Abstract
A major challenge in the care of patients with heart failure and preserved ejection fraction (HFpEF) is the lack of proven therapies due to disappointing results from randomized controlled trials (RCTs). The heterogeneity of the HFpEF syndrome and the use of conventional RCT designs are possible reasons underlying the failure of these trials. There are several factors— including the widespread adoption of electronic health records, decreasing costs of obtaining high-dimensional data, and the availability of a wide variety of potential therapeutics—that have evolved to enable more innovative clinical trial designs in HFpEF. Here we review the current landscape of HFpEF RCTs and present several innovative RCT designs that could be implemented in HFpEF, including enrichment trials, adaptive trials, umbrella trials, basket trials, and machine learning-based trials (including examples for each). Our hope is that the description of the aforementioned innovative trial designs will stimulate new approaches to clinical trials in HFpEF.
Keywords: heart failure with preserved ejection fraction, precision medicine, personalized medicine, clinical trials, treatment
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a common and increasingly prevalent clinical syndrome that has proven difficult to treat effectively [1]. Clinical trials for HFpEF have been largely disappointing, with only a few therapies such as spironolactone and implantable hemodynamic monitoring shown to reduced heart failure (HF) hospitalizations in randomized controlled trials [2–4]. As covered in detail in the introductory (overview) article of this series on precision medicine in HFpEF [5], the heterogeneity of the HFpEF syndrome may be a major factor underlying the failure of these prior clinical trials, which have thus far essentially used a one-size-fits-all approach [6,7]. Furthermore, major Phase 3 clinical trials in HFpEF and other common cardiovascular syndromes incorporate very large numbers of patients (typically > 3,000–4,000 participants) in order to ensure a sufficient number of events, but also to account for the inherent heterogeneity present in large-scale, broad-based trials. As part of this trend, so-called “pragmatic trials” have increased in popularity as a way of enrolling even larger numbers of patients for common diseases and clinical syndromes, in order to determine the efficacy of a treatment in a low-cost, efficient manner [8,9].
But there may be alternate ways to improve the track record of clinical trials in HFpEF. Given the heterogeneity of the HFpEF syndrome, with its varying etiologies, pathophysiologies, and clinical presentations, it is not surprising that several investigators have advocated improved “match-making” of patients, treatments, and trial endpoints as a way of moving forward with HFpEF [6,10]. Such an approach is at the heart of a “precision medicine” or “personalized medicine” approach. There are several factors that have evolved to enable more innovative clinical trial designs that leverage a precision medicine approach in HFpEF. Examples include the widespread adoption of electronic health records (which are searchable and analyzable using text mining approaches such as natural language processing [11]); the decreasing costs of obtaining high-dimensional data (e.g., genomics, proteomics, metabolomics, activity trackers, continuous heart rate and blood pressure monitors, deep phenotyping of cardiac imaging data, etc.) on patients; and the availability of a wide variety of potential therapeutics for HFpEF.
In this review, we cover several aspects of innovative clinical trials for precision medicine in HFpEF: (1) overview of the unmet need for new approaches to clinical trials in HFpEF; (2) a brief description of the current landscape of HFpEF clinical trials; (3) an analysis of various innovative clinical trial designs and how they could be applied to HFpEF; (4) statistical considerations for novel clinical trial designs; and (5) potential challenges and hurdles for innovative clinical trial designs in HFpEF.
The Unmet Need for New Approaches to Clinical Trials in HFpEF
In the past 5 years (May 2012-May 2017), 199 review articles have been published on the topic of HFpEF [12]. Common to nearly all of these reviews is the acknowledgement that HFpEF is one of the greatest unmet challenges in cardiovascular medicine today given the lack of available therapies. Despite many advances in HF with reduced ejection fraction (HFrEF), there has been little progress in HFpEF, with the vast majority of clinical trials returning neutral or negative results. It is therefore clear that new approaches to HFpEF are sorely needed.
Several authors have published strategies for improving the track record of clinical trials in HFpEF [13,14], but few have focused on the design of the HFpEF clinical trials themselves. Over the past 10–15 years, important strides have been made in the understanding of the pathophysiology of the HFpEF syndrome due to innovative basic science and clinical studies of the HFpEF syndrome [15–17]. In addition, there has been great interest in the development of novel therapies for HFpEF, with several novel medications and devices currently in various stages of development. Thus, the time is ripe to innovate in the clinical trial space in HFpEF.
The Current Landscape of Clinical Trials for HFpEF
The medical community, payers, regulatory agencies, funding agencies, and pharmaceutical and medical device industries have all embraced the need to find better therapies for HFpEF [13]. Thus, there are several drugs and devices in development for HFpEF, and there are several ongoing HFpEF clinical trials (Table 1), which address various etiologies and pathophysiologies of HFpEF (Figure 1). While some of these trials are targeted to specific etiologies of HFpEF (e.g., transthyrtein cardiac amyloidosis [18], hypertrophic cardiomyopathy [19]) or specific pathophysiologies present in HFpEF (inflammation [20], iron deficiency), for the most part they are non-targeted clinical trials that attempt to treat HFpEF patients using a one-size-fits-all approach.
Table 1.
Current Landscape of Multi-Center Randomized Controlled Trials in Heart Failure with Preserved Ejection Fraction
| Trial name | Sponsor | Treatment | Study design | Targeted approach |
|---|---|---|---|---|
| ATTR-ACT | Pfizer | Tafamadis (TTR stabilizer) | Phase 3 RCT + extension study | TTR cardiac amyloidosis |
| BeAT-HF | CVRx | Barostim Neo (carotid baroreceptor stimulator) | Phase 3 RCT | None |
| D-HART2 | NIH | Anakinra (IL-1 blocker) | Phase 2 RCT | High-sensitivity CRP > 2.0 mg/dl |
| EMPEROR-Preserved | Boehringer-Ingelheim | Empaglaflozin (SGLT-2 inhibitor) | Phase 3 RCT | None |
| ENDEAVOUR | Alnylam | Resuviran (TTR RNAi) | Phase 2 RCT | TTR cardiac amyloidosis |
| FAIR-HFpEF | Investigator-initiated | Intravenous iron (ferric carboxymaltose) | Phase 2 RCT | Iron deficiency (ferritin <100 ng/mL or ferritin 100–299 ng/mL plus transferrin saturation <20%) |
| INDIE-HFpEF | NIH, Aries | Inhaled nitrite | Phase 2 RCT, cross-over | None |
| KNO3CK-OUT | NIH | Potassium nitrate (inorganic nitrate) | Phase 2 RCT, cross-over | None |
| LIBERTY | Gilead | Eleclazine (late INa+ inhibitor) | Phase 2 RCT | Hypertrophic cardiomyopathy |
| PANACHE | Bayer | Neladenoson bialanate (adenosine partial A1 receptor agonist) | Phase 2 RCT | None |
| PARAGON | Novartis | Sacubitril/valsartan (angiotensin receptor-neprilysin inhibitor) | Phase 3 RCT | None |
| PARALLAX | Novartis | Sacubitril/valsartan (angiotensin receptor-neprilysin inhibitor) | Phase 3 RCT | None |
| PRESERVED-HF | AstraZeneca* | Dapaglaflozin (SGLT-2 inhibitor) | Phase 3 RCT | Diabetic/pre-diabetic HFpEF |
| REDUCE LAP-HF I | Corvia | Interatrial shunt device | Phase 2 RCT, cross-over | Left heart failure-predominant HFpEF (PCWP≫RAP) |
| REDUCE LAP-HF II | Corvia | Interatrial shunt device | Phase 3 RCT, cross-over | Left heart failure-predominant HFpEF (PCWP≫RAP) |
| SERENADE | Actelion | Macitentan (endothelin receptor antagonist) | Phase 2 RCT | CpcPH-HFpEF |
| SOUTHPAW | United Therapeutics | Oral treprostinil (prostacyclin analogue) | Phase 2 RCT | CpcPH-HFpEF |
Investigator-initiated trial via Mid-America Heart Institute, Kansas City, Missouri, USA
Note: the trials listed above are not meant to be a comprehensive list; rather they represent the majority of the major randomized, multi-center HFpEF clinical trials that were ongoing at the time of publication of the manuscript.
TTR = transthyrtein; IL-1 = interleukin-1; SGLT-2 = sodium-glucose transporter-2; RNAi = RNA interference; INa+ = inward sodium current; RCT = randomized controlled trial; CRP = C-reactive protein; HFpEF = heart failure with preserved ejection fraction; PCWP≫RAP = pulmonary capillary wedge pressure greater than right atrial pressure; CpcPH= combined post- and pre-capillary pulmonary hypertension.
Figure 1. Etiological and Pathophysiological Model of Heart Failure with Preserved Ejection Fraction.
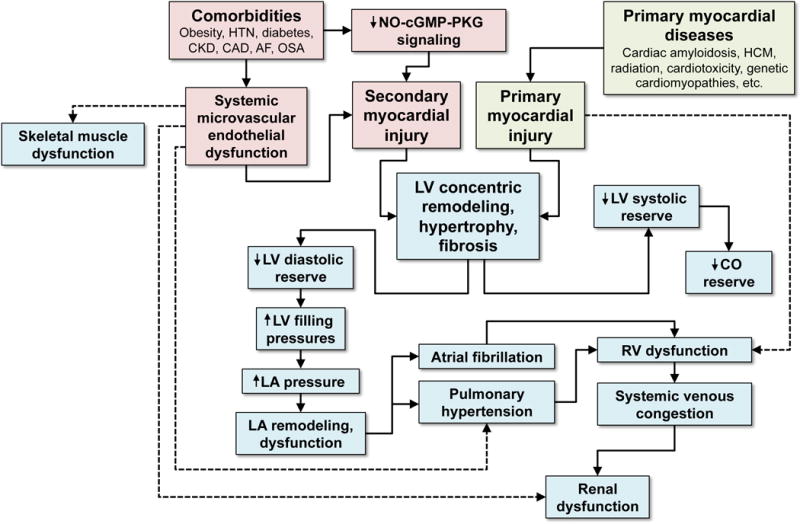
HFpEF can result from primary or secondary myocardial injury, with the latter being more typical of the syndrome. Comorbidities are thought to lead to systemic inflammation, which is associated with systemic endothelial dysfunction and decreased NO-cGMP-PKG signaling which result in secondary myocardial injury. Other diseases such as cardiac amyloidosis and HCM result in primary myocardial injury. Regardless of the cause, LV concentric remodeling and hypertrophy ensues, which results in a cascade of LV dysfunction, LA dysfunction, and RV dysfunction, ultimately leading to pulmonary and systemic venous congestion. Systemic endothelial dysfunction also likely contributes to skeletal muscle dysfunction and renal dysfunction, both of which are present in the vast majority of HFpEF patients. HTN = hypertension; CKD = chronic kidney disease; CAD = coronary artery disease; AF = atrial fibrillation; OSA = obstructive sleep apnea; NO = nitric oxide; cGMP = cyclic guanosine monophosphate; PKG = protein kinase G; HCM = hypertrophic cardiomyopathy; LV = left ventricular; LA = left atrial; RV = right ventricular; CO = cardiac output.
Although the development of multiple new therapies for HFpEF is quite exciting, the sheer number of trials is making it more and more challenging to effectively enroll patients into clinical trials for HFpEF, especially considering that enrollment for HFpEF trials was quite dismal when HFpEF trials were much more rare (e.g., during the TOPCAT trial—which was conducted from 2006–2012—there were very few other HFpEF clinical trials, and yet the TOPCAT enrollment rate was still only 2.6 patients per site per year). Furthermore, if HFpEF trials became increasingly more targeted in their approach, the number of available patients eligible for each trial will continue to get smaller and smaller. For this reason, new approaches are urgently needed.
A similar dilemma has occurred in oncology: the stakes are typically high, with high morbidity and mortality for many cancers; numerous potential therapies are in development; and more precise classification of cancers using molecular diagnostics means that the available sample sizes for clinical trials is quite low. The oncology field has responded by incorporating innovative clinical trial designs (e.g., adaptive, biomarker-based clinical trials; and sequential, multi-drug, goal-directed clinical trials [21–23]) to confront these challenges, and the same approach can be applied to HFpEF. Indeed, other fields such as aging have also embraced new approaches, recognizing that heterogeneous, multi-system diseases require getting away from a myopic approach to clinical syndromes. For example, in the Targeting Aging with Metfromin (TAME) trial, subjects ages 65–79 will be enrolled in a large randomized controlled trial to study the effects of metformin on a variety of aging-related disorders, with a composite endpoint that includes incident cardiovascular events, cancer, dementia, and mortality [24].
Innovative Clinical Trial Designs for HFpEF
In order to start creating novel clinical trials in HFpEF, we must start to think about HFpEF and related syndromes in new ways, similar to what is being done in oncology. Currently, most cancer drugs are approved based on tumor location, not a deranged molecular pathway, but this is changing. Similarly, in HFpEF, clinical trials could be focused on a molecular or pathophysiologic abnormality known to lead to HFpEF and related diseases of other organs. Thinking about clinical syndromes and treatments in this manner makes it clear that most diagnoses are man-made creations that help with aggregating patients who look alike based on a definition of a particular syndrome (such as HFpEF). This would explain why medications seem to only work in a subset of patients instead of all patients with HFpEF. Thus, we may need to consider deconstructing the definition of common diseases such as HFpEF and start focusing instead of common pathophysiologies or molecular signatures that are present across diseases.
Innovation in clinical trial designs has a long history, and multiple novel designs are now available and have been utilized in a variety of medical fields, including cardiology. Thus, the clinical trial designs described here are not really novel; however, their application to HFpEF (and HF in general) would be innovative because the current landscape of HFpEF clinical trials (as described above) are nearly all traditional designs. Here we outline several novel clinical trial designs, including enrichment trials, adaptive trials, umbrella trials, basket trials, and the application of machine learning to clinical trials (summarized in Table 2).
Table 2.
Summary of Innovative Clinical Trial Designs in Heart Failure with Preserved Ejection Fraction
| Trial design | Description | When to use | Pros | Cons |
|---|---|---|---|---|
| Enrichment | An enrichment trial involves assaying the therapeutic target (e.g., a biomarker) or some other factor that is thought to indicate an increased likelihood of responsiveness to the therapy. In this way, the clinical trial can be enriched for patients who are most likely to respond to the treatment. | When there is a clear HFpEF subgroup that can be identified based on a particular biomarker, and this subgroup is thought to be more responsive to the therapy. |
|
|
| Adaptive | Adaptive trials involve flexible trial design and the use of accumulated data to change aspects of the trial without undermining the validity and integrity of the trial. Several parameters (e.g., inclusion/exclusion criteria, sample size, drug dose and treatment schedule, endpoints, etc.) can be modified in a pre-specified fashion based on data collected and analyzed during the trial. | When there is a uncertainty regarding the optimal design of the trial, and when data can be collected and analyzed in a continuous fashion during the trial in order to determine whether certain features of the trial can be adapted. |
|
|
| Umbrella | In an umbrella trial design, a variety of targeted treatments are tested in parallel. | When multiple treatment options exist, and when enough patients can be recruited and targeted towards the various treatments in the umbrella trial. |
|
|
| Basket | Basket trials are focused on the underlying target and not the disease or clinical syndrome per se. | When the therapeutic target is clearly defined and can be assayed in a wide variety of patients with multiple different clinical diseases or syndromes. |
|
|
| Machine learning | There are several applications of machine learning to clinical trials, including unsupervised learning to identify patient clusters that may have differential treatment responses; supervised learning which may be able to determine treatment responders; and reinforcement learning, involves a computer learning decision-making by repeatedly walking through win-lose scenarios (such as a patient who does vs. does not respond to a particular therapy). | When a large amount of data collected in a previous trial or current trial is available for analysis. |
|
|
Enrichment trials
Current HFpEF clinical trials typically use an untargeted design. Patients are randomized to either the active treatment or control arm of the study, without pre-trial screening for an abnormality in the target of the drug or device that is being tested. An alternate approach would be to enrich the trial by assaying patients for the target of the drug, after which there are several options (Figure 2) [21]: (1) randomize all patients and evaluate the subgroup that expresses the target in pre-specified subgroup analyses; (2) randomize only those patients that express the target; or (3) split the patients into 2 groups (those that express the target and those who do not express the target), and then randomize and analyze each group separately. By assaying the target, the clinical trial can be enriched for the patients thought to be the ones who are most likely to respond to the treatment.
Figure 2. Biomarker-based Enrichment Trial Designs.
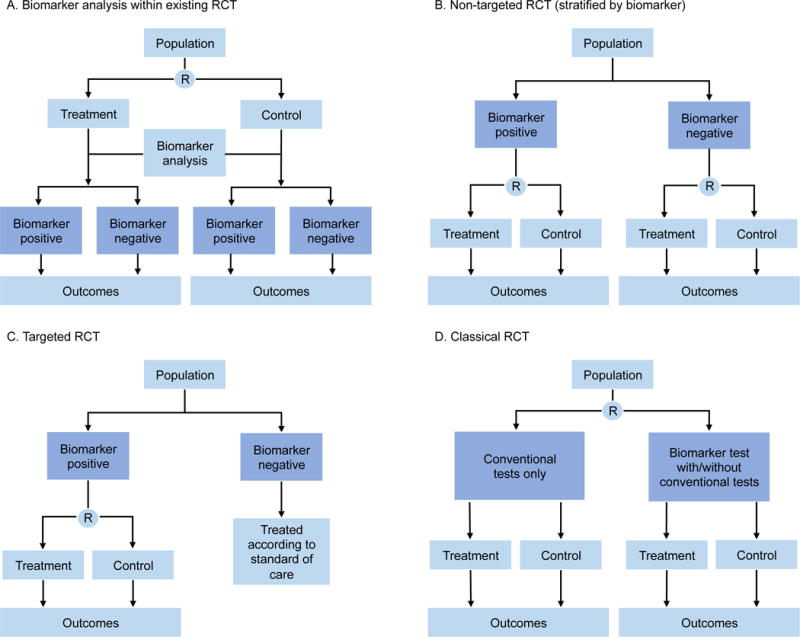
A. Biomarker discovery is performed in a trial that is used to address a therapeutic question but patient recruitment and treatment allocation are not informed by the marker status. B. A non-targeted biomarker study in which the trial is designed and powered to address the biomarker hypothesis to ensure adequate biomarker representation and distribution between arms. C. Biomarker-targeted randomized controlled trial (RCT) in which the presence of the selection marker guides patient allocation. D. RCT that compares biomarker directed therapy with conventional therapy, which allows the overall concept of the biomarker approach to be tested as a whole. R = randomization. Note: for HFpEF trials, potential “biomarkers” for use in RCTs include physiologic markers and cardiac imaging markers. Reproduced with permission from Ref. #21 (Biankin AV, et al. Patient-centric trials for therapeutic development in precision oncology. Nature 2015; 526:361–370).
In HFpEF, the REDUCE LAP-HF I and II trials (randomized controlled trials of an interatrial shunt device vs. placebo sham procedure) could be viewed as enrichment trials [25]. Patients undergo non-invasive and invasive hemodynamic screening to ensure that only those patients that express the target (elevated left atrial [LA] pressure compared to right atrial [RA] pressure at rest or during exercise) are included into the trial, thereby enriching the trial for those patients who are most likely to respond to a device that creates a shunt between the LA and RA. For the interatrial shunt device, where it is clear that patients without the target (i.e., those HFpEF patients with RA pressure ≥ LA pressure) will not benefit from the device, the choice of the aforementioned enrichment trial designs is obvious. However, this is not always the case, especially for pharmacological therapies that may have off-target effects. For example, one could imagine that neprilysin inhibition with sacubitril/valsartan (which is currently being tested in HFpEF in the PARAGON trial) would be most efficacious in patients with overactive neprilysin and/or deficiency in the production of cyclic GMP (cGMP) via the natriuretic peptide, particulate guanylate cyclase pathway. However, neprilysin inhibitors are known to affect the breakdown of a variety of vasoactive peptides and therefore may have off-target beneficial effects in patients who do not meet criteria for expression of the target (augmented neprilysin or deficient cGMP). Thus, for trials of sacubitril/valsartan an enrichment trial design that includes HFpEF patients who do and do not express the target would be a reasonable approach.
The design of enrichment trials depends on several factors. First, there must be a way to determine expression of the target. In the case of HFpEF, that could mean a biomarker (e.g., cGMP levels), cardiac imaging marker (e.g., extracellular volume fraction [a marker of diffuse interstitial fibrosis] on cardiac magnetic resonance imaging T1 mapping), invasive hemodynamic measure (e.g., LA-RA pressure gradient), or even an extra-cardiac parameter (e.g., skeletal muscle perfusion during exercise). Second, the proportion of the patients who test positive for expression of the target and the relative effectiveness of the treatment (compared to control) for test negative patients is also a major consideration. When less than half of patients are test negative and the investigational treatment has little or no benefit for test negative patients, the enrichment (targeted) design requires dramatically fewer randomized patients but may require a greater number of screened patients.
Adaptive trials
In HFpEF, not only have clinical trials had a disappointing track record, but the timeline for therapeutic development has also been quite prolonged. Over the past ~15 years, there have only been 5 large-scale randomized trials in HFpEF (PEP-CHF [26], CHARM-Preserved [27], I-PRESERVE [28], TOPCAT [4], and the ongoing PARAGON trial). For patients, healthcare providers, and pharmaceutical and device companies, speeding up the therapeutic development process in HFpEF would therefore be a major advance. Adaptive clinical trial designs [29] may be the answer to these unmet needs.
The guiding tenets of adaptive clinical trials are flexibility in trial design and the use of accumulated data to hone aspects of the trial without undermining the validity and integrity of the trial [30]. In adaptive trials, several parameters (e.g., inclusion/exclusion criteria, sample size, drug dose and treatment schedule) can be modified in a pre-specified fashion to allow for faster evaluation of treatments. Furthermore, the data collected during the conduct of the trial can allow for “smarter” trial design by using lessons learned from that data to adapt the trial with the goal of making the trial more successful. Statistical considerations for adaptive trials can be complex, though several approaches have been published [31–33]. Nevertheless, adaptive trials can be complex for investigators, study coordinators, and trialists (given the need for detailed, active follow-up during the trial), and may be difficult for clinicians to interpret once results are reported.
A key aspect of adaptive trial designs is that only prospectively planned changes in trial design can be implemented, which ensures that bias is not introduced into the trial, and that the statistical inferences are valid. Several study features can be modified in adaptive trials, including eligibility criteria, randomization ratios, analytic methods, and endpoints. In addition, treatment arms can be dropped, added to, or modified. Furthermore, adaptive designs could be used to combine 2 phases of development of a treatment (e.g., “seamless” design combining phase 2 and phase 3 of drug development [34]).
There are several advantages and disadvantages to consider when evaluating whether an adaptive design is the best for a particular therapeutic in development for HFpEF. Advantages of an adaptive design include increase speed and efficiency (with fewer patients needed), which could potentially lead to lower costs. In addition, in an adaptive design fewer patients are exposed to ineffective or harmful treatments. Disadvantages include the need for rigorous planning and complex statistical methods; preservation of trial integrity by only allowing pre-specified trial adaptations and maintaining strict blinding; and potential difficulties in terms of regulatory acceptance of the trial design and results.
However, despite these potential hurdles, adaptive trials could be advantageous in HFpEF. For example, in the TOPCAT trial, at the point of 75% of total enrollment, the interim analysis showed that the results looked promising but fell in a zone that would have benefited from a increased sample size. If an adaptive design had been incorporated into TOPCAT, a pre-specified design rule at the point of 75% enrollment would have called for increasing the sample size, and that could have led to a positive overall trial [35].
The SOCRATES-Preserved trial (Phase 2b dose finding study of vericiguat, an oral soluble guanylate cyclase [sGC] stimulator in worsening HFpEF) [36,37] is a good example of a trial that may have benefited from an adaptive design. In SOCRATES-Preserved, after 12 weeks of treatment there was no difference in the co-primary endpoint (change in N-terminal pro-B-type natriuretic peptide [NTproBNP] and change in LA volume) among those randomized to vericiguat vs. placebo. In hindsight, 4 aspects of the trial were noteworthy: (1) sGC stimulation with vericiguat (which increases cGMP levels) could have beneficial effects in several non-cardiac tissues (including the vasculature, lungs, adipose tissue, skeletal muscle, and kidneys) that have been implicated in the HFpEF syndrome; (2) SOCRATES-Preserved required patients to be enrolled within 30 days after HF hospitalization, which meant that they were a high-risk, frail group of patients, who commonly had atrial fibrillation; (3) the co-primary endpoint (change in NTproBNP and LA volume) was based on the successful PARAMOUNT trial (Phase 2 trial of sacubitril/valsartan vs. valsartan in HFpEF) [38] and not necessarily on the mechanism of action of vericiguat; and (4) a total of 4 vericiguat dose groups were tested in the trial (and were compared to placebo) which meant that the 5 randomization groups were quite small (n=93–96 patients in each group). Each of these 4 a priori decisions about the SOCRATES-Preserved trial design could have led to its failure to demonstrate a beneficial effect of vericiguat in HFpEF.
In an adaptive design, each of these 4 aspects of the trial could have been analyzed continuously throughout the trial and pre-specified as modifiable. First, because sGC stimulation could have a variety of effects in multiple organs, detailed readout of major organs involved in HFpEF during the trial could have assisted with the determination that physical functioning appeared to improve the most, which (in the absence of a detectable effect on cardiac function) suggests that skeletal muscle function improved significantly with vericiguat treatment. Second, trial enrollment was difficult at some sites because of the unwillingness of sick HFpEF patients to be enrolled in such close proximity to their HF hospitalization; given the mechanism of action of the drug there was no compelling reason why it would be more efficacious in the “worsening” HFpEF patient vs. the chronic stable HFpEF patient. Third, given the high proportion of patients with atrial fibrillation, and the fact that post-HF hospitalization NTproBNP values likely decrease in the majority patients, the endpoints became questionable. Patients with atrial fibrillation are unlikely to achieve LA remodeling with reduction in LA size within a short time frame, and it would be difficult to demonstrate a difference in reduction in NTproBNP in the post-hospitalization given the decline in NTproBNP levels in the majority of HFpEF patients after hospitalization, a dynamic period with several potential continued treatment modifications and the potential for repeat hospitalization. Finally, despite a priori power calculations, the inclusion of a relatively large number of dose finding groups in the SOCRATES-Preserved trial meant that the study could have been underpowered.
Had an adaptive design been implemented in SOCRATES-Preserved, one could imagine that the trial design would have changed with the cumulative knowledge gained during the conduct of the trial. A post-hospitalization, worsening HFpEF group of patients randomized to 4 dose groups vs. placebo, with biomarker, echocardiography, and quality of life testing, and the co-primary endpoint of change in LA volume and change in NTproBNP could have been adapted into a chronic, stable HFpEF population, with collapsing of the 4 dose groups into the 2 highest dose groups, and addition of exercise testing (e.g., 6-minute walk testing or cardiopulmonary exercise testing) to the endpoint. Such as an adaptive design could have meant the difference between a failed Phase 2b trial and a positive trial that led to a further development into a Phase 3 trial.
The challenge of adaptive designs is that we cannot predict the future; thus, investigative groups that design adaptive trials must think carefully and try to consider all of the potential scenarios during the development of the study protocol. Furthermore, even if an adaptive design such as the aforementioned one for SOCRATES-Preserved had been implemented, the statistical considerations would have likely been quite complex. Nevertheless, HFpEF clinical trialists must challenge themselves to consider adaptive designs to improve the drug development process. Table 3 lists several potential adaptive clinical trial designs that could be applied to HFpEF [39].
Table 3.
Summary of Adaptive Clinical Trial Designs
| Design | When to use | Merits | Limitations |
|---|---|---|---|
| Adaptive Accrual Design | Used when biomarker-based subgroups could be predefined, but with uncertainty on the best possible endpoint and population | Allows interim modification of patient population to accrual, higher probability of trial success, enhancement of benefit-risk relationship | Increased complexity to avoid type I error inflation due to interim adaptations and multiplicity |
| Biomarker-Adaptive Threshold Design | Use when the threshold of biomarker for defining a positive or negative biomarker status is not clear | Combines test of overall treatment effect with the establishment and validation of a cut point for a pre-specified biomarker; increased efficiency | Increase complexity to avoid type I error inflation due to multiplicity |
| Adaptive Signature Design | Used when both the potential biomarkers and the cut off are unknown | Combines test of overall treatment effect with identification and validation of a biomarker signature for sensitive patient population; ability to de-risk losing the label of broader population | Increased complexity to avoid type I error inflation due to multiplicity Large sample size may need as only half patients used for development or validation of signature from a large number of potential biomarkers |
| Cross-Validated Adaptive Signature Design | Used when both the potential biomarkers and the cut off are unknown | An cross-validation extension of adaptive signature design use entire study population for signature development and validation enhanced efficiency | Increased complexity to avoid type I error inflation due to multiplicity difficulty in interpreting the significant results in sensitive subgroup |
| Bayesian Adaptive Randomization Enrichment Design | Used when performing adaptive randomization according to patients biomarker status and when endpoint can be assess in a relative short period of time | Refines the estimation and randomization of the patients as trial progresses | Increased complexity requires a quick turnaround time |
Umbrella trials
With the advent of a large number of potential therapies for HFpEF, coupled with the high morbidity of the HFpEF syndrome, there is a pressing need to test a variety of therapies as efficiently as possible. In an umbrella trial design, a variety of targeted treatments are tested in parallel [21,23]. In oncology, an umbrella design typically starts with a single tumor type. Patients are then tested for a panel of genetic markers in the tumor tissue (or circulating biomarkers), after which they are matched up with specific trials that fit best with the underlying molecular aberration that is the cause of their tumor. A similar approach can be applied to HFpEF, as is being done in the Northwestern University HFpEF program (Figure 3A). Patients with HFpEF are first interrogated in observational HFpEF studies that involve deep phenotyping (e.g., biomarkers, cardiac imaging with echocardiography and cardiac MRI, exercise testing, body MRI to evaluate adipose tissue distribution, arterial tonometry, coronary flow reserve testing, and/or invasive hemodynamics). Next, once the particular pathophysiologic abnormality or phenotype is ascertained, patients are targeted toward the specific HFpEF clinical trial that is the best match for them. It is important to note that the HFpEF trials shown in Figure 3A are not necessarily mutually exclusive; the decision to target a HFpEF patient to a particular clinical trial is based on a combination of the underlying etiology and pathophysiology of HFpEF, clinical presentation phenotype, and clinical judgment of the site investigator.
Figure 3. Umbrella Trial Design and Infrastructure.
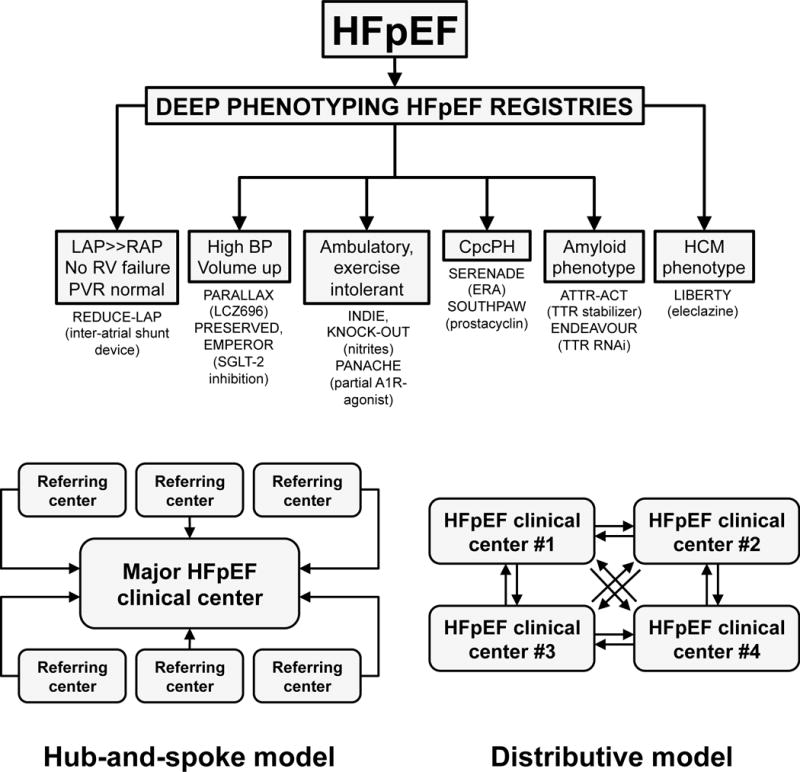
Top panel: Umbrella design approach for HFpEF clinical trials in the Northwestern University HFpEF Program. Once the diagnosis of HFpEF is confirmed, patients are enrolled into HFpEF registries that involve deep phenotyping, after which patients are directed to the most appropriate HFpEF clinical trial based on their underlying etiology or pathophysiology. It is important to note that the inclusion/exclusion criteria for these trials are not necessarily mutually exclusive. Thus, currently the assignment of patients into particular trials is based on clinical judgment, in addition to the inclusion/exclusion criteria. Bottom panel: An umbrella design requires a large number of patients in order to enroll in each of the HFpEF clinical trials. Two potential models are a “hub-and-spoke” model (left) and a “distributive” model (right). In the hub-and-spoke model, referring hospitals and clinics would send HFpEF patients to a major regional HFpEF clinical center that conducts each of the HFpEF clinical trials. In the distributive model, multiple centers with expertise in HFpEF in a given region would be involved in the umbrella trial design. Each center would be responsible for a 1 or more of the trials involved in the umbrella design. HFpEF patients would then be transferred between centers depending on the trial that best fits their underlying HFpEF subtype.
LAP = left atrial pressure; RAP = right atrial pressure; BP = blood pressure; CpcPH = combined post- and pre-capillary pulmonary hypertension; HCM = hypertrophic cardiomyopathy.
Potential advantages of umbrella designs include less screen failures because a variety of trials are available for patients. For example, HFpEF trials typically exclude patients with cardiac amyloidosis or genetic forms of hypertrophic cardiomyopathies. In the Northwestern HFpEF Program, the ability to enroll HFpEF patients with these etiologies in alternate clinical trials (e.g., ATTR-ACT and ENDEAVOUR for transthyretin cardiac amyloidosis, and LIBERTY for hypertrophic cardiomyopathy) provided options for these patients and enhanced clinical trial enrollment overall. In addition, the umbrella design allows for a more targeted approach that comes closer to the goal of achieving precision medicine.
The major pitfall of an umbrella design is that a large number of patients with HFpEF at sites are needed to successfully enroll in the several HFpEF clinical trials that are being conducted simultaneously. Solutions to this problem are as follows: (1) a large-scale, dedicated HFpEF program [40] that cares for a large number of patients at a single institution; (2) a regional network of sites that can conduct several HFpEF trials either in a “hub-and-spoke” model or a “distributive” model (Figure 3B).
Basket trials
The application of a basket trial design [22] to HFpEF is perhaps the most “outside-the-box” trial design of those discussed thus far. Basket trials are focused on the underlying target and not the disease or clinical syndrome per se. In oncology, basket trials are histology-independent—patients with cancers of different organs and histologic types are united based on underlying, specific molecular abnormality. These patients of a variety of tumor types, but with a specific somatic mutation, for example, are treated with a drug that targets that mutation.
How could a basket trial design be applied to HFpEF and related syndromes? Two examples are provided here: (1) obesity-related syndromes and (2) cardiac microvascular dysfunction. Obesity is highly prevalent in patients with HFpEF, and HFpEF in the setting of morbid obesity (i.e., body mass index > 35 kg/m2) may be a unique phenotype that specifically requires weight loss treatment [41,42]. Obese HFpEF patients often have other obesity-related diseases such as obstructive sleep apnea, obesity-hypoventilation syndrome, non-alcoholic fatty liver disease, and even obesity-related glomerulomegaly with resultant kidney disease [42]. In a basket design, patients with a variety of overlapping disorders related to morbid obesity would be grouped together and treated in a single trial. For example, a randomized controlled trial involving a weight loss drug could be tested in patients with BMI > 40 kg/m2 and HFpEF or obstructive sleep apnea or non-alcoholic fatty liver disease. Patients would then be followed for a variety of endpoints, some specific to the diseases and clinical syndromes included in the study (e.g., exercise testing, apnea-hypopnea index, and liver fat by computed tomography, respectively), and some that evaluate overall effectiveness of the medication (e.g., quality of life metrics). Figure 4 presents a hypothetical example of such an approach using the weight loss drug lorcaserin, a serotonin receptor subtype (5-HT2c) agonist [43,44]. Morbidly obese patients with evidence of unoccupied 5-HT2c receptors (which, in the future, could be quantified using positron emission tomography-computed tomograph [PET-CT] scanning of the brain [45], Figure 4 [top panel]) and one of the aforementioned obesity-related conditions would be enrolled in the trial in a basket design (Figure 4, bottom panel) with a variety of endpoints.
Figure 4. Hypothetical Basket Trial Design for Heart Failure with Preserved Ejection Fraction.
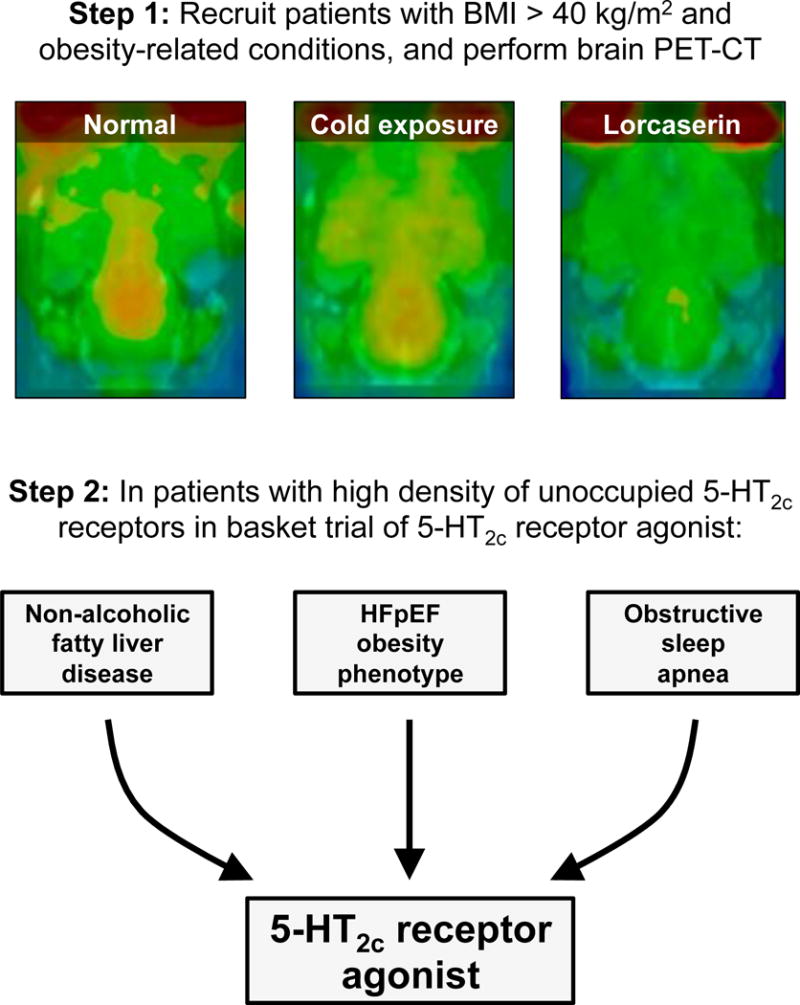
Potential basket clinical trial design of lorcaserin (a weight loss drug that targets the 5-HT2c receptor in the brain) for the obese HFpEF phenotype and other obesity-related conditions (non-alcoholic fatty liver disease and obstructive sleep apnea). In this example basket trial design, morbidly obese patients (BMI [body mass index] > 40 kg/m2) first undergo 4- (3- [18F]fluorophenethoxy)pyrimidine PET-CT scanning of the brain to identify those with a high density of unoccupied 5-HT2c receptors (particularly in the hypothalamus, where they are associated with satiety). Those patients who meet these criteria and have obesity-related HFpEF, non-alcoholic fatty liver disease, and/or obesity-induced obstructive sleep apnea all enter a single trial with multiple different outcomes that account for the variety of obesity-related clinical syndromes included in the trial. Top panel: Brain 4- (3- [18F]fluorophenethoxy)pyrimidine PET-CT examples in normal rats (left); rats treated with cold exposure (middle); and rats treated with lorcaserin (right). Orange-yellow signal in the PET-CT scan represents [18F] uptake. Bottom panel: Basket clinical trial design. Top panel PET-CT images reproduced from Ref. #45 (under the open access American Chemical Society AuthorChoice License).
In the second example, the treatment target would be cardiac microvascular dysfunction, which is thought to be a major underlying pathophysiologic abnormality in HFpEF [46–49]. Patients with HFpEF with evidence of cardiac microvascular dysfunction could be grouped under a basket design with other patients who have evidence of cardiac microvascular dysfunction, including patients with ischemia and no obstructive coronary disease (INOCA) [50] and patients with systemic sclerosis [51]. In this example, there would be a common pathophysiologic endpoint (coronary flow reserve) but different functional endpoints (e.g., exercise capacity, angina questionnaires, quality of life, and extracardiac assessment of microvascular dysfunction [e.g., Raynaud’s phenomenon]) that may be differentially applicable to the underlying diseases and clinical syndromes included in the basket design.
A key advantage of a basket trial design is the ability to target a molecular or pathophysiologic abnormality shared by several different diseases and clinical syndromes. Since HFpEF and other clinical syndromes (and even diseases) are often simply man-made constructs, treating a single target across diseases enables a more holistic therapeutic approach. However, there are several potential challenges posed by an basket design, including (1) the fact that different diseases may respond differently to targeting the same underlying molecular or pathophysiologic etiology; (2) the investigational therapeutic may have a variety of effects, including off-target effects that may be beneficial or harmful depending on the disease being treated; (3) the basket design requires the ability to precisely test for the target of the therapeutic (e.g., molecular pathway) in patients; and (4) determining optimal strategies for patient recruitment across subspecialty silos, and the appropriate outcomes for each disease/syndrome in the basket trial is likely to be difficult. Basket designs are currently in use in oncology because a specific somatic mutation can be targeted across different tumor types, and the outcome (e.g., tumor size and extent) is easier to envision. Application of the basket design to other fields in medicine is likely to be more challenging. Nevertheless, the basket design gives investigators a chance to broaden the scope of potential clinical trials beyond man-made silos of disease.
Machine learning to enhance clinical trials
Three common uses of machine learning that are germane to clinical trial design include pattern recognition (unsupervised learning), prediction of treatment response (supervised learning), and decision-making (reinforcement learning) [52,53]. All 3 could be used to provide a more precision medicine approach to HFpEF. In a study that focused on personalized nutrition, Zeevi et al. used both unsupervised and supervised learning, coupled with high-dimensional data (activity, lifestyle, nutrition, genomics, and microbiome) to develop a more personalized approach to nutrition, with the goal of improving post-prandial glucose responses (i.e., to prevent hyperglycemia) [54]. Patterns of dietary response were first determined using unsupervised learning, followed by prediction of post-prandial glucose responses using supervised learning. A similar approach could be used in HFpEF, with patients classified into subtypes using unsupervised learning, and then monitored for different treatment responses to a variety of interventions with supervised learning to predict future treatment responses. The availability of both invasive and non-invasive continuous monitoring devices for HF patients could expedite such studies by providing real-time feedback on treatment efficacy.
Reinforcement learning involves a computer learning decision-making by repeatedly walking through win-lose scenarios, learning from wins and losses, and replaying the scenarios [53]. This type of machine learning approach has been successfully used to play games, such as DeepMind’s AlphaGo, which has mastered the game of Go [55]. Clinical medicine can also be viewed in such a context, and thus it is not surprising the reinforcement learning has been advocated as a way to improve clinical trials [56,57]. The idea is that if there is enough available information from prior clinical trials, including positive and negative results, a computer could use that data to improve the conduct of the trial as it is being conducted. Furthermore, the treatments could be personalized based on each patient’s “scenario” that had been modeled by the reinforcement learning algorithm.
Developing a Pipeline for HFpEF Therapeutics
A precision medicine framework for HFpEF clinical trials will require several key steps, as outlined in Table 4: efficient participant screening and recruitment into trials, deep phenotyping of study patients, flexibility of clinical trial protocols, and availability of a wide variety of potential therapies [21]. A pipeline approach to developing therapeutics, as outlined for the oncology field by Trusheim and colleagues [58], could also be used in HFpEF. Clinical trials could be organized in such a way that hypothesis-generating innovative trials (e.g., adaptive designs, basket designs) are followed systematically by confirmatory trials, and then, for approved drugs and devices, by real-world evidence. Operational innovations would also be necessary for a successful pipeline, include: master protocols; systematic screening and identification of patients (ideally through automated algorithms); and standardized repositories (including phenotyping [e.g., cardiac imaging] and biobanks). Together, these aspects of a pipeline for precision medicine clinical trials could allow for increased efficiency and scale, with faster trials that increase availability of novel therapies to patients; and better and more comprehensive evidence for targeted therapeutics.
Table 4.
Delivering Multi-Drug Portfolio Studies
| 1. Participant screening and recruitment in to trials | |
| Requires a viable means to identify low prevalence subpopulations and direct individuals to an appropriate clinical trial, through a patient-centric approach whereby each individual can have access to many options via a single screening process |
|
| 2. Deep phenotyping | |
| Requires a system (platform, screening/selection algorithm) that enables broad but robust patient profiling and provides viable development routes for larger studies and regulatory interactions |
|
| 3. Protocol flexibility | |
| Requires an early development protocol that is flexible allowing change to emerging science and understanding of patient markers and novel sub-phenotypes, and/or a confirmatory development protocol permitting regulatory interactions using different types of datasets |
|
| 4. Availability and delivery of therapies | |
| Requires an operational machinery that allows studies to be conducted over diverse groups and geographical areas with aligned and efficient regulatory and ethics processes, patient screening/recruitment and ability to distribute multiple candidate drugs to multiple sites in a cost-effective and efficient manner |
|
Successful delivery of multidrug portfolio studies requires innovation across clinical design and implementation; key aspects to consider for diagnostics, clinical protocol operational delivery. Reproduced with permission from Ref. #21 (Biankin AV, et al. Patient-centric trials for therapeutic development in precision oncology. Nature 2015; 526:361–370).
Statistical considerations
Although methods for statistical analysis of the aforementioned innovative clinical trial designs are beyond the scope of this review, there are some key aspects to consider when designing precision medicine trials. Ma et al. [59] have reviewed a process for using statistical inference to establish personalized treatment rules: (1) acquire the training data, ideally from an previously conducted randomized controlled trial; (2) chose a method of inference based on clinical endpoints (e.g., binary, continuous, survival) and data dimensionality (i.e., low- vs. high-dimensional data); (3) identify an individual treatment rule based on derived treatment contrasts, and select a clinically relevant decision threshold; (4) fit the model to the training data, ensuring appropriate selection of covariates and estimation of model parameters; (5) evaluate model performance, which should include measuring the extent of clinical benefit; and (6) apply the treatment rule to future patients, with continual improvement of the model based on its performance and additional collected data.
In addition to developing a process to develop statistical inference for a particular precision medicine therapeutic, the issue of subgroup analyses in clinical trials must be considered carefully. As explained above, a key tenet of precision or personalized medicine is determining which patients do and do not benefit from a particular therapeutic in the context of a randomized controlled trial, which often involves subgroup analyses. However, it is well known that subgroup analyses have serious limitations. False positives can occur due to multiple comparisons, and false negatives are common due to limited power. Perhaps most importantly, personalization of therapy is more complex than simple subgroup analyses because multiple characteristics can vary simultaneously [60]. Burke et al. advocate for pre-specified primary subgroup analyses that are hypothesis-testing and can guide clinical care [61]. Secondary subgroup analyses are hypothesis-generating and should stimulate further research, but should not guide clinical care. These authors advocate following Bayes’s rule: interpretation of a subgroup analysis is analogous to rigorously evaluating a diagnostic test. Bayes’s rule: posterior odds = sensitivity/(1-specificity)*prior odds. Statistical power = sensitivity of the trial. 1-Alpha = specificity of the trial (usually set at 95%).
By ensuring that precision medicine trials in HFpEF have a pre-specified, well-thought process for statistical analyses, and carefully considering subgroup analyses of trials from their outset, HFpEF clinical trialists can help ensure that the trial results are valid and justified.
Potential Challenges and Hurdles for Innovative Clinical Trial Designs in HFpEF
Although newer trial designs appear promising for HFpEF, there are several hurdles that stand in the way of their implementation. First, many of these designs require adequate markers (biomarkers, pathophysiologic markers) that define who will benefit most from the investigational therapeutic, and such markers may not be available. Second, large numbers of HFpEF patients will need to undergo screening to find enough patients for some of the trial types described above. Current enrollment in HFpEF clinical trials, while improving, is still quite low, and there is an unmet need for improved automated screening of the electronic health record and/or dedicated, large-scale HFpEF programs. Third, some designs (e.g., umbrella trials) will require collaborative networks among institutions and cooperation among the companies making the investigational treatments. Finally, the notion of precision medicine (more precise therapies for smaller numbers of patients) creates a real tension for companies, payers, healthcare providers, and patients because the smaller number of treatable patients could mean lower profits and higher prices for these treatments. However, for common clinical syndromes such as HFpEF that lack effective therapies, a novel and effective therapeutic that is only applicable to a fraction of the patients would be a major advance and would still likely be profitable given the high prevalence of HFpEF (~3–4 million in the US and ~15 million worldwide).
In addition to the several potential challenges for innovative clinical trial designs in HFpEF reviewed above, there are some overarching challenges and hurdles to consider when designing new trials for HFpEF. First, given the immense costs of drug development, pharmaceutical and device companies may be reluctant to embrace newer designs due to the notion that they are riskier than traditional designs. Second, clinical trialists (and site investigators) may also be reticent to utilize novel trial designs due to increase complexities in the operation of such trials. There may also be fear that even if regulatory bodies accept these novel designs that once the trial has been conducted approval of the new therapeutic will be hampered by lack of understanding of the novel clinical trial design. Finally, even for approved therapeutics, if the innovative trial that led to approval is not clear in its clinical benefit, payers and clinicians may not use the therapeutic due to perceived lack of efficacy. For each of these challenges, examples of success in other fields (e.g., oncology) will help prove the utility of these novel trial designs so that companies, investigators, regulators, payers, and clinicians will be more willing to accept the incorporation of novel trial designs for HFpEF.
Conclusions
Given the disappointing track record of prior HFpEF clinical trials, coupled with the emerging understanding of HFpEF as a heterogeneous clinical syndrome, it is imperative that innovative clinical trials designs are considered during the development process for novel therapeutics. Here we have reviewed some of the more popular novel clinical trial designs that have been used in other fields of medicine. Aspects of these innovative designs are currently being employed in HFpEF clinical trials (e.g., enrichment designs to increase the number of patients most likely to respond to the investigational treatment). However, there is considerable room for increased innovation in HFpEF clinical trials, and hopefully the incorporation of newer trial designs in HFpEF will lead to a more personalized therapeutic approach and better results for HFpEF clinical trials, which ultimately could lead to improved patient care.
Acknowledgments
Funding: Dr. Shah is supported by National Institutes of Health R01 HL107577 and R01 HL127028; and American Heart Association #16SFRN28780016 and #15CVGPSD27260148.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of interest: Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis; and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, and United Therapeutics.
Ethical approval: This review article does not contain any primary data from studies with human participants or animals performed by the author.
Informed consent: Not applicable (this paper is a review article with no primary research involved).
References
- 1.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134(1):73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(6):935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 5.Shah SJ. Precision Medicine for Heart Failure with Preserved Ejection Fraction: An Overview. J Cardiovasc Transl Res. 2017 doi: 10.1007/s12265-017-9756-y. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SJ. Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. J Am Coll Cardiol. 2013;62(15):1339–1342. doi: 10.1016/j.jacc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10(3):407–418. doi: 10.1016/j.hfc.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford I, Norrie J. Pragmatic Trials. N Engl J Med. 2016;375(5):454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 9.Lund LH, Oldgren J, James S. Registry-Based Pragmatic Trials in Heart Failure: Current Experience and Future Directions. Curr Heart Fail Rep. 2017;14(2):59–70. doi: 10.1007/s11897-017-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Diez J, Solomon SD, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35(40):2797–2815. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonnalagadda SR, Adupa AK, Garg RP, Corona-Cox J, Shah SJ. Text Mining of the Electronic Health Record: An Information Extraction Approach for the Automated Identification and Subphenotyping of HFpEF Patients for Clinical Trials. J Cardiovasc Transl Res. 2017 doi: 10.1007/s12265-017-9752-2. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Website. http://www.pubmed.gov (search term: (hfpef [ti] or “diastolic heart failure” [ti] or “preserved ejection fraction” [ti]) and review [pt])). Accessed 20 May 2017.
- 13.Butler J, Fonarow GC, Zile MR, Lam CS, Roessig L, Schelbert EB, et al. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail. 2014;2(2):97–112. doi: 10.1016/j.jchf.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senni M, Greene SJ, Butler J, Fonarow GC, Gheorghiade M. Drug Development for Heart Failure With Preserved Ejection Fraction: What Pieces Are Missing From the Puzzle? Can J Cardiol. 2017;33(6):768–776. doi: 10.1016/j.cjca.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11(9):507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 16.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 18.Maurer MS, Elliott P, Merlini G, Shah SJ, Waddington Cruz M, Flynn A, et al. Design and Rationale of the Phase 3 Tafamidis in ATTR-ACT (Transthyretin Cardiomyopathy Clinical Trial) Circ Heart Fail. 2017 doi: 10.1161/CIRCHEARTFAILURE.116.003815. (in press) [DOI] [PubMed] [Google Scholar]
- 19.Olivotto I, Hellawell JL, Farzaneh-Far R, Blair C, Coppini R, Myers J, et al. Novel Approach Targeting the Complex Pathophysiology of Hypertrophic Cardiomyopathy: The Impact of Late Sodium Current Inhibition on Exercise Capacity in Subjects with Symptomatic Hypertrophic Cardiomyopathy (LIBERTY-HCM) Trial. Circ Heart Fail. 2016;9(3):e002764. doi: 10.1161/CIRCHEARTFAILURE.115.002764. [DOI] [PubMed] [Google Scholar]
- 20.Van Tassell BW, Buckley LF, Carbone S, Trankle CR, Canada JM, Dixon DL, et al. Interleukin-1 blockade in heart failure with preserved ejection fraction: rationale and design of the Diastolic Heart Failure Anakinra Response Trial 2 (D-HART2) Clin Cardiol. 2017 doi: 10.1002/clc.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biankin AV, Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature. 2015;526(7573):361–370. doi: 10.1038/nature15819. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth SJ, Biankin AV. The Challenges of Precision Oncology Drug Development and Implementation. Public Health Genomics. 2015;18(6):338–348. doi: 10.1159/000441557. [DOI] [PubMed] [Google Scholar]
- 23.Jurgensmeier JM, Eder JP, Herbst RS. New strategies in personalized medicine for solid tumors: molecular markers and clinical trial designs. Clin Cancer Res. 2014;20(17):4425–4435. doi: 10.1158/1078-0432.CCR-13-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman T, Komtebedde J, Burkhoff D, Massaro J, Maurer MS, Leon MB, et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure: Rationale and Design of the Randomized Trial to REDUCE Elevated Left Atrial Pressure in Heart Failure (REDUCE LAP-HF I) Circ Heart Fail. 2016;9(7) doi: 10.1161/CIRCHEARTFAILURE.116.003025. [DOI] [PubMed] [Google Scholar]
- 26.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 28.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt DL, Mehta C. Adaptive Designs for Clinical Trials. N Engl J Med. 2016;375(1):65–74. doi: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]
- 30.Gallo P, Chuang-Stein C, Dragalin V, Gaydos B, Krams M, Pinheiro J, et al. Adaptive designs in clinical drug development–an Executive Summary of the PhRMA Working Group. J Biopharm Stat. 2006;16(3):275–283. doi: 10.1080/10543400600614742. discussion 285-291, 293-278, 311-272. [DOI] [PubMed] [Google Scholar]
- 31.Hung HM, Wang SJ, O'Neill R. Statistical considerations for testing multiple endpoints in group sequential or adaptive clinical trials. J Biopharm Stat. 2007;17(6):1201–1210. doi: 10.1080/10543400701645405. [DOI] [PubMed] [Google Scholar]
- 32.Thall P, Fox P, Wathen J. Statistical controversies in clinical research: scientific and ethical problems with adaptive randomization in comparative clinical trials. Ann Oncol. 2015;26(8):1621–1628. doi: 10.1093/annonc/mdv238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SJ. Perspectives on the use of adaptive designs in clinical trials. Part I. Statistical considerations and issues. J Biopharm Stat. 2010;20(6):1090–1097. doi: 10.1080/10543406.2010.514446. [DOI] [PubMed] [Google Scholar]
- 34.Stallard N, Todd S. Seamless phase II/III designs. Stat Methods Med Res. 2011;20(6):623–634. doi: 10.1177/0962280210379035. [DOI] [PubMed] [Google Scholar]
- 35.Bristow MR, Enciso JS, Gersh BJ, Grady C, Rice MM, Singh S, et al. Detection and Management of Geographic Disparities in the TOPCAT Trial: Lessons Learned and Derivative Recommendations. JACC Basic Transl Sci. 2016;1(3):180–189. doi: 10.1016/j.jacbts.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38(15):1119–1127. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pieske B, Butler J, Filippatos G, Lam C, Maggioni AP, Ponikowski P, et al. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES) Eur J Heart Fail. 2014;16(9):1026–1038. doi: 10.1002/ejhf.135. [DOI] [PubMed] [Google Scholar]
- 38.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Chow SC, Chang M. Biomarker-Driven Adaptive Design for Precision Medicine. J Translational Biomarkers Diagn. 2016;2:15–24. [Google Scholar]
- 40.Shah SJ, Cogswell R, Ryan JJ, Sharma K. How to Develop and Implement a Specialized Heart Failure with Preserved Ejection Fraction Clinical Program. Curr Cardiol Rep. 2016;18(12):122. doi: 10.1007/s11886-016-0802-1. [DOI] [PubMed] [Google Scholar]
- 41.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68(2):200–203. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Meltzer HY, Roth BL. Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest. 2013;123(12):4986–4991. doi: 10.1172/JCI70678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shukla AP, Kumar RB, Aronne LJ. Lorcaserin Hcl for the treatment of obesity. Expert Opin Pharmacother. 2015;16(16):2531–2538. doi: 10.1517/14656566.2015.1096345. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Moon BS, Lee BC, Lee HY, Kim HJ, Choo H, et al. A Potential PET Radiotracer for the 5-HT2C Receptor: Synthesis and in Vivo Evaluation of 4-(3-[18F]fluorophenethoxy)pyrimidine. ACS Chem Neurosci. 2017;8(5):996–1003. doi: 10.1021/acschemneuro.6b00445. [DOI] [PubMed] [Google Scholar]
- 46.Lim SL, Lam CS, Segers VF, Brutsaert DL, De Keulenaer GW. Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur Heart J. 2015;36(31):2050–2060. doi: 10.1093/eurheartj/ehv132. [DOI] [PubMed] [Google Scholar]
- 47.Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, et al. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J. 2017;38(7):473–477. doi: 10.1093/eurheartj/ehw461. [DOI] [PubMed] [Google Scholar]
- 48.Lam CS, Lund LH. Microvascular endothelial dysfunction in heart failure with preserved ejection fraction. Heart. 2016;102(4):257–259. doi: 10.1136/heartjnl-2015-308852. [DOI] [PubMed] [Google Scholar]
- 49.Giamouzis G, Schelbert EB, Butler J. Growing Evidence Linking Microvascular Dysfunction With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016;5(2) doi: 10.1161/JAHA.116.003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desai CS, Lee DC, Shah SJ. Systemic sclerosis and the heart: current diagnosis and management. Curr Opin Rheumatol. 2011;23(6):545–554. doi: 10.1097/BOR.0b013e32834b8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deo RC. Machine Learning in Medicine. Circulation. 2015;132(20):1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mnih V, Kavukcuoglu K, Silver D, Rusu AA, Veness J, Bellemare MG, et al. Human-level control through deep reinforcement learning. Nature. 2015;518(7540):529–533. doi: 10.1038/nature14236. [DOI] [PubMed] [Google Scholar]
- 54.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163(5):1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Silver D, Huang A, Maddison CJ, Guez A, Sifre L, van den Driessche G, et al. Mastering the game of Go with deep neural networks and tree search. Nature. 2016;529(7587):484–489. doi: 10.1038/nature16961. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, Kosorok MR, Zeng D. Reinforcement learning design for cancer clinical trials. Stat Med. 2009;28(26):3294–3315. doi: 10.1002/sim.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y, Zeng D, Socinski MA, Kosorok MR. Reinforcement learning strategies for clinical trials in nonsmall cell lung cancer. Biometrics. 2011;67(4):1422–1433. doi: 10.1111/j.1541-0420.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trusheim MR, Shrier AA, Antonijevic Z, Beckman RA, Campbell RK, Chen C, et al. PIPELINEs: Creating Comparable Clinical Knowledge Efficiently by Linking Trial Platforms. Clin Pharmacol Ther. 2016;100(6):713–729. doi: 10.1002/cpt.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma J, Hobbs BP, Stingo FC. Statistical Methods for Establishing Personalized Treatment Rules in Oncology. Biomed Res Int. 2015;2015:670691. doi: 10.1155/2015/670691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365(9454):176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 61.Burke JF, Sussman JB, Kent DM, Hayward RA. Three simple rules to ensure reasonably credible subgroup analyses. BMJ. 2015;351:h5651. doi: 10.1136/bmj.h5651. [DOI] [PMC free article] [PubMed] [Google Scholar]


