Abstract
Hypertensive patients usually have a higher risk of new‐onset diabetes mellitus (NOD) which may trigger cardiovascular diseases. In this study, the effectiveness of six antihypertensive agents with respect to NOD prevention in hypertensive patients was assessed. A network meta‐analysis was conducted to compare the efficacy of specific drug classes. PubMed and Embase databases were searched for relevant articles. Results of the pairwised meta‐analysis were illustrated by odd ratios (OR) and a corresponding 95% confidence interval (CI). The probabilities and outcome of each treatment were ranked and summarized using the surface under the cumulative ranking curve (SUCRA).Twenty‐three trials were identified, including 224,832 patients with an average follow‐up period of 3.9 ± 1.0 years. The network meta‐analysis showed that patients treated by angiotensin II receptor blockers (ARBs) were associated with a lower risk of NOD compared to placebo (PCB), calcium channel blockers (CCBs) and β‐blockers, while diuretic appeared to be ineffective for NOD prevention. Network meta‐analysis results of specific drugs showed that enalapril exhibited distinct advantages and hydrochlorothiazide also exhibited a reliable performance. Our results suggested that both ARBs and angiotensin converse enzyme inhibitors (ACEIs), especially candesartan and enalapril, were preferable for NOD prevention in hypertensive patients. Hydrochlorothiazide also exhibited a reliable performance in comparison with other agents.
Keywords: new‐onset diabetes, angiotensin II receptor blockers, angiotensin II converse enzyme inhibitors, calcium channel blockers, β‐blockers, diuretic, hypertension
Introduction
NOD and hypertension often co‐existed, and thereby, the risk of cardiovascular diseases is substantially increased 1, 2. And previous evidence showed that this clinical dilemma was associated with an increased risk of hepatitis C virus infection and graft rejection and loss and thereby affected patient survival quality 3. Also, it was reported that diabetes may be prevented by renin–angiotensin blockers 4, 5. Recently, a number of trials on antihypertensive medications such as angiotensin‐converting enzyme inhibitors (ACEIs), ARBs, CCBs, diuretics and β‐blockers have explored whether these medications influenced NOD development 2, 6, 7.
Studies showed that ACEI or ARB could reduce the incidence of NOD in patients 8. ACEI therapy contributed to significant reduction compared with diuretics and β‐blockers 9, and it is potentially effective in reducing hypertension and cardiovascular risks 9. ARB is an antihypertensive agent which is able to reduce NOD development by enhancing the insulin sensitivity 10. However, it did not improve clinical outcome of cardiovascular diseases and no significant evidence was revealed from former trials 11.
Clinical trials reviewed that the use of valsartan in patients with cardiovascular disease resulted in a 14% reduction in NOD, while no significant therapeutic improvement for cardiovascular disease was confirmed 12. CCB could significantly reduce the incidence of NOD, and CCB combined with ARB had metabolically neutral effects 13. Effects of β‐blockers on NOD patient are controversial which might contribute to a reduced mortality and morbidity of heart failure among patients with NOD 14, while it might trigger the development of NOD 6. It was also indicated that the use of diuretic is associated with a decreased incidence of NOD 15 and prolonged diuretic treatment may result in an increased fasting glucose 15. In addition, the overall glycemic status was affected when both diuretics and β‐blockers are combined together 15. However, a study based on Indian population suggested that diuretics might increase the risk of NOD; that is, hypertensive patients treated with β‐blockers and diuretics exhibited higher incidence of diabetes mellitus 13.
Although previous meta‐analyses concluded that some antihypertensive medications were effective in NOD prevention, uncertain and controversy still remained to be clarified 4, 8, 9, 10, 16, 17, 18, 19, 20, 21, 22, 23, 24, besides, meta‐analyses were limited by few trials with direct comparisons between two treatments. Instead, a network meta‐analysis (NMA) can be conducted if both treatments have been compared to a common comparator. Formally, NMA can be defined as a statistical combination of all available evidence for an outcome from several studies across multiple treatments to generate estimates of pairwise comparison of each intervention to every other intervention within a network 25. As a result, we performed this NMA to compare the relative effectiveness of several antihypertensive medications including ACEIs (enalapril, lisinopril, perindopril, quinapril, ramipril, trandolapril), ARBs (include candesartan, losartan, telmisartan, valsartan), CCBs (amlodipine, verapamil), diuretics (bendrofluazide, chlorthalidone, hydrochlorothiazide) and β‐blockers (atenolol, propranolol).
Materials and methods
Data search
PubMed and Embase were searched, and studies from January 1985 up to June 2016 were identified to evaluate the efficacy of ACEIs, ARBs, CCBs, β‐blockers and diuretic. ARBs, ACEIs, CCBs, β‐blockers or diuretics and individual agent names within the medication classes were combined with ‘diabetes’, ‘pre‐diabetes’, ‘new‐onset diabetes mellitus’, ‘new‐onset diabetes’, ‘NOD’, ‘hypertension’, ‘high risk’, and ‘randomized, controlled, trials’’. Reference lists of identified articles including previous meta‐analyses and reviews were evaluated for additional relevant studies and information.
Selection criteria
Studies were included if they fulfilled all the criteria as following: (i) comparison among ARBs, ACEIs, CCBs, β‐blockers, diuretics and PCB or other routine treatments; (ii) inclusion of individuals with hypertension or other high‐risk factors; (iii) the incidence of NOD as primary end‐point; (iv) average follow‐up over 1 year, recruiting more than 100 patients. Trials which did not meet above requirements were excluded.
Data extraction and quality assessment
For each trial, all data derived from the published tables or texts were tabulated into a Microsoft Excel spreadsheet and the corresponding study characteristics were reviewed. In all data derived from each trial, we included total number of patients (overall population), number of patients with NOD at baseline, type and dosage of medications (ARBs, ACEIs, CCBs, β‐blockers, diuretics), follow‐up duration and other key study information.
Statistical analysis
The incidence of NOD was treated as a dichotomous variable and assessed by odds ratios (ORs) with 95% confidential intervals (CIs) for six antihypertensive. Then, a subgroup analysis was conducted to compare the efficacy of specific drugs using NMA. Pooled ORs were calculated using the DerSimonian and Laird random‐effects model 26 or the Mantel–Haenszel fixed‐effects model 27, depending on the heterogeneity of treatment effects across studies. Bayesian statistical model was used. The percentage variability of the pooled ORs attributable to heterogeneity among the selected studies was quantified using the I 2 statistic test 28. Typically, values above 50% were deemed to suggest large between‐study heterogeneity. Under such a circumstance, the random‐effects model was used to improve accuracy of research. Ranking of medication with respect to the effectiveness of NOD prevention was achieved using the surface under the cumulative ranking area (SUCRA). A higher SUCRA value indicates a more desirable property with respect to a certain end‐point. Statistical analyses were conducted using R version 3.1.3 (R Project for Statistical Computing, Vienna, Austria). P < 0.05 was considered significantly different.
Results
Study selection
As schematically shown in Figure 1, among the 396 potentially eligible trials, 52 duplicates were removed, 297 studies were excluded by screening titles and abstracts and 24 full‐text articles were ruled out as their outcome did not contain NOD data or medications were not properly compared. Thus, there were 23 totally randomized clinical trials, including a total of 224,832 patients, following as the selected criteria and selected in this meta‐analysis study 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51.
Figure 1.
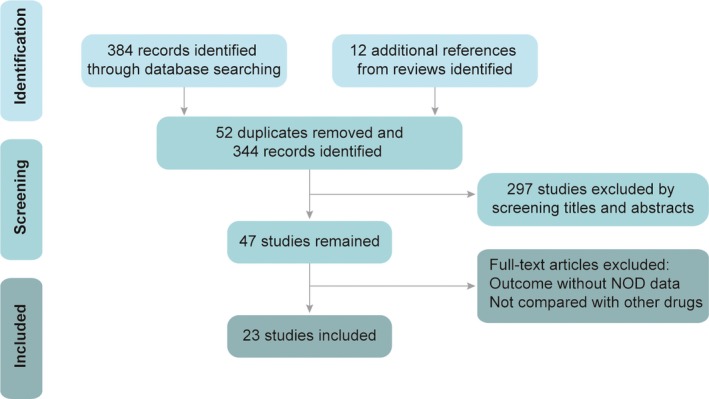
Study flow diagram. NOD: new‐onset diabetes.
Population characteristics
The general characteristics of the identified trials are shown in Table 1. Eight trials were designed to compare ARBs‐based treatments against PCB; six trials were aimed to compare ACEIs‐based treatments against PCB; and nine trials were designed to compare to each other among the five different treatments, namely ARBs, ACEIs, CCBs, β‐blockers or diuretic. A total of 224,832 hypertensive patients were involved in our study. A total of 53,719 (23.9%) patients were treated by PCB, 42,422 (18.9%) patients were randomized to receive ARBs, 39,899 (17.7%) patients received CCBs, 33,645 (15.0%) patients were treated by β‐blockers, 29,259 (13.0%) were treated by ACEIs and 25,888 (11.5%) were treated by diuretics. Network plots of six different kinds of medications and 18 agents were shown in Figure 2 and Figure S1. The mean age of these identified patients ranged from 51 to 72 years, and the duration was over an average follow‐up period of 3.9 ± 1.0 years.
Table 1.
Characteristics of studies included in the network meta‐analysis
| Study | Blinding | Duration (years) | Treatment class | Treatment drugs | Mean age | BP (mmHg) | Sample size |
|---|---|---|---|---|---|---|---|
| MRC trail,1985 | Single‐blind | 4.9 | β‐Blocker/diuretic/PCB | Propranolol/bendrofluazide/Placebo | 51/51/51 | 158/98 | 4403/4297/8654 |
| Wilhelmsen,1987 | – | 3.8 | β‐Blocker/diuretic | Atenolol/metoprolol/bendrofluazide/hydrochlorothiazide | 52.3/52.2 | 166/107 | 3727/3297 |
| Yusuf,2001 | Double‐blind | 4.5 | ACEI/PCB | Ramipril/placebo | 66.3/65.9 | 136.4/78.2 | 2837/2883 |
| ALLHAT officers,2002 | Double‐blind | 4.9 | ACEI/diuretic/CCB | Lisinopril/chlorthalidone/amlodipine | 67/67/67 | 146/84 | 9054/15,255/9048 |
| Lindholm,2002 | Double‐blind | 4.8 | ARB/β‐blocker | Losartan/atenolol | 66.9/66.9 | 174.3/97.9 | 4605/4588 |
| Fox,2003 | Double‐blind | 4.3 | ACEI/PCB | Perindopril/placebo | 60/60 | 137/82 | 6110/6108 |
| Vermes,2003 | Double‐blind | 2.9 | ACEI/PCB | Enalapril/placebo | 56.1/56.8 | 127.4/77.8 | 153/138 |
| Wing,2003 | – | 4.1 | ACEI/diuretic | Enalapril/hydrochlorothiazide | 72/71.9 | 167/91 | 3044/3039 |
| Pfeffer,2003 | Double‐blind | 3.1 | ARB/PCB | Candesartan/placebo | 65.9/66 | 130.6/76.6 | 3803/3796 |
| Littell,2003 | Double‐blind | 3.7 | ARB/PCB | Candesartan/placebo | 76.4/76.4 | 166/90.3 | 2477/2460 |
| Granger,2003 | Double‐blind | 2.8 | ARB/PCB | Candesartan/placebo | 66.3/66.8 | 127/78 | 1013/1015 |
| Yusuf,2003 | Double‐blind | 3.1 | ARB/PCB | Candesartan/placebo | 67.2/67.1 | 130.6/76.6 | 1514/1509 |
| Pepine,2003 | – | 2.7 | CCB/β‐blocker | Verapamil/atenolol | 66/66 | 149.5/86.3 | 11,267/11,309 |
| McMurray,2003 | Double‐blind | 3.4 | ARB/PCB | Candesartan/placebo | 64/64.1 | 166/90.3 | 1276/1272 |
| Braunwald,2004 | Double‐blind | 4.8 | ACEI/PCB | Trandolapril/placebo | 64/64 | 134/78 | 4158/4132 |
| Julius,2004 | Double‐blind | 4.2 | ARB/CCB | Valsartan/amlodipine | 67.2/67.3 | 154.5/87.4 | 7649/7596 |
| Dahlof,2005 | Single‐blind | 5.5 | β‐Blocker/CCB | Atenolol/amlodipine | 63/63 | 130/‐ | 9618/9639 |
| Dream Investigators,2006 | Double‐blind | 3 | ACEI/PCB | Ramipril/placebo | 54.7/54.7 | 136.1/83.4 | 2623/2646 |
| Yusuf,2008 | – | 2.5 | ARB/PCB | Telmisartan/placebo | 66.1/66.2 | 144.1/83.8 | 10,146/10,186 |
| Ogihara,2008 | – | 3.2 | ARB/CCB | Candesartan/amlodipine | 63.8/63.9 | 162.5/91.6 | 2354/2349 |
| TRANSCEND,2008 | Double‐blind | 4.7 | ARB/PCB | Telmisartan/placebo | 66.9/66.9 | 140.7/81.8 | 2954/2972 |
| Rouleau,2008 | Double‐blind | 2.95 | ACEI/PCB | Quinapril/placebo | 61/61 | 122/70 | 1280/1273 |
| NAVIGAROR group,2010 | Double‐blind | 6 | ARB/PCB | Valsartan/placebo | 63.7/63.8 | 139.4/82.5 | 4631/4675 |
BP: blood pressure; PCB: placebo; ACEI: angiotensin converse enzyme inhibitor; CCB: calcium channel blockers; ARB: angiotensin II receptor blockers.
Figure 2.
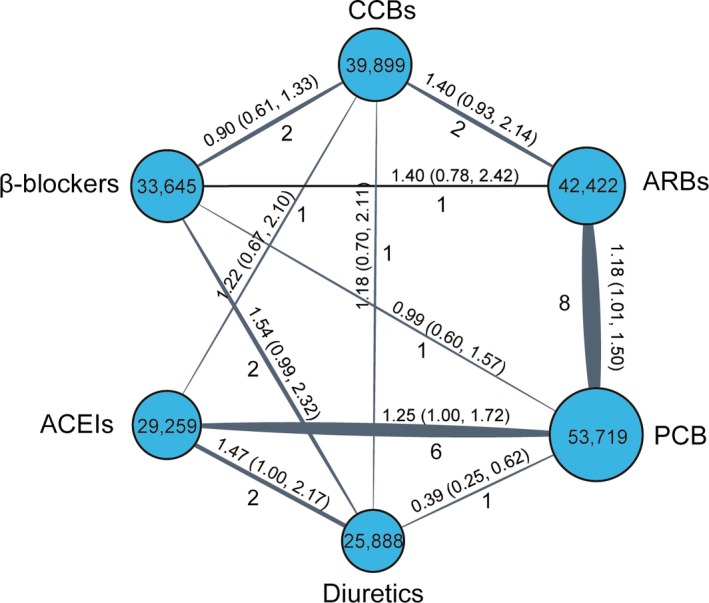
Network plot of eligible comparisons of categorized six different medications in NOD in the meta‐analysis. The width of the lines represents the total number of trials for each comparison. ACEIs: angiotensin converse enzyme inhibitors; ARBs: angiotensin II receptor blockers; CCBs: calcium channel blockers; NOD: new‐onset diabetes.
Incidence of NOD
As shown in Table 2, both ACEIs and ARBs showed a significant decline in the incidence of NOD compared to PCB, (OR = 0.82, 95% CrI = 0.64–0.99; OR = 0.81, 95% CrI = 0.66–0.96).And diuretics were associated with a higher risk of NOD compared with PCB (OR = 1.44 95% CrI = 1.06–1.94). Treatment of β‐blockers and diuretics showed a higher incidence of NOD than ACEIs (OR = 1.38, 95% CrI = 1.00–1.93; OR = 1.75, 95% CrI = 1.31–2.41), whereas β‐blockers, CCBs and diuretics also showed a significant elevation in the incidence of NOD compared to ARBs (OR = 1.40, 95% CrI = 1.04–1.88; OR = 1.33, 95% CrI = 1.00–1.75; OR = 1.78, 95% CrI = 1.30–2.46). Figure 3 illustrated the forest plot of network results.
Table 2.
Results of six interventions for the incidence of new‐onset diabetes (NOD) from network meta‐analysis
| Treatment | PCB | Diuretics | CCBs | β‐Blockers | ARBs | ACEIs |
|---|---|---|---|---|---|---|
| PCB | PCB | 1.44 (1.06, 1.94) | 1.07 (0.80, 1.43) | 1.13 (0.83, 1.53) | 0.81 (0.67, 0.96) | 0.82 (0.65, 1.00) |
| Diuretics | 0.70 (0.52, 0.95) | Diuretics | 0.74 (0.54, 1.03) | 0.79 (0.57, 1.07) | 0.56 (0.41, 0.77) | 0.57 (0.42, 0.76) |
| CCBs | 0.94 (0.70, 1.26) | 1.34 (0.97, 1.87) | CCBs | 1.06 (0.80, 1.39) | 0.75 (0.57, 1.00) | 0.77 (0.56, 1.04) |
| β‐Blockers | 0.89 (0.65, 1.21) | 1.27 (0.93, 1.76) | 0.94 (0.72, 1.26) | β‐Blockers | 0.71 (0.53, 0.96) | 0.73 (0.52, 1.00) |
| ARBs | 1.24 (1.04, 1.49) | 1.78 (1.30, 2.46) | 1.33 (1.00, 1.75) | 1.40 (1.04, 1.88) | ARBs | 1.02 (0.77, 1.31) |
| ACEIs | 1.22 (1.00, 1.54) | 1.75 (1.31, 2.41) | 1.31 (0.97, 1.80) | 1.38 (1.00, 1.93) | 0.98 (0.76, 1.30) | ACEIs |
Bold values mean statistic difference.
Figure 3.
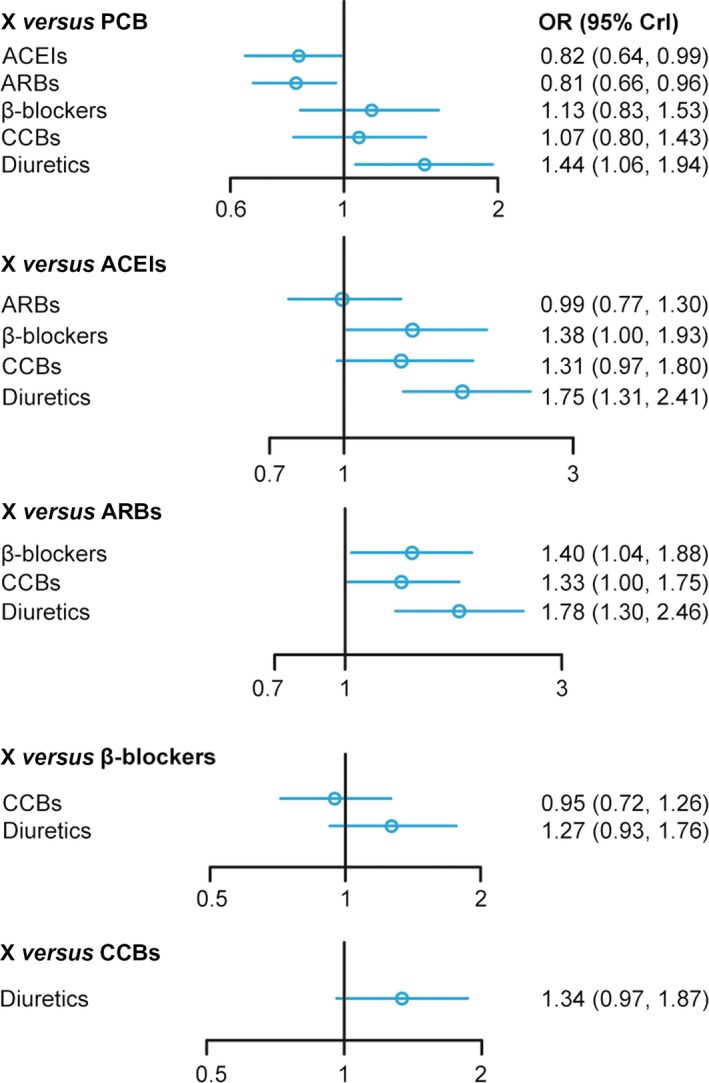
Forest plot for ARBs, ACEIs, CCBs, β‐blockers, diuretic or PCB‐based strategy on the incidence of NOD. ACEIs: angiotensin converse enzyme inhibitors; ARBs: angiotensin II receptor blockers; CCBs: calcium channel blockers; NOD: new‐onset diabetes.
Ranking of antihypertensive medications by SUCRA
The probability of six antihypertensive medications having specific rank (1–6) and the probability of three kinds of ARBs having each specific rank (1–4) for the incidence of NOD are presented in Figure 4. SUCRA showed that both ARBs (SUCRA = 0.894) and ACEIs (SUCRA = 0.880) exhibited distinct advantages compared to the other four treatments and diuretics (SUCRA = 0.022) exhibited the last least reliable performance in comparison with other medications. Candesartan was considered to be more desirable than other ARBs (Table 3 and Table S1).
Figure 4.
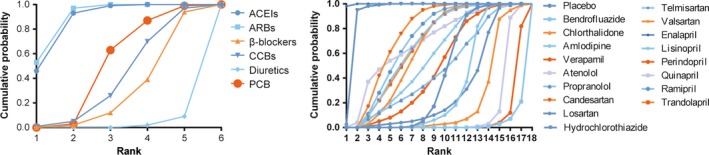
Rank graphs showing the probability of six different kinds of medications having each specific rank (1–6) and the probability of three kinds of ARBs having each specific rank (1–4) for end‐points. Ranking suggests the probability to be the best intervention treatment, the second best and so on. Rank 1st is best and Rank 6th is worst.
Table 3.
Surface under the cumulative ranking curve (SUCRA) of 18 treatments according to NOD
| Classification | Drugs | SUCRA | Mean rank |
|---|---|---|---|
| ACEI | Enalapril | 0.998 | 1 |
| ACEI | Ramipril | 0.700 | 4 |
| ACEI | Quinapril | 0.698 | 5 |
| ACEI | Trandolapril | 0.661 | 7 |
| ACEI | Lisinopril | 0.558 | 9 |
| ACEI | Perindopril | 0.485 | 10 |
| ARB | Candesartan | 0.735 | 3 |
| ARB | Telmisartan | 0.675 | 6 |
| ARB | Valsartan | 0.628 | 8 |
| ARB | Losartan | 0.306 | 14 |
| β‐Blocker | Propranolol | 0.475 | 11 |
| β‐Blocker | Atenolol | 0.122 | 16 |
| CCB | Amlodipine | 0.326 | 13 |
| CCB | Verapamil | 0.059 | 17 |
| Diuretic | Hydrochlorothiazide | 0.938 | 2 |
| Diuretic | Chlorthalidone | 0.203 | 15 |
| Diuretic | Bendrofluazide | 0.020 | 18 |
| Placebo | Placebo | 0.424 | 12 |
Assessing inconsistency between direct and indirect evidence
One fundamental assumption in our NMA is the adoption of a consistency model in which the extent of consistency is validated using the node splitting method. Results of direct, indirect and network comparisons of these interventions were displayed in node splitting forest plots as shown in Figure 5 and a P‐value of less than 0.05 suggests potentially significant inconsistency and hence, the consistency model assumption may be violated. The inconsistency only exists in comparison between PCB and diuretics (P‐value = 0.001). As it is the only case in which potential significant inconsistency may arise from, we did not replace the consistency model in our NMA.
Figure 5.
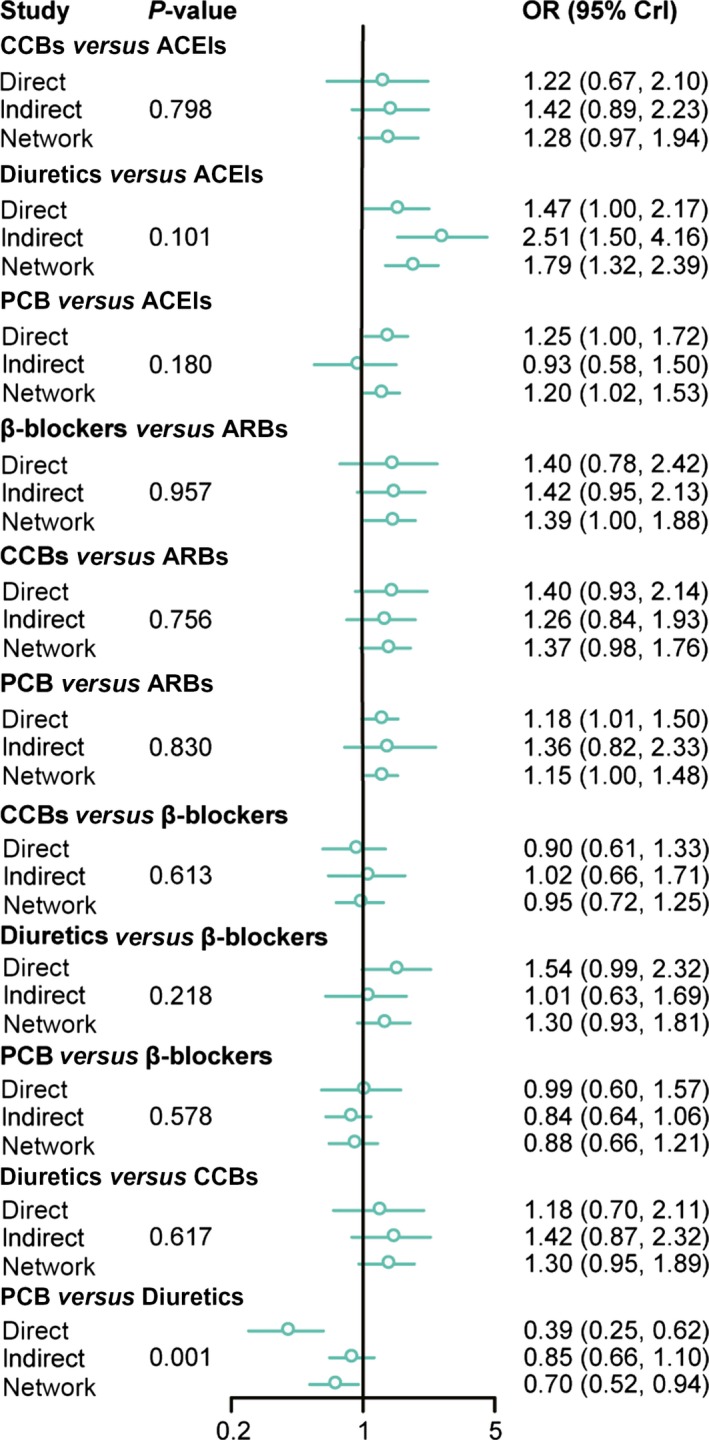
Summarized results of direct and indirect comparisons between ARBs, ACEIs, CCBs, β‐blockers, diuretic or PCB‐based strategy on the incidence of NOD. ACEIs: angiotensin converse enzyme inhibitors; ARBs: angiotensin II receptor blockers; CCBs: calcium channel blockers; NOD: new‐onset diabetes.
Discussion
Patients with hypertension usually have a higher risk of NOD which may trigger cardiovascular diseases 52. Preventing NOD among patients with hypertension has been considered as a prioritized task by clinicians. Current antihypertensive medications are generally divided into several classes, namely thiazide diuretic, ACEIs, ARBs, CCBs and β‐blockers 53. In this study, we collected data from 23 NOD studies which investigated six antihypertensive medications in order to assess their efficacy with respect to NOD prevention. We aimed to provide conclusive evidence for ranking these medications so that potential guidance with respect to medication selection can be recommended to clinicians.
In this study, the results of NMA showed that patients treated by ARBs or ACEIs were associated with a reduced risk of NOD compared to those with PCB, while diuretic appeared to be ineffective with respect to NOD prevention. ARBs also exhibited a better performance with respect to NOD prevention compared to CCBs or β‐blockers. As suggested by the overall rank, both ARBs and ACEIs, especially enalapril and candesartan, were more preferable than other treatments and hydrochlorothiazide also exhibited a reliable performance in comparison with other agents.
Previous studies demonstrated that the renin–angiotensin system was activated in all insulin resistant states in which type II diabetes or hypertension may be involved 54. Blocking the RAS not only improved blood circulation and cellular ionic balance of pancreatic and skeletal muscle cells, but also enhanced the effects of peripheral insulin and insulin secretion by promoting the recruitment and differentiation of adipocytes in diabetes 55. Recent studies showed that hypertensive patients treated by ACEIs or ARB were associated with a lower risk of NOD or adverse cardiovascular events 56, 57, 58 and such a mechanism may involve the improvements in both insulin sensitivity and secretion 59. However, ARBs and ACEIs have different mechanisms with respect to preventing insulin resistance. For instance, ACEIs inhibits the conversion from Ang I to Ang II and blocks the degradation of bradykinin whereas ARBs suppresses Ang II by selectively binding to the corresponding receptor site 60. In this study, both direct and indirect evidence confirmed that patients treated by ARBs or ACEIs were associated with a reduced risk of NOD compared to those with PCB, while diuretics appeared to be ineffective with respect to NOD prevention and ARBs also exhibited better performance with respect to NOD prevention compared to CCBs or β‐blockers. Compared to diuretics, ACEIs is potentially more cost‐effective for elderly hypertensive patients 1. The corresponding mechanism of ACEIs may be linked with the lack of major sympathoexcitatory effects improvements in insulin sensitivity 61.
Diuretics have been widely used for managing salt‐sensitive hypertension, and they are divided into diuretics, such as hydrochlorothiazide and thiazide‐like diuretics such as chlorthalidone and bendrofluazide 62. A recent study showed that hydrochlorothiazide was inferior to indapamide for improving endothelial functions and ventriculoarterial coupling in patients who suffered from both hypertension and diabetes 63. In this study, patients treated by ARBs or ACEIs were associated with a significant reduction in the incidence of NOD compared to those treated by bendrofluazide or chlorthalidone. However, patients treated by hydrochlorothiazide exhibited a significant reduction in the risk of NOD compared to those treated by ARBs, ACEIs, CCBs, β‐blockers or other diuretics except enalapril. CCBs and β‐blockers, as two other first‐line antihypertensive medications, are effective in preventing cardiovascular events 64 and are generally prescribed by clinicians as hypertension therapies 65. It has been shown that CCBs or β‐blockers had mild or no impact on the risk of NOD 66. Results from our study indicated that patients treated by CCBs or β‐blockers seemed to have equivalent risk of NOD.
As the first Bayesian NMA, our study compared six antihypertensive medications that were used in hypertensive patients for preventing NOD and the corresponding data were synthesized from the current literature. However, a few limitations contained in this study should be concerned and addressed in the future. Firstly, there was significant variation in the number of studies with respect to each comparison. For instance, the number of studies which compared losartan, propranolol, chlorthalidone or bendrofluazide was significantly less than that of others and this may result in wide confidence interval for summary statistics when data were synthesized. Secondly, variation in the sample size and study duration within each individual study as well as variation in other study characteristics may cause significant heterogeneity and thereby pooling evidence from individual studies with significant heterogeneity may not be comparable.
For summary, both ARBs and ACEIs exhibited compelling results with respect to the prevention of NOD and such a trend is even more significant in patients treated by enalapril (ACEIs) or candesartan (ARBs). Apart from that, hydrochlorothiazide also exhibited reliable performance in comparison with other agents. Large‐scale randomized trials should be designed and implemented to confirm the above conclusions.
Disclosure of interest
The authors declare that they have no conflict of interests concerning this article.
Supporting information
Figure S1. Network plot of eligible studies comparing 18 agents included in six different kinds of medications in NOD. The width of the lines represents the total number of trials for each comparison.
Table S1. Results of 17 antihypertensive agents and placebo for the incidence of new onset diabetes (NOD) from net‐work meta‐analysis.
References
- 1. Chowdhury EK, Ademi Z, Moss JR, et al Cost‐utility of angiotensin‐converting enzyme inhibitor‐based treatment compared with thiazide diuretic‐based treatment for hypertension in elderly Australians considering diabetes as comorbidity. Medicine. 2015; 94: e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liou YS, Chen HY, Tien L, et al Antihypertensive drug use and new‐onset diabetes in female patients with coronary artery disease: a population‐based longitudinal cohort study. Medicine (Baltimore). 2015; 94: e1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palepu S, Prasad GV. New‐onset diabetes mellitus after kidney transplantation: current status and future directions. World J Diabetes. 2015; 6: 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andraws R, Brown DL. Effect of inhibition of the renin‐angiotensin system on development of type 2 diabetes mellitus (meta‐analysis of randomized trials). Am J Cardiol. 2007; 99: 1006–12. [DOI] [PubMed] [Google Scholar]
- 5. Hassanin A, Malek HA. Effect of renin inhibition on adipokines in diabetic rats. Pak J Pharm Sci. 2014; 27: 767–72. [PubMed] [Google Scholar]
- 6. Vardeny O, Uno H, Braunwald E, et al Opposing effects of beta blockers and angiotensin‐converting enzyme inhibitors on development of new‐onset diabetes mellitus in patients with stable coronary artery disease. Am J Cardiol. 2011; 107: 1705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liou YS, Ma T, Tien L, et al The relationship between antihypertensive combination therapies comprising diuretics and/or beta‐blockers and the risk of new‐onset diabetes: a retrospective longitudinal cohort study. Hypertens Res. 2009; 32: 496–9. [DOI] [PubMed] [Google Scholar]
- 8. Tocci G, Paneni F, Palano F, et al Angiotensin‐converting enzyme inhibitors, angiotensin ii receptor blockers and diabetes: a meta‐analysis of placebo‐controlled clinical trials. Am J Hypertens. 2011; 24: 582–90. [DOI] [PubMed] [Google Scholar]
- 9. Geng DF, Jin DM, Wu W, et al Angiotensin converting enzyme inhibitors for prevention of new‐onset type 2 diabetes mellitus: a meta‐analysis of 72,128 patients. Int J Cardiol. 2013; 167: 2605–10. [DOI] [PubMed] [Google Scholar]
- 10. Song HF, Wang S, Li HW. Effect of angiotensin receptor blockers in the prevention of type 2 diabetes and cardiovascular events: a meta‐analysis of randomized trials. Chin Med J. 2012; 125: 1804–10. [PubMed] [Google Scholar]
- 11. Ricci F, Di Castelnuovo A, Savarese G, et al Ace‐inhibitors versus angiotensin receptor blockers for prevention of events in cardiovascular patients without heart failure ‐ a network meta‐analysis. Int J Cardiol. 2016; 217: 128–34. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJ, Holman RR, Haffner SM, et al Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010; 362: 1477–90. [DOI] [PubMed] [Google Scholar]
- 13. Ahmad MA, Kapur P, Khanam R, et al Comparative effect of antihypertensive therapy on blood glucose level in hypertensive patients in an Indian population. Drug Res (Stuttg). 2014; 64: 276–80. [DOI] [PubMed] [Google Scholar]
- 14. Garcia‐Egido A, Andrey JL, Puerto JL, et al Beta‐blocker therapy and prognosis of heart failure patients with new‐onset diabetes mellitus. Int J Clin Pract. 2015; 69: 550–9. [DOI] [PubMed] [Google Scholar]
- 15. Karnes JH, Gong Y, Arwood MJ, et al Alteration in fasting glucose after prolonged treatment with a thiazide diuretic. Diabetes Res Clin Pract. 2014; 104: 363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abuissa H, Jones PG, Marso SP, et al Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta‐analysis of randomized clinical trials. J Am Coll Cardiol. 2005; 46: 821–6. [DOI] [PubMed] [Google Scholar]
- 17. Bangalore S, Kumar S, Wetterslev J, et al Angiotensin receptor blockers and risk of myocardial infarction: meta‐analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ. 2011; 342: d2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bangalore S, Parkar S, Grossman E, et al A meta‐analysis of 94,492 patients with hypertension treated with beta blockers to determine the risk of new‐onset diabetes mellitus. Am J Cardiol. 2007; 100: 1254–62. [DOI] [PubMed] [Google Scholar]
- 19. Cheung BMY, Cheung GTY, Lauder IJ, et al Meta‐analysis of large outcome trials of angiotensin receptor blockers in hypertension. J Hum Hypertens. 2006; 20: 37–43. [DOI] [PubMed] [Google Scholar]
- 20. Geng DF, Jin DM, Wu W, et al Angiotensin receptor blockers for prevention of new‐onset type 2 diabetes: a meta‐analysis of 59,862 patients. Int J Cardiol. 2012; 155: 236–42. [DOI] [PubMed] [Google Scholar]
- 21. Gillespie EL, White CM, Kardas M, et al The impact of ace inhibitors or angiotensin ii type 1 receptor blockers on the development of new‐onset type 2 diabetes. Diabetes Care. 2005; 28: 2261–6. [DOI] [PubMed] [Google Scholar]
- 22. Mason JM, Dickinson HO, Nicolson DJ, et al The diabetogenic potential of thiazide‐type diuretic and beta‐blocker combinations in patients with hypertension. J Hypertens. 2005; 23: 1777–81. [DOI] [PubMed] [Google Scholar]
- 23. Saha SA, Molnar J, Arora RR. Tissue ace inhibitors for secondary prevention of cardiovascular disease in patients with preserved left ventricular function: a pooled meta‐analysis of randomized placebo‐controlled trials. J Cardiovasc Pharmacol Ther. 2007; 12: 192–204. [DOI] [PubMed] [Google Scholar]
- 24. Savarese G, Costanzo P, Cleland JGF, et al A meta‐analysis reporting effects of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. J Am Coll Cardiol. 2013; 61: 131–42. [DOI] [PubMed] [Google Scholar]
- 25. Snedecor SJ, Ph D, Patel DA, et al From pairwise to network meta‐analyses. In: Biondi‐Zoccai G, editor. 2014. pp. 21–41.
- 26. Jackson D, White IR, Thompson SG. Extending dersimonian and laird's methodology to perform multivariate random effects meta‐analyses. Stat Med. 2010; 29: 1282–97. [DOI] [PubMed] [Google Scholar]
- 27. Day NE, Byar DP. Testing hypotheses in case‐control studies–equivalence of mantel‐haenszel statistics and logit score tests. Biometrics. 1979; 35: 623–30. [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 29. Braunwald E, Domanski MJ, Fowler SE, et al Angiotensin‐converting‐enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004; 351: 2058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dahlof B, Sever PS, Poulter NR, et al Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian cardiac outcomes trial‐blood pressure lowering arm (ascot‐bpla): a multicentre randomised controlled trial. Lancet. 2005; 366: 895–906. [DOI] [PubMed] [Google Scholar]
- 31. Fox KM, Investigators EUtOrocewPiscAd . Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double‐blind, placebo‐controlled, multicentre trial (the Europa study). Lancet. 2003; 362: 782–8. [DOI] [PubMed] [Google Scholar]
- 32. Granger CB, McMurray JJ, Yusuf S, et al Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function intolerant to angiotensin‐converting‐enzyme inhibitors: the charm‐alternative trial. Lancet. 2003; 362: 772–6. [DOI] [PubMed] [Google Scholar]
- 33. Group NS , McMurray JJ, Holman RR, et al Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010; 362: 1477–90. [DOI] [PubMed] [Google Scholar]
- 34. Investigators DT, Bosch J, Yusuf S, et al Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006; 355: 1551–62. [DOI] [PubMed] [Google Scholar]
- 35. Julius S, Kjeldsen SE, Weber M, et al Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the value randomised trial. Lancet. 2004; 363: 2022–31. [DOI] [PubMed] [Google Scholar]
- 36. Lindholm LH, Ibsen H, Dahlof B, et al Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction in hypertension study (life): a randomised trial against atenolol. Lancet. 2002; 359: 1004–10. [DOI] [PubMed] [Google Scholar]
- 37. Lithell H, Hansson L, Skoog I, et al The study on cognition and prognosis in the elderly (scope): principal results of a randomized double‐blind intervention trial. J Hypertens. 2003; 21: 875–86. [DOI] [PubMed] [Google Scholar]
- 38. McMurray JJ, Ostergren J, Swedberg K, et al Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function taking angiotensin‐converting‐enzyme inhibitors: the charm‐added trial. Lancet. 2003; 362: 767–71. [DOI] [PubMed] [Google Scholar]
- 39. Party MRCW . Mrc trial of treatment of mild hypertension: principal results. Medical research council working party. Br Med J (Clin Res Ed) 1985; 291: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Officers A, Coordinators for the ACRGTA, Lipid‐Lowering Treatment to Prevent Heart Attack T . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (allhat). JAMA. 2002; 288: 2981–97. [DOI] [PubMed] [Google Scholar]
- 41. Ogihara T, Nakao K, Fukui T, et al Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension. 2008; 51: 393–8. [DOI] [PubMed] [Google Scholar]
- 42. Pepine CJ, Handberg EM, Cooper‐DeHoff RM, et al A calcium antagonist vs a non‐calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The international verapamil‐trandolapril study (invest): a randomized controlled trial. JAMA. 2003; 290: 2805–16. [DOI] [PubMed] [Google Scholar]
- 43. Pfeffer MA, Swedberg K, Granger CB, et al Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the charm‐overall programme. Lancet. 2003; 362: 759–66. [DOI] [PubMed] [Google Scholar]
- 44. Rouleau JL, Warnica WJ, Baillot R, et al Effects of angiotensin‐converting enzyme inhibition in low‐risk patients early after coronary artery bypass surgery. Circulation. 2008; 117: 24–31. [DOI] [PubMed] [Google Scholar]
- 45. Telmisartan Randomised AssessmeNt Study in ACEiswcDI , Yusuf S, Teo K, et al Effects of the angiotensin‐receptor blocker telmisartan on cardiovascular events in high‐risk patients intolerant to angiotensin‐converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008; 372: 1174–83. [DOI] [PubMed] [Google Scholar]
- 46. Vermes E, Ducharme A, Bourassa MG, et al Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the studies of left ventricular dysfunction (solvd). Circulation. 2003; 107: 1291–6. [DOI] [PubMed] [Google Scholar]
- 47. Wilhelmsen L, Berglund G, Elmfeldt D, et al Beta‐blockers versus diuretics in hypertensive men: main results from the happhy trial. J Hypertens. 1987; 5: 561–72. [DOI] [PubMed] [Google Scholar]
- 48. Wing LM, Reid CM, Ryan P, et al A comparison of outcomes with angiotensin‐converting–enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003; 348: 583–92. [DOI] [PubMed] [Google Scholar]
- 49. Yusuf S, Diener HC, Sacco RL, et al Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008; 359: 1225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yusuf S, Gerstein H, Hoogwerf B, et al Ramipril and the development of diabetes. JAMA. 2001; 286: 1882–5. [DOI] [PubMed] [Google Scholar]
- 51. Yusuf S, Pfeffer MA, Swedberg K, et al Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the charm‐preserved trial. Lancet. 2003; 362: 777–81. [DOI] [PubMed] [Google Scholar]
- 52. Alderman MH. New onset diabetes during antihypertensive therapy. Am J Hypertens. 2008; 21: 493–9. [DOI] [PubMed] [Google Scholar]
- 53. Virk SA, Donaghue KC, Wong TY, et al Interventions for diabetic retinopathy in type 1 diabetes: systematic review and meta‐analysis. Am J Ophthalmol. 2015; 160: e4. [DOI] [PubMed] [Google Scholar]
- 54. Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013; 15: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444: 840–6. [DOI] [PubMed] [Google Scholar]
- 56. Mancia G, Grassi G, Zanchetti A. New‐onset diabetes and antihypertensive drugs. J Hypertens. 2006; 24: 3–10. [DOI] [PubMed] [Google Scholar]
- 57. Chowdhury EK, Owen A, Ademi Z, et al Short‐ and long‐term survival in treated elderly hypertensive patients with or without diabetes: findings from the second Australian national blood pressure study. Am J Hypertens. 2014; 27: 199–206. [DOI] [PubMed] [Google Scholar]
- 58. Gupta AK, Dahlof B, Dobson J, et al Determinants of new‐onset diabetes among 19,257 hypertensive patients randomized in the Anglo‐Scandinavian cardiac outcomes trial–blood pressure lowering arm and the relative influence of antihypertensive medication. Diabetes Care. 2008; 31: 982–8. [DOI] [PubMed] [Google Scholar]
- 59. Scheen AJ. Renin‐angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta‐analysis of randomised clinical trials. Diabetes Metab. 2004; 30: 487–96. [DOI] [PubMed] [Google Scholar]
- 60. Basile JN. Antihypertensive therapy, new‐onset diabetes, and cardiovascular disease. Int J Clin Pract. 2009; 63: 656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seravalle G, Brambilla G, Pizzalla DP, et al Differential effects of enalapril‐felodipine versus enalapril‐lercanidipine combination drug treatment on sympathetic nerve traffic and metabolic profile in obesity‐related hypertension. J Am Soc Hypertens. 2016; 10: 244–51. [DOI] [PubMed] [Google Scholar]
- 62. Roush GC, Sica DA. Diuretics for hypertension: a review and update. Am J Hypertens. 2016; 29: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 63. Vinereanu D, Dulgheru R, Magda S, et al The effect of indapamide versus hydrochlorothiazide on ventricular and arterial function in patients with hypertension and diabetes: results of a randomized trial. Am Heart J. 2014; 168: 446–56. [DOI] [PubMed] [Google Scholar]
- 64. Costanzo P, Perrone‐Filardi P, Petretta M, et al Calcium channel blockers and cardiovascular outcomes: a meta‐analysis of 175,634 patients. J Hypertens. 2009; 27: 1136–51. [DOI] [PubMed] [Google Scholar]
- 65. Yamamoto Y, Sonoyama K, Matsubara K, et al The status of hypertension management in Japan in 2000. Hypertens Res. 2002; 25: 717–25. [DOI] [PubMed] [Google Scholar]
- 66. Taylor EN, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006; 29: 1065–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Network plot of eligible studies comparing 18 agents included in six different kinds of medications in NOD. The width of the lines represents the total number of trials for each comparison.
Table S1. Results of 17 antihypertensive agents and placebo for the incidence of new onset diabetes (NOD) from net‐work meta‐analysis.


