, Xiong X, et al Remote ischemic preconditioning protects against liver ischemia‐reperfusion injury via heme oxygenase‐1‐induced autophagy. PLoS One. 2014; 9: e98834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008; 8: 279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen HH, Chen YT, Yang CC, et al Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia‐reperfusion injury in rats through suppression of mitochondrial permeability transition. J Pineal Res. 2016; 61: 52–68. [DOI] [PubMed] [Google Scholar]
- 20. van Wijk SJ, Hageman GJ. Poly(ADP‐ribose) polymerase‐1 mediated caspase‐independent cell death after ischemia/reperfusion. Free Radic Biol Med. 2005; 39: 81–90. [DOI] [PubMed] [Google Scholar]
- 21. Kim SJ, Eum HA, Billiar TR, et al Role of heme oxygenase 1 in TNF/TNF receptor‐mediated apoptosis after hepatic ischemia/reperfusion in rats. Shock. 2013; 39: 380–8. [DOI] [PubMed] [Google Scholar]
- 22. Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995; 146: 3–15. [PMC free article] [PubMed] [Google Scholar]
- 23. Knudsen AR, Andersen KJ, Hamilton‐Dutoit S, et al Correlation between liver cell necrosis and circulating alanine aminotransferase after ischaemia/reperfusion injuries in the rat liver. Int J Exp Pathol. 2016; 97: 133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chattopadhyay P, Chaudhury P, Wahi AK. Ca2+ concentrations are key determinants of ischemia‐reperfusion‐induced apoptosis: significance for the molecular mechanism of Bcl‐2 action. Appl Biochem Biotechnol. 2010; 160: 1968–77. [DOI] [PubMed] [Google Scholar]
- 25. Gujral JS, Bucci TJ, Farhood A, et al Mechanism of cell death during warm hepatic ischemia‐reperfusion in rats: apoptosis or necrosis? Hepatology. 2001; 33: 397–405. [DOI] [PubMed] [Google Scholar]
- 26. Yang M, Antoine DJ, Weemhoff JL, et al Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2014; 20: 1372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lemasters JJV. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol. 1999; 276: G1–6. [DOI] [PubMed] [Google Scholar]
- 28. Rosentreter D, Funken D, Reifart J, et al RIP1‐dependent programmed necrosis is negatively regulated by caspases during hepatic ischemia‐reperfusion. Shock. 2015; 44: 72–6. [DOI] [PubMed] [Google Scholar]
- 29. Peralta C, Hotter G, Closa D, et al Protective effect of preconditioning on the injury associated to hepatic ischemia‐reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997; 25: 934–7. [DOI] [PubMed] [Google Scholar]
- 30. Chu MJ, Vather R, Hickey AJ, et al Impact of ischaemic preconditioning on experimental steatotic livers following hepatic ischaemia‐reperfusion injury: a systematic review. HPB (Oxford). 2015; 17: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richards JA, Wigmore SJ, Devey LR. Heme oxygenase system in hepatic ischemia‐reperfusion injury. World J Gastroenterol. 2010; 16: 6068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rudiger HA, Graf R, Clavien PA. Sub‐lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol. 2003; 39: 972–7. [DOI] [PubMed] [Google Scholar]
- 33. Ben Mosbah I, Duval H, Mbatchi SF, et al Intermittent selective clamping improves rat liver regeneration by attenuating oxidative and endoplasmic reticulum stress. Cell Death Dis. 2014; 5: e1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rolo AP, Teodoro JS, Peralta C, et al Prevention of I/R injury in fatty livers by ischemic preconditioning is associated with increased mitochondrial tolerance: the key role of ATPsynthase and mitochondrial permeability transition. Transpl Int. 2009; 22: 1081–90. [DOI] [PubMed] [Google Scholar]
- 35. O'Neill S, Leuschner S, McNally SJ, et al Meta‐analysis of ischaemic preconditioning for liver resections. Br J Surg. 2013; 100: 1689–700. [DOI] [PubMed] [Google Scholar]
- 36. Eipel C, Glanemann M, Nuessler AK, et al Ischemic preconditioning impairs liver regeneration in extended reduced‐size livers. Ann Surg. 2005; 241: 477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ye B, Zhao H, Hou H, et al Ischemic preconditioning provides no additive clinical value in liver resection of cirrhotic and non‐cirrhotic patients under portal triad clamping: a prospective randomized controlled trial. Clin Res Hepatol Gastroenterol. 2014; 38: 467–74. [DOI] [PubMed] [Google Scholar]
- 38. Longo L, Sinigaglia‐Fratta LX, Weber GR, et al Hypothermia is better than ischemic preconditioning for preventing early hepatic ischemia/reperfusion in rats. Ann Hepatol. 2016; 15: 110–20. [DOI] [PubMed] [Google Scholar]
- 39. Lai IR, Ma MC, Chen CF, et al The protective role of heme oxygenase‐1 on the liver after hypoxic preconditioning in rats. Transplantation. 2004; 77: 1004–8. [DOI] [PubMed] [Google Scholar]
- 40. Kim JS, Ohshima S, Pediaditakis P, et al Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology. 2004; 39: 1533–43. [DOI] [PubMed] [Google Scholar]
- 41. Guimaraes Filho MA, Cortez E, Garcia‐Souza EP, et al Effect of remote ischemic preconditioning in the expression of IL‐6 and IL‐10 in a rat model of liver ischemia‐reperfusion injury. Acta Cir Bras. 2015; 30: 452–60. [DOI] [PubMed] [Google Scholar]
- 42. Xue TM, Tao LD, Zhang J, et al Intestinal ischemic preconditioning reduces liver ischemia reperfusion injury in rats. Mol Med Rep. 2016; 13: 2511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zapletal C, Fallsehr C, Reidel M, et al Induction of HSP70 shows differences in protection against I/R injury derived by ischemic preconditioning and intermittent clamping. Microvasc Res. 2010; 80: 365–71. [DOI] [PubMed] [Google Scholar]
- 44. Jin C, Zhang PJ, Wu XM, et al Impact of hypoxic preconditioning on apoptosis and its possible mechanism in orthotopic liver autotransplantation in rats. Hepatobiliary Pancreat Dis Int. 2009; 8: 40–5. [PubMed] [Google Scholar]
- 45. Chouker A, Ohta A, Martignoni A, et al In vivo hypoxic preconditioning protects from warm liver ischemia‐reperfusion injury through the adenosine A2B receptor. Transplantation. 2012; 94: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gamdzyk M, Malek M, Bratek E, et al Hyperbaric oxygen and hyperbaric air preconditioning induces ischemic tolerance to transient forebrain ischemia in the gerbil. Brain Res. 2016; 1648: 257–65. [DOI] [PubMed] [Google Scholar]
- 47. Han G, Ma L, Guo Y, et al Hyperbaric oxygen therapy palliates lipopolysaccharide‐induced acute lung injury in rats by upregulating AQP1 and AQP5 expression. Exp Lung Res. 2015; 41: 444–9. [DOI] [PubMed] [Google Scholar]
- 48. Margarido MR, Silveira MR, Vanni JC, et al Hyperoxic preconditioning in partial liver ischemia. Acta Cir Bras. 2014; 29 19–23. [DOI] [PubMed] [Google Scholar]
- 49. Caldeira DE, Souza ME, Gomes MC, et al Effects of hyperbaric oxygen (HBO), as pre‐conditioning in liver of rats submitted to periodic liver ischemia/reperfusion. Acta Cir Bras. 2013; 28: 66–71. [DOI] [PubMed] [Google Scholar]
- 50. Losada DM, Chies AB, Feres O, et al Effects of hyperbaric oxygen therapy as hepatic preconditioning in rats submitted to hepatic ischemia/reperfusion injury. Acta Cir Bras. 2014; 29: 61–6. [DOI] [PubMed] [Google Scholar]
- 51. Caldeira DE, Silveira MR, Margarido MR, et al Effect of hyperbaric hepatic hyperoxia on the liver of rats submitted to intermittent ischemia/reperfusion injury. Acta Cir Bras. 2014; 29: 24–8. [DOI] [PubMed] [Google Scholar]
- 52. Liu Y, Sun XJ, Liu J, et al Heme oxygenase‐1 could mediate the protective effects of hyperbaric oxygen preconditioning against hepatic ischemia‐reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2011; 38: 675–82. [DOI] [PubMed] [Google Scholar]
- 53. Losada DM, Jordani ME, Jordani MC, et al Should preconditioning hyperbaric oxygenation protect the liver against ischemia‐reperfusion injury? An experimental study in a rat model. Transplant Proc. 2014; 46: 56–62. [DOI] [PubMed] [Google Scholar]
- 54. Yu SY, Chiu JH, Yang SD, et al Preconditioned hyperbaric oxygenation protects the liver against ischemia‐reperfusion injury in rats. J Surg Res. 2005; 128: 28–36. [DOI] [PubMed] [Google Scholar]
- 55. Schwartz J, Holmuhamedov E, Zhang X, et al Minocycline and doxycycline, but not other tetracycline‐derived compounds, protect liver cells from chemical hypoxia and ischemia/reperfusion injury by inhibition of the mitochondrial calcium uniporter. Toxicol Appl Pharmacol. 2013; 273: 172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim JS, Wang JH, Biel TG, et al Carbamazepine suppresses calpain‐mediated autophagy impairment after ischemia/reperfusion in mouse livers. Toxicol Appl Pharmacol. 2013; 273: 600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ohana G, Cohen S, Rath‐Wolfson L, et al A3 adenosine receptor agonist, CF102, protects against hepatic ischemia/reperfusion injury following partial hepatectomy. Mol Med Rep. 2016; 14: 4335–4341. [DOI] [PubMed] [Google Scholar]
- 58. Rehman H, Sun J, Shi Y, et al NIM811 prevents mitochondrial dysfunction, attenuates liver injury, and stimulates liver regeneration after massive hepatectomy. Transplantation. 2011; 91: 406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hataji K, Watanabe T, Oowada S, et al Effects of a calcium‐channel blocker (CV159) on hepatic ischemia/reperfusion injury in rats: evaluation with selective NO/pO2 electrodes and an electron paramagnetic resonance spin‐trapping method. Biol Pharm Bull. 2010; 33: 77–83. [DOI] [PubMed] [Google Scholar]
- 60. McDonald KA, Huang H, Tohme S, et al Toll‐like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high‐mobility group box protein B1 (HMGB1) signaling. Mol Med. 2014; 20: 639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ouzounidis N, Giakoustidis A, Poutahidis T, et al Interleukin 18 binding protein ameliorates ischemia/reperfusion‐induced hepatic injury in mice. Liver Transpl. 2016; 22: 237–46. [DOI] [PubMed] [Google Scholar]
- 62. Li JY, Gu X, Zhang WH, et al GdCl3 abates hepatic ischemia‐reperfusion injury by inhibiting apoptosis in rats. Hepatobiliary Pancreat Dis Int. 2009; 8: 518–23. [PubMed] [Google Scholar]
- 63. Yuan L, Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med. 2009; 30: 29–41. [DOI] [PubMed] [Google Scholar]
- 64. Jaeschke H, Farhood A. Neutrophil and Kupffer cell‐induced oxidant stress and ischemia‐reperfusion injury in rat liver. Am J Physiol. 1991; 260: G355–62. [DOI] [PubMed] [Google Scholar]
- 65. Tanemura A, Kuriyama N, Azumi Y, et al Thrombomodulin administration attenuates ischemia‐reperfusion injury of the remnant liver after 70% hepatectomy in rats: simulated model of small‐for‐size graft in living donor liver transplantation. Transplant Proc. 2014; 46: 1107–11. [DOI] [PubMed] [Google Scholar]
- 66. Joe Y, Zheng M, Kim HJ, et al Cilostazol attenuates murine hepatic ischemia and reperfusion injury via heme oxygenase‐dependent activation of mitochondrial biogenesis. Am J Physiol Gastrointest Liver Physiol. 2015; 309: G21–9. [DOI] [PubMed] [Google Scholar]
- 67. Yucel AF, Pergel A, Aydin I, et al Effect of infliximab on acute hepatic ischemia/reperfusion injury in rats. Int J Clin Exp Med. 2015; 8: 21287–94. [PMC free article] [PubMed] [Google Scholar]
- 68. Zaki HF, Abdelsalam RM. Vinpocetine protects liver against ischemia‐reperfusion injury. Can J Physiol Pharmacol. 2013; 91: 1064–70. [DOI] [PubMed] [Google Scholar]
- 69. Sawada T, Inoue K, Tanabe D, et al Experimental studies on protective effects of FK506 against hepatic ischemia‐reperfusion injury. J Med Invest. 2016; 63: 262–9. [DOI] [PubMed] [Google Scholar]
- 70. Zhao G, Shen X, Nan H, et al Remifentanil protects liver against ischemia/reperfusion injury through activation of anti‐apoptotic pathways. J Surg Res. 2013; 183: 827–34. [DOI] [PubMed] [Google Scholar]
- 71. Bellanti F, Mirabella L, Mitarotonda D, et al Propofol but not sevoflurane prevents mitochondrial dysfunction and oxidative stress by limiting HIF‐1alpha activation in hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2016; 96: 323–33. [DOI] [PubMed] [Google Scholar]
- 72. Zhao G, Ma H, Shen X, et al Role of glycogen synthase kinase 3beta in protective effect of propofol against hepatic ischemia‐reperfusion injury. J Surg Res. 2013; 185: 388–98. [DOI] [PubMed] [Google Scholar]
- 73. Li J, Yuan T, Zhao X, et al Protective effects of sevoflurane in hepatic ischemia‐reperfusion injury. Int J Immunopathol Pharmacol. 2016; 29: 300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chattopadhyay P, Verma N, Verma A, et al Calcium antagonist prevents calcium flux induced necrosis and apoptosis in ischemic reperfusion of rat liver. Indian J Clin Biochem. 2008; 23: 356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Onody P, Stangl R, Fulop A, et al Levosimendan: a cardiovascular drug to prevent liver ischemia‐reperfusion injury? PLoS One. 2013; 8: e73758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Grossini E, Pollesello P, Bellofatto K, et al Protective effects elicited by levosimendan against liver ischemia/reperfusion injury in anesthetized rats. Liver Transpl. 2014; 20: 361–75. [DOI] [PubMed] [Google Scholar]
- 77. Nogueira MA, Coelho AM, Sampietre SN, et al Beneficial effects of adenosine triphosphate‐sensitive K+ channel opener on liver ischemia/reperfusion injury. World J Gastroenterol. 2014; 20: 15319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mahfoudh Boussaid A, Selmi R, Bejaoui M, et al Effectiveness of a single versus repeated administration of trimetazidine in the protection against warm ischemia/reperfusion injury of rat liver. Turk J Med Sci. 2016; 46: 1258–64. [DOI] [PubMed] [Google Scholar]
- 79. Sheth H, Hafez T, Glantzounis GK, et al Glycine maintains mitochondrial activity and bile composition following warm liver ischemia‐reperfusion injury. J Gastroenterol Hepatol. 2011; 26: 194–200. [DOI] [PubMed] [Google Scholar]
- 80. Kim JS, He L, Qian T, et al Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003; 3: 527–35. [DOI] [PubMed] [Google Scholar]
- 81. Zhang X, Lemasters JJ. Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic Biol Med. 2013; 63: 243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pediaditakis P, Kim JS, He L, et al Inhibition of the mitochondrial permeability transition by protein kinase A in rat liver mitochondria and hepatocytes. Biochem J. 2010; 431: 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jiang Q, Pan Y, Cheng Y, et al Protection of rat liver against hepatic ischemia‐reperfusion injury by a novel selenocysteine‐containing 7‐mer peptide. Mol Med Rep. 2016; 14: 2007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Katsumi H, Nishikawa M, Hirosaki R, et al Development of PEGylated cysteine‐modified lysine dendrimers with multiple reduced thiols to prevent hepatic ischemia/reperfusion injury. Mol Pharm. 2016; 13: 2867–73. [DOI] [PubMed] [Google Scholar]
- 85. Lin FS, Shen SQ, Chen ZB, et al 17beta‐estradiol attenuates reduced‐size hepatic ischemia/reperfusion injury by inhibition apoptosis via mitochondrial pathway in rats. Shock. 2012; 37: 183–90. [DOI] [PubMed] [Google Scholar]
- 86. Tao X, Sun X, Xu L, et al Total flavonoids from Rosa laevigata Michx fruit ameliorates hepatic ischemia/reperfusion injury through inhibition of oxidative stress and inflammation in rats. Nutrients. 2016; 8: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tao YE, Wen Z, Song Y, et al Paeoniflorin attenuates hepatic ischemia/reperfusion injury via anti‐oxidative, anti‐inflammatory and anti‐apoptotic pathways. Exp Ther Med. 2016; 11: 263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. He D, Guo Z, Pu JL, et al Resveratrol preconditioning protects hepatocytes against hepatic ischemia reperfusion injury via Toll‐like receptor 4/nuclear factor‐kappaB signaling pathway in vitro and in vivo . Int Immunopharmacol. 2016; 35: 201–9. [DOI] [PubMed] [Google Scholar]
- 89. Cheng F, Li Y, Feng L, et al Effects of tetrandrine on ischemia/reperfusion injury in mouse liver. Transplant Proc. 2008; 40: 2163–6. [DOI] [PubMed] [Google Scholar]
- 90. Curek GD, Cort A, Yucel G, et al Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology. 2010; 267: 147–53. [DOI] [PubMed] [Google Scholar]
- 91. Liang R, Nickkholgh A, Kern M, et al Green tea extract ameliorates reperfusion injury to rat livers after warm ischemia in a dose‐dependent manner. Mol Nutr Food Res. 2011; 55: 855–63. [DOI] [PubMed] [Google Scholar]
- 92. Chi X, Zhang R, Shen N, et al Sulforaphane reduces apoptosis and oncosis along with protecting liver injury‐induced ischemic reperfusion by activating the Nrf2/ARE pathway. Hepatol Int. 2015; 9: 321–9. [DOI] [PubMed] [Google Scholar]
- 93. Sun PP, Yuan F, Xu J, et al Cryptotanshinone ameliorates hepatic normothermic ischemia and reperfusion injury in rats by anti‐mitochondrial apoptosis. Biol Pharm Bull. 2014; 37: 1758–65. [DOI] [PubMed] [Google Scholar]
- 94. Lee HM, Jang HJ, Kim SS, et al Protective effect of eupatilin pretreatment against hepatic ischemia‐reperfusion injury in mice. Transplant Proc. 2016; 48: 1226–33. [DOI] [PubMed] [Google Scholar]
- 95. Konig K, Granja T, Eckle VS, et al Inhibition of plexin C1 protects against hepatic ischemia‐reperfusion injury. Crit Care Med. 2016; 44: e625–32. [DOI] [PubMed] [Google Scholar]
- 96. Zhang J, Xu P, Song P, et al CCL2‐CCR2 signaling promotes hepatic ischemia/reperfusion injury. J Surg Res. 2016; 202: 352–62. [DOI] [PubMed] [Google Scholar]
- 97. Sass G, Shembade ND, Haimerl F, et al TNF pretreatment interferes with mitochondrial apoptosis in the mouse liver by A20‐mediated down‐regulation of Bax. J Immunol. 2007; 179: 7042–9. [DOI] [PubMed] [Google Scholar]
- 98. Theruvath TP, Snoddy MC, Zhong Z, et al Mitochondrial permeability transition in liver ischemia and reperfusion: role of c‐Jun N‐terminal kinase 2. Transplantation. 2008; 85: 1500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shi Y, Rehman H, Ramshesh VK, et al Sphingosine kinase‐2 inhibition improves mitochondrial function and survival after hepatic ischemia‐reperfusion. J Hepatol. 2012; 56: 137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee LY, Harberg C, Matkowskyj KA, et al Cell‐specific overactivation of nuclear erythroid 2 p45‐related factor 2‐mediated gene expression in myeloid cells decreases hepatic ischemia/reperfusion injury. Liver Transpl. 2016; 22: 1115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shi J, Shao W, Yang D, et al Hydrodynamics‐based transfection of plasmid encoding receptor activator for nuclear factor kappa B‐Fc protects against hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2010; 16: 611–20. [DOI] [PubMed] [Google Scholar]
- 102. Roy A, Srivastava M, Saqib U, et al Potential therapeutic targets for inflammation in toll‐like receptor 4 (TLR4)‐mediated signaling pathways. Int Immunopharmacol. 2016; 40: 79–89. [DOI] [PubMed] [Google Scholar]
- 103. Jiang N, Zhang X, Zheng X, et al Targeted gene silencing of TLR4 using liposomal nanoparticles for preventing liver ischemia reperfusion injury. Am J Transplant. 2011; 11: 1835–44. [DOI] [PubMed] [Google Scholar]
- 104. Li L, Li G, Yu C, et al A role of microRNA‐370 in hepatic ischaemia‐reperfusion injury by targeting transforming growth factor‐beta receptor II. Liver Int. 2015; 35: 1124–32. [DOI] [PubMed] [Google Scholar]
- 105. Ar'Rajab A, Ahren B, Sundberg R, et al The function of a colloid in liver cold‐storage preservation. Transplantation. 1991; 52: 34–8. [DOI] [PubMed] [Google Scholar]
- 106. Morariu AM, Vd Plaats A, V Oeveren W, et al Hyperaggregating effect of hydroxyethyl starch components and University of Wisconsin solution on human red blood cells: a risk of impaired graft perfusion in organ procurement? Transplantation. 2003; 76: 37–43. [DOI] [PubMed] [Google Scholar]
- 107. Baskin‐Bey ES, Washburn K, Feng S, et al Clinical trial of the pan‐caspase inhibitor, IDN‐6556, in human liver preservation injury. Am J Transplant. 2007; 7: 218–25. [DOI] [PubMed] [Google Scholar]
- 108. Cui LZ, Wang B, Chen LY, et al The effect of ischemic precondition to IL‐6 on rat liver ischemia‐reperfusion injury in transplantation. Asian Pac J Trop Med. 2013; 6: 395–9. [DOI] [PubMed] [Google Scholar]
- 109. Leal AJ, Tannuri AC, Belon AR, et al Effects of ischemic preconditioning in a pig model of large‐for‐size liver transplantation. Clinics (Sao Paulo). 2015; 70: 126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhuonan Z, Sen G, Zhipeng J, et al Hypoxia preconditioning induced HIF‐1alpha promotes glucose metabolism and protects mitochondria in liver I/R injury. Clin Res Hepatol Gastroenterol. 2015; 39: 610–9. [DOI] [PubMed] [Google Scholar]
- 111. Guan L, Liu H, Fu P, et al The protective effects of trypsin inhibitor on hepatic ischemia‐reperfusion injury and liver graft survival. Oxid Med Cell Longev. 2016; 2016: 1429835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stoffels B, Yonezawa K, Yamamoto Y, et al Meloxicam, a COX‐2 inhibitor, ameliorates ischemia/reperfusion injury in non‐heart‐beating donor livers. Eur Surg Res. 2011; 47: 109–17. [DOI] [PubMed] [Google Scholar]
- 113. Zaouali MA, Bardag‐Gorce F, Carbonell T, et al Proteasome inhibitors protect the steatotic and non‐steatotic liver graft against cold ischemia reperfusion injury. Exp Mol Pathol. 2013; 94: 352–9. [DOI] [PubMed] [Google Scholar]
- 114. Padrissa‐Altes S, Zaouali MA, Boncompagni E, et al The use of a reversible proteasome inhibitor in a model of Reduced‐Size Orthotopic Liver transplantation in rats. Exp Mol Pathol. 2012; 93: 99–110. [DOI] [PubMed] [Google Scholar]
- 115. Kuriyama N, Isaji S, Hamada T, et al The cytoprotective effects of addition of activated protein C into preservation solution on small‐for‐size grafts in rats. Liver Transpl. 2010; 16: 1–11. [DOI] [PubMed] [Google Scholar]
- 116. Pantazi E, Zaouali MA, Bejaoui M, et al Sirtuin 1 in rat orthotopic liver transplantation: an IGL‐1 preservation solution approach. World J Gastroenterol. 2015; 21: 1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wu K, Ma L, Xu T, et al Protective effects against hepatic ischemia‐reperfusion injury after rat orthotopic liver transplantation because of BCL‐2 overexpression. Int J Clin Exp Med. 2015; 8: 13818–23. [PMC free article] [PubMed] [Google Scholar]
- 118. Fukazawa K, Vitin AA, Pretto EA Jr. Serum acidosis prior to reperfusion facilitates hemodynamic recovery following liver transplantation. J Anesth. 2016; 30: 80–8. [DOI] [PubMed] [Google Scholar]
- 119. Pantazi E, Bejaoui M, Zaouali MA, et al Losartan activates sirtuin 1 in rat reduced‐size orthotopic liver transplantation. World J Gastroenterol. 2015; 21: 8021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhuang Z, Lian P, Wu X, et al Abate cytochrome C induced apoptosome to protect donor liver against ischemia reperfusion injury on rat liver transplantation model. Am J Transl Res. 2016; 8: 1738–47. [PMC free article] [PubMed] [Google Scholar]
- 121. Bejaoui M, Pantazi E, De Luca V, et al Acetazolamide protects steatotic liver grafts against cold ischemia reperfusion injury. J Pharmacol Exp Ther. 2015; 355: 191–8. [DOI] [PubMed] [Google Scholar]
- 122. Fayed NA, Sayed EI, Saleh SM, et al Effect of dexmedetomidine on hepatic ischemia‐reperfusion injury in the setting of adult living donor liver transplantation. Clin Transplant. 2016; 30: 470–82. [DOI] [PubMed] [Google Scholar]
- 123. Cheng MX, Chen ZZ, Cai YL, et al Astragaloside IV protects against ischemia reperfusion in a murine model of orthotopic liver transplantation. Transplant Proc. 2011; 43: 1456–61. [DOI] [PubMed] [Google Scholar]
- 124. Theruvath TP, Czerny C, Ramshesh VK, et al C‐Jun N‐terminal kinase 2 promotes graft injury via the mitochondrial permeability transition after mouse liver transplantation. Am J Transplant. 2008; 8: 1819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Li JQ, Qi HZ, He ZJ, et al Cytoprotective effects of human interleukin‐10 gene transfer against necrosis and apoptosis induced by hepatic cold ischemia/reperfusion injury. J Surg Res. 2009; 157: e71–8. [DOI] [PubMed] [Google Scholar]
- 126. Si ZZ, Li JQ, Qi HZ, et al Recombinant adenovirus vector Ad‐hIL‐10 protects grafts from cold ischemia‐reperfusion injury following orthotopic liver transplantation in rats. Hepatobiliary Pancreat Dis Int. 2010; 9: 144–8. [PubMed] [Google Scholar]
- 127. Bhogal RH, Curbishley SM, Weston CJ, et al Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 2010; 16: 1303–13. [DOI] [PubMed] [Google Scholar]
- 128. Fu H, Xu H, Chen H, et al Inhibition of glycogen synthase kinase 3 ameliorates liver ischemia/reperfusion injury via an energy‐dependent mitochondrial mechanism. J Hepatol. 2014; 61: 816–24. [DOI] [PubMed] [Google Scholar]
- 129. Nativ NI, Yarmush G, So A, et al Elevated sensitivity of macrosteatotic hepatocytes to hypoxia/reoxygenation stress is reversed by a novel defatting protocol. Liver Transpl. 2014; 20: 1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gupta NA, Kolachala VL, Jiang R, et al Mitigation of autophagy ameliorates hepatocellular damage following ischemia‐reperfusion injury in murine steatotic liver. Am J Physiol Gastrointest Liver Physiol. 2014; 307: G1088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Llacuna L, Fernandez A, Montfort CV, et al Targeting cholesterol at different levels in the mevalonate pathway protects fatty liver against ischemia‐reperfusion injury. J Hepatol. 2011; 54: 1002–10. [DOI] [PubMed] [Google Scholar]
- 132. Chu MJ, Hickey AJ, Tagaloa S, et al Ob/ob mouse livers show decreased oxidative phosphorylation efficiencies and anaerobic capacities after cold ischemia. PLoS One. 2014; 9: e100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chu MJ, Hickey AJ, Jiang Y, et al Mitochondrial dysfunction in steatotic rat livers occurs because a defect in complex i makes the liver susceptible to prolonged cold ischemia. Liver Transpl. 2015; 21: 396–407. [DOI] [PubMed] [Google Scholar]
- 134. He S, Rehman H, Wright GL, et al Inhibition of inducible nitric oxide synthase prevents mitochondrial damage and improves survival of steatotic partial liver grafts. Transplantation. 2010; 89: 291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chu MJ, Premkumar R, Hickey AJ, et al Steatotic livers are susceptible to normothermic ischemia‐reperfusion injury from mitochondrial complex‐I dysfunction. World J Gastroenterol. 2016; 22: 4673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cahova M, Palenickova E, Dankova H, et al Metformin prevents ischemia reperfusion‐induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am J Physiol Gastrointest Liver Physiol. 2015; 309: G100–11. [DOI] [PubMed] [Google Scholar]
- 137. Matsuda A, Kuriyama N, Kato H, et al Comparative study on the cytoprotective effects of activated protein C treatment in nonsteatotic and steatotic livers under ischemia‐reperfusion injury. Biomed Res Int. 2015; 2015: 635041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Okatani Y, Wakatsuki A, Enzan H, et al Edaravone protects against ischemia/reperfusion‐induced oxidative damage to mitochondria in rat liver. Eur J Pharmacol. 2003; 465: 163–70. [DOI] [PubMed] [Google Scholar]
- 139. Fu H, Chen H, Wang C, et al Flurbiprofen, a cyclooxygenase inhibitor, protects mice from hepatic ischemia/reperfusion injury by inhibiting GSK‐3beta signaling and mitochondrial permeability transition. Mol Med. 2012; 18: 1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


