Abstract
The aim of the study was to compare clinical outcomes and toxicity between 3D conformal radiotherapy (3DCRT) and image-guided intensity-modulated radiotherapy (IG-IMRT) administered through helical tomotherapy in locally advanced rectal cancer (LARC) patients receiving preoperative chemoradiotherapy. We reviewed 144 patients with Stage II–III rectal cancer receiving preoperative fluoropyrimidine-based chemoradiotherapy followed by radical resection. Tumor responses following chemoradiotherapy were evaluated using the Dworak tumor regression grade (TRG). Of the 144 patients, 45 received IG-IMRT and 99 received 3DCRT. A significant reduction in Grade 3 or 4 acute gastrointestinal toxicity (IG-IMRT, 6.7%; 3DCRT, 15.1%; P = 0.039) was observed by IG-IMRT. The pathologic complete response (pCR) rate did not differ between the IG-IMRT and the 3DCRT group (17.8% vs 15.1%, P = 0.52). Patients in the IG-IMRT group had the trend of favorable tumor regressions (TRG 3 or 4) compared with those in the 3DCRT group (66.7% vs 43.5%, P = 0.071). The median follow-up was 53 months (range, 18–95 months) in the 3DCRT group and 43 months (range, 17–69 months) in the IG-IMRT group. Four-year overall, disease-free, and local failure–free survival rates of the IG-IMRT and 3DCRT groups were 81.6% and 67.9% (P = 0.12), 53.8% and 51.8% (P = 0.51), and 88% and 75.1% (P = 0.031), respectively. LARC patients treated with preoperative IG-IMRT achieved lower acute gastrointestinal adverse effects and a higher local control rate than those treated with 3DCRT, but there was no prominent difference in distant metastasis rate and overall survival between two treatment modalities.
Keywords: image guidance, locally advanced rectal cancer, tomotherapy, conformal, toxicity, chemoradiotherapy
INTRODUCTION
Preoperative concurrent chemoradiotherapy (CCRT) followed by radical resection is currently the standard treatment for locally advanced rectal cancer (LARC) [1–3]. Although CCRT improves local control, the multimodality therapy contributes to several treatment-related toxic events. Of the numerous toxicities, some adverse events have been attributed to radiotherapy (RT) [4–6].
With advances in RT, high conformal radiation coverage of the target volumes with sparing of the surrounding normal tissues can be achieved through intensity-modulated radiotherapy (IMRT). Moreover, with the integration of image-guided radiotherapy (IGRT) and IMRT, a small planning target volume (PTV) margin can be applied, consequently further reducing potential radiation toxicity. Numerous dosimetric studies have reported that in pelvic malignancy irradiation, radiation to the small bowel, bladder, and rectum is lower in IMRT than in 3D conformal radiotherapy (3DCRT) [7–11]. However, few studies have compared the clinical outcomes directly between image-guided IMRT (IG-IMRT) and 3DCRT in LARC patients receiving preoperative CCRT. Therefore, we retrospectively analyzed the clinical outcomes and toxicities between IG-IMRT and 3DCRT in LARC patients at a single institution.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the medical records of LARC patients treated with preoperative CCRT followed by radical resection between May 2006 and January 2015. The inclusion criteria were as follows: (i) pathologically proven rectal adenocarcinoma, (ii) locally advanced diseases (clinical T3–4 or nodal involvement), (iii) tumors located within 15 cm of the anal verge, (iv) patients receiving preoperative CCRT followed by intentional radical resection, and (v) no evidence of distant metastasis at diagnosis. The exclusion criteria were local excision of tumor, history of prior pelvic irradiation, and history of malignancies other than rectal cancer. The study was approved by the institutional ethics committee of our hospital.
Pretreatment evaluation entailed a complete history review and physical examination, colonoscopy, tumor biopsy, chest radiography, abdominal computed tomography (CT) and pelvic magnetic resonance imaging, serum carcinoembryonic antigen (CEA) level assessment, and routine laboratory studies. The tumor stage was classified according to the seventh edition of the AJCC Cancer Staging Manual and Handbook [12].
RT technique
All patients received planning CT in the supine position after they were immobilized using custom thermoplastic immobilization devices. All patients were asked to void and then drink 200 ml of water 30 min before CT simulation and each treatment. 3DCRT was delivered using the 2100 C/D linear accelerator (Varian Medical system, Palo Alto, CA). For the 3DCRT plan, a three-field technique included two opposed lateral fields and one posterior–anterior field with wedges and photon energy of 10 MV was used. RT was administered to the whole pelvis at a dose of 45 Gy in 25 fractions. Radiation portal fields were determined using the following rules: (i) superior border: L5–S1 interspace, (ii) inferior border: 3–4 cm below the primary tumor, (iii) lateral border: 1.5 cm outside the true bony pelvis, (iv) posterior margin: 1.5 cm behind the anterior bony sacral margin, and (v) anterior border: posterior border of the symphysis pubis.
IG-IMRT was administered using a Hi-Art system (TomoTherapy Inc., Madison, WI). A fixed-jaw mode with a field width of 2.5 or 5 cm was used for treatment planning, depending on overall treatment time. Pitch varied from 0.215 to 0.287. The modulation factor ranged from 2 to 3, depending on homogeneity and conformity index. The gross tumor volume was defined as rectal tumors and clustered lymph nodes or lymph nodes with diameter >1 cm. The clinical target volume (CTV_45 Gy) included the primary tumor, the mesorectum, the sacral canal, and the following lymph nodes: perirectal, presacral, hypogastric, obturator, and internal iliac. If the tumor had invaded the prostate gland, bladder, or vagina, the CTV_45 Gy was extended to cover the involved organ and the external iliac nodal regions. For lesions with anal invasion, the perineum and bilateral inguinal lymph nodes were included in the CTV_45 Gy. A total dose of 45 Gy in 25 fractions was delivered to the planning target volume (PTV_45 Gy), with a simultaneous integrated boost of 0.2 Gy per day for the primary tumor up to a total dose of 50 Gy. PTV with a superior, inferior, and radical margin of 5–7 mm was added to the CTV_45 Gy. A more conservative PTV margin of 3 mm was added to the CTV_50 Gy as a boost to the primary tumor. The treatment plan was accepted if ≥95% of the PTV received the prescribed dose. The objective of sparing normal tissue was to reduce the volume of the small bowel irradiated with doses >15 Gy and to maintain mean bladder doses at <21 Gy. The bowel volume included individual bowel loops extending to 1.5 cm above the field edge. Before each treatment, patients were repositioned according to daily image guidance through megavoltage CT, which was coregistered with planning kilovoltage CT.
Chemotherapy and surgery
Concomitant with RT, chemotherapy, including bolus infusional 5-fluorouracil (5-FU) at 350 mg/m2 during the first and fifth weeks of radiotherapy or capecitabine at 850 mg/m2 twice daily for 5 days/week during the radiotherapy, was administered. Patients underwent total mesorectal excision (TME) 6–8 weeks after CCRT completion. Patients were recommended to receive adjuvant chemotherapy if they had one of the following risk factors: (i) pathologic lymph node metastasis, (ii) positive resection or circumferential margin, or (iii) pathologic T3–4 lesion [13]. Adjuvant 5-FU–based chemotherapy consisted of one of the following two regimens: (i) four cycles of monthly bolus injections of 5-FU (350 mg/m2/day) on Day 1–5; or (ii) six cycles of capecitabine 850 mg/m2 twice daily for 14 days, followed by 7 days rest after each cycle.
Pathology review
Two experienced pathologists analyzed all resected specimens, using the standard method. The pathologic stage (ypT and ypN), histologic grade, lymphovascular invasion, perineural invasion, circumferential resection margin status, and tumor regression grade were documented. The tumor response following chemoradiotherapy was evaluated according to the Dworak tumor regression grade (TRG) [14]. Responders were defined as patients with a TRG of 3 or 4 and non-responders as those with a TRG of 0–2.
Toxicity evaluation
Acute toxicity was evaluated weekly according to the Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/reporting/ctc.html). Late toxicity was graded according to the objective criteria of the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer scale, depending on the late adverse effects of RT [15].
Statistical analysis
The continuous parameters were represented as the median and range, while frequency and percentage were given for the categorical variables. The Chi-square test and the paired t test were used for comparison of categorical and continuous variables, respectively. Survival times were estimated using the Kaplan–Meier method and compared using the log-rank test. A Cox proportional hazards model was used to evaluate the association between clinical parameters and survival using the backward stepwise procedure. Data analyses were performed using the JMP software (version 9.0, SAS Institute Inc., Cary, NC). Results were considered significant at P < 0.05.
RESULTS
Patient demographics
The medical files of 172 LARC patients undergoing CCRT were retrieved for an initial analysis between May 2006 and January 2015. Of the 172 patients, 7 were lost to follow-up, 10 presented evidence of distant metastases at the initial diagnosis, 5 refused to undergo radical resection, and 6 received RT because of recurrent rectal cancer; these patients were excluded. Thus, 144 patients were enrolled into our study. Among the 144 patients in the current study, 45 patients received IG-IMRT and 99 patients received 3DCRT. The patient characteristics by treatment group are summarized in Table 1. No significant differences were observed between the IG-IMRT and 3DCRT groups.
Table 1.
Patient characteristics in IG-IMRT and 3DCRT groups
| Characteristics | IG-IMRT n = 45 |
3DCRT n = 99 |
P |
|---|---|---|---|
| Age, median, year (range) | 64 (37–87) | 61 (34–85) | 0.183 |
| Gender | 0.189 | ||
| Female | 23 (51.1) | 39 (39.4) | |
| Male | 22 (48.9) | 60 (60.6) | |
| Clinical tumor depth | 0.118 | ||
| T2 | 5 (11.1) | 9 (9.1) | |
| T3 | 35 (77.8) | 74 (74.7) | |
| T4 | 5 (11.1) | 16 (16.2) | |
| Clinical lymph node metastasis | 0.731 | ||
| N0 | 12 (26.6) | 22 (22.2) | |
| N1 | 19 (42.2) | 40 (40.4) | |
| N2 | 14 (31.2) | 37 (37.4) | |
| Distance from anal verge (cm) | 0.073 | ||
| <5 | 32 (71.1) | 55 (55.5) | |
| 5–10 | 12 (26.7) | 34 (34.3) | |
| 11–15 | 1 (2.2) | 10 (10.2) | |
| Tumor differentiation | 0.524 | ||
| Well | 4 (8.8) | 4 (4) | |
| Moderately | 38 (84.4) | 88 (88.9) | |
| Poorly | 3 (6.8) | 7 (7.1) | |
| Pretreatment CEA (ng/ml) | 0.745 | ||
| ≤5 | 16 (35.6) | 38 (38.4) | |
| >5 | 29 (64.4) | 61 (61.6) | |
| Concurrent chemotherapy | 0.093 | ||
| Infusion 5-FU | 15 (33.3) | 54 (54.5) | |
| Capecitabine | 30 (66.7) | 45 (45.5) | |
| Type of surgery | 0.773 | ||
| Lower anterior resection | 39 (88.9) | 86 (86.8) | |
| Abdominoperineal resection | 5 (11.1) | 13 (13.2) | |
| Anal-preserving surgery (for low rectal tumor) | |||
| Yes | 27 (84.4) | 42 (76.4) | 0.366 |
| No | 5 (15.6) | 13 (23.6) | |
| Median RT dose, Gy (range) | 50 (45–50) | 50.4 (41.4–50.4) | 0.692 |
| Adjuvant chemotherapy | 0.186 | ||
| Yes | 21 (46.7) | 53 (53.5) | |
| No | 24 (53.3) | 46 (46.5) | |
| Median follow-up, month (range) | 43 (17–69) | 53 (18–95) |
Data are presented as n (%), unless otherwise indicated. IG-IMRT = image-guided intensity-modulated radiotherapy, 3DCRT = 3D conformal radiotherapy, CEA = carcinoembryonic antigen, 5-FU = 5-fluorouracil, RT = radiotherapy.
Dose–volume histogram and acute toxicity
Table 2 presents the dose–volume histogram data between the two treatment groups. IG-IMRT significantly decreased the high dose to the small bowel and the mean bladder dose compared with 3DCRT. Acute and late toxicities are summarized in Table 3. Only one patient experienced Grade 4 toxicity in the study. In Grade 3 or 4 (Grade 3+) overall acute toxicities, IG-IMRT resulted in significantly less overall Grade 3+ toxicity than 3DCRT (8.9% vs 20.2%, P = 0.042). The decreased incidence of Grade 3 or 4 acute gastrointestinal (GI) toxicity in the IG-IMRT group contributed to this reduction in overall Grade 3+ toxicity (6.7% vs 15.1%; P = 0.039). Of the three patients experiencing Grade 3 acute GI toxicity in the IG-IMRT group, two had diarrhea and one patient had proctitis. Of the 15 patients experiencing Grade 3+ acute GI toxicity in the 3DCRT group, 9 had diarrhea, 5 had proctitis, and 1 had nausea. The two treatment groups did not differ significantly in Grade 3+ acute hematologic and genitourinary (GU) toxicity.
Table 2.
Dose–volume histogram data between IG-IMRT and 3DCRT
| IG-IMRT | 3DCRT | P | |
|---|---|---|---|
| GTV volume (cm3) | 67 ± 59 | 72 ± 62 | 0.362 |
| PTV volume (cm3) | 799 ± 355 | 812 ± 383 | 0.428 |
| SB volume (cm3) | 611 ± 314 | 682 ± 364 | 0.246 |
| bladder volume (cm3) | 162 ± 103 | 132 ± 98 | 0.193 |
| V5-SB (cm3) | 482 ± 244 | 372 ± 262 | 0.003 |
| V10-SB (cm3) | 392 ± 173 | 353 ± 218 | 0.089 |
| V15-SB (cm3) | 226 ± 99 | 255 ± 185 | 0.032 |
| V20-SB (cm3) | 97 ± 49 | 188 ± 126 | 0.012 |
| V25-SB (cm3) | 43 ± 26 | 176 ± 131 | 0.008 |
| V30-SB (cm3) | 18 ± 18 | 161 ± 118 | 0.002 |
| V35-SB (cm3) | 10 ± 14 | 157 ± 112 | 0.001 |
| V40-SB (cm3) | 4 ± 9 | 145 ± 120 | <0.001 |
| V45-SB (cm3) | 0.7 ± 2 | 137 ± 104 | <0.001 |
| Mean bladder dose (Gy) | 18.6 ± 6 | 27.2 ± 14.9 | 0.015 |
| V21-bladder (%) | 36 ± 27 | 50 ± 36 | 0.023 |
Data are presented as mean ± standard deviation. IG-IMRT = image-guided intensity-modulated radiotherapy, 3DCRT = 3D conformal radiotherapy, GTV = gross tumor volume, PTV = planning target volume, SB = small bowel, V dose = the percentage of the organ at least covered by each dose.
Table 3.
Comparison of toxicity and treatment breaks in IG-IMRT and 3DCRT groups
| IG-IMRT n = 45 |
3DCRT n = 99 |
P | |
|---|---|---|---|
| Acute toxicity | |||
| Overall Grade 3 or 4 toxicity | 4 (8.9) | 20 (20.2) | 0.042 |
| Skin | 96 (97.0) | 0.132 | |
| Grade 0–2 | 44 (97.8) | 96 (97.0) | |
| Grade 3/4 | 1 (2.2)/0 (0) | 3 (3.0)/0 (0) | |
| GI | 0.039 | ||
| Grade 0–2 | 42 (93.3) | 84 (84.8) | |
| Grade 3/4 | 3 (6.7)/0 (0) | 14 (14.1)/1 (1.1) | |
| GU | 0.618 | ||
| Grade 0–2 | 45 (100) | 96(97.0) | |
| Grade 3/4 | 0 (0)/0 (0) | 3 (3.0)/0 (0) | |
| Hematological | 0.234 | ||
| Grade 0–2 | 45 (100) | 97 (98.9) | |
| Grade 3/4 | 0 (0)/0 (0) | 2 (2.0)/0 (0) | |
| Treatment break | 1 (2.2) | 8 (8.1) | 0.178 |
| Postoperative complications | |||
| Anastomotic leakage | 2 (5.1) | 7 (8.1) | 0.313 |
| Pelvic abscess | 1 (2.5) | 3 (3.5) | 0.209 |
| Late toxicity | |||
| Overall Grade 3 or 4 toxicity | 4 (8.9) | 13 (13.1) | 0.216 |
| GI | 0.071 | ||
| Grade 1–2 | 43 (95.6) | 89 (89.9) | |
| Grade 3/4 | 2 (4.4)/0 (0) | 10 (10.1)/0 (0) | |
| GU | 0.781 | ||
| Grade 1–2 | 45 (100) | 96 (96.9) | |
| Grade 3/4 | 0 (0)/0 (0) | 3 (3.1)/0 (0) |
Data are presented as n (%). IG-IMRT = image-guided intensity-modulated radiotherapy, 3DCRT = 3D conformal radiotherapy, GI = gastrointestinal, GU = genitourinary.
All but two patients completed the planned radiation regimen in the current study, and those two patients were treated with 3DCRT. The RT course of one patient in the IG-IMRT group was interrupted because of Grade 3 diarrhea, and the RT course of eight patients in the 3DCRT group was interrupted: five for Grade 3 diarrhea, two for Grade 3 dermatitis, and one for Grade 3 leukopenia. The median RT duration was significantly shorter in the IG-IMRT group compared with that in the 3DCRT group (35 days vs 40 days, P = 0.016).
Post-operative complication and late toxicity
Among patients receiving low anterior resection, 2 of the 39 patients (5.1%) in the IM-IGRT group and 7 of the 86 patients (8.1%) in the 3DCRT group had anastomotic leakage. Of these patients, one in the IG-IMRT group and three in the 3DCRT group had pelvic abscess requiring percutaneous CT-guided drainage. All patients recovered uneventfully after medical or surgical treatments. No treatment-related deaths were reported.
Grade 3 or higher late GI toxicity was reduced in the IG-IMRT group (4.4%) versus in the 3DCRT group (10.1%), but this did not reach statistical significance (P = 0.071; Table 3). In the IG-IMRT group, one patient with Grade 3 small bowel obstruction 18 months after operation underwent surgical resection. Surgical intervention was performed on one patient who developed a colovaginal fistula 11 months postoperatively. No patient in the IG-IMRT group experienced Grade 3 or 4 late GU toxicity. In the 3DCRT group, five patients had Grade 3 small bowel obstructions, which were observed 12, 16, 21, 25 and 26 months after operation, respectively. Two patients had a Grade 3 anastomotic stenosis 11 and 19 months postoperatively. Grade 3 colitis was observed in two patients 13 and 22 months postoperatively. Two patients in the 3DCRT group had Grade 3 ureter strictures with symptomatic hydronephrosis requiring long-term double J catheter insertion; one had ureter stricture 20 months after surgery, and the other had the toxicity 27 months postoperatively. One patient had a Grade 3 rectovesical fistula 22 months postoperatively and required surgery.
Surgery and pathologic response
All patients completed TME. The median time from the end of RT to surgery was 7 weeks (range, 6–8 weeks). The median time interval between radiotherapy and surgery was no different between the IMRT group and the 3DCRT group (7 vs 7 weeks, P = 1.00). The pathologic characteristics are detailed in Table 4. There were more ypT3 and ypT4 patients in the 3DCRT group compared with in the IG-IMRT group (51.4% vs 40%, P = 0.22); however, the difference did not reach statistical significance. In addition, IG-IMRT resulted in favorable tumor regressions (TRG 3 or 4) compared with 3DCRT (66.7% vs 43.5%, P = 0.071). Downstaging of the T category from cT3–4 to ypT0–2 was observed in 24 (60%) and 53 (58.8%) patients in the IG-IMRT and 3DCRT groups, respectively (P = 0.91). Nodal downstaging from the clinically positive lymph node status to ypN0 was observed in 25 (75.7%) and 62 (79.4%) patients in the IG-IMRT and 3DCRT groups, respectively (P = 0.67). Of the 144 patients in this study, a pathologic complete response (pCR) was achieved in 23 patients (16%). The pCR rate did not differ between the IG-IMRT and 3DCRT groups (17.8% vs 15.1%, P = 0.53). In the 3DCRT group, 2 patients exhibited ypT0N1a and ypT0N1c, and neither of them developed recurrence after 2 years of follow-up.
Table 4.
Pathology characteristics in IG-IMRT and 3DCRT groups
| Characteristics | IG-IMRT n = 45 |
3DCRT n = 99 |
P |
|---|---|---|---|
| Pathologic tumor depth | 0.221 | ||
| ypT0 | 8 (17.8) | 17 (17.2) | |
| ypT1 | 2 (4.4) | 5 (5.1) | |
| ypT2 | 17 (37.8) | 26 (26.3) | |
| ypT3 | 17 (37.8) | 44 (44.4) | |
| pT4 | 1 (2.2) | 7 (7) | |
| Pathologic lymph node metastasis | 0.832 | ||
| pN0 | 34 (75.6) | 70 (70.7) | |
| ypN1 | 8 (17.8) | 21 (21.2) | |
| ypN2 | 3 (6.6) | 8 (8.1) | |
| Pathologic complete response | 8 (17.8) | 15 (15.1) | 0.527 |
| Median number of resected nodesa | 12 (0–21) | 10 (0–28) | 0.795b |
| Median number of involved nodesa | 0 (0–9) | 0 (0–13) | 0.321b |
| Tumor regression grade | 0.092 | ||
| 0 | 1 (2.2) | 8 (8.1) | |
| 1 | 6 (13.3) | 24 (24.2) | |
| 2 | 8 (17.8) | 24 (24.2) | |
| 3 | 22 (48.9) | 26 (26.3) | |
| 4 | 8 (17.8) | 17 (17.2) | |
| Treatment responsec | 0.071 | ||
| Favorable | 30 (66.7) | 43 (43.5) | |
| Unfavorable | 15 (33.3) | 56 (56.5) | |
| Surgical distal margin | 0.383 | ||
| Negative | 42 (93.3) | 92 (92.9) | |
| Positive | 3 (6.7) | 7 (7.1) | |
| Circumferential resection margin | 0.376 | ||
| Negative | 43 (95.6) | 96 (96.9) | |
| Positive | 2 (4.4) | 3 (3.1) | |
| Perineural invasion | 0.136 | ||
| Negative | 35 (77.8) | 65 (65.6) | |
| Positive | 10 (22.2) | 34 (34.4) | |
| Lymphovascular invasion | 0.699 | ||
| Negative | 38 (84.4) | 86 (86.8) | |
| Positive | 7 (15.6) | 13 (13.2) | |
| Tumor differentiation | 0.823 | ||
| Well | 6 (13.3) | 20 (20.2) | |
| Moderately | 36 (80.0) | 77 (77.8) | |
| Poorly | 3 (6.7) | 2 (2.0) |
Data are presented as n (%), unless otherwise indicated.
aMedian (range).
bt-test.
cFavorable treatment responses included TRG 3 or 4; unfavorable treatment responses included TRG 0–2. IG-IMRT = image-guided intensity-modulated radiotherapy, 3DCRT = 3D conformal radiotherapy.
Failure patterns and survival data
The median follow-up was 53 months (range, 18–95 months) in the 3DCRT group and 43 months (range, 17–69 months) in the IG-IMRT group. There were 5 patients in the IG-IMRT group who died; 4 deaths were due to tumor progressions and one died from a heart attack. In the 3DCRT groups, 22 patients died from recurrent tumors, 3 patients died from cardiovascular events and one patient had a fatal car accident. The most common site for local relapse was the presacral space (56.5%), followed by the anastomotic site (39.1%); however, there was no significant difference in local failure sites between the two treatment groups.
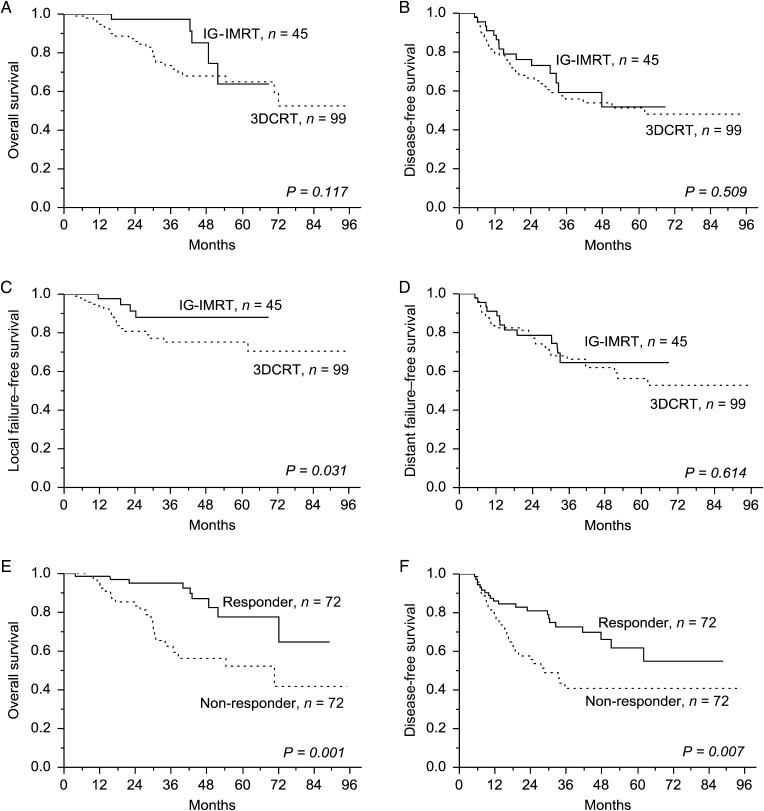
The 4-year overall survival (OS), disease-free survival (DFS), local failure–free survival (LFFS), and distant failure–free survival (DFFS) rates of patients in the IG-IMRT and 3DCRT groups were 81.6% and 67.9% (P = 0.12, Fig. 1A), 53.8% and 51.8% (P = 0.51, Fig. 1B), 88% and 75.1% (P = 0.031, Fig. 1C) and 64.5% and 62% (P = 0.61, Fig. 1D), respectively. Table 5 lists the Cox-proportional hazards analysis for LFFS. Multivariate analysis demonstrated use of IG-IMRT was an independent predictor of LFFS. The 4-year OS rates were 85.4% and 56.2% for responders and non-responders, respectively (P = 0.001, Fig. 1E). The 4-year DFS rates were 66.2% and 40.8% for responders and non-responders, respectively (P = 0.007, Fig. 1F).
Fig. 1.
Comparison of the overall (A), disease-free (B), local failure-free (C), and distant failure-free (D) survival rates between the image-guided intensity-modulated radiotherapy (IG-IMRT) and 3D conformal radiotherapy (3DCRT) groups. The 4-year overall (E) and disease-free (F) survival rates for responders and non-responders.
Table 5.
Prognostic factor analysis for local-failure-free survival
| Variables | 4-year local-failure-free survival (%) | P value | |
|---|---|---|---|
| Univariate | HR (95% CI; P) | ||
| Type of radiotherapy | |||
| IG-IMRT vs 3DCRT | 88 vs 75.1 | 0.031 | 0.35 (0.11–0.94; 0.042) |
| Age (year) | |||
| ≤64 vs >64 | 71.1 vs 82.6 | 0.648 | |
| Gender | |||
| Female vs male | 86.3 vs 73.5 | 0.153 | |
| Distance from anal verge (cm) | |||
| ≤5 vs >5 | 81.2 vs 76.4 | 0.791 | |
| Clinical tumor depth | |||
| T2–3 vs T4 | 85.6 vs 33.8 | 0.001 | 0.15 (0.06–0.94; 0.012) |
| Clinical lymph node metastasis | |||
| N0 vs N1–2 | 81.8 vs 72.2 | 0.128 | |
| Type of chemotherapy | |||
| 5-FU vs capecitabine | 75.9 vs 82.7 | 0.463 | |
| Adjuvant chemotherapy | |||
| Yes vs no | 83.5 vs 76.3 | 0.243 | |
| Tumor response | |||
| pCR vs non-pCR | 100 vs 74.2 | 0.045 | 0.76 (0.10–1.73; 0.121) |
| ypT0–2 vs ypT3–4 | 88.8 vs 66.9 | 0.031 | 0.52 (0.25–2.61; 0.092) |
| ypN0 vs ypN1–2 | 81.5 vs 71.6 | 0.156 | |
| Favorable vs unfavorablea | 92.1 vs 62.4 | 0.002 | 0.18 (0.02–0.79; 0.012) |
| Surgical distal margin | |||
| Negative vs positive | 83.4 vs 49.6 | 0.009 | 0.26 (0.07–0.88; 0.026) |
| CRM | |||
| Negative vs positive | 86.2 vs 40.9 | 0.001 | 0.16 (0.03–0.80; 0.021) |
aFavorable: tumor regression Grade 3–4; unfavorable: tumor regression Grade 0–2. IG-IMRT = image-guided intensity-modulated radiotherapy, 3DCRT = 3D conformal radiotherapy, HR = hazard ratio, CI = confidence interval, P = P-value, 5-FU = 5-fluorouracil, pCR = pathologic complete response, CRM = circumferential resection margin.
DISCUSSION
Numerous dosimetric comparisons between IMRT and conventional RT techniques in rectal cancer patients have shown that IMRT entails higher target conformity and lower bowel and bladder exposure [7, 8, 16]. However, reports of clinical outcome and toxicity comparisons between IMRT and 3DCRT have rarely been published, particularly comparisons with IG-IMRT. Table 6 summarizes the efficacy and adverse effects of previously published studies of comparisons between IMRT and 3DCRT for rectal cancer patients. Four retrospective series compared the use of IMRT with 3DCRT [4, 5, 17, 18]. Droge et al. compared volumetric-modulated arc therapy (VMAT) with 3DCRT in patients homogeneously treated according to the CAO/ARO/AIO-04 trial and reported that VMAT reduced acute and late adverse effects [19]. However, the median follow-up time was 18.3 months in the VMAT group, which was too short to represent cumulative rates of late toxicity, and survival or disease control status comparisons between the VMAT and 3DCRT groups were not reported. To the best of our knowledge, this is the first comprehensive study comparing clinical outcomes and toxicities of IG-IMRT and 3DCRT with a longer follow-up time.
Table 6.
Summary of studies comparing the use of IMRT with 3DCRT
| n | Stage | Median RT dose | Chemotherapy | Acute toxicity | Treatment break (%) | Late toxicity | Anus-preserving surgery | pCR rate (%) | Tumor control | |
|---|---|---|---|---|---|---|---|---|---|---|
| Parekh et al. [4] | IMRT: 20 | All Stage II or III, except 2 with Stage I; 6 with Stage IVa | IMRT: 50 Gy/25 Fr (SIB) | 5-FU (300 mg/m2): 67% Cap (825 mg/m2 twice daily): 33% |
IMRT: (Grade 2+) *GI 30%; Hema: 10%; skin 35%; GU 0% |
IMRT: 0 | IMRT: none | IMRT: 70% | IMRT: 21.4 | NR |
| 3DCRT: 28 | 3D-CRT: 50.4 Gy/28 Fr | 3DCRT: (Grade 2+) *GI 60.7%; Hema 28.6%; skin 39.3%; GU 7.4% | 3D-CRT: 7.1 | 3DCRT: Grade 3 small bowel obstruction 3.6% | 3DCRT: 64.3% | 3DCRT: 16.7 | NR | |||
| Yang et al. [17] 8% in adjuvant setting | IMRT: 98 | All Stage II or III except 14 with Stage I; 13 with Stage IV | IMRT: 50 Gy/25 Fr (SIB) | 5-FU (225 mg/m2): 93% Cap (875 mg/m2 twice daily): 7% |
IMRT (Grade 2+) *Diarrhea 10.8%; proctitis: 23%. |
NR | NR | NR | NR | NR |
| 3DCRT: 79 | 3DCRT: 50.4 Gy/28 Fr | 3DCRT (Grade 2+) *Diarrhea 32.3%; proctitis: 38% |
NR | NR | NR | NR | NR | |||
| Jabbour et al. [16] | IMRT: 30 | All Stage II or III except 7 with Stage IV | IMRT: 50.4 Gy/28 Fr | 5-FU (225 mg/m2) or Cap (825 mg/m2 twice daily): 83.7% Cap/oxaliplatin: 11.6% |
IMRT (Grade 3+): diarrhea 3%; GU 0% | IMRT: 0 | NR | NR | IMRT: 20 | cLRR: 6.7% |
| cDMR: 6.7% | ||||||||||
| 3DCRT: 56 | 3DCRT: 50.4 Gy/ 28 Fr | 3DCRT (Grade 3+): diarrhea 9%; GU 2% | 3DCRT: 20 | NR | NR | 3DCRT: 21 | cLRR: 7% | |||
| cDMR: 12.5% | ||||||||||
| Samuelian et al. [5] | IMRT: 31 | All Stage II or III, but 22 with recurrent disease 11 for postoperative RT | IMRT: 50 Gy/25 Fr (SIB) | 5-FU (250 mg/m2): 43.5% Cap (875 mg/m2 twice daily): 54.3% |
IMRT (Grade 3+): GI 3%; Hema: 3%; skin: 3.2%. (Grade 2+): *GI: 32%; Hema: 45%; GU 16%; skin 10% |
IMRT: 6.5% | aIMRT: 5.3% | IMRT: 82% | IMRT: 19 | NR |
| 3DCRT: 61 | 3DCRT: 50.4 Gy/28 Fr | 3DCRT (Grade 3+): GI 10%; Hema: 5%; skin: 1.6%. (Grade 2+): *GI: 62%; Hema 44%; GU 21%; skin 3% | 3DCRT: 16.4% | a3DCRT: 15% | 3DCRT: 84% | 3DCRT: 28 | NR | |||
| Droge et al. [18] | VMRT: 81 | Stage II or III | VMAT and 3DCRT: 50.4 Gy/28 Fr | 5-FU (1000 mg/m2 on Day 1–5 and 29–33 of the RT) | VMAT (Grade 3+): *proctitis 2%; Hema 3%; GU 1%; *skin 0% | NR | VMAT (Grade 3+): proctitis 3%; GU 3%; skin 0% | VMAT: 31%b | VMAT: 20 | NR |
| 3DCRT: 107 | 3DCRT (Grade 3+): *proctitis 12%; Hema 4%; GU 3%; *skin 7% | NR | 3DCRT (Grade 3+) proctitis 8%; GU 10%; skin 2% | 3DCRT: 23%b | 3DCRT: 13 | NR | ||||
| Present study | HT: 45 | Stage II or III | HT: 50 Gy/25 Fr (SIB) | 5-FU (350 mg/m2): 47.9% Cap (850 mg/m2 twice daily): 52.1% |
HT (Grade 3+): *GI 6.7%; GU 0%; Hema: 0%; skin: 2.2% | HT: 2.2% | HT (Grade 3+): GI 4.4%; GU 0% | HT: 84.4%b | HT: 17.8 | dLRR: 8.9% |
| dDMR: 26.7% | ||||||||||
| 3DCRT: 99 | 3DCRT: 50.4 Gy/28 Fr | 3DCRT (Grade 3+): *GI 15.1%; GU 3%; Hema: 2%; skin: 3% | 3DCRT: 8.1% | 3DCRT(Grade 3+): GI 10.1%; GU 3.1% | 3DCRT: 76.4%b | 3DCRT: 15.1 | dLRR: 19.2% | |||
| dDMR: 33.3% |
*Statistically significant difference between IMRT and 3DCRT.
aPostoperative complications.
bIn tumors located within 0–5 cm from anal verge.
cMedian follow-up time was 23 months in the 3DCRT group compared with 11 months in the IMRT group.
dMedian follow-up time was 53 months in the 3DCRT group and 43 months in the IG-IMRT group.
NR = not reported, IMRT = intensity-modulated radiotherapy, 3DCRT = 3D conformal radiotherapy, pCR = pathological complete response, RT = radiotherapy, GI = gastrointestinal, Hema = hematological, GU = genitourinary, Cap = capecitabine, Fr = fraction, SIB = simultaneous integral boost, VMAT = volumetric-modulated arc therapy, HT = helical tomotherapy, LRR = local recurrence rate, DMR = distant metastasis rate.
In our study, patients receiving IG-IMRT had significantly reduced severe acute GI toxicity compared with those receiving 3DCRT (6.7% vs 15.1%, P = 0.039). In our cohort, diarrhea was the most common acute GI toxicity and the most common reason for RT interruption. Samuelian et al. retrospectively compared the acute toxicity of IMRT and 3DCRT in combination with chemotherapy in rectal cancer patients and reported that IMRT resulted in lower Grade ≥2 acute GI toxicity than did 3DCRT (32% vs 62%) [5]. Although the reduction in GI toxicity obtained using IMRT is consistent with our results, Samuelian et al. included patients with recurrent disease who received RT postoperatively and who were treated with non-curative intent, which might influence target delineation and the dosage of radiotherapy or chemotherapy and consequently the clinical outcome and toxicity. Parekh et al. retrospectively analyzed 48 rectal cancer patients receiving either IMRT- or 3DCRT-based preoperative chemoradiotherapy and reported a significant reduction in Grade ≥2 GI toxicity (30% vs 60.7%) [4]. Our results demonstrated that IG-IMRT significantly reduced Grade 2 and 3 GI toxicity. The possible reason for the reduced Grade 3 GI toxicity is the combination of daily IGRT with IMRT in this study. In general, the incidence of Grade 3 diarrhea and proctitis in rectal cancer patients receiving conventional RT combined with 5-FU is 12–36%, [1–3] which is considerably higher than observed in our current results. With reduced irradiated bowel volumes through the use of IMRT and minimized planning target margins through the use of IGRT, Grade 3 or 4 GI toxicity was lower than with 3DCRT. In addition, with the dynamic-jaw mode of tomotherapy, we may improve the longitudinal dose conformity, and we can reduce the penumbra superiorly and inferiorly to the target, resulting in less integral dose and toxicity [20–22]. We did not use dynamic-jaw mode in the current study because the dynamic-jaw technique has only been available at our institute since September 2015. Further investigations on association between use of the dynamic-jaw mode and pelvic radiation–related toxicity are warranted.
In this study, treatment responders (TRG 3–4) had a higher OS and DFS than did the non-responders (TRG 0–2) (77.6% vs 52.2% and 61.7% vs 40.8%, respectively; Fig. 1E, F). Treatment response to neoadjuvant CCRT is an early indicator of the long-term prognosis in LARC patients [23, 24]. In the CAO/ARO/AIO-94 trial, patients with complete (TRG 4) or intermediate pathologic responses (TRG 2 and 3) had improved DFS after preoperative CCRT than those with poor responses [25]. Furthermore, Ark et al. analyzed 725 rectal cancer patients receiving neoadjuvant CCRT followed by radical resection and they demonstrated that tumor response (complete vs intermediate vs poor) was associated with 5-year recurrence-free survival, distant metastasis, and local recurrence rates [26]. The findings indicate that treatment response is closely correlated to the oncologic prognosis. In this study, patients receiving IG-IMRT had the trend of favorable tumor regressions compared with those receiving 3DCRT (66.7% vs 43.5%, P = 0.071). In addition, patients receiving IG-IMRT exhibited improved LFFS rates; however, no significant difference was observed in OS and DFS between the treatment groups. This finding is consistent with other studies that suggest that RT usually contributes to improved locoregional control [1, 3].
In the present study, RT duration was shorter in the IG-IMRT group compared with that in the 3DCRT group (median time, 35 days vs 40 days). Prolonged RT duration has been associated with poor tumor responses. The CAO/AIO/ARO-94 trial demonstrated that prolonged radiation was correlated with higher locoregional recurrence [27]. Parekh et al. analyzed 48 LARC patients undergoing preoperative CCRT and showed that RT duration was significantly shorter in the IMRT group compared with that in the 3DCRT group. Moreover, patients receiving IMRT had favorable pathologic downstaging profiles [4]. In this study, patients requiring treatment interruptions were less in the IG-IMRT group compared with in the 3DCRT group. Therefore, a shorter RT duration in the IG-IMRT group may contribute to favorable tumor regressions.
Lymph node status is a significant prognostic factor in LARC patients undergoing neoadjuvant CCRT [28, 29]. Because the presacral space was the most common site of local failure in this study, we hypothesize that a higher boost dose to the presacral space might improve oncologic outcomes. Additional prospective randomized trials are required to verify this hypothesis.
Because IMRT enables us to gain steep dose gradients around target volumes, the potential for missing or underdosing tumors always raises concerns [30, 31]. Our study demonstrated the efficacy of T or N downstaging seemed to be similar between IG-IMRT and 3DCRT groups. We further evaluated those patients with locoregional recurrences and found that none of them were marginal failures. This implied that IMRT cooperated with IGRT would not compromise outcomes by potentially missing targets. However, further research is necessary to confirm the indications of IG-IMRT. Given the potential for marginal failures in rectal cancer patients treated with IMRT, we applied RTOG guidelines and indications in the anorectal contouring atlas to each patient receiving IMRT.
In the current study, patients underwent radiotherapy in the supine position, which could reduce set-up uncertainty. Certain studies have suggested that the prone position for the treatment of rectal cancer patients had the advantage of small-bowel sparing [4, 8, 32]. However, Beriwal et al. demonstrated no difference existed in GI toxicity between prone and supine IMRT [33]. In the Mayo Clinic, rectal cancer patients undergoing IMRT in the supine position experienced less GI toxicity [5]. The optimal positioning for pelvic irradiation remains controversial. Accordingly, we treated rectal cancer patients in the supine position because of reproducibility and tolerability. However, the question needs further investigations.
The current study has some limitations. First, this study had a retrospective design. Second, two chemotherapy regimens (infusional 5-FU and oral capecitabine) were used in combination with RT in this study. Evidence has not demonstrated any significant difference in tumor downstaging, survival profile and toxicity between capecitabine and infusional 5-FU in LARC patients undergoing preoperative CCRT [34, 35]. Therefore, the two different chemotherapy regimens had a limited effect on oncologic outcome.
CONCLUSIONS
In summary, IG-IMRT with a simultaneous integrated boost resulted in a lower GI toxicity and more favorable local control than did 3DCRT in patients with LARC, but no difference in distant metastasis rate or OS was observed between the two treatment modalities. Therefore, prospective, randomized studies are necessary to validate our observational study.
ACKNOWLEDGEMENTS
The authors acknowledge the contribution made to data collection by the Colorectal Cancer Group from the Cancer Center of Kaohsiung Medical University Hospital. The data was presented at the 56th Annual Meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO), 14–17 September 2014, San Francisco, CA.
FUNDING
This work was supported by grants from the Excellence for Cancer Research Center (MOST104-2325-B-037-001); the Ministry of Health and Welfare (MOHW 105-TDU-B-212-134007), Taiwan, Republic of China; Kaohsiung Medical University Hospital (KMUH102-2M47, KMUH104-4M44, KMUH104-4R19, KMUH-S10418-2, KMUH-S104105); the Center for Biomarkers and Biotech Drugs, Kaohsiung Medical University (KMU-TP104C00, KMU-TP104C03, KMU-TP104C04, KMU-TP104C07, KMU-TP103H10, KMU-TP103H11, KMU-PT104002, KMU-DK105001, KMU-DK106005, D08-00005-10401). This work was partly supported by a grant from the Research Center for Environmental Medicine, Kaohsiung Medical University (KMU-TP104A11), Kaohsiung, Taiwan, the Health and Welfare Surcharge on Tobacco Products and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1. Bosset JF, Collette L, Calais G, et al. . Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23. [DOI] [PubMed] [Google Scholar]
- 2. Roh MS, Colangelo LH, O'Connell MJ, et al. . Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sauer R, Becker H, Hohenberger W, et al. . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- 4. Parekh A, Truong MT, Pashtan I, et al. . Acute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancer. Gastrointest Cancer Res 2013;6:137–43. [PMC free article] [PubMed] [Google Scholar]
- 5. Samuelian JM, Callister MD, Ashman JB, et al. . Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:1981–7. [DOI] [PubMed] [Google Scholar]
- 6. Swellengrebel HA, Marijnen CA, Verwaal VJ, et al. . Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br J Surg 2011;98:418–26. [DOI] [PubMed] [Google Scholar]
- 7. Engels B, De Ridder M, Tournel K, et al. . Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: impact on the irradiated volume of small bowel. Int J Radiat Oncol Biol Phys 2009;74:1476–80. [DOI] [PubMed] [Google Scholar]
- 8. Arbea L, Ramos LI, Martinez-Monge R, et al. . Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol 2010;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan P, Yeo I, Perkins G, et al. . Dosimetric comparison of intensity-modulated, conformal, and four-field pelvic radiotherapy boost plans for gynecologic cancer: a retrospective planning study. Radiat Oncol 2006;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luxton G, Hancock SL, Boyer AL. Dosimetry and radiobiologic model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2004;59:267–84. [DOI] [PubMed] [Google Scholar]
- 11. Mell LK, Tiryaki H, Ahn KH, et al. . Dosimetric comparison of bone marrow–sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:1504–10. [DOI] [PubMed] [Google Scholar]
- 12. Greene FL. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer-Verlag, 2010. [Google Scholar]
- 13. Huang CM, Huang MY, Tsai HL, et al. . An observational study of extending FOLFOX chemotherapy, lengthening the interval between radiotherapy and surgery, and enhancing pathological complete response rates in rectal cancer patients following preoperative chemoradiotherapy. Therap Adv Gastroenterol 2016;9:702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19–23. [DOI] [PubMed] [Google Scholar]
- 15. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6. [DOI] [PubMed] [Google Scholar]
- 16. Guerrero Urbano MT, Henrys AJ, Adams EJ, et al. . Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys 2006;65:907–16. [DOI] [PubMed] [Google Scholar]
- 17. Jabbour SK, Patel S, Herman JM, et al. . Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits. Int J Surg Oncol 2012;2012:891067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang TJ, Oh JH, Son CH, et al. . Predictors of acute gastrointestinal toxicity during pelvic chemoradiotherapy in patients with rectal cancer. Gastrointest Cancer Res 2013;6:129–36. [PMC free article] [PubMed] [Google Scholar]
- 19. Droge LH, Weber HE, Guhlich M, et al. . Reduced toxicity in the treatment of locally advanced rectal cancer: a comparison of volumetric modulated arc therapy and 3D conformal radiotherapy. BMC Cancer 2015;15:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manabe Y, Shibamoto Y, Sugie C, et al. . Helical and static-port tomotherapy using the newly-developed dynamic jaws technology for lung cancer. Technol Cancer Res Treat 2015;14:583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krause S, Beck S, Schubert K, et al. . Accelerated large volume irradiation with Dynamic Jaw/Dynamic Couch Helical Tomotherapy. Radiat Oncol 2012;7:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterzing F, Uhl M, Hauswald H, et al. . Dynamic jaws and dynamic couch in helical tomotherapy. Int J Radiat Oncol Biol Phys 2010;76:1266–73. [DOI] [PubMed] [Google Scholar]
- 23. Huang MY, Lin CH, Huang CM, et al. . Relationships between SMAD3 expression and preoperative fluoropyrimidine-based chemoradiotherapy response in locally advanced rectal cancer patients. World J Surg 2015;39:1257–67. [DOI] [PubMed] [Google Scholar]
- 24. Tsai H-L, Wang J-Y. Predictors of response in locally advanced rectal cancer following concurrent chemoradiotherapy. Biomark Genom Med 2013;5:18–22. [Google Scholar]
- 25. Rodel C, Martus P, Papadoupolos T, et al. . Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688–96. [DOI] [PubMed] [Google Scholar]
- 26. Park IJ, You YN, Agarwal A, et al. . Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fietkau R, Rodel C, Hohenberger W, et al. . Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control: results of study CAO/ARO/AIO-94. Int J Radiat Oncol Biol Phys 2007;67:1008–19. [DOI] [PubMed] [Google Scholar]
- 28. Huang CM, Huang CW, Huang MY, et al. . Coexistence of perineural invasion and lymph node metastases is a poor prognostic factor in patients with locally advanced rectal cancer after preoperative chemoradiotherapy followed by radical resection and adjuvant chemotherapy. Med Princ Pract 2014;23:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moureau-Zabotto L, Farnault B, de Chaisemartin C, et al. . Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2011;80:483–91. [DOI] [PubMed] [Google Scholar]
- 30. Chen AM, Farwell DG, Luu Q, et al. . Marginal misses after postoperative intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2011;80:1423–9. [DOI] [PubMed] [Google Scholar]
- 31. David MB, Eisbruch A. Delineating neck targets for intensity-modulated radiation therapy of head and neck cancer. What we learned from marginal recurrences. Front Radiat Ther Oncol 2007;40:193–207. [DOI] [PubMed] [Google Scholar]
- 32. Zhu J, Liu F, Gu W, et al. . Concomitant boost IMRT-based neoadjuvant chemoradiotherapy for clinical stage II/III rectal adenocarcinoma: results of a phase II study. Radiat Oncol 2014;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beriwal S, Jain SK, Heron DE, et al. . Dosimetric and toxicity comparison between prone and supine position IMRT for endometrial cancer. Int J Radiat Oncol Biol Phys 2007;67:485–9. [DOI] [PubMed] [Google Scholar]
- 34. Chen CF, Huang MY, Huang CJ, et al. . A observational study of the efficacy and safety of capecitabine versus bolus infusional 5-fluorouracil in pre-operative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 2012;27:727–36. [DOI] [PubMed] [Google Scholar]
- 35. Chan AK, Wong AO, Jenken DA. Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer—is it equivalent to 5-FU infusion plus leucovorin and radiotherapy. Int J Radiat Oncol Biol Phys 2010;76:1413–9. [DOI] [PubMed] [Google Scholar]