Abstract
Rationale:
Perimesencephalic nonaneurysmal subarachnoid hemorrhage (PNSAH) is characterized by a pattern of extravasated blood restricted to the perimesencephalic cisterns, normal angiographic findings, and an excellent prognosis with an uneventful course and low risks of complication. The precise etiology of bleeding in patients with PNSAH has not yet been established. The most common hypothesis is that PNSAH is venous in origin. Intracranial venous hypertension has been considered as the pivotal factor in the pathogenesis of PNSAH. The underlying venous pathology such as straight sinus stenosis, jugular vein occlusion may contribute to PNSAH. We describe a patient in whom transverse sinus thrombosis preceded intracranial venous hypertension and PNSAH. These findings supported that the source of the subarachnoid hemorrhage is venous in origin.
Patient concerns and diagnoses:
A 45-year-old right-handed man was admitted to the hospital with a sudden onset of severe headache associated with nausea, vomiting, and mild photophobia for 6 hours. The patient was fully conscious and totally alert. An emergency brain computed tomography (CT) revealed an acute subarachnoid hemorrhage restricted to the perimesencephalic cisterns. CT angiography revealed no evidence of an intracranial aneurysm or underlying vascular malformation. Digital subtraction angiography of arterial and capillary phases confirmed the CT angiographic findings. Assessment of the venous phase demonstrated right transverse sinus thrombosis. Magnetic resonance imaging confirmed the diagnosis of cerebral venous sinus thrombosis (CVST). Lumbar puncture revealed an opening pressure of 360 mmH2O, suggestive of intracranial venous hypertension. Grave disease was diagnosed by endocrinological investigation.
Interventions:
Low-molecular-weight heparin, followed by oral warfarin, was initiated immediately as the treatment for cerebral venous sinus thrombosis and PNSAH.
Outcomes:
The patient discharged without any neurologic defect after 3 weeks of hospital stay. MR venography revealed recanalization of right transverse sinus at the 6-month follow-up. No clinical or neuroimaging evidence of relapse was detected at 12 months follow-up.
Lessons:
Hyperthyroidism may contribute to the development of CVST. The presence of acute transverse sinus thrombosis, as a cause of PNSAH, provides further support for the hypothesis that the source of PNSAH is venous in origin and intracranial venous hypertension plays a critical role in the pathogenesis of PNSAH.
Keywords: cerebral venous sinus thrombosis, hyperthyroidism, subarachnoid hemorrhage, venous hypertension, venous pathology
1. Introduction
Perimesencephalic nonaneurysmal subarachnoid hemorrhage (PNSAH), identified by van Gijn et al. in 1985,[1] has been well recognized as a distinct clinical and imaging entity that contributes approximately 8% to 11% of the patients with subarachnoid hemorrhage (SAH) and constitutes 21% to 68% of those with a negative angiogram.[2] Radiographically, PNSAH is characterized by normal 4-vessel angiography in combination with a pattern of extravasated blood restricted to the perimesencephalic cisterns or prepontine cisterns, without the extension of blood to lateral Sylvian fissures, anterior interhemispheric fissures, and lateral ventricles on computed tomographic imaging.[3] In contradistinction to aneurysmal SAH, PNSAH has an excellent prognosis with an uneventful clinical course and a low risk of vasospasm, rebleeding, hydrocephalus, and delayed cerebral ischemia.[4,5] However, many patients with PNSAH complained of functional decline which prevented them from returning to work.[6] The pathogenesis and mechanisms of the PNSAH are controversial. Theories include rupture of perforating arteries or capillary, basilar artery dissection, thrombosed arterial aneurysms, cavernous malformations, and dural arteriovenous fistula.[7] Recently, several studies have reported an association between intracranial venous hypertension and PNSAH.[7–11] This presumption is supported by reports on PNSAH induced by straining and physical exertion,[11] as well as more recent studies highlighting abnormal venous drainage patterns of the basal vein of Rosenthal.[7,12–14] The presence of underlying anomaly in cerebral venous circulation, such as stenosis of straight sinus,[9] jugular vein,[8] and vein of Galen,[12,15,16] has been implicated in several case reports as an important predisposing factor in the pathogenesis of PNSAH.[9,15–17]
Herein, we described a case of rarely reported transverse sinus thrombosis producing intracranial venous hypertension and the occurrence of PNSAH. It provided strong support to the hypothesis that the source of PNSAH is venous in origin.[7] Then, a review of published literature was undertaken to reveal the association between underlying venous pathology and PNSAH.
2. Consent
Written informed consent was obtained from the patient before and after all procedures.
3. Case report
A 45-year-old right-handed Chinese man presented to our hospital with a 6-hour history of sudden onset of a severe headache in the occipital-temporal orientation, associated with nausea, vomiting, and mild photophobia. The headache was persistent and unresponsive to non-steroid analgesics. He had no relevant family history of thrombosis or hemorrhage. His past medical history was unremarkable without risk factors such as diabetes, hypertension, hypercholesteremia, autoimmune disorders, alcohol consumption, cigarette smoking, or drug abuse. Upon arrival to the emergency department, the patient was fully conscious and totally alert, presenting apparently a normal higher cortical function, but suffering from severe headache, photophobia, stiff neck and vomiting with a Glasgow Coma Scale of 15/15. The severity of SAH was assessed as grade 2 on the Hunt & Hess scale and grade 1 on World Federation of Neurological Surgeons (WFNS) scale. No abnormalities or focal neurologic deficits were remarkable except for stiff neck in a general physical examination.
An emergency noncontrast brain computed tomography (CT) revealed an acute subarachnoid hemorrhage restricted to the perimesencephalic and prepontine cisterns. There was no extension of the hemorrhage to interhemispheric fissure and the Sylvian fissures. This pattern of hemorrhage was well known as a perimesencephalic SAH (Fig. 1A–C). Followed CT angiography did not reveal any evidence of an intracranial aneurysm or underlying vascular malformation. The patient was admitted to the neurology intensive care unit for making preparations for digital subtraction angiography (DSA). On the second day after hospitalization, DSA showed no evidence of an aneurysm and vascular malformation in arterial and capillary phases (Fig. 2A and B). Assessment of the venous phase of left internal carotid artery angiogram showed normal cortical veins, superior sagittal sinus, and left transverse sinus (Fig. 2D and F). But the assessment of the venous phase of right internal carotid artery angiogram demonstrated an absence of normal venous outflow of the right transverse sinus and prominent cerebral veins as extensive collateralizations (Fig. 2C and E). The findings suggested right transverse sinus thrombosis. Brain magnetic resonance images were conducted to confirm the diagnosis of cerebral sinus thrombosis 3 days after admission, which revealed an image of normal gray and white matter, without lesions in the brain parenchyma. However, the images of the right transverse sinus revealed the presence of a hyperintense lesion on both T1-weighted image and T2-weighted image inside the sinus (Fig. 3A–C), suggestive of the replacement of signal void by thrombus. Axial gadolinium-enhanced T1-weighted images demonstrated filling defects in the right transverse sinus and sigmoid sinus (Fig. 3D), compatible with the absence of flow on contrast MR venography (Fig. 3E and F).
Figure 1.

Axial sections of noncontrast brain computed tomographic imaging at symptom onset demonstrated acute hemorrhage (yellow arrow) localized in the left perimesencephalic cistern (A) and prepontine cistern (B–D), suggesting perimesencephalic subarachnoid hemorrhage. There were mild hyperdensity lesions in right transverse sinus location (D, red arrow), suggestive of transverse sinus thrombosis.
Figure 2.
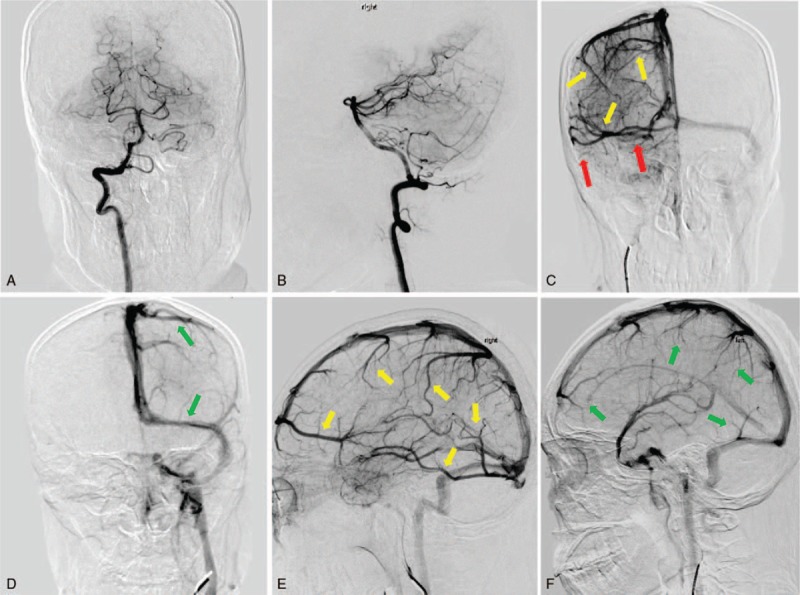
The 4-vessels digital subtraction angiograms on the second day after admission showed no evidence of an intracranial aneurysm or underlying vascular malformation in vertebrobasilar artery system (A and B). Assessment of the venous phase of left internal carotid artery angiogram showed normal cortical veins, superior sagittal sinus, and left transverse sinus (D and F, green arrow). Venous phase of right internal carotid artery angiogram demonstrated prominent cerebral veins as extensive collateralizations (C and E, yellow arrow) and an absence of normal venous outflow of the right transverse sinus (C, red arrow).
Figure 3.
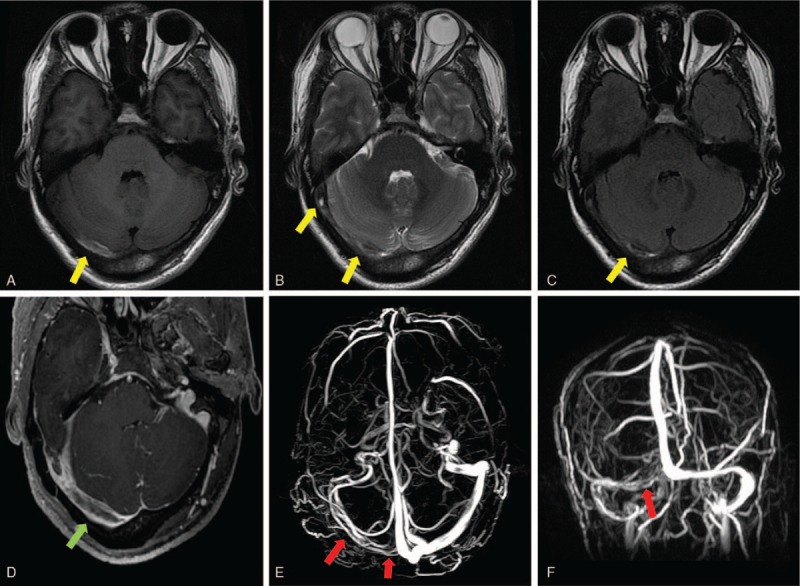
Magnetic resonance imaging performed on the third day after admission showed an absence of flowing avoid in the area of right transverse sinus and sigmoid sinus (A–C). Axial sections of T1-weighted image (Fig. 4A), T2-weighted images (Fig. 4B), and fluid attenuated inversion recovery (FLAIR) images (Fig. 4C) demonstrated the presence of a hyperintense lesion on all of the 3 sequences inside the right transverse sinus, suggesting thrombosis in the right transverse sinus (A–C, yellow arrow). Axial gadolinium-enhanced T1-weighted images demonstrated filling defects in the right transverse sinus and sigmoid sinus (D, green arrow). Magnetic resonance venography demonstrated right transverse and sigmoid sinus thrombosis (E and F, red arrow).
Routine laboratory examinations, including complete blood count, liver and renal function tests, serum electrolytes, erythrocyte sedimentation rate (ESR), and routine urine test were all within normal limits. There was not any significant abnormality in routine investigations except for an increased D-dinner level (21.7 mg/L, reference range 0.1–5 mg/L) and elevated activity of factor VIII (212%, reference range 50–150%). Regarding the etiology of the cerebral venous thrombosis, different markers of prothrombotic conditions on laboratory examinations were performed including antithrombin III, protein S, protein C, factor V Leiden, homocysteine, antiphospholipid antibodies, anti-β2-glycoprotein-1 IgM, and lupus-like anticoagulant. These results failed to provide evidence for hereditary or acquired coagulation defect. Other immune tests such as anti-nuclear antibody, anti-extractable nuclear antigen, anti-dsDNA, anti-neutrophil cytoplasmic antibodies, anti-smooth muscle antibody were all negative. Hepatitis panels and tumor biomarkers tests were all negative. Endocrinological work-up revealed hyperthyroidism on the basis of serum FT3 (14.8 pmol/L, reference range 3.60–10.82 pmol/L), serum FT4 (33.5 pmol/L, reference range 6.7–21.4 pmol/L), serum thyroid stimulating hormone (TSH) (below 0.02 mIU/L, reference range 0.9–7.10 mIU/L), antithyroglobulin antibody 1:320, and anti-TSH receptor antibody 56.5% (reference range < 10%).
Lumbar puncture in the third day after admission demonstrated xanthochromia cerebrospinal fluid with an opening pressure of 360 mmH2O. Cerebrospinal fluid (CSF) test revealed 87 mg/dL of glucose, 59 mg/dL of protein, 1447 cells/mm3 of red blood cell and 127 cells/mm3 of white blood cell (52% polymorphonuclear leukocytes, 31% lymphocytes, 17% monocytes). Other inspections of CSF including gram stains, India ink stain, acid fast bacilli stain, and virological panels were all negative. Mannitol and glycerol were initiated to control the increased intracranial pressure after lumbar puncture.
The diagnosis of PNSAH caused by cerebral venous sinus thrombosis was established based on the clinical course and radiological images 3 days after admission. The diagnosis of Graves disease was established based on the results of laboratory investigations 4 days after admission. Graves disease was treated with propylthiouracil and propranolol. After informed consent was obtained on the fourth day after admission, therapy for cerebral venous thrombosis started with low-molecular weight heparin (Nadroparin, 6150AXaIU, subcutaneous injection, twice daily). Subsequent oral anticoagulant therapy with warfarin (target International normalized ratio = 2–3) was started 14 days later. The clinical symptoms were markedly improved at 10 days of posttreatment (day 14 after admission). Brain CT (Fig. 4A–C) on day 16 provided an apparently normal image of the perimesencephalic cistern, demonstrating a complete absorption of hemorrhage. His headache subsided on the same day with a mildly elevated CSF pressure of 200 mmH2O in the re-examination of lumbar puncture. The patient discharged without any neurologic defect after 3 weeks of hospital stay. At his 6-month follow-up, he had no complaints. MR venography revealed recanalization of sinus thrombosis in the corresponding area (Fig. 5A and B). Laboratory findings indicated normal values of free T3, free T4 level, and activity of factor VIII except for a mildly decreased TSH (0.62 mIU/L, reference range 0.9–7.10 mIU/L). Then oral anticoagulant therapy of warfarin discontinued. At his 1-year follow-up, he had no clinical relapses.
Figure 4.
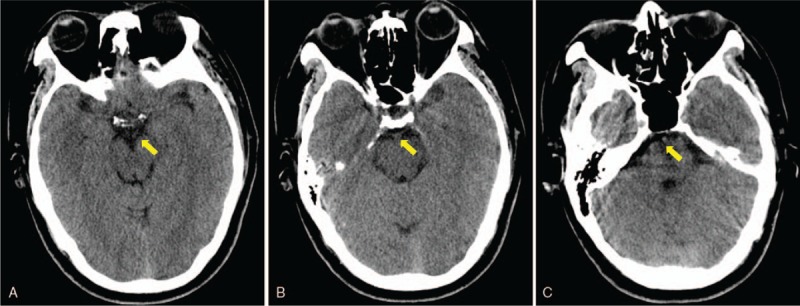
Brain computed tomographic imaging performed on day 11 provided an apparently normal image of perimesencephalic and prepontine cisterns, demonstrating a complete absorption of hemorrhage (A, B and C, yellow arrow).
Figure 5.
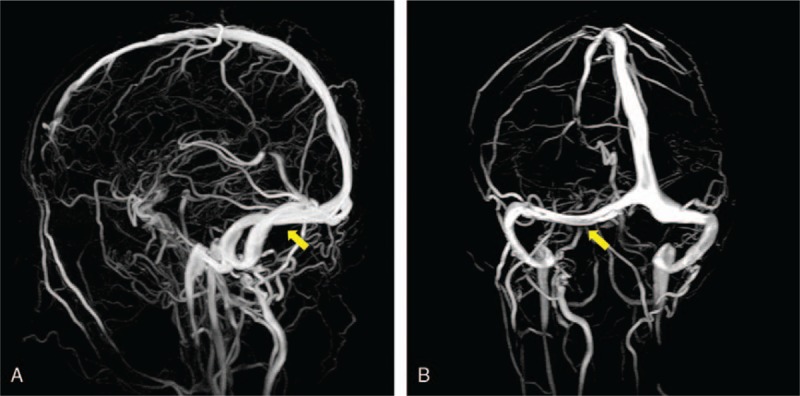
MR venography at 6-month follow-up revealed a recanalization of the right transverse sinus (A and B, yellow arrow).
Timeline of clinical course, interventions and outcome shows in Figure 6.
Figure 6.
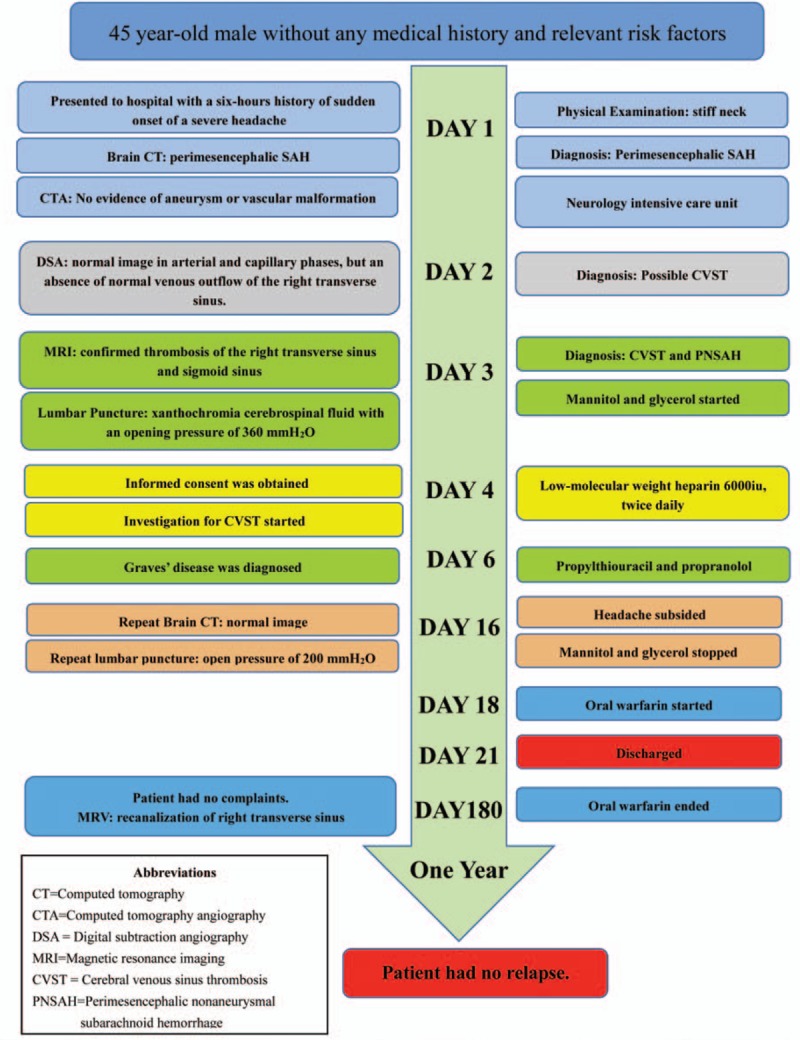
Timeline of clinical coure, interventions, and outcome.
4. Discussion
Perimesencephalic nonaneurysmal subarachnoid hemorrhage was first described as a distinct sub-type of SAH with a characteristic subarachnoid blood distribution pattern and the absence of an angiographically confirmed aneurysm.[1,3,7] PNSAH was identified by computed tomographic imaging evidence of the accumulation blood around the perimesencephalic and prepontine cisterns with minimal intraventricular extension.[3] Compared with the patients with aneurysmal SAH, patients with PNSAH typically present with a similar initial presentation of a sudden onset of an excruciating headache.[18,19] However, patients with PNSAH were associated with an excellent prognosis with an uncomplicated illness course and a low risk of rebleeding, cerebral vasospasm, hydrocephalus, delayed cerebral ischemia, no decrease in quality of life and death.[4,5,7,18–20] But it has been reported that cognitive impairment was common in this cohort.[6] Several risk factors for PNSAH have been suggested such as younger age, female sex, and smoking.[21,22] Although the only risk factor consistently present in previous studies is hypertension, patients with PNSAH are more likely to have a lower frequency of hypertension than patients with aneurysm SAH.[21,22]
While arterial and venous origins have already been both postulated as the source of perimesencephalic subarachnoid hemorrhage,[7,23,24] the precise etiology and anatomical source of PNSAH still remain enigmatic despite recent advances in the association between venous drainage pattern of the basal vein of Rosenthal and PNSAH.[7,12–14] The most common hypothesis is that PNSAH is venous in origin due to the invariably mild initial presentation, its benign clinical course, limited extension of hemorrhage, low incidence of rebleeding and vasospasm.[1,4,7] Although rupture of arterial perforators and small aneurysms has been reported sporadically,[25,26] a recent study failed to find any lesion of the basilar artery in 7 patients with PNSAH using 7-tesla vessel wall imaging technology, supporting the hypothesis that PNSAH has a venous origin.[27] Intracranial venous hypertension has been considered as the pivotal factor in the pathogenesis of PNSAH.[8,11,13,14,17] Elevated intracranial venous pressure can lead to vessel engorgement and venous rupture. This hypothesis is supported by reports on PNSAH induced by straining, physical exertion,[11] performing Valsalva maneuvers, and hypoxic training during swimming.[28] These behaviors will produce increased intrathoracic pressure and block venous flow from the brain to the right atrium, which leads to venous congestion, increased intracranial venous pressure and venous breakdown.[11,28] On the other hand, this presumption is supported by reports on more recent studies highlighting aberrant venous drainage patterns of the basal vein of Rosenthal.[7,12–14,29] Van der Schaaf et al[29] and colleagues reported that primitive drainage patterns of the basal vein of Rosenthal (BVR) directly into dural sinuses or the internal cerebral veins instead of into the Galenic venous system were much common in patients with PNSAH than those in patients with aneurysmal SAH. Furthermore, in the patients who have primitive BVR drainage in one hemisphere, hemorrhage was likely to present on the same side of the abnormal BVR drainage.[29,30] This phenomenon suggested an association between the primitive BVR drainage and PNSAH. Similar findings have been reported by different studies.[7,12,14,16,29,30] A recent meta-analysis included 8 studies investigating the prevalence of primitive BVR drainage patterns in patients with PNSAH and aneurysmal SAH.[13] It concluded that patients with PNSAH have a higher prevalence of primitive BVR drainage than patients with aneurysmal SAH, suggested a venous origin of some PNSAH.[13] The direct connection of the basal vein of Rosenthal with the dural sinuses may predispose to a sudden increase in intracranial venous pressure with venous engorgement and rupture as a result.
Since intracranial venous hypertension is a characteristic clinical feature of cerebral venous and sinus thrombosis, reports of associated venous rupture such as PNSAH and convexal SAH should not be surprising.[17,31–34] In our case, the occurrence of PNSAH was associated with transverse sinus thrombosis, an occurrence not yet previously reported. In our case, the venous phase of DSA demonstrated an absence of normal venous outflow of the right transverse sinus and prominent cerebral veins as extensive collateralizations. This exerted intracranial venous hypertension and retrograde hydrostatic pressure into the cerebral deep veins system which bridge the perimesencephalic and prepontine subarachnoid space that resulted in a rupture of perimesencephalic veins.[17,34] The veins in the corresponding anatomic location have relatively thin vessel walls without smooth muscle fibers and venous valves, also contributing to the rupture of venous.
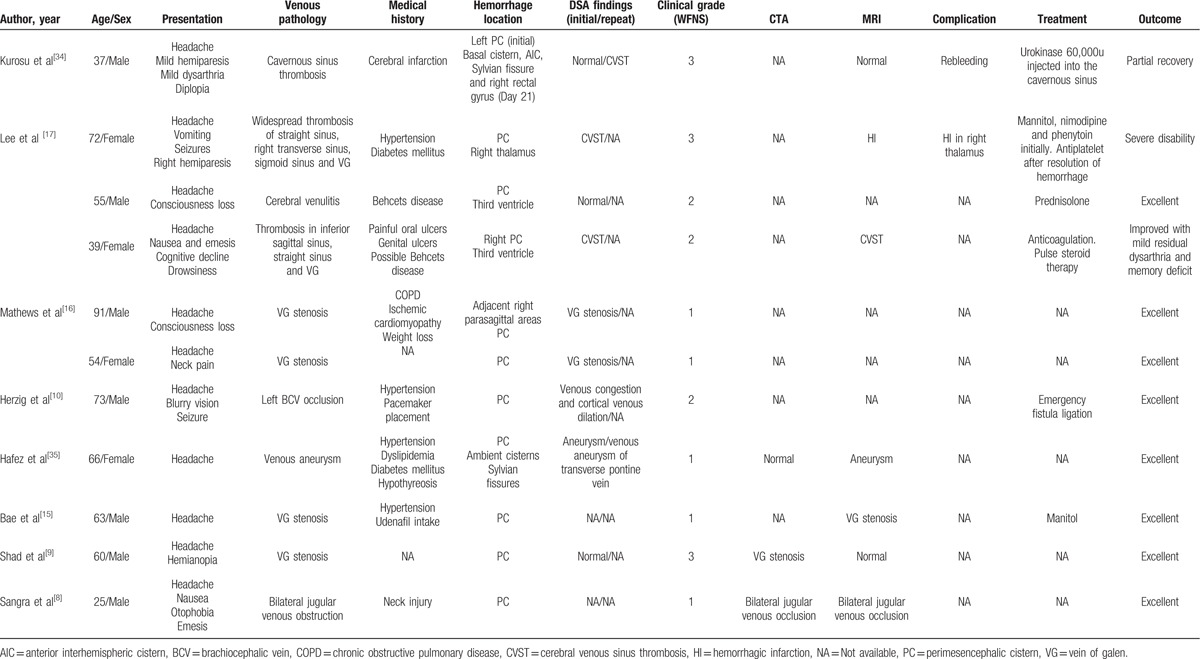
Our case of PNSAH strengthens the evidence linking PNSAH with venous hypertension associated with underlying venous pathology in the cerebral venous circulation. We searched for case reports of venous abnormalities associated with PNSAH by conducting a search of PubMed. Table 1 showed the results: 11 patients with underlying venous pathology associated with PNSAH. Venous abnormalities, including venous aneurysm,[35] venulitis associated with Behcet disease,[17] stenosis or occlusion of vein of Galen, straight sinus, jugular vein and brachiocephalic vein, and cerebral venous thrombosis of cavernous sinus,[34] widespread sinuses,[17] straight sinus, and the vein of Galen,[17] have been implicated in various case reports as potential sources of the bleeding. So, underlying venous pathology may be identified as an important predisposing factor in the pathogenesis of PNSAH. Most of the cases with PNSAH caused by underlying venous pathology had an excellent outcome without any complication.[8,9,15,17,35] However, patients with cerebral venous thrombosis were likely to have poorer neurological symptoms, higher scores of WFNS scale, longer duration of hospital stay together with higher frequencies of initial neurologic deficits, poor outcome relevant complication including rebleeding and hemorrhagic infarction.[17,34] The prognosis of patients with PNSAH caused by CVST is not as good as those of patients with PNSAH caused by other etiology. Although the prognosis of CVST is usually favorable and the mortality is relatively low, the functional outcome and mortality may vary in different condition. Data from the largest CVST registry of the National Inpatient Sample revealed that intracranial hemorrhage (ICH) was an independent predictor of increased risk of mortality (adjusted OR = 5.4, 95% CI = 4.38–7.96).[36] Motor deficits and ICH were also strongly associated with poor outcome in the International Study on Cerebral Vein and Dural Sinus Thrombosis study (ISCVT) cohort and a Pakistani series.[37] In the ICH subgroup analysis of ISCVT cohort study, patients were older, and more likely to have severe clinical presentations and a much worse 6-month prognosis than those in CVST patients without ICH. Having a thrombosis in the deep cerebral venous system (adjusted odds ratio [adjusted OR] = 5.43; 95% confidence interval [95% CI] = 1.67–17.61), or a right transverse sinus thrombosis (adjusted OR = 2.56; 95% CI = 1.03–6.40), and having a motor deficit (adjusted OR = 2.94; 95% CI = 1.21–7.10) were the key predictors of poor prognosis.[38] Since SAH and deep cerebral venous system involved were the characteristic clinical features of PNSAH, poor prognosis in patients with PNSAH caused by CVST should not be surprising. On the other hand, patients complicated with CVST might have acute and progressive intracranial venous hypertension compared with patients with PNSAH caused by other etiology, this might contribute to the development of poor outcome. So, early evaluation of CVST is very important for the treatment of PNSAH. In addition to the evidence from digital subtraction venography, there are some case reports that reveal intracranial venous hypertension associated with spinal arteriovenous fistula and thoracolumbar spinal vascular malformation,[39] may lead to PNSAH. We suggest a comprehensive analysis of cerebral veins must be performed in patients with PNSAH to determine whether there is an underlying venous pathology or a primitive BVR drainage pattern.
Table 1.
Cases of perimesencephalic nonaneurysmal subarachnoid hemorrhage caused by underlying venous pathology.
In this case, there was no significant abnormality in coagulative investigations except for an increased D-dinner level and elevated activity of factor VIII. Connective tissue disease, antiphospholipid syndrome, deficiencies of antithrombin, protein C or protein S, or factor V Leiden were excluded by the relevant laboratory examination. CVST has been reported as a cerebrovascular complication of hyperthyroidism in a number of previous studies.[40–42] Hyperthyroidism may be a predisposing factor for CVST in our case through a hypercoagulable state including increase in acute-phase reactants, elevated activity of thrombin and fibrinogen, and decrease in fibrinolytic activity.[40–42] But the available literature does not provide an answer to the need for prolonged anticoagulant therapy in patients with CVST and hyperthyroidism, we discounted anticoagulant therapy at 6-month follow-up according to the available guidelines.
The management of CVST with subarachnoid hemorrhage is controversial and not well established. The most recent guidelines proposed by the AHA for CVST state that once the diagnosis of CVST has been confirmed, anticoagulation treatment should be initiated immediately even in the setting of intracerebral hemorrhage.[43] The recommendation for patients with CVST and intracerebral hemorrhage is based on the assumption that the treatment of anticoagulation can prevent thrombus formation and promote spontaneous recanalization of the existing thrombus more than it promotes the development of bleeding.[44] However, there is only rare evidence on the benefit and bleeding risk of systemic anticoagulation in patients with SAH and PNASH induced by CVST.[17,31–33,43] On the base of pathological assumption of PNSAH, anticoagulation is likely to be effective without extension of bleeding in the current case combined with these previous reports. However, the optimal anticoagulation dosing, initiation time of anticoagulation, and monitoring tests for anticoagulation have not been well established.[43,45,46] For patients with CVST and SAH, intracranial aneurysm must be ruled out before anticoagulation by using magnetic resonance angiography or digital subtraction angiography. Meanwhile, repeating CT after at least 1 day from onset of symptoms is recommended to confirm that hemorrhage is reducing or at least not progressing before starting anticoagulation.[46] Initial anticoagulation is generally with adjusted-dose unfractionated heparin (UFH) or weight-based low molecular weight heparin (LMWH) in full anticoagulant doses.[40] Recent studies demonstrated LMWH had better efficacy and safety in comparison with UFH, with lower risk of major hemorrhage, thrombotic complications, and death.[47] As the pharmacokinetic parameter of LMWH is well defined for normal weight patients, monitoring is not required as a routine test for LMWH according to the American college of chest physicians (ACCP) and British society for haematology guidelines.[48] In some special condition such as obese patients, pregnant patients, patients with renal impairment, and patients with high risk of hemorrhage, anti-Xa activity assay may be of some value in the investigation of pharmacokinetics of LMWH.[48,49] However, anti-Xa activity assay seems to have poor predictive value for risk of hemorrhage and antithrombotic efficacy.[48,49] Other parameters, including thrombin generation assay, platelet contractile force, clot elastic modulus, and thromboelastography, have been applied to monitor pharmacokinetics of LMWH, but still lack of evidence.[49] The treating dosage of LMWH for patients with increased bleeding risk suggested by Babin et al[49] is 86 units/kg twice daily with a recommended therapeutic anti-Xa level from 0.5 to 1.1 U/mL. In clinical practice, repeat CT scan when necessary, platelet count detection together with the careful clinical observation of conscious state, neurologic impairment, skin or mucous hemorrhage, symptoms and signs of intracranial hypertension were the method to monitor the drug effect of anticoagulants.
5. Conclusion
The present case is the first to describe the occurrence of transverse sinus thrombosis in relation to PNSAH. Hyperthyroidism related to Graves disease may contribute to the development of CVST. The pathophysiologic mechanism of PNSAH in our case could provide further support to the hypothesis that the source of PNSAH is venous in origin and intracranial venous hypertension may play a key role in the pathogenesis of PNSAH. Our case combined with the underlying venous pathology of PNSAH from recent reports provides a better understanding the pathogenesis and management of PNSAH. Comprehensive analysis of cerebral veins may help to establish the cause of PNSAH.
Footnotes
Abbreviations: AHA = American Heart Association, BVR = basal vein of rosenthal, CSF = cerebrospinal fluid, CT = computed tomography, CTA = computed tomography angiography, CVST = cerebral venous sinus thrombosis, DSA = digital subtraction angiography, FT3 = free triiodothyronine, FT4 = free thyroxine, MRI = magnetic resonance imaging, PNSAH = perimesencephalic nonaneurysmal subarachnoid hemorrhage, SAH = subarachnoid hemorrhage, TSH = thyroid stimulating hormone, WFNS = World Federation of Neurological Surgeons.
F-WF, JR, and Y-YZ have contributed equally to this article as the co-first authors.
Authors’ contributions: JR was the chief physician for this patient and collected the data of this patient. F-WF and Y-YZ drafted the manuscript and made a substantial contribution to the concept and design. WC analyzed the imaging data. LS, Q-HZ, J-GY, and J-QK collected the clinical data and searched published literature. G-QZ conceived and designed the study, critically reviewed and revised the manuscript. All authors have read and approved the final submission.
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- [1].van Gijn J, van Dongen KJ, Vermeulen M, et al. Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology 1985;35:493–7. [DOI] [PubMed] [Google Scholar]
- [2].Kleinpeter G, Lehr S. Characterization of risk factor differences in perimesencephalic subarachnoid hemorrhage. Minim Invasive Neurosurg 2003;46:142–8. [DOI] [PubMed] [Google Scholar]
- [3].Rinkel GJ, Wijdicks EF, Vermeulen M, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol 1991;12:829–34. [PMC free article] [PubMed] [Google Scholar]
- [4].Kong Y, Zhang JH, Qin X. Perimesencephalic subarachnoid hemorrhage: risk factors, clinical presentations, and outcome. Acta Neurochir Suppl 2011;110(Pt):197–201. [DOI] [PubMed] [Google Scholar]
- [5].Patel NB, Patel AD, Wilkinson J, et al. An analysis of 88 patients with diffuse and “benign” perimesencephalic subarachnoid hemorrhage. J Neurol Surg A Cent Eur Neurosurg 2014;75:299–304. [DOI] [PubMed] [Google Scholar]
- [6].Krajewski K, Dombek S, Martens T, et al. Neuropsychological assessments in patients with aneurysmal subarachnoid hemorrhage, perimesencephalic SAH, and incidental aneurysms. Neurosurg Rev 2014;37:55–62. [DOI] [PubMed] [Google Scholar]
- [7].Kapadia A, Schweizer TA, Spears J, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurg 2014;82:1131–43. [DOI] [PubMed] [Google Scholar]
- [8].Sangra MS, Teasdale E, Siddiqui MA, et al. Perimesencephalic nonaneurysmal subarachnoid hemorrhage caused by jugular venous occlusion: case report. Neurosurgery 2008;63:E1202–3. discussion E1203. [DOI] [PubMed] [Google Scholar]
- [9].Shad A, Rourke TJ, Hamidian Jahromi A, et al. Straight sinus stenosis as a proposed cause of perimesencephalic non-aneurysmal haemorrhage. J Clin Neurosci 2008;15:839–41. [DOI] [PubMed] [Google Scholar]
- [10].Herzig DW, Stemer AB, Bell RS, et al. Neurological sequelae from brachiocephalic vein stenosis. J Neurosurg 2013;118:1058–62. [DOI] [PubMed] [Google Scholar]
- [11].Matsuyama T, Okuchi K, Seki T, et al. Perimesencephalic nonaneurysmal subarachnoid hemorrhage caused by physical exertion. Neurol Med Chir (Tokyo) 2006;46:277–81. discussion 281–272. [DOI] [PubMed] [Google Scholar]
- [12].Watanabe A, Hirano K, Kamada M, et al. Perimesencephalic nonaneurysmal subarachnoid haemorrhage and variations in the veins. Neuroradiology 2002;44:319–25. [DOI] [PubMed] [Google Scholar]
- [13].Rouchaud A, Lehman VT, Murad MH, et al. Nonaneurysmal perimesencephalic hemorrhage is associated with deep cerebral venous drainage anomalies: A systematic literature review and meta-analysis. AJNR Am J Neuroradiol 2016;37:1657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Buyukkaya R, Yildirim N, Cebeci H, et al. The relationship between perimesencephalic subarachnoid hemorrhage and deep venous system drainage pattern and calibrations. Clin Imaging 2014;38:226–30. [DOI] [PubMed] [Google Scholar]
- [15].Bae EK, Ahn JH, Park JJ. Nonaneurysmal subarachnoid hemorrhage after udenafil intake. J Stroke Cerebrovasc Dis 2013;22:e647–9. [DOI] [PubMed] [Google Scholar]
- [16].Mathews MS, Brown D, Brant-Zawadzki M. Perimesencephalic nonaneurysmal hemorrhage associated with vein of Galen stenosis. Neurology 2008;70(Pt):2410–1. [DOI] [PubMed] [Google Scholar]
- [17].Lee J, Koh E-M, Chung C-S, et al. Underlying venous pathology causing perimesencephalic subarachnoid hemorrhage. Can J Neurol Sci 2014;36:638–42. [DOI] [PubMed] [Google Scholar]
- [18].Hurley TR, Balandrin J. Perimesencephalic nonaneurysmal subarachnoid hemorrhage: review of the literature. Neurosurgery 1997;40:885. [DOI] [PubMed] [Google Scholar]
- [19].Akcakaya MO, Aydoseli A, Aras Y, et al. Clinical course of nontraumatic nonaneurysmal subarachnoid hemorrhage: A single institution experience over 10 years and review of the contemporary literature. Turk Neurosurg 2016;doi: 10.5137/1019-5149.JTN.18359-16.2. [DOI] [PubMed] [Google Scholar]
- [20].Flaherty ML, Haverbusch M, Kissela B, et al. Perimesencephalic subarachnoid hemorrhage: incidence, risk factors, and outcome. J Stroke Cerebrovasc Dis 2005;14:267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mensing LA, Ruigrok YM, Greebe P, et al. Risk factors in patients with perimesencephalic hemorrhage. Eur J Neurol 2014;21:816–9. [DOI] [PubMed] [Google Scholar]
- [22].Canhao P, Falcao F, Pinho e Melo T, et al. Vascular risk factors for perimesencephalic nonaneurysmal subarachnoid hemorrhage. J Neurol 1999;246:492–6. [DOI] [PubMed] [Google Scholar]
- [23].Kalra VB, Wu X, Matouk CC, et al. Use of follow-up imaging in isolated perimesencephalic subarachnoid hemorrhage: a meta-analysis. Stroke 2015;46:401–6. [DOI] [PubMed] [Google Scholar]
- [24].Sahin S, Delen E, Korfali E. Perimesencephalic subarachnoid hemorrhage: Etiologies, risk factors, and necessity of the second angiogram. Asian J Neurosurg 2016;11:50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maslehaty H, Mahvash M, Doukas A, et al. Basilar tip aneurysm in perimesencephalic like subarachnoid hemorrhage. Importance of exact diagnosis and the role of delayed repeated digital subtraction angiography. Acta Neurochir (Wien) 2013;155:595–6. [DOI] [PubMed] [Google Scholar]
- [26].Park SQ, Kwon OK, Kim SH, et al. Pre-mesencephalic subarachnoid hemorrhage: rupture of tiny aneurysms of the basilar artery perforator. Acta Neurochir (Wien) 2009;151:1639–46. [DOI] [PubMed] [Google Scholar]
- [27].Vergouwen MD, Hendrikse J, van der Kolk AG, et al. 7 Tesla vessel wall imaging of the basilar artery in perimesencephalic hemorrhage. Int J Stroke 2015;10:E31. [DOI] [PubMed] [Google Scholar]
- [28].Blandford J, Chalela JA. Perimesencephalic subarachnoid hemorrhage triggered by hypoxic training during swimming. Neurocrit Care 2013;18:395–7. [DOI] [PubMed] [Google Scholar]
- [29].van der Schaaf IC, Velthuis BK, Gouw A, et al. Venous drainage in perimesencephalic hemorrhage. Stroke 2004;35:1614–8. [DOI] [PubMed] [Google Scholar]
- [30].Yamakawa H, Ohe N, Yano H, et al. Venous drainage patterns in perimesencephalic nonaneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 2008;110:587–91. [DOI] [PubMed] [Google Scholar]
- [31].Arevalo-Lorido JC, Carretero-Gomez J. Cerebral venous thrombosis with subarachnoid hemorrhage: a case report. Clin Med Res 2015;13:40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khatib KI, Baviskar AS. Treatment of cerebral venous sinus thrombosis with subdural hematoma and subarachnoid hemorrhage. J Emerg Trauma Shock 2016;9:155–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anderson B, Sabat S, Agarwal A, et al. Diffuse subarachnoid hemorrhage secondary to cerebral venous sinus thrombosis. Pol J Radiol 2015;80:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kurosu A, Suzukawa K, Amo M, et al. Perimesencephalic non-aneurysmal subarachnoid hemorrhage caused by cavernous sinus thrombosis: case report. Neurol Med Chir (Tokyo) 2007;47:258–60. [DOI] [PubMed] [Google Scholar]
- [35].Hafez A, Numminen J, Rahul R, et al. Perimesencephalic subarachnoid hemorrhage with a positive angiographic finding: case report and review of the literature. Acta Neurochir (Wien) 2016;158:1045–9. [DOI] [PubMed] [Google Scholar]
- [36].Nasr DM, Brinjikji W, Cloft HJ, et al. Mortality in cerebral venous thrombosis: results from the national inpatient sample database. Cerebrovasc Dis 2013;35:40–4. [DOI] [PubMed] [Google Scholar]
- [37].Borhani Haghighi A, Edgell RC, Cruz-Flores S, et al. Mortality of cerebral venous-sinus thrombosis in a large national sample. Stroke 2012;43:262–4. [DOI] [PubMed] [Google Scholar]
- [38].Girot M, Ferro JM, Canhao P, et al. Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage. Stroke 2007;38:337–42. [DOI] [PubMed] [Google Scholar]
- [39].Fassett DR, Rammos SK, Patel P, et al. Intracranial subarachnoid hemorrhage resulting from cervical spine dural arteriovenous fistulas: literature review and case presentation. Neurosurg Focus 2009;26:E4. [DOI] [PubMed] [Google Scholar]
- [40].Kim DD, Chunilal S, Young S, et al. A study of venous thrombosis incidence in patients with acute hyperthyroidism. Intern Med J 2013;43:361–5. [DOI] [PubMed] [Google Scholar]
- [41].Migeot M, Rutgers MP, Gille M. Puerperal cerebral sinus venous thrombosis and acute hyperthyroidism in Graves’ disease. Acta Neurol Belg 2013;113:331–3. [DOI] [PubMed] [Google Scholar]
- [42].Elbers LP, van Zaane B, Gerdes VE, et al. Venous thromboembolism in overt hyperthyroidism—a direct association with clinical implications? Neth J Med 2014;72:242–4. [PubMed] [Google Scholar]
- [43].Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:1158–92. [DOI] [PubMed] [Google Scholar]
- [44].de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke 1999;30:484–8. [DOI] [PubMed] [Google Scholar]
- [45].Martinelli I, Franchini M, Mannucci PM. How I treat rare venous thromboses. Blood 2008;112:4818–23. [DOI] [PubMed] [Google Scholar]
- [46].Hegazi MO, Ahmed S, Sakr MG, et al. Anticoagulation for cerebral venous thrombosis with subarachnoid hemorrhage: a case report. Med Princ Pract 2010;19:73–5. [DOI] [PubMed] [Google Scholar]
- [47].Qureshi A, Perera A. Low molecular weight heparin versus unfractionated heparin in the management of cerebral venous thrombosis: A systematic review and meta-analysis. Ann Med Surg (Lond) 2017;17:22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kitchen S, Gray E, Mackie I, et al. Measurement of non-coumarin anticoagulants and their effects on tests of haemostasis: Guidance from the British Committee for Standards in Haematology. Br J Haematol 2014;166:830–41. [DOI] [PubMed] [Google Scholar]
- [49].Babin JL, Traylor KL, Witt DM. Laboratory monitoring of low-molecular-weight heparin and fondaparinux. Semin Thromb Hemost 2017;43:261–9. [DOI] [PubMed] [Google Scholar]


