Abstract
Background: Rosmarinic acid (RA) is a polyphenol present in members of the Lamiaceae family. In this study, yhe anti-inflammatory and anti-glycative effects of RA in the livers of type 1 diabetic mice were examined.
Methods: The diabetic mice were divided into three groups: diabetic mice with 0, low dose RA (25 mg/ml), and high dose RA (50 mg/ml). One group of non-diabetic mice was used as a control for comparison. RA was supplied via daily 200 μL oral injections for 9 weeks. The level of interleukin (IL)-6, the tumor necrosis factor (TNF)-alpha, the prostaglandin E2 (PGE2), and the activity of cyclooxygenase (COX)-2 in the livers were measured. The hepatic receptor of advanced glycative endproduct (RAGE), the sorbitol levels, and the glyoxalase 1 (GLO-1) activity were also determined.
Results: Compared with diabetic group that received no RA, the groups with RA supplements at both levels of dosages had increased body weight and had both decreased water intake and feed intake (p < 0.05). RA intake was found to reduce plasma glucose level and elevate plasma insulin level when compared with the diabetic group that received no RA (p < 0.05). RA treatments lowered the hepatic level of IL-6, TNF-alpha, and PGE2, as well as the activity of COX-2 (p < 0.05). RA administration also decreased hepatic RAGE and sorbitol levels, and GLO-1 activity when compared with the diabetic group that received no RA (P < 0.05).
Conclusion: These findings support the conclusion that rosmarinic acid (RA) could be a potent protective agent for the liver against diabetic injury.
Keywords: Rosmarinic acid, Diabetes, RAGE, PGE2
1. Introduction
Rosmarinic acid (RA) is a main polyphenol present in Rosmarinus Officinalis L., Coleus aromaticus, and members of the Lamiaceae family. It has been documented that RA has many bio-activities including anti-oxidative, anti-microbial, anti-inflammatory, anti-metastatic, neuroprotective, and immunomodulatory effects [1–3]. Furthermore, RA has been considered to be a potent agent for chronic disease prevention and/or alleviation [3–5]. The anti-diabetic effects of RA in rodents have been examined, and the authors of those studies have indicated that RA could improve glycemic control, oxidative stress and vascular dysfunction, which in turn can attenuate the progression of diabetes, as well as delay the occurrence of diabetic complications [6–8].
Inflammation and glycation are two major pathological characteristics of diabetes types 1 and 2. Although the antiinflammatory effect of RA has been reported [9], it is still unclear whether RA intake could alleviate hepatic inflammation under diabetic conditions. The liver preserves many nutrients and exerts many crucial physical functions. If RA does protect the liver against diabetes-related inflammation, it may be a beneficial nutritional support for diabetic subjects. Thus, in our present study we examined the impact of RA upon a variety of inflammatory factors including interleukin (IL)-6, prostaglandin E2 (PGE2), and cyclooxygenase (COX)-2 in the livers of type 1 diabetic mice to evaluate the anti-inflammatory effects of RA. Thus far, less attention has been paid to the anti-glycative activities of RA, especially in the liver. The increase in the receptors of advanced glycative endproducts (RAGE) plays an important role in diabetic progression because RAGE reacts with AGEs and other ligands such as beta-amyloid, and the interactions of RAGE and its ligands further activates other signal pathways responsible for the production of oxidative, inflammatory, or angiogenic factors [10–12]. Thus, the decline of RAGE formation due to RA definitely contributes to diminishing glycative injury and other diabetic pathological stress. In addition, sorbitol level and glyoxalase 1 (GLO-1) activity are two biomarkers used for evaluating glycative stress. If RA decreases sorbitol generation and/or increases GLO-1 activity, it subsequently may alleviate glycative injury. In our present study, type 1 diabetes was induced in mice, and followed with RA treatment. The anti-inflammatory and anti-glycative effects of RA in the livers of these mice was examined.
2. Materials and Methods
2.1. Materials
RA (97%), propylene glycol (PG, 98%), and streptozotocin (STZ, 99.5%) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RA was dissolved in PG at 50 mg/ml.
2.2. Animals and diet
Male Balb/c mice at 5-wk old were obtained from the National Laboratory Animal Center (Taipei City, Taiwan). The mice were housed in a 12-h light and 12-h dark cycle. A standard diet and water were supplied ad libitum. Mice with a body weight of 24.2 ± 1.6 g were selected to be induced with diabetes by a single IV injection of STZ at 50 mg/kg into the tail vein after mice had been fasting for 12 h. At day 10, the blood glucose level of 12-h fasted mice was measured via a one-touch blood glucose meter (Lifescan Inc., Milpitas, CA, USA). Mice with a blood glucose level ≥ 200 mg/dl were used. Use of those mice was approved by the China Medical University Animal Care and Use Committee, and the permission number was 104-305.
2.3. Animal Experiment
The diabetic mice were divided into three groups: diabetic mice with 0, low dose RA (25 mg/ml), and high dose RA (50 mg/ ml). One group of non-diabetic mice was used as a control for comparison. RA at 200 μL was supplied every day via oral injection. Body weight and glucose level were recorded every week. After a 9-week supplementation, mice were fasted for 12 h and then killed with carbon dioxide. Both blood and liver from each mouse were collected. The protein concentration of liver homogenate was measured by a commercial kit (Pierce Biotechnology Inc., Rockford, IL, USA).
2.4. Insulin analysis
Plasma insulin level (μg/L) was assayed by an insulin radioimmunoassay kit purchased from Linco Research Inc. (St. Charles, MO, USA).
2.5. Measurement of inflammatory factors
The hepatic levels of IL-6 and tumor necrosis factor (TNF)-alpha were measured by using cytoscreen immunoassay kits (BioSource International, Camarillo, CA, USA). The PGE2 level and COX-2 activity were determined by kits purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). The COX-2 activity was assayed by monitoring the variation of absorbance at 590 nm, which indicated the generation of oxidized N, N, N’, N’-tetramethyl-p- phenylenediamine.
2.5. Determination of glycative factors
The hepatic RAGE and sorbitol levels were determined by a colorimetric assay kit (BioVision, Mountain View, CA, USA) and a SEA645Mu ELISA kit (USCNK Life Science Inc., Hubei, China), respectively. The GLO-1 activity was quantified by a commercial kit obtained from Bioassay Co. (Hayward, CA, USA).
2.6. Statistical analyses
Data were expressed as mean ± standard deviation (SD). Each group had eight mice (n = 8). Statistical analysis was processed by using a one-way analysis of variance, and Dunnett’s t-test was used for Post-hoc comparisons. A P value < 0.05 was defined as significant.
3. Results
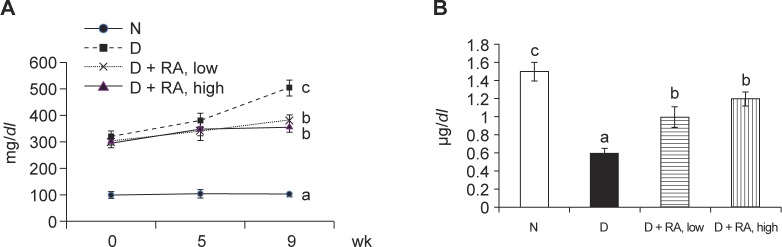
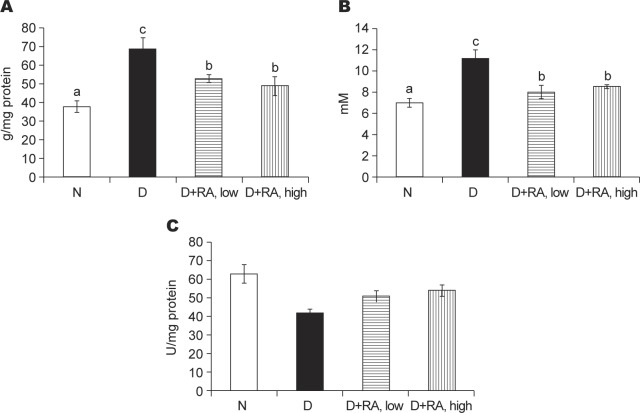
Compared with the diabetic group of mice that received no RA, RA supplements at both low and high doses increased body weight, and decreased water intake and feed intake (Table 1, p < 0.05). As shown in Fig. 1, RA intake reduced the plasma glucose level (a), and elevated the plasma insulin level (b) when compared with the diabetic group of mice that received no RA (p < 0.05). RA supplements reduced the hepatic level of IL-6, TNF-alpha and PGE2, and the activity of COX-2 (Table 2, p < 0.05). As shown in Fig. 2, RA intake lowered the hepatic level of RAGE (a) and sorbitol (b), and raised the GLO-1 activity (c) when compared with the diabetic group of mice that received no RA (p < 0.05).
Table 1.
body weight (BW), water intake (WI, ml/mouse/day), feed intake (FI, g/mouse/day) in normal (N) or diabetic mice (D) treated with RA at 0, low, or high dose for 9 weeks. Values are mean ± SD, n = 8. a-cmeans in a row without a common letter differ statistically p < 0.05.
| N | D | D + RA, low | D + RA, high | |
|---|---|---|---|---|
| BW | 32.4 ± 0.9c | 20.1 ± 1.1a | 23.5 ± 0.6b | 22.7 ± 0.8b |
| WI | 2.3 ± 0.3a | 6.5 ± 1.0c | 5.1 ± 0.5b | 4.5 ± 0.7b |
| FI | 2.1 ± 0.5a | 6.8 ± 0.8c | 5.3 ± 0.6b | 4.7 ± 0.4b |
Fig. 1.
Plasma glucose level (mg/dl, a) and plasma insulin level (μg/l, b) in normal (N) or diabetic mice (D) treated with RA at 0, low, or high dose for 9 weeks. Values are mean ± sd, n = 8. a-cmeans among bars without a common letter differ, p < 0.05.
Table 2.
The hepatic level of IL-6 (pg/mg protein), TNF-alpha (pg/mg protein), PGE2 (μg/mg protein), and COX-2 activity (nmol/min/mg protein) in normal (N) or diabetic mice (D) treated with RA at 0, low, or high dose for 9 weeks. Values are mean ± SD, n = 8. a-dmeans in a row without a common letter differ statistically, p < 0.05.
| N | D | D + RA, low | D + RA, high | |
|---|---|---|---|---|
| IL-6 | 40 ± 5a | 103 ± 9b | 54 ± 8a | 46 ± 4a |
| TNF-alpha | 4.8 ± 0.6a | 8.3 ± 1.0b | 5.2 ± 0.3a | 5.1 ± 0.5a |
| PGE2 | 6.4 ± 0.4a | 19.5 ± 1.1c | 14.4 ± 0.7b | 12.9 ± 0.9b |
| COX-2 | 1.8 ± 0.2a | 16.8 ± 0.8d | 11.1 ± 1.0c | 5.7 ± 0.6b |
Fig. 2.
The hepatic level of RAGE (μg/mg protein, a) and sorbitol (mM, b), and GLO-1 activity (U/mg protein, c) in normal (N) or diabetic mice (D) treated with RA at 0, low, or high dose for 9 weeks. Values are mean ± SD, n = 8. a-emeans among bars without a common letter differ, p < 0.05.
4. Discussion
As reported by others [6–8] and observed by our present study, RA supplement attenuates the pathological progression of diabetes, which is evidenced by decreased water and feed intake, lowered blood glucose level, and increased insulin level. Furthermore, we found that RA intake effectively alleviated hepatic inflammatory and glycative stress via decreasing the production or activity of inflammatory and glycative factors such as IL-6, TNF-alpha, PGE2, COX-2, RAGE and sorbitol, and increasing the GLO-1 activity. These novel findings support the notion that RA protects the liver against inflammatory and glycative injury from diabetes, which in turn benefits the hepatic functions.
Both IL-6 and TNF-alpha are inflammatory cytokines and are commonly used as biomarkers for evaluating inflammatory status, especially under a diabetic condition [13]. The results of our present study indicate that RA treatments effectively decrease the hepatic release of IL-6 and TNF-alpha. These data support the anti-inflammatory activity of RA and show that the anti-inflammatory protection of RA extends to the liver under a diabetic condition. In addition, COX-2 and PGE2, which is a metabolite of COX-2, are important pathological mediators that contribute to the progression of inflammatory disorders including diabetes [14, 15]. Thus, the decline in COX-2 activity and PGE2 formation due to RA treatments observed in our present study suggest the attenuation in hepatic inflammatory stress. These results also explain the anti-inflammatory actions of RA that were observed.
The pathological importance of RAGE has attracted more attention because RAGE can bind many ligands including AGEs, high-mobility group box protein 1, β-amyloid and even lipopolysaccharide [16, 17]. Our present study found that RAGE production in the liver becomes elevated due to diabetes. It is possible that the increased RAGE in the liver not only favors glycative reactions by reacting with AGEs, but also promots inflammatory and/or oxidative response by reacting with other ligands. Our data revealed that RA treatments at both low and high doses lowered hepatic RAGE formation, which subsequently decreased the interaction between RAGE and its ligands, and finally they mitigated RAGE-associated diabetic pathological development. The polyol pathway is responsible for the conversion of glucose to sorbitol through the action of aldose reductase [18]. The activation of this pathway facilitates the pathogenesis of diabetic microvascular complications because the excessive sorbitol and its downstream products promote AGEs formation and enhance glycative stress [19, 20]. In our present study, an increased hepatic sorbitol level was found, which implies that the polyol pathway was activated in the livers of the diabetic mice. However, RA treatments at both low and high doses markedly reduced hepatic sorbitol generation. It is thus possible that RA limited aldose reductase activity in the livers of diabetic mice, which consequently suppressed sorbitol production and diminished hepatic glycative stress. On the other hand, it is known that glycative products and their precursors that are formed due to diabetic stress can be metabolized by the glyoxalase system to less toxic compounds, in which the glycative injury or stress can be attenuated [21, 22]. GLO-1 is the rate-limiting enzyme of the glyoxalase system, and it is considered to be an important anti-glycation enzyme because the enhancement of this enzyme enhances the host’s defensive capability against glycation [23]. Thus, the raised hepatic GLO-1 activity from RA treatments observed in our present study might be responsible for contributing to a decrease in the production of hepatic glycative products and their precursors. These results suggest that RA may ameliorate glycative injuries in the liver under a diabetic condition through regulating polyol and glyoxalase pathways.
In summary, a supplementation of rosmarinic acid (RA) decreased the inflammatory and glycative stress in the livers of diabetic mice via decreasing the generation of IL-6, TNF-alpha, PGE2, RAGE, and sorbitol, as well as reducing COX-2 activity and raising GLO-1 activity. Such findings support that this polyphenol could be considered as a potent protective agent for the liver against diabetic injury.
Conflicts of Interest Statement
None.
References
- 1. Amoah SK, Sandjo LP, Kratz JM, Biavatti MW. Rosmarinic Acid-Pharmaceutical and Clinical Aspects. Planta Med. 2016; 82: 388–406. [DOI] [PubMed] [Google Scholar]
- 2. González-Vallinas M, Reglero G. Ramírez de Molina A. Rosemary (Rosmarinus officinalis L.) extract as a potential complementary agent in anticancer therapy. Nutr Cancer. 2015; 67: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 3. Nabavi SF, Tenore GC, Daglia M, Tundis R, Loizzo MR, Nabavi SM. The cellular protective effects of rosmarinic acid: from bench to bedside. Curr Neurovasc Res. 2015; 12: 98–105. [DOI] [PubMed] [Google Scholar]
- 4. Shan Y, Wang DD, Xu YX, Wang C, Cao L, Liu YS, Zhu CQ. Aging as a precipitating factor in chronic restraint stress-induced Tau aggregation pathology, and the protective effects of rosmarinic acid. J Alzheimers Dis. 2016; 49: 829–844. [DOI] [PubMed] [Google Scholar]
- 5. Ferreira LG, Celotto AC, Capellini VK, Albuquerque AA, Nadai TR, Carvalho MT, Evora PR.. Is rosmarinic acid underestimated as an experimental cardiovascular drug? Acta Cir Bras. 2013; 28 Suppl 1: 83–87. [DOI] [PubMed] [Google Scholar]
- 6. Runtuwene J, Cheng KC, Asakawa A, Amitani H, Amitani M, Morinaga A, et al Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des Devel Ther. 2016; 10: 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mushtaq N, Schmatz R, Ahmed M, Pereira LB, da Costa P, Reichert KP, et al Protective effect of rosmarinic acid against oxidative stress biomarkers in liver and kidney of strepotozotocin-induced diabetic rats. J Physiol Biochem. 2015; 71: 743–751. [DOI] [PubMed] [Google Scholar]
- 8. Sotnikova R, Okruhlicova L, Vlkovicova J, Navarova J, Gajdacova B, Pivackova L, et al Rosmarinic acid administration attenuates diabetes-induced vascular dysfunction of the rat aorta. J Pharm Pharmacol. 2013; 65: 713–723. [DOI] [PubMed] [Google Scholar]
- 9. Luan H, Kan Z, Xu Y, Lv C, Jiang W. Rosmarinic acid protects against experimental diabetes with cerebral ischemia: relation to inflammation response. J Neuroinflammation. 2013; 10: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leung SS, Forbes JM, Borg DJ. Receptor for advanced glycation end products (RAGE) in type 1 diabetes pathogenesis. Curr Diab Rep. 2016; 16: 100. [DOI] [PubMed] [Google Scholar]
- 11. Takeda S, Sato N, Rakugi H, Morishita R. Molecular mechanisms linking diabetes mellitus and Alzheimer disease: beta-amyloid peptide, insulin signaling, and neuronal function. Mol Biosyst. 2011; 7: 1822–1827. [DOI] [PubMed] [Google Scholar]
- 12. Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009; 52: 2251–2263. [DOI] [PubMed] [Google Scholar]
- 13. Govindaraj J, Sorimuthu Pillai S. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic rats. Mol Cell Biochem. 2015; 404: 143–159. [DOI] [PubMed] [Google Scholar]
- 14. Gordillo-Moscoso A, Ruiz E, Carnero M, Reguillo F, Tejerina E, et al Relationship between serum levels of triglycerides and vascular inflammation, measured as COX-2, in arteries from diabetic patients: a translational study. Lipids Health Dis. 2013; 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohamed R, Jayakumar C, Ranganathan PV, Ganapathy V, Ramesh G. Kidney proximal tubular epithelial-specific overexpression of netrin-1 suppresses inflammation and albuminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am J Pathol. 2012; 181: 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basta G, Navarra T, De Simone P, Del Turco S, Gastaldelli A, Filipponi F. What is the role of the receptor for advanced glycation end products-ligand axis in liver injury?. Liver Transpl. 2011; 17: 633–640. [DOI] [PubMed] [Google Scholar]
- 17. Ramasamy R, Shekhtman A, Schmidt AM. The multiple faces of RAGE–opportunities for therapeutic intervention in aging and chronic disease. Expert Opin Ther Targets. 2016; 20: 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grewal AS, Bhardwaj S, Pandita D, Lather V, Sekhon BS. Updates on aldose reductase inhibitors for management of diabetic complications and non-diabetic diseases. Mini Rev Med Chem. 2016; 16: 120–162. [DOI] [PubMed] [Google Scholar]
- 19. Obrosova IG, Kador PF. Aldose reductase / polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol. 2011; 12: 373–385. [DOI] [PubMed] [Google Scholar]
- 20. Setter SM, Campbell RK, Cahoon CJ. Biochemical pathways for microvascular complications of diabetes mellitus. Ann Pharmacother. 2003; 37: 1858–1866. [DOI] [PubMed] [Google Scholar]
- 21. Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci (Lond). 2015; 128: 839–861. [DOI] [PubMed] [Google Scholar]
- 22. Rabbani N, Xue M, Thornalley PJ. Activity, regulation, copy number and function in the glyoxalase system. Biochem Soc Trans. 2014; 42: 419–424. [DOI] [PubMed] [Google Scholar]
- 23. Hirakawa Y, Inagi R. Glycative stress and its defense machinery glyoxalase 1 in renal pathogenesis. Int J Mol Sci. 2017; 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]