Abstract
In participation of the fifth statistical assessment of modeling of proteins and ligands (SAMPL5), the strength of association of six guests (3–8) to two hosts (1 and 2) were measured by 1H NMR and ITC. Each host possessed a unique and well-defined binding pocket, whilst the wide array of amphiphilic guests possessed binding moieties that included: a terminal alkyne, nitro-arene, alkyl halide and cyano-arene groups. Solubilizing head groups for the guests included both positively charged trimethylammonium and negatively charged carboxylate functionality. Measured association constants (Ka) covered five orders of magnitude, ranging from 56 M−1 for guest 6 binding with host 2 up to 7.43 × 106 M−1 for guest 6 binding to host 1.
Introduction
The hosts octa-acid (OA) 1 (Figure 1) and its tetra-endo-methyl octa-acid derivative (TEMOA) 2, are water-soluble deep-cavity cavitands that contain a hydrophobic binding pocket and an exterior coating of eight carboxylic acid groups arranged in a square anti-prism array; the former engenders guest binding, whilst the latter – because it maximizes the distance and distribution of points on the surface of a sphere – bestows these hosts with good aqueous solution solubility under basic conditions.
Figure 1.
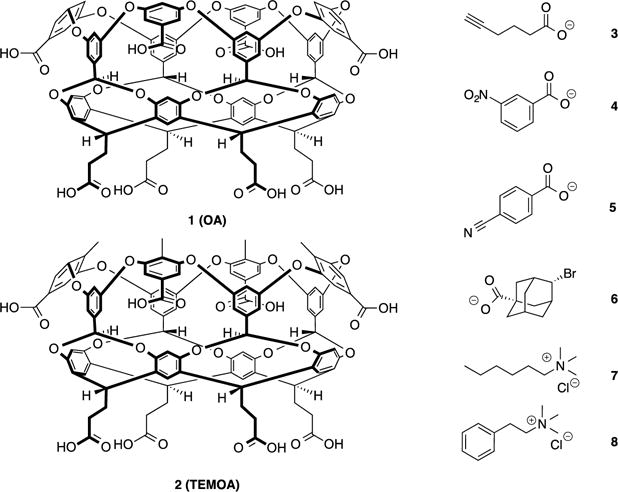
Chemical structures of hosts 1 and 2 and guests 3–8.
The well-defined binding pockets of these hosts are formed primarily by their eight “lower” aromatic rings, and include four benzal protons that project into the midsection of the cavity and act as weak hydrogen bond donors.1 Both hosts are obtained via essentially the same synthetic approach.2 In addition to these eight aromatic rings, there are four “upper” aromatic rings at the rim of each host that bestow rigidity to their hydrophobic pockets. This third row of rings also promotes assembly of these hosts in the presence of certain guests.3 Host 1 has only been seen to form dimeric assemblies. In contrast, the endo-methyls of 2 project upwards from the rim somewhat, reducing the predisposition of the host to dimerize, but promoting its assembly into tetrameric and hexameric complexes instead.2b, 4
In addition to these self-assembling properties, both hosts form 1:1 host-guest complexes in the presence of amphiphiles5 or small, anionic guests.6 For the former, binding of the hydrophobic tail leaves the polar head-group well solvated at the entrance of the binding pocket, and as with small anion binding to 1,6 this reduces the hydrophobicity of the rim/portal region and inhibits self-assembly.
As part of the SAMPL5 exercise we sought to compare the complexation properties of both 1 and 2 using six different amphiphilic guests 3–8 (Figure 1). Guest choice mainly focused on establishing a broad range of structure types for the challenge, but also had to consider host and guest limitations even in this relatively straightforward system. For example, our initial set of guests included 6-nitrohexanoic acid and n-decyl trimethylammonium. However, it was necessary to run all experiments at pH = 11.3–11.5 for good host solubility, and under these conditions the acidic α-proton of 6-nitrohexanoic acid was deprotonated. This equilibrium and the resulting change in heat caused prevented the analysis of guest binding. In the case of n-decyl trimethylammonium, although amphiphilic, this guest still triggered dimerization of the host. Evidently, binding to the pocket of either hosts led to a length of the large hydrophobic chain protruding from the cavity and exposed to the aqueous environment. This led to an overall relatively hydrophobic rim region that promoted the assembly of a 2:1 host-guest complex. Although it was determined that dimerization could be prevented by use of very dilute solutions, insufficient heat was liberated under such conditions and noise interference became significant. For these very different reasons, these two guests were dropped from the analysis.
Binding affinities for the host-guest systems involving 1 and 2 and 3–8 were determined through Isothermal Titration Calorimetry (ITC) and 1H NMR. To maintain the integrity essential to the SAMPL projects, the results of the experiments were withheld from the from the SAMPL organizers and participants until all calculations were completed. Herein, we present the thermodynamic parameters resulting from these empirical experiments; the corresponding computational predictions can be found in the other papers submitted to this special issue.
Experimental
Full experimental details are given in the Supporting Information. Hosts 1 and 2 were synthesized following previously reported procedures.2 All reagents and guests 3–5 were purchased from Aldrich and were used without purification. Guest 6 was synthesized from commercially available 2-adamantanone by following a slightly modified literature procedure. Its structure was confirmed by single-crystal X-ray crystallography. Guest 7 was synthesized from commercially available hexyltrimethylammonium bromide via chloride ion exchange (Dowex 1 × 8 chloride resin). Guest 8 was synthesized from commercially available N,N-dimethylphenethylamine by methylation with methyl iodide followed by chloride ion exchange (Dowex 1 × 8 chloride resin).
All NMR spectra were recorded on a Bruker 500 MHz spectrometer at 25 °C, with the exception of the complex between 2 and 6, which was carried out at 5 °C. Spectral processing was carried out using Mnova software (Mestrelab Research S.L). All NMR titrations were carried out in 10 mM phosphate buffer (pH = 11.3) prepared with D2O (Cambridge Isotopes, 99.9%+), with each solution prepared on the day of titration. The determination of the corresponding association constants (Ka) followed standard titration and curve fitting procedures.7
Isothermal Titration Calorimetric (ITC) analyses of the binding events were performed in 50 mM phosphate buffer (pH = 11.5) at 25 °C using a VP-ITC MicroCalorimeter from Microcal, USA. Curve fitting of the binding isotherms were processed using ORIGIN 7.0 software included with the instrument.
Results and discussion
Association constants (Ka) were determined for each host-guest pair using ITC and 1H NMR analytical techniques, but host-guest behavior and instrumental limitations prevented both techniques being used for all complexes.
With NMR analysis, host-guest exchange processes can be either fast or slow relative to the NMR timescale. Slow exchanging systems, e.g. guest 6 (Figure 2), give resolution of two distinct signals for a single proton or a set of equivalent protons representing the free and bound states. With known initial concentrations in the sample, the peaks for free host, free guest, and host-guest complex were simply integrated to determine the corresponding Ka value.
Figure 2.
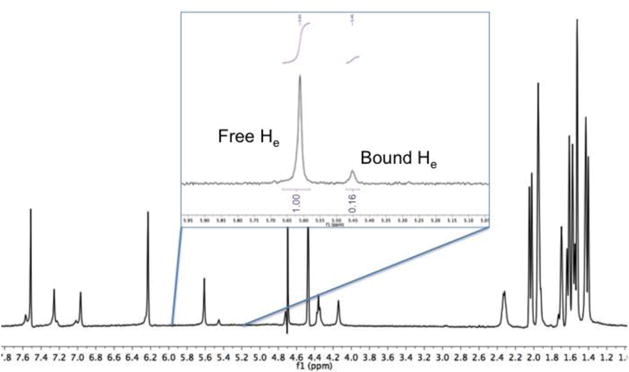
Slow-exchange complex. Ka value determination based on integration of host 2 free and bound ‘He’ protons (see Figure S1, Supporting information) signal intensity with twenty equivalents of 6.
Fast exchange between the free and the bound state (Figure 3) results in only one peak of interest – a weighted average – of the free and bound signals. With these conditions the NMR spectrum does not possess sufficient information for a Ka determination and a titration experiment must be carried out that tracks the changes in chemical shift (Δ∂) of a signal as a function of changing host-guest ratio. Binding affinities were determined using NMR for all host-guest complexes, except the host-guest pair 1 and 6, which had an association constant beyond the upper limit of the technique.
Figure 3.
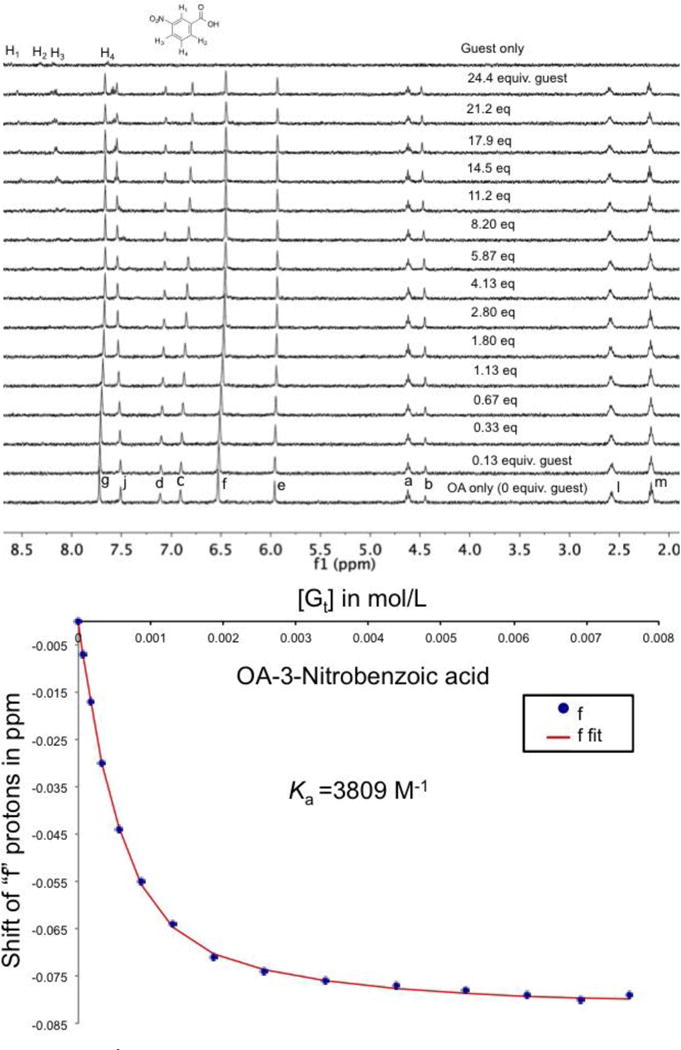
Top: Stacked 1H NMR spectra for the titration of 4 to 1 in 10 mM phosphate buffer (pH = 11.3). Bottom: The corresponding binding isotherm fitting the signal from the Hf proton (See Figure S1 for proton assignments) to a 1:1 binding model.
ITC is capable of measuring not only larger Ka values than NMR, but also all principle thermodynamic parameters (ΔG, ΔH, and –TΔS) of the binding event in a single experiment. Binding affinities are determined by measuring the amount of heat released or absorbed (ΔH) as a function of host-guest ratio, and fitting those values to a 1:1 binding model. For host 1, the complexation of guest 3–5 and 7 and 8 gave relatively low Wiseman parameters and were subsequently analyzed as described by Turnbull8 and Tellinghusen.9 Furthermore, to ensure a measurable amount of heat from these systems relatively concentrated guest (titrant) solutions were used. These high concentrations resulted in measurable heats of dilution, and consequently for these binding processes control experiments titrating the guest into buffer solution alone were carried out (Figure 4). Standard ITC titrations were performed for host 2 with guests 3–5 and 7. However, the complexes between this host and guests 6 and 8 failed to produce sufficient heat and Ka values had to be determined solely by NMR. In all cases the resulting data was fitted to a 1:1 binding model for determination of thermodynamic parameters.
Figure 4.

a) ITC data for the complex formed between 1 and 5. b) ITC titration of 5 into buffer alone. c) An overlay of the data from a) and b). d) The resultant final binding curve after subtracting (b) from (a).
The thermodynamic data obtained from ITC and NMR is shown in Tables 1 and 2 respectively. Note that the two techniques give slightly different Ka values. This is to be expected considering the differences between the two techniques. First and foremost, NMR values are obtained from changes to the local electron density around a specific proton, whereas the ITC value is a measure of global changes to the system. Second, the ITC experiments were performed in 50 mM phosphate buffered H2O, whilst the NMR experiments are performed in 10 mM phosphate buffered D2O. Ionic strength has a considerable effect on the binding of guests.6b, 6c Third, there is a known isotope effect in binding affinity determinations in aqueous supramolecular chemistry grounded in the differences in hydrogen bond strengths in H2O verses D2O.10
Table 1.
Thermodynamic data from ITC for the binding of guests 3–8 to hosts 1 and 2.a
| Guests | OA (1)b | TEMOA (2)c | ||||||
|---|---|---|---|---|---|---|---|---|
| Ka (M−1) | ΔG° cal/mol |
ΔH° cal/mol |
-TΔS cal/mol/K | Ka (M−1) | ΔG° cal/mol |
ΔH° cal/mol |
-TΔS cal/mol/K | |
| 3 | 9040 (±40) |
−5398 (±3) |
−7713 (±48) |
2315 (±45) |
10150 (±50) |
−5476 (±10) |
−9961 (±6) |
4485 (±15) |
| 4 | 8140 (±60) |
−5335 (±5) |
−5669 (±7) |
334 (±12) |
2050 (±70) |
−4521 (±19) |
−9051 (±130) |
4530 (±150) |
| 5 | 2890 (±20) |
−4731 (±14) |
−4445 (±81) |
−286 (±79) |
7085 (±155) |
−5255 (±14) |
−7559 (±103) |
2304 (±117) |
| 6 | 7.43 × 106 (±4 × 104) |
−9369 (±7) |
−14783 (±23) |
5414 (±20) |
– | – | – | – |
| 7 | 1960 (±20) |
−4492 (±10) |
−5913 (±95) |
1421 (±102) |
16000 (±1500) |
−5732 (±55) |
−6619 (±180) |
887 (±234) |
| 8 | 535 (±7) |
−3724 (±9) |
−9962 (±108) |
6238 (±99) |
– | – | – | – |
25 °C, 50 mM sodium phosphate buffer, pH = 11.5.
The average of three experiments.
The average of two experiments.
Table 2.
Binding constant (Ka) data from NMR for the binding of guests 3–8 to hosts 1 and 2.a
| Guests | OA (1) | TEMOA (2) |
|---|---|---|
| Ka (M−1) | Ka (M−1) | |
| 3 | 5002 | 6941 |
| 4 | 3935 (Hf) | 1817 (CH3) |
| 5 | 1307 | 4956 |
| 6 | – | 56 (He)b |
| 7 | 5164 | 2.31 × 104 |
| 8 | 1997 (Hc) | 729 |
Values are the averages of two experiments and are calculated using the Hb proton signal (Figure S1, Supporting Information) unless otherwise noted in parenthesis. Experimental conditions were: 25 °C, 10 mM sodium phosphate buffer, pH = 11.3.
Measured at 5 °C.
Key aspects controlling the strength of association are the complementarity between host and guest and changes in host and guest solvation. However, although the latter plays a significant contribution,11 it is not directly addressable by NMR. In contrast, NMR analysis of the complexes described here – as well as previous studies5 – readily allow interpretation in terms of the structures of the hosts and guests.
The guests can be divided into two categories: carboxylates (3–6) and ammonium salts (7 and 8). However the relatively small set of examples precludes any conclusions pertaining to how Coulombic forces contribute to complexation. That stated, it is interesting to note in passing that where Ka determinations by NMR and ITC could be compared, the NMR determinations were always lower for the carboxylates, and always higher for the ammonium guests.
The carboxylate guests offer a variety of binding moieties. Guest 3 contains a terminal alkyne, 4 and 5 are both substituted benzoic acids with meta-nitro and para-cyano groups respectively, and 6 is a bulky adamantane brominated at the 4-position. It was assumed that with its terminal alkyne group, 3 would bind deeply into the pocket of the two hosts with the alkyne anchored to the base of their tapering pockets. Guest 5 is also shaped to allow its cyano group to fill the very bottom of the pocket, whilst having its carboxylate at the portal region. However, in the case of 4, the nitro group is both polar and located meta- to the carboxylate. Thus, even if the nitro group had a preference for desolvation and burial within the host, it would be forced to impact the sides of either host if the C-CO2− bond of the guest lies on the C4 axis of the host for maximal solvation. In the case of 6, the rotund nature of the guest suggested it might be incongruous to the binding pocket of 2. Moreover, the bromo-substituent was considered to impact the walls of each of the hosts and lead to sub-optimal binding. The trimethylammonium guests contain a hexyl chain (7) and a phenethyl group (8). In each case these bulkier chains were assumed to bind preferentially to the hydrophobic pockets of the hosts rather than the trimethylammonium groups. As with the carboxylates, NMR shift data confirmed this to be the case; these hosts do not form strong cation-π interactions in water.
As shown in Table 1 (ITC) and Table 2 (NMR), the binding constants of guests 3–8 to hosts 1 and 2 covered a range of five orders of magnitude. A noteworthy observation is the very different selectivity demonstrated by the two structurally related hosts. For example, complexes between 2 and guests 3, 5, and 7 are stronger than the corresponding complexes with host 1, but it is host 1 that forms the stronger complexes with guests 8 and 6.
The alkyne carboxylate guest 3 exhibited a similar affinity for both hosts. NMR chemical shifts reveal that the alkyne anchors to the base of the host allowing the carboxylate to reside at the portal of the pocket. The thermodynamic parameters for hexanoate binding to 1 were recently reported.12 The weaker measured affinity for hexanoate (Ka = 6,750 verses 9,040 M−1) suggests a preference for these cavitands binding alkynes over alkanes. Furthermore, the ΔH for the complexation of the hexanoate and its alkyne counterpart 3 were respectively −5.13 and −7.71 kcal mol−1, suggesting a high complementarity between the alkyne and the host. The corresponding –TΔS values for hexanoate and 3 were a barely favorable −0.10 and unfavorable 2.31 kcal mol−1, suggesting a tighter alkyne complex and enthalpy-entropy compensation.13 Overall, these results suggest that the small, rigid alkyne group is able to bind deeply into the cavity and form a relatively stronger complex.
Guest 3 forms an even stronger complex with host 2. It is difficult to see how the methyl groups of 2 could directly interact with the guest and lead to the much higher enthalpy of complexation observed (ΔH = −7.71 verses −9.96 kcal mol−1), so differences in solvation of the hosts may contribute to these observed differences.
Guest 4 contains meta-oriented polar substituents. The Ka value for the binding to 1 is almost four times higher than for 2, but the enthalpy of complexation is much higher for host 2; it is a much larger entropic penalty for 2 binding 4 that leads to weaker complexation. For this guest we presume it is the highly polar carboxylate that dominates guest orientation within the pocket and that the less polar nitro group is, at least in part, buried within the pocket. If this is the case, this nitro group location has the potential to contact the endo-methyl groups of 2 and lead to interactions that may dominate the observed differences between the two hosts.
Guest 5 also contains two polar groups, but this time they are para-oriented so that the cyano group can bind down into the base of the tapering pocket. The overall strength of the complexation of 5 to 1 is not exceptionally high, but it is the only host-guest combination studied here exhibiting both favorable enthalpy and entropy of binding. In contrast, the affinity of 5 for 2 is more than twice as strong as for 1, and in large part this is an enthalpic phenomenon; the binding of 5 to 2 is enthalpically driven and entropically unfavorable, but this enthalpic influence is so strong that the overall association is much stronger than 1 binding guest 5.
Both p-ethyl- and p-chloro-benzoates have been studied binding to host 1 previously.5b Although a different buffer was used in this study, the larger, more than one order of magnitude higher Ka values for these guests suggests that the cyano group of 5 is not an efficient anchor for promoting guest complexation.
Whereas the entropic cost of binding cyano guest 5 to 1 is very similar to nitro-arene 4, in the case of host 2 the entropic cost of binding of guest 5 is half that of 4. This is presumably linked to the idea that the para-substituent is remote from the endo-methyl groups whereas in the case of 4 the meta-nitro group is forced to interact with these host groups.
Note the common theme for guests 3–5: in all cases binding to 2 is more exothermic and suffers a greater entropic penalty than binding to host 1. This suggests two general possibilities: 1) that the endo-methyls of host 2 can form better and/or more numerous non-covalent contacts with the guests; 2) there is a fundamental difference in the solvation of the pockets of hosts 1 and 2.
Guest 6 provides the most interesting data in this series, with the largest difference in affinity between the two hosts (five orders of magnitude). The rotund nature of the adamantane cage complements the pocket of 1, and previous studies reveal this moiety to be among the strongest binders to 1.14 For example, the complexation of adamantane carboxylate to host 1 releases 9.1 kcal mol−1 of free energy.6a Correspondingly, guest 6 was expected to form a strong complex with 1, even though the bromine substituent might impact with the wall of the pocket. The association of 1 and 6 proved to have the largest ΔH of complexation in the series, and was accompanied by an unexceptional entropic penalty. As a result, overall, binding of 6 was noted to be slightly stronger than the parent adamantane carboxylate. Presumably this is because although the bromine substituent can impact the sides of the host when the C-CO2− bond of 6 is maximally solvated and co-axial with the C4 axis of the host, moving the carboxylate bond out of the C4 axis towards the wall of the host allows the bromine of the guest to form C–H∙∙∙X–R hydrogen bonds with the benzal protons of the host.1 Indeed, this possibility was strongly suggested by a ~ 0.2 ppm downfield shift of the benzal signal of the host upon guest binding.
Previous examinations in organic solvents have revealed no complexation between adamantane derivatives and hosts analogous to 2, a fact attributed to the hindering methyl groups around the rim of this host and the rotund nature of the adamantane cage.14a However, guest associations are much stronger in water, and weak binding of 6 to 2 was indeed confirmed by NMR (binding was too weak to determine by ITC). NMR revealed that in addition to the hydrophobic effect binding was also promoted by the formation of C–H∙∙∙Br–R hydrogen bonds between host and guest. Thus the titration experiment revealed a large upfield shift of ~ 0.25 ppm for the Hb proton of the host. This experiment also revealed an exchange process between the free and the bound state that was on the NMR timescale at 25 °C, and so the determination of the Ka value was carried out at 5 °C (Figure 2).
Guest 7 contains a hydrophobic hexyl tail for complexation with the pocket and a polar trimethylammonium head group to be solvated at the mouth of the cavity. Surprisingly, the affinity of 7 for 2 is nearly an order of magnitude stronger than it is for 1. As with most complexes examined here, the binding to both hosts is enthalpically driven with a relatively low entropic penalization; in the case of 2, binding is only slighter more favorable enthalpically, but the entropic penalty for complexation is much smaller.
In comparing the binding of 7 to 1 with that of previously determined hexanoate,12 it is evident that the ammonium guest bound more weakly and that this was primarily because of a larger entropic penalty to complexation (a barely favorable −0.10 verses unfavorable 1.42 kcal mol−1).
Related guest 8 possesses a phenyl group as its hydrophobic tail. ITC measurements revealed weak binding to host 1, but a comparable experiment with 2 released no measurable heat. Binding was however observed by NMR, suggesting that this host-guest complexation is entropically dominated. This guest was the weakest binder to 1, and the second weakest binder to 2.
Conclusions
We have measured the thermodynamic data for the binding of six guests to two hosts. Ten of the host-guest pairs proved amenable to ITC analysis, with the remaining two pairs being analyzable only by NMR. The association constants of guests 3–8 to hosts 1 and 2 in aqueous solution covered a range of five orders of magnitude: from as low as Ka = 56 M−1 for the host-guest pair 2 and 6, to as high as Ka = 7.43 × 106 M−1 for 1 and 6. Host 1 has been studied previously, and where comparisons could be made it was possible to extend our knowledge about the ability of different moieties to promote complexation to this family of hosts. The steric hindrance induced by the methylated rim of host 2, and/or the changes they induce in pocket solvation, were shown to have a complex effect on guest binding. In some cases binding was enhanced relative to 1, whilst in other cases the affinity was lower. However, in all cases that provided direct comparison by ITC, guest binding to 2 was always more exothermic than binding to 1.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the National Institutes of Health (GM 098141). The authors also acknowledge the Louisiana Board of Regents enhancement grant (Grant LEQSF-(2002-03)-ENH-TR-67) used to purchase the X-ray diffractometer.
References
- 1.Gibb CLD, Stevens ED, Gibb BC. C-H···X-R (X = Cl, Br, and I) Hydrogen Bonds Drive the Complexation Properties of a Nanoscale Molecular Basket. J Am Chem Soc. 2001;123(24):5849–5850. doi: 10.1021/ja005931p. [DOI] [PubMed] [Google Scholar]
- 2.(a) Liu S, Whisenhunt-Ioup SE, Gibb CLD, Gibb BC. An improved synthesis of ‘octa-acid’ deep-cavity cavitand. Supramol Chem. 2011;23(6):480–485. doi: 10.1080/10610278.2010.550290. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gan H, Benjamin CJ, Gibb BC. Nonmonotonic Assembly of a Deep-Cavity Cavitand. J Am Chem Soc. 2011;133(13):4770–4773. doi: 10.1021/ja200633d. [DOI] [PubMed] [Google Scholar]
- 3.(a) Gibb CLD, Gibb BC. Well-Defined, Organic Nanoenvironments in Water: The Hydrophobic Effect Drives a Capsular Assembly. J Am Chem Soc. 2004;126(37):11408–11409. doi: 10.1021/ja0475611. [DOI] [PubMed] [Google Scholar]; (b) Jordan JH, Gibb BC. Molecular containers assembled through the hydrophobic effect. Chem Soc Rev. 2015;44(2):547–585. doi: 10.1039/c4cs00191e. [DOI] [PubMed] [Google Scholar]; (c) Sullivan MR, Gibb BC. Differentiation of small alkane and alkyl halide constitutional isomers via encapsulation. Org Biomol Chem. 2015;13(6):1869–1877. doi: 10.1039/c4ob02357a. [DOI] [PubMed] [Google Scholar]
- 4.Gan H, Gibb BC. Guest-mediated switching of the assembly state of a water-soluble deep-cavity cavitand. Chem Commun (Cambridge, U K) 2013;49(14):1395–1397. doi: 10.1039/c2cc38227j. [DOI] [PubMed] [Google Scholar]
- 5.(a) Gibb CLD, Gibb BC. Binding of cyclic carboxylates to octa-acid deep-cavity cavitand. J Comput-Aided Mol Des. 2014;28(4):319–325. doi: 10.1007/s10822-013-9690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun H, Gibb CLD, Gibb BC. Calorimetric analysis of the 1:1 complexes formed between a water-soluble deep-cavity cavitand, and cyclic and acyclic carboxylic acids. Supramol Chem. 2008;20(1&2):141–147. [Google Scholar]
- 6.(a) Gibb CLD, Gibb BC. Anion binding to hydrophobic concavity is central to the salting-in effects of Hofmeister chaotropes. J Am Chem Soc. 2011;133(19):7344–7347. doi: 10.1021/ja202308n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Carnegie RS, Gibb CLD, Gibb BC. Anion Complexation and The Hofmeister Effect. Angew Chem, Int Ed. 2014;53(43):11498–11500. doi: 10.1002/anie.201405796. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gibb CLD, Oertling EE, Velaga S, Gibb BC. Thermodynamic Profiles of Salt Effects on a Host-Guest System: New Insight into the Hofmeister Effect. J Phys Chem B. 2015;119(17):5624–5638. doi: 10.1021/acs.jpcb.5b01708. [DOI] [PubMed] [Google Scholar]; (d) Sokkalingam P, Shraberg J, Rick SW, Gibb BC. Binding Hydrated Anions with Hydrophobic Pockets. J Am Chem Soc. 2016;138(1):48–51. doi: 10.1021/jacs.5b10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale PA, Steed JW, editors. Supramolecular Chemistry: From Molecules to Nanomaterials, Volume 1: Concepts. John Wiley & Sons Ltd; 2012. p. 235. [Google Scholar]
- 8.Turnbull WB, Daranas AH. On the Value of c: Can Low Affinity Systems Be Studied by Isothermal Titration Calorimetry? J Am Chem Soc. 2003;125(48):14859–14866. doi: 10.1021/ja036166s. [DOI] [PubMed] [Google Scholar]
- 9.Tellinghuisen J. Isothermal titration calorimetry at very low c. Anal Biochem. 2008;373(2):395–397. doi: 10.1016/j.ab.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Rekharsky MV, Inoue Y. Solvent and Guest Isotope Effects on Complexation Thermodynamics of Alpha-, Beta-, and 6-Amino-6-deoxy-beta-cyclodextrins. J Am Chem Soc. 2002;124(41):12361–12371. doi: 10.1021/ja027031+. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Amotz D, Underwood R. Unraveling Water’s Entropic Mysteries: A Unified View of Nonpolar, Polar, and Ionic Hydration. Acc Chem Res. 2008;41(8):957–967. doi: 10.1021/ar7001478. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Sokkalingam P, Gibb BC. ITC and NMR analysis of the encapsulation of fatty acids within a water-soluble cavitand and its dimeric capsule. Supramol Chem. 2016;28(1–2):84–90. doi: 10.1080/10610278.2015.1082563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chodera JD, Mobley DL. Entropy-enthalpy compensation: role and ramifications in biomolecular ligand recognition and design. Annu Rev Biophys. 2013;42:121–142. doi: 10.1146/annurev-biophys-083012-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Laughrey ZR, Gibb CLD, Senechal T, Gibb BC. Guest binding and orientation within open nanoscale hosts. Chem - Eur J. 2003;9(1):130–139. doi: 10.1002/chem.200390008. [DOI] [PubMed] [Google Scholar]; (b) Gibb CLD, Xi H, Politzer PA, Concha M, Gibb BC. The synthesis and binding properties of nano-scale hydrophobic pockets. Tetrahedron. 2002;58(4):673–681. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


