Abstract
Background
Most odour baits designed to attract host-seeking mosquitoes contain carbon dioxide (CO2), which enhances trap catches, given its role as a mosquito flight activator. However, the use of CO2 is expensive and logistically demanding for prolonged area-wide use.
Methods
This study explored the possibility of replacing organically-produced CO2 with 2-butanone in odour blends targeting host-seeking malaria mosquitoes. During semi-field and field experiments MM-X traps were baited with a human odour mimic (MB5 blend) plus CO2 or 2-butanone at varying concentrations. Unbaited traps formed a control. The attraction of Anopheles gambiae s.s., Anopheles arabiensis and Anopheles funestus to these differently baited traps was measured and mean catch sizes were compared to determine whether 2-butanone could form a viable replacement for CO2 for these target species.
Results
Under semi-field conditions significantly more female An. gambiae mosquitoes were attracted to a reference attractant blend (MB5 + CO2) compared to MB5 without CO2 (P < 0.001), CO2 alone (P < 0.001), or a trap without a bait (P < 0.001). Whereas MB5 + CO2 attracted significantly more mosquitoes than its variants containing MB5 plus different dilutions of 2-butanone (P = 0.001), the pure form (99.5%) and the 1.0% dilution of 2-butanone gave promising results. In the field mean indoor catches of wild female An. gambiae s.l. in traps containing MB5 + CO2 (5.07 ± 1.01) and MB5 + 99.5% 2-butanone (3.10 ± 0.65) did not differ significantly (P = 0.09). The mean indoor catches of wild female An. funestus attracted to traps containing MB5 + CO2 (3.87 ± 0.79) and MB5 + 99.5% 2-butanone (3.37 ± 0.70) were also similar (P = 0.635). Likewise, the mean outdoor catches of An. gambiae and An. funestus associated with MB5 + CO2 (1.63 ± 0.38 and 0.53 ± 0.17, respectively) and MB5 + 99.5% 2-butanone (1.33 ± 0.32 and 0.40 ± 0.14, respectively) were not significantly different (P = 0.544 and P = 0.533, respectively).
Conclusion
These results demonstrate that 2-butanone can serve as a good replacement for CO2 in synthetic blends of attractants designed to attract host-seeking An. gambiae s.l. and An. funestus mosquitoes. This development underscores the possibility of using odour-baited traps (OBTs) for monitoring and surveillance as well as control of malaria vectors and potentially other mosquito species.
Background
Malaria vectors require a blood meal to develop their eggs [1] and the process of finding blood hosts is primarily mediated by host odour [2–5]. Carbon dioxide (CO2) is one of the important components of human host odour affecting mosquito host-seeking behaviour [6]. It is thought that this gas activates mosquitoes by eliciting take-off behaviour. The presence of CO2 then sustains the mosquitoes in host-seeking flight [6, 7], guiding them towards their blood meal hosts [3]. It is not surprising, therefore, that CO2 is a key ingredient of synthetic mosquito attractants for host-seeking mosquitoes [8]. The application of this gas from pressurized cylinders, fermenting sugar (i.e., sucrose) or molasses and/or the use of dry ice present major challenges to the use of CO2 -based mosquito attractants under field conditions. The gas cylinders are heavy, bulky, expensive and prone to leakages [9] and dry ice can be difficult to obtain, transport and store [9–12]. Whilst CO2 produced by fermenting refined sugar or molasses can offer a solution to these problems [13] this method of CO2 production is also expensive and presents logistical challenges when used on a large scale because the gas is only produced over one trapping night (ca ten hours) and must be replenished daily.
In a study by Turner et al. 2-butanone was identified as a potential replacement for CO2 in a synthetic blend of mosquito attractants [14]. These authors demonstrated the capacity of 2-butanone to induce a dose-dependent activation of the cleavage product A (cpA) CO2 receptor neuron in the maxillary palps of Anopheles gambiae, Aedes aegypti and Culex quinquefasciatus. This simulated the activity of CO2. In related studies, acetone and cyclopentanone have also been tested as substitutes for CO2 [15–17] but with little success under field conditions [16, 17]. The current study sought to: (a) evaluate the synergistic importance of CO2 as a mosquito attractant in counter-flow MM-X traps; (b) assess the attraction of mosquitoes to different concentrations of 2-butanone; (c) determine the optimal concentration of 2-butanone for attracting mosquitoes; and, (d) evaluate the attraction of mosquitoes to odour baits containing 2-butanone in the field.
Methods
Mosquito rearing
All semi-field experiments reported in this article utilized laboratory colonies of the Mbita strains of An. gambiae or Anopheles arabiensis. The mosquitoes were reared under ambient environmental conditions at the Thomas Odhiambo Campus of the International Centre of Insect Physiology and Ecology (icipe-TOC) located near Mbita Point Township in western Kenya. Mosquito eggs were placed in plastic trays containing water from Lake Victoria. Water was filtered through charcoal to remove sediments. Larvae were fed on GO-CAT® Complete cat food (Purina, Nestle S.A) supplied three times a day (0.03 mg/larva/day). Pupae were collected in clean cups daily. Collected pupae were transferred to an adult holding room and placed in mesh-covered cages (30 × 30 × 30 cm) prior to adult emergence. The adults were fed on 6% glucose solution through wicks made from absorbent tissue paper. The mosquitoes were randomly aspirated from the cages into paper cups using hand-held mouth aspirators and were starved for 8 h prior to experiments. The mosquitoes were only supplied with water from a wet cotton cloth material placed on top of holding cups during starvation.
Synthetic mosquito attractants
The combination of CO2 and a synthetic mosquito attractant blend, referred to as the Mbita Blend 5 or MB5 [18] was used as a reference standard. The chemical constituents of MB5 included ammonia (2.5%), lactic acid (85%), tetradecanoic acid (0.00025%), 3-methyl-1-butanol (0.000001%), and 1-butylamine (0.001). All these chemical compounds were purchased from Sigma Aldrich Chemicals GmbH (Germany). Carbon dioxide was produced at the rate of 80.63 ± 2.82 ml/min by mixing 250 g of molasses (Mumias Sugar Company Ltd, Kenya), 17.5 g dry yeast (Angel® Yeast Company Ltd, China) and 2 l of water [13]. Each component of MB5 was dispensed from individual strips (measuring 26.5 cm × 1.0 cm) of nylon stockings [19, 20]. The nylon strips (15 denier microfibres, 90% polyamide, 10% spandex) were purchased from Bata Shoe Company Ltd, Kenya.
Semi-field experimental set up
All semi-field experiments were carried out inside screen-walled greenhouses at icipe-TOC (00°25′S, 34°13′E). The floor of the screen house (11.5 m × 7.3 m) was covered with sand, and watered daily to keep the microclimate humid and avert deaths of experimental mosquitoes due to desiccation. Each semi-field experiment was run between 20.00 and 06.30 h by utilizing 200 adult female mosquitoes. The mosquitoes, aged three to 6 days post-emergence and which had no prior access to a blood meal, were released at the centre of the screen-house in all replicates. MM-X traps containing four different test odours were placed at the four corners of the screen-walled greenhouse in four choice tests (Fig. 1a) or at two diagonal corners of the screen-house in two choice tests (Fig. 1b). The treatments were rotated in sequence until each had occupied every corner of the screen-house four times or two opposite corners of the greenhouse along a diagonal axis two times. The traps were rotated to eliminate any positional bias. During an experimental replicate the mosquitoes freely accessed all treatments, which were availed simultaneously in the screen-house enclosure on each experimental night. Mosquitoes attracted to specific treatments were caught in MM-X traps which functioned to both disperse the odours as well as capture attracted mosquitoes [21]. At the end of each experiment, trapped mosquitoes were taken to the laboratory, immobilized by freezing at −20°C and counted.
Fig. 1.
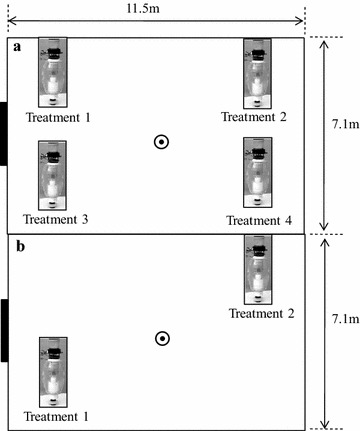
Four-choice (a) and two-choice (b) semi-field experimental set ups used to evaluate attraction of malaria mosquitoes to attractant blends containing 2-butanone in place of carbon dioxide. The position of the MMX-traps, the mosquito release point  and the entrance to the screen-house (
and the entrance to the screen-house ( ) are shown
) are shown
Field study site
Field studies were conducted at Kigoche village near Ahero Town (00°081S, 034°551E) in Kisumu County, Western Kenya. Kigoche receives long rains from April to June and short rains from September to October yearly. The village lies at an altitude of 1160 m above sea level with an average relative humidity of 65% and an average annual rainfall range of 1000–1800 mm. Most residents in Kigoche engage in irrigated rice farming, which creates breeding sites for malaria mosquitoes. The houses are mainly covered with corrugated iron sheet roofs and have mud walls with open eaves through which mosquitoes enter [22]. Anopheles arabiensis and Anopheles funestus are the principal vectors of malaria in the area [23].
Synergistic importance of carbon dioxide as a mosquito attractant
The primary aim of this baseline study was to examine the synergistic attraction of malaria mosquitoes to CO2 when augmented with other chemical compounds. This was achieved with a laboratory colony of An. gambiae mosquitoes using a fully replicated 4 × 4 Latin Square experimental design. The design included four treatments (Fig. 1): (a) no bait (the control); (b) the MB5 reference attractant blend plus CO2 (MB5 + CO2); (c) MB5 alone; and, (d) CO2 alone. The experiments were carried out for 16 nights.
Attraction of malaria mosquitoes to different concentrations of 2-butanone
Semi-field behavioural experiments were carried out to determine the best concentration at which 2-butanone attracts mosquitoes. Dual-choice assays compared behavioural responses of An. gambiae mosquitoes towards a reference treatment (MB5 + CO2) versus a test treatment (i.e., MB5 augmented with various dilutions of 2-butanone in distilled water, namely 99.5, 10, 1.0, 0.1, 0.01, 0.004, 0.001, and 0.0004%). Thus, a total of eight dual-choice experiments were carried out. The different dilutions of 2-butanone in the test treatments acted as substitutes for CO2. Each dual-choice assay was carried out over a period of four nights (Fig. 1).
The optimal concentration of 2-butanone that attracts malaria mosquitoes
The aim of this set of experiments was to determine if 2-butanone can mimic the synergistic effects of CO2 and, therefore, act as a substitute for this gas in synthetic attractants for mosquitoes. The experiments aimed to determine which of the two most promising concentrations of 2-butanone, (99.5 and 1% as determined in the previous experiments), served better as a replacement for CO2 in attractants for mosquitoes. The treatments included: (a) no bait (the control); (b) MB5 + CO2; (c) MB5 + 99.5% 2-butanone; and, (d) MB5 + 1.0% 2-butanone (Fig. 1). Female An. gambiae or An. arabiensis mosquitoes were released in separate screen houses on each experimental night for 16 nights each.
Attraction of malaria mosquitoes to 2-butanone-based odour baits in the field
Indirect experimental comparisons were carried out indoors and outdoors in Kigoche village in western Kenya to evaluate the capacity of 2-butanone-based odour baits to attract mosquitoes under field conditions. In the indoor scenario, six houses each separated by a distance of ≥25 m were selected. All houses in the village were entered into an Excel spread sheet and computer-generated random numbers were used to select the six houses to be used in experiments. All selected houses measured between 15.0 and 20.0 sq m ground surface area. The six houses all had mud walls and floors with open eaves, corrugated iron-sheet roofs, no ceiling, and were either single or double roomed [24]. They were located on a transect oriented east–west along the northern edge of the Ahero rice irrigation scheme, approximately 28–150 m apart, 10–20 m away from cowsheds and within a range of 100 m from irrigation water channels and rice paddies [13, 25]. The selected houses had no occupants during experiments. Each house was assigned one of six treatments per night on a strict rotational basis to exclude positional bias. In the outdoor scenario, six open sites separated by a distance of ≥25 m were selected. The outdoor experimental sites had only short grass with no tall vegetation, were situated ≥25 m from the nearest house or cowshed and were located ≥100 m from the nearest mosquito larval breeding habitat. Each outdoor site was assigned one of six treatments per night on a rotational basis to exclude positional bias.
The six treatments which were allocated to the indoor and outdoor sites included: (a) no bait (i.e. the negative control); (b) CO2; (c) 99.5% 2-butanone; (d) MB5; (e) MB5 + CO2; and (f) MB5 + 99.5% 2-butanone. All chemical constituents of the odour baits, except CO2, were released using nylon strips [19, 23, 26]. All treatments were simultaneously dispensed using MM-X traps, which were hung 15 cm above the ground from a roof pole in indoor experiments and on a tripod stand in the outdoor scenario. The two studies were run from 18.00 to 06.00 h concurrently for 30 nights. All the traps were collected the following morning and transported to a field laboratory located at the Ahero Multipurpose Development Training Institute (AMDTI) where the mosquitoes were immobilized by freezing at −20 °C prior to counting. All female Anopheles mosquitoes were preserved in Eppendorf tubes containing 80% ethanol. Subsamples of collected mosquitoes belonging to the An. gambiae complex and the An. funestus group were identified to species level using molecular tools [27, 28].
Ethical considerations
This study was approved by the ethical review committee of the Kenya Medical Research Institute (KEMRI/RES/7/3/1). The purpose and procedures of the study were explained to local leaders, household heads and resident volunteers in Kigoche village. The houses for the study were selected randomly and permission sought from the household head before experiments were rolled out.
Data analysis
Mean mosquito catches were calculated in all experiments. The effect of a treatment as a major predictor of the number of mosquitoes caught in a trap under semi-field conditions was modelled using generalized linear models (GLM) with a Poisson distribution and a log link function. Data collected during field experiments were analysed using GLM fitted with negative binomial distribution and a log link function. The effects of treatments and house position on mosquito catches were tested as parameters in the model while night effect was captured as a covariate. All analyses were performed using IBM SPSS Statistics, version 20.0.
Results
The work reported in this paper was carried out between August 2012 and August 2013. The semi-field experiments used a total of 16,000 female mosquitoes comprising 12,800 An. gambiae and 3200 An. arabiensis.
Synergistic importance of carbon dioxide as a mosquito attractant
The semi-field experiments carried out to measure the synergistic effect of CO2 as an ingredient in mosquito attractants were conducted over a period of 16 nights. Out of the 3200 female An. gambiae mosquitoes released, 1743 (54.5%) were recaptured. Mosquito catches differed significantly among the four treatments (P = 0.001). The MB5 reference attractant blend with CO2 (MB5 + CO2) attracted significantly more mosquitoes (n = 1053; mean = 65.81 ± 2.03) than MB5 alone (n = 264; mean = 16.5 ± 1.02; P = 0.001), CO2 alone (n = 388; mean = 24.25 ± 1.23; P = 0.001) and a trap with no bait (n = 38; mean = 2.38 ± 0.39; P = 0.001) (Fig. 2).
Fig. 2.
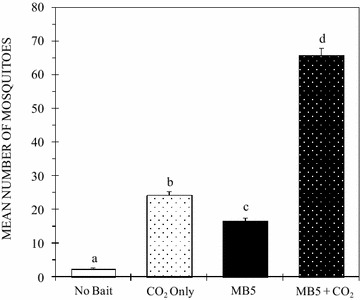
Mean numbers of Anopheles gambiae mosquitoes caught in an MM-X trap without a bait or in a trap baited with either CO2 alone, Mbita blend alone (MB5) or MB5 + CO2. Error bars denote the standard error of the mean number of mosquitoes trapped. Bars with different letters denote significant differences in the number of mosquitoes caught in traps containing each of the lures
Attraction of mosquitoes to different concentrations of 2-butanone
These experiments were carried out over a period of 32 nights. Of the 6400 female An. gambiae mosquitoes released, 4271 (66.7%) were recaptured. The mosquitoes trapped in each complete dual-choice comparison ranged from 51 to 77% of the 200 that were released in each replicate. In all cases the reference treatment (MB5 + CO2) used as a positive control, attracted a significantly more mosquitoes than the test treatments (P = 0.001 in all cases; Table 1). The pure form (99.5%) and the 1.0% dilution of 2-butanone were the most promising concentrations and were subsequently used to evaluate whether 2-butanone could substitute CO2 in a blend of synthetic mosquito attractants.
Table 1.
Mean number (±SE) of Anopheles gambiae mosquitoes attracted to MM-X traps containing the reference (MB5 + CO2) and test treatments (MB5 + dilution ‘X’ of 2-butanone) of candidate synthetic mosquito attractant blends
| Exp | Dilution of 2-butanone (‘X’), % | N | n | %Response | Mean (±SE) mosquito catches | Exp (B) | |
|---|---|---|---|---|---|---|---|
| Reference (MB5 + CO2) | Test treatment (MB5 + ‘X’) | ||||||
| 1 | 99.5 | 4 | 622 | 77.75 | 122.75 ± 5.54 | 32.75 ± 2.86 | 3.748* |
| 2 | 10 | 4 | 559 | 69.88 | 122.75 ± 5.54 | 17.00 ± 2.06 | 7.221* |
| 3 | 1.0 | 4 | 588 | 73.50 | 118.75 ± 5.45 | 28.25 ± 2.66 | 4.204* |
| 4 | 0.1 | 4 | 526 | 65.75 | 115.00 ± 5.36 | 16.50 ± 2.03 | 6.970* |
| 5 | 0.01 | 4 | 415 | 51.88 | 89.75 ± 4.74 | 14.00 ± 1.87 | 6.411* |
| 6 | 0.004 | 4 | 543 | 67.88 | 118.50 ± 5.44 | 17.25 ± 2.08 | 6.870* |
| 7 | 0.001 | 4 | 535 | 66.88 | 118.00 ± 5.43 | 15.75 ± 1.98 | 7.492* |
| 8 | 0.0004 | 4 | 483 | 60.38 | 106.50 ± 5.16 | 14.25 ± 1.89 | 7.474* |
The number of replicates (N), the number (n) and percentage (%) of trapped mosquitoes, test statistic {Exp(B)} and the level of statistical significance (* indicates P < 0.001) in each dual choice experiment is shown. 800 female An. gambiae were released across each series of four replicates
The optimal concentration of 2-butanone that attracts mosquitoes
Tests with An. arabiensis were conducted in May 2013. A total of 585 female An. arabiensis mosquitoes were recaptured out of the 3200 released. The mean numbers of mosquitoes that were caught in the trap with no bait, or the traps baited with MB5 + 1.0% 2-butanone, MB5 + 99.5% 2-butanone and MB5 + CO2 were 0.56 ± 0.19 (n = 9), 4.9 ± 0.51 (n = 67), 4.88 ± 0.55 (n = 78) and 26.94 ± 1.3 (n = 431), respectively (Fig. 3a). Whereas MB5 + CO2 attracted the majority of An. arabiensis (P = 0.001), the numbers of mosquitoes attracted to MB5 + 1.0% 2-butanone and MB5 + 99.5% 2-butanone did not differ significantly (P = 0.361).
Fig. 3.
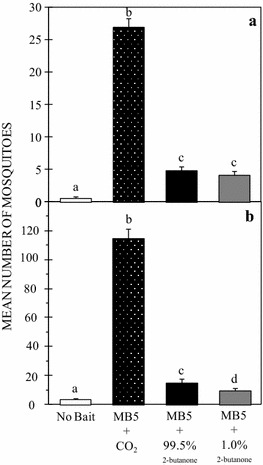
Mean number of Anopheles arabiensis (a) and Anopheles gambiae (b) mosquitoes caught in an MM-X trap with no bait, in a trap baited with either MB5 + CO2, MB5 + 99.5% 2-butanone or MB5 + 1.0% 2-butanone. Error bars denote the standard error of the mean number of mosquitoes trapped. Bars with different letters denote statistically significant differences in the number of mosquitoes trapped
Tests with An. gambiae were conducted in December 2012. For this species 2294 out of the 3200 mosquitoes released were recaptured. The mean numbers of An. gambiae mosquitoes caught in the trap with no bait, or the traps baited with MB5 + 1.0% 2-butanone, MB5 + 99.5% 2-butanone and MB5 + CO2 were 3.8 ± 0.49 (n = 61), 9.63 ± 0.78 (n = 154), 15.25 ± 0.98 (n = 244) and 114.69 ± 2.68 (n = 1835), respectively (Fig. 3b). The treatment containing MB5 + CO2 lured higher numbers of mosquitoes than all the other treatments (P = 0.001). A significantly higher number of the mosquitoes was attracted to the blend containing MB5 + 99.5% 2-butanone than that containing MB5 + 1.0% 2-butanone (P = 0.001). The blend with MB5 + 99.5% 2-butanone also attracted a significantly higher number of mosquitoes than the trap with no bait (P = 0.001).
Attraction of malaria mosquitoes to 2-butanone based odour baits in the field
Indoor mosquito catches
All field studies were conducted during the dry season (from July to August 2013). The adult female mosquitoes collected indoors included An. gambiae s.l. (55.1%; n = 466), An. funestus (37.4%; n = 316), Culex spp (4.5%; n = 38), Mansonia spp (0.7%; n = 6) and other anopheline species (2.36%; n = 20) (Fig. 4). A total of 456 male adult mosquitoes comprising 76.1% An. gambiae s.l., 19.3% An. funestus, 2.6% Culex and 2.0% Mansonia were collected indoors.
Fig. 4.
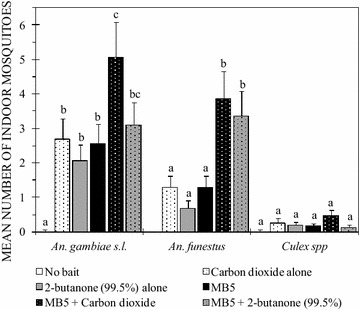
Mean number of wild female mosquitoes caught indoors in MM-X traps with no bait or in a trap baited with either CO2 alone, 2-butanone (99.5%) alone, MB5, MB5 + CO2, or MB5 + 2-butanone (99.5%). Error bars denote the standard error of the mean number of mosquitoes trapped. Bars with different letters within a given mosquito species denote significant differences in the number of mosquitoes trapped. No An. funestus were caught with the unbaited trap
There was no significant difference between the attraction of female An. gambiae s.l. mosquitoes to MB5 + CO2 and MB5 + 99.5% 2-butanone (P = 0.090) (Fig. 4), and each of the two treatments was significantly more attractive to female An. gambiae s.l. compared to a trap with no bait (P = 0.001 for each). MB5 + CO2 attracted significantly more An. gambiae s.l. mosquitoes than either CO2 alone (P = 0.031), 99.5% 2-butanone alone (P = 0.003) or MB5 alone (P = 0.021). However, there was no difference between the capture rate of An. gambiae s.l. in traps containing MB5 + 99.5% 2-butanone compared with 99.5% 2-butanone alone, MB5 alone, or CO2 alone (P ≥ 0.05). The 466 An. gambiae s.l. mosquitoes trapped were 53.7% unfed; 12.2% blood-fed and 34.1% gravid.
Similarly, there was no difference in the response of female An. funestus mosquitoes to MB5 + CO2 or MB5 + 99.5% 2-butanone (P = 0.635), but each of the two treatments was significantly more attractive than any of the other blends (P = 0.001) (Fig. 4). The responses of An. funestus mosquitoes to CO2 alone, 2-butanone (99.5%) alone and MB5 alone were similar (P = 0.098). Of the 316 female An. funestus mosquitoes trapped, 97.2 were unfed, 0.3% were blood-fed and 2.5% were gravid.
Outdoor mosquito catches
The adult female mosquitoes collected outdoors included An. gambiae s.l. (3%; n = 127), An. funestus (1.1%; n = 46), Culex (68.2%; n = 2889), Mansonia (17.2%; n = 730) and other Anopheles mosquito species (10.5%; n = 446) (Fig. 5). The 656 adult male mosquitoes trapped outdoors comprised 7.2% An. gambiae s.l., 1.7% An. funestus, 76.2% Culex, 12.8% Mansonia and 2.1% other Anopheles mosquito species.
Fig. 5.
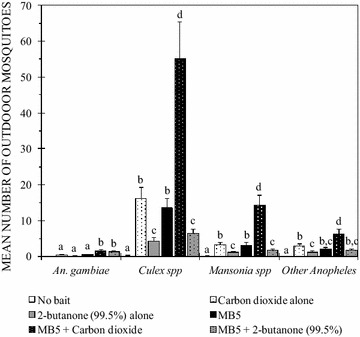
Mean number of wild female mosquitoes caught outdoors in MM-X traps with no bait or in a traps baited with either CO2 alone, 2-butanone (99.5%) alone, MB5, MB5 + CO2, or MB5 + 2-butanone (99.5%). Error bars denote the standard error of the mean number of mosquitoes trapped. Bars with different letters within a given mosquito species denote significant differences in the number of mosquitoes trapped. The numbers of An. funestus are not shown due to the very low catches
There were no significant differences in the responses of female An. gambiae s.l. mosquitoes to MB5 + CO2 and MB5 + 99.5% 2-butanone (P = 0.544), but each of the two treatments was significantly more attractive than a trap with no bait (P = 0.001 for both). There were fewer An. gambiae s.l. mosquitoes collected in a trap baited with MB5 alone than MB5 + CO2 (P = 0.004) or MB5 + 99.5% of 2-butanone (P = 0.02). The responses of An. gambiae s.l. to CO2 alone were similar to 99.5% of 2-butanone alone (P = 0.147) and MB5 alone (0.884) but lower than MB5 + CO2 (P = 0.003) or MB5 + 99.5% of 2-butanone (P = 0.014). The female An. gambiae s.l. mosquitoes trapped were either unfed (83.5%; n = 106), blood fed (4.7%; n = 6) or gravid (11.8%; n = 15).
The response of female An. funestus mosquitoes to MB5 + CO2 and MB5 + 99.5% 2-butanone did not differ (P = 0.533). Likewise, there were no significant differences between the responses of An. funestus mosquitoes to CO2 alone and 2-butanone (99.5%) alone (P = 1.000). All the female An. funestus mosquitoes trapped were unfed.
Identity of female An. gambiae s.l. and An. funestus mosquitoes by PCR
The 240 specimens of An. gambiae s.l. that were randomly selected from the 593 collected in total and analysed by PCR indicated the presence of 94.2% An. arabiensis and 5.8% An. gambiae s.s. Of the 105 random samples of An. funestus analysed out of the 362 collected in total, 97.3% were An. funestus s.s. while 2.7% could not be identified even after conducting repeated runs.
Discussion
In this study, it was observed that the responses of laboratory-reared An. gambiae s.s. mosquitoes to the MB5 reference attractant blend with CO2 were significantly higher compared to MB5 alone, CO2 alone or a trap without a bait. In all semi-field investigations MB5 + CO2 attracted a significantly higher number of mosquitoes than its variants containing the different dilutions of 2-butanone used to replace CO2. When using the blends of MB5 + 2-butanone, the highest catches were associated with the 99.5% concentration of 2-butanone and the 1.0% concentration of 2-butanone. Overall catches of An. arabiensis were far much lower than those of An. gambiae under semi-field conditions, probably because the human-mimicking attractant blends were developed and customized using An. gambiae as the test organism [25, 26] and that An. arabiensis has a more opportunistic host preference [1]. In the field study, An. gambiae s.l., An. funestus and Culex species were attracted to both MB5 + CO2 and MB5 + 99.5% 2-butanone with no significant difference in catch size between the blends. These results demonstrate that 2-butanone can be used as a replacement for CO2 under field conditions.
The finding that significantly more laboratory-reared mosquitoes were attracted to MB5 + CO2 than to MB5 alone (P < 0.001), CO2 alone (P < 0.001) or a trap without a bait (P < 0.001) underscores the action of CO2 as a synergist in mosquito attractants [21, 29]. The gas is known to activate mosquitoes by eliciting take-off behaviour and sustaining them in host-seeking flight [6, 7, 30]. These findings are in line with the results of studies which demonstrated that compounds are more attractive to host-seeking mosquitoes when blended than when applied alone [31].
It was observed that the pure (99.5%) form of 2-butanone is a potential replacement for CO2 in mosquito attractants. Under field conditions there were no differences between the numbers of An. gambiae s.l. and An. funestus mosquitoes attracted to MB5 + CO2 compared with MB5 + 99.5% 2-butanone. 2-butanone is a natural product identified in the emanations of various vertebrates and arthropods [32, 33] and several insects express a behavioural response upon exposure to this compound. Two separate studies [34, 35] have reported that the olfactory receptor cell of the fruit fly Bactrocera tyoni that responds to CO2 also responds to 2-butanone. And more recently, Turner et al. [14] demonstrated the capacity of 2-butanone to induce a dose-dependent activation of the CO2 receptor neuron in the maxillary palps of An. gambiae, Aedes aegypti and Culex quinquefasciatus. Failure to observe this effect under semi-field conditions may imply that 2-butanone acts as a long range rather than a short or medium-range cue or that the proximity of a more attractive alternative (MB5 + CO2) was preferred when mosquitoes were presented with a direct choice. Furthermore, there were no statistical differences in the numbers of An. funestus attracted to MB5 + CO2 or MB5 + 2-butanone (99.5%) under field conditions, but because a colony of this mosquito species has not been established at the research station in Mbita the response of this species under semi-field conditions could not be tested.
The relatively high number of wild male An. gambiae s.l. and An. funestus mosquitoes in traps baited with synthetic odour blends is contrary to expectations because males are phytophagous and are thought unlikely to respond to host-seeking odour blends compared to female mosquitoes [3, 37]. It may be assumed that the males were pursuing the females for mating, if this life history trait ever occurs indoors without swarming. Currently, there is an urgent need for potent synthetic odour blends for sampling and control of male mosquitoes. Such blends could be deployed to reduce mating success, and also to reduce the number of gravid female mosquitoes and prevalence of mosquito-borne diseases. Because the prospects of eliminating malaria are largely threatened by rapid development of drug-resistant Plasmodium parasites and insecticide-resistant malaria vectors, novel tools are needed. Odour-baited trapping technology has been used successfully in western Kenya to reduce mosquito bites and malaria prevalence [38]. The number of mosquitoes around houses was reduced by mass deployment of outdoor traps that were baited with MB5 augmented with 2-butanone instead of CO2 [36, 38]. The baited traps were effectively and repeatedly used for removal trapping of outdoor-biting mosquito vectors. Although residual attraction of mosquitoes to synthetic compounds impregnated on nylon strips has been reported [23], similar studies are needed for mosquito attractants that are augmented with 2-butanone. An odour bait with a residual activity of long duration without the need for frequent replenishment would be convenient for both monitoring as well as removal trapping of mosquitoes in remote areas of sub-Saharan Africa.
Human landing catches, light traps, bed nets occupied by humans, pyrethrum spray catches, and man-landing catches are commonly used for sampling of malaria vectors and estimation of malaria transmission intensity [39]. The methods vary in terms of reliability and efficacy, hence the need for standardized tools that are sensitive, specific, reliable and ethically acceptable for trapping and sampling malaria vectors. Recent studies indicate that synthetic odour baits dispensed by MM-X traps can be used reliably to collect live and species specific samples of both indoor and outdoor biting malaria and other mosquito vectors, particularly those which are host-seeking [13, 18, 29]. Thus, it is important to compare the odour blend, putative CO2 replacement and odour baited traps that were used in the current study with the common trapping tools outlined above.
Conclusion
This study demonstrates that 2-butanone has the potential to serve as a substitute for carbon dioxide in synthetic mosquito attractants, which is an essential step towards the development of sustainable, olfactory-based tools for mosquito vector control and surveillance [36]. The study further emphasizes the possibility of using OBTs for monitoring, surveillance and control of malaria and other mosquito vectors. Further studies are needed to evaluate the residual activity of 2-butanone in lures for mosquitoes as well as to understand more about its mode of action.
Authors’ contributions
MMM, CKM, WT, and WRM designed the study. MMM, AH and WRM analysed the data. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Mr. David Odhiambo Alila for rearing mosquitoes used in semi-field experiments. Bruno Otieno is thanked for teaching the principal author basic bioassay techniques. Annette Busula, Anthony Mutai and Andrew Abuya are acknowledged for helping out with 4 × 4 semi-field experiments seeking to evaluate whether 2-butanone can act as a substitute for carbon dioxide in synthetic mosquito attractants. Mr. Charles Oketch assisted with field experiments. We thank Mr. Fred Kisanya for hosting our laboratory activities at the Ahero Multipurpose Development Training Institute. Our special thanks go to the inhabitants of Kigoche Village, Kisumu County, Kenya, for allowing us to work in their homesteads. This work was supported by grants from the COmON Foundation, the Netherlands, through the Food For Thought campaign of Wageningen University Fund, and through funds from the Foundation for the National Institutes of Health (FNIH) through the Grand Challenges in Global Health initiative (GCGH #121).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Monicah M. Mburu, Email: monicahmirai@yahoo.com
Collins K. Mweresa, Email: collins.mweresa@yahoo.com
Philemon Omusula, Email: pomusulabtr@yahoo.com.
Alexandra Hiscox, Email: alexandra.hiscox@wur.nl.
Willem Takken, Email: willem.takken@wur.nl.
Wolfgang R. Mukabana, Email: rmukabana@yahoo.co.uk
References
- 1.Clements AN. The biology of mosquitoes, vol. 2 sensory reception and behaviour. London: Chapman and Hall; 1999. [Google Scholar]
- 2.Takken W. The role of olfaction in host-seeking of mosquitoes: a review. Insect Sci Appl. 1991;12:287–295. [Google Scholar]
- 3.Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 4.Smallegange RC, Takken W. Host-seeking behaviour of mosquitoes: responses to olfactory stimuli in the laboratory. Olfaction Vector Host Interact. 2010;2:143–180. [Google Scholar]
- 5.Lehane M. Location of the host. In: Lehane M, editor. Biology of blood-sucking insects. Cambridge: Cambridge University Press; 1991. pp. 25–51. [Google Scholar]
- 6.Gillies M. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull Entomol Res. 1980;70:525–532. doi: 10.1017/S0007485300007811. [DOI] [Google Scholar]
- 7.Dekker T, Takken W, Carde RT. Structure of host-odour plumes influences catch of Anopheles gambiae s.s. and Aedes aegypti in a dual-choice olfactometer. Physiol Entomol. 2001;26:124–134. doi: 10.1046/j.1365-3032.2001.00225.x. [DOI] [Google Scholar]
- 8.Costantini C, Gibson G, Sagnon NF, Torre AD, Brady J, Coluzzi M. Mosquito responses to carbon dioxide in B West African Sudan savanna village. Med Vet Entomol. 1996;10:220–227. doi: 10.1111/j.1365-2915.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 9.Saitoh Y, Hattori J, Chinone S, Nihei N, Tsuda Y, Kurahashi H, Kobayashi M. Yeast-generated CO2 as a convenient source of carbon dioxide for adult mosquito sampling. J Am Mosq Control Assoc. 2004;20:261–264. [PubMed] [Google Scholar]
- 10.van den Hurk AF, Hall-Mendelin S, Johansen CA, Warrilow D, Ritchie SA. Evolution of mosquito-based arbovirus surveillance systems in Australia. J BioMed Biotechnol. 2012;2012:325659. doi: 10.1155/2012/325659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J, Mukabana WR, et al. Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar J. 2010;9:292. doi: 10.1186/1475-2875-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oli K, Jeffery J, Vythilingam I. A comparative study of adult mosquito trapping using dry ice and yeast generated carbon dioxide. Trop Biomed. 2005;22:249–251. [PubMed] [Google Scholar]
- 13.Mweresa CK, Omusula P, Otieno B, van Loon JJ, Takken W, Mukabana WR. Molasses as a source of carbon dioxide for attracting the malaria mosquitoes Anopheles gambiae and Anopheles funestus. Malar J. 2014;13:160. doi: 10.1186/1475-2875-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner SL, Li N, Guda T, Githure J, Cardé RT, Ray A. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature. 2011;474:87–91. doi: 10.1038/nature10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tauxe GM, MacWilliam D, Boyle SM, Guda T, Ray A. Targeting a dual detector of skin and CO 2 to modify mosquito host seeking. Cell. 2013;155:1365–1379. doi: 10.1016/j.cell.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mboera L, Takken W, Sambu E. The response of Culex quinquefasciatus (Diptera: Culicidae) to traps baited with carbon dioxide, 1-octen-3-ol, acetone, butyric acid and human foot odour in Tanzania. Bull Entomol Res. 2000;90:155–159. doi: 10.1017/S0007485300000262. [DOI] [PubMed] [Google Scholar]
- 17.Philippe-Janon JCD, van den Hurk AF, Francis DP, Shivas MA, Jansen CC. Field comparison of cyclopentanone versus carbon dioxide as an attractant for adult mosquitoes in Southeast Queensland, Australia. J Med Entomol. 2015;52:483. doi: 10.1093/jme/tjv011. [DOI] [PubMed] [Google Scholar]
- 18.Menger DJ, Otieno B, de Rijk M, Mukabana WR, van Loon JJ, Takken W. A push-pull system to reduce house entry of malaria mosquitoes. Malar J. 2014;13:119. doi: 10.1186/1475-2875-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukabana WR, Mweresa CK, Omusula P, Orindi BO, Smallegange RC, van Loon JJ, et al. Evaluation of low density polyethylene and nylon for delivery of synthetic mosquito attractants. Parasit Vectors. 2012;5:202. doi: 10.1186/1756-3305-5-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumu F, Biswaro L, Mbeleyela E, Killeen GF, Mukabana R, Moore SJ. Using nylon strips to dispense mosquito attractants for sampling the malaria vector Anopheles gambiae s.s. J Med Entomol. 2010;47:274–282. doi: 10.1093/jmedent/47.2.274. [DOI] [PubMed] [Google Scholar]
- 21.Njiru BN, Mukabana WR, Takken W, Knols BG. Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar J. 2006;5:39. doi: 10.1186/1475-2875-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Njie M, Dilger E, Lindsay SW, Kirby M. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol. 2009;46:505–510. doi: 10.1603/033.046.0314. [DOI] [PubMed] [Google Scholar]
- 23.Mweresa CK, Otieno B, Omusula P, Weldegergis BT, Verhulst NO, Dicke M, et al. Understanding the long-lasting attraction of malaria mosquitoes to odor baits. PLoS ONE. 2015;10:e0121533. doi: 10.1371/journal.pone.0121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atieli H, Menya D, Githeko A, Scott T. House design modifications reduce indoor resting malaria vector densities in rice irrigation scheme area in western Kenya. Malar J. 2009;8:108. doi: 10.1186/1475-2875-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukabana WR, Mweresa CK, Otieno B, Omusula P, Smallegange RC, van Loon JJ, et al. A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J Chem Ecol. 2012;38:235–244. doi: 10.1007/s10886-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okumu FO, Killeen GF, Ogoma S, Biswaro L, Smallegange RC, Mbeyela E, et al. Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE. 2010;5:e8951. doi: 10.1371/journal.pone.0008951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 28.Koekemoer L, Kamau L, Hunt R, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 29.Jawara M, Smallegange RC, Jeffries D, Nwakanma DC, Awolola TS, Knols BGJ, et al. Optimizing odor-baited trap methods for collecting mosquitoes during the malaria season in The Gambia. PLoS ONE. 2009;4:e8167. doi: 10.1371/journal.pone.0008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster B, Lacey E, Cardé R. Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J Chem Ecol. 2015;41:59–66. doi: 10.1007/s10886-014-0542-x. [DOI] [PubMed] [Google Scholar]
- 31.Smallegange RC, Qiu YT, van Loon JJ, Takken W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) Chem Senses. 2005;30:145–152. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- 32.Le Danvic C, Gérard O, Sellem E, Ponsart C, Chemineau P, Humblot P, et al. Enhancing bull sexual behavior using estrus-specific molecules identified in cow urine. Theriogenology. 2015;83:1381–1388. doi: 10.1016/j.theriogenology.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Manrique G, Vitta AC, Ferreira RA, Zani CL, Unelius CR, Lazzari CR, et al. Chemical communication in Chagas disease vectors. Source, identity, and potential function of volatiles released by the metasternal and Brindley’s glands of Triatoma infestans adults. J Chem Ecol. 2006;32:2035–2052. doi: 10.1007/s10886-006-9127-7. [DOI] [PubMed] [Google Scholar]
- 34.Hull C, Cribb B. Olfaction in the Queensland fruit fly, Bactrocera tryoni. I: identification of olfactory receptor neuron types responding to environmental odors. J Chem Ecol. 2001;27:871–887. doi: 10.1023/A:1010374617409. [DOI] [PubMed] [Google Scholar]
- 35.Inouchi J. Speices specific odor responses in the insects. Aroma Res. 2005;6:8. [Google Scholar]
- 36.Killeen GF. Mass trapping of malaria vector mosquitoes. Lancet. 2016;388:1136–1137. doi: 10.1016/S0140-6736(16)30674-2. [DOI] [PubMed] [Google Scholar]
- 37.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Ann Rev Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 38.Homan T, Hiscox A, Mweresa CK, Masiga D, Mukabana WR, Oria P, et al. The effect of mass mosquito trapping on malaria transmission and disease burden (SolarMal): a stepped-wedge cluster randomized trial. Lancet. 2016;388:1193–1201. doi: 10.1016/S0140-6736(16)30445-7. [DOI] [PubMed] [Google Scholar]
- 39.Kelly-Hope L, Mckenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;8:19. doi: 10.1186/1475-2875-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]


