Abstract
Background:
Aim of this study was to evaluate the effectiveness of various surgical interventions for the management of cervical spondylosis due to the ossification of posterior longitudinal ligament (OPLL).
Methods:
After a comprehensive literature search in electronic databases, studies were selected by following pre-determined eligibility criteria. Random effects meta-analyses were performed to estimate the effect sizes of various surgical approaches in improving Japanese Orthopedic Association (JOA) scores at latest follow-up and meta-regression analyses were carried out to examine the factors affecting the change in JOA score.
Results:
Twenty-three studies [1576 patients; 57.83 (95% confidence interval, 95% CI: 55.98–59.68] years of age; and 73 (70–76) % males; follow-up 55.4 ± 25.7 (range 12–170) months] were included in the meta-analysis. All surgical interventions significantly (P < .00001) improved JOA score. Anterior surgical approaches had an effect size of 4.80 [4.10–5.50] and posterior approaches with laminectomy and laminoplasty improved JOA score by 3.57 [2.39–4.75] and 3.99 [3.65–4.32], respectively. Improvement in JOA score was significantly inversely related to the preoperative JOA score (P < .00001). Surgical interventions did not significantly affect cervical lordosis at the latest follow-up.
Conclusion:
Surgical interventions for cervical spondylosis due to OPLL significantly improve JOA score as observed at the latest follow-up and this is found to be significantly inversely associated with preoperative JOA score.
Keywords: cervical spine, follow-up, ossification of posterior longitudinal ligament, surgery
1. Introduction
Ossification of the posterior longitudinal ligament (OPLL) is a heterotopic hyperostotic state due to the ectopic calcification [1] that can develop at various locations of the spine requiring radiological diagnosis for which computed tomography (CT) has better diagnostic accuracy than plain radiography.[2] Prognosis and surgical outcomes remain poor in patients with multiple-region OPLL.[3]
OPLL is one of the main causes of cervical myelopathy and an important factor for disease progression.[1,4] The prevalence may differ in different geographic regions. Whereas the prevalence of OPLL in Japanese populations is reported to be 1.9% to 4.3% [1] in the United States, its prevalence is estimated at 0.1% to 1.3%.[5] Although not fully understood, the etiology of OPLL is multifactorial with both genetic and environmental associations. Genes implicated in its pathogenesis include BMP4, BMP9, COL6A1,[3–6]HAO1A, HAO1, RSPO2, and CCDC91, RSPH9, and STK38L.[6–9] Increase in bone mineral density is also reported in patients with OPLL.[10]
There are no effective conservative treatments for OPLL. Surgical options include anterior corpectomy and fusion, laminectomy and fusion, and laminoplasty.[11] Anterior decompression to remove OPLL directly is the prime choice, as anterior compression of the spinal cord is the major source of pathology.[12,13] However, significant complications are associated with anterior corpectomy and fusion.[14] Moreover, when OPLL spreads overs multiple vertebrae or occupies much area of the canal, anterior decompression becomes difficult. Because of these constraints, posteriorly approached procedures including laminoplasty and laminectomy and fusion are frequently used, although their use is also not cost-free. Patients with straight or kyphotic preoperative cervical curvature usually have poor outcomes after laminoplasty and the incidence of progressive ossification and kyphotic deformity may increase in the long-term follow-up.[15,16]
Several studies have reported the outcomes of surgical interventions for the treatment of OPLL with anterior or posterior surgical approaches. However, no clear difference in outcomes can be noted from the outcomes of individual studies. We have carried out a systematic review of relevant studies and have performed a meta-analysis of the change in Japanese Orthopedic Association (JOA) score as an outcome measure to assess the overall effect size of the change at the latest follow-up and outcomes with respect to various surgical approaches/techniques. An attempt is also made to identify the factors affecting the change in JOA scores by testing various explanatory variables in the meta-regression analyses.
2. Methods
This systematic review was performed in accordance with the Cochrane Collaboration guidelines[17] and is reported in accordance with PRSIMA statement.[18]
2.1. Eligibility and meta-analysis endpoint/variables
Eligible studies of this meta-analysis were those reporting the outcomes of a surgical intervention for the treatment of cervical spondylosis due to OPLL with a follow-up duration of at least 1 year and measured change in JOA score preoperatively, after surgery, and at latest follow-up.
Meta-analysis endpoint (outcome measure) was the change in JOA score from baseline through latest follow-up. For meta-regression analyses, the change in JOA score at the latest follow-up (dependent variable) was tested to evaluate the relationships with several explanatory variables, including the number of subjects in the study, follow-up duration, year of publication, age of subjects at the time of surgery, gender (percentage of males), number of spondylotic levels involved in surgery, duration of surgery, blood loss during surgery, hospital stay duration, preoperative JOA score, and preoperative lordosis.
2.2. Data acquisition
For the acquisition of data, literature search was undertaken in electronic databases (Embase, Google Scholar, Ovid SP, PubMed/Medline, and ASI Web of Science). Medical Subject Headings (MeSH) and keywords specific to this research were used as different combinational phrases. For primary search, “ossification of posterior longitudinal ligament-cervical spondylosis surgery” combination was used. For secondary searches, several MeSH and keywords, including range of motion (ROM), lordosis, myelopathy, radiculopathy, arthroplasty, corpectomy, discectomy, laminectomy, and laminoplasty were used in combination with primary phrase. Search encompassed original research articles published in English language. Bibliographies of retrieved research articles and software indicated corroborations were also considered.
2.3. Data and analyses
Relevant data pertaining to the patients’ demography, clinical and orthopedic characteristics, surgery type/technique and device, study population size and follow-up duration, study design and analytical methods, outcome assessment tools and outcomes, and adverse events were obtained from the retrieved research articles and organized in specialized datasheets.
For the estimation of effect size of change in JOA score, data were either extracted raw from the respective research articles or calculated by using latest follow-up and baseline values. Inverse variance weighted effect sizes of overall (all surgical interventions) and subgroups (various surgery types) were obtained under random effects meta-analyses that were carried out with Stata software (version 12; Stata Corporation, College Station, TX). Subgroup analyses were carried out with regards to surgery type, age at surgery, and follow-up (JOA outcomes: postoperative vs latest follow-up).
Meta-regression analyses were also carried out with Stata software by using restricted maximum likelihood method. A P value of less than .05 was considered to show a significant relationship. Between-study variance was tested with tau2 index and the percentage of between-study heterogeneity (outcome inconsistency) was assessed with I2 index. For the assessment of publication bias, Begg funnel plot and Egger precision plot were examined and trim and fill method was used to estimate the number of missing studies.
3. Results
Twenty-three studies[19–41] reporting surgical interventions in 1576 patients were selected by following the eligibility criteria (Fig. 1). This patient population was followed for 52.60 (95% confidence interval (95% CI) 42.54–62.65] months after surgery. Average age (weighted) was 57.83 (95% CI 55.98–59.68) years and 73 [70–76] % were males. There was no significant publication bias as assessed with Begg test (adjusted Kendall score = − 68; P = .202; Fig. 2A) and Egger test (coefficient bias = − 1.884; P = .201; Fig. 2B) as well as with trim and fill method.
Figure 1.
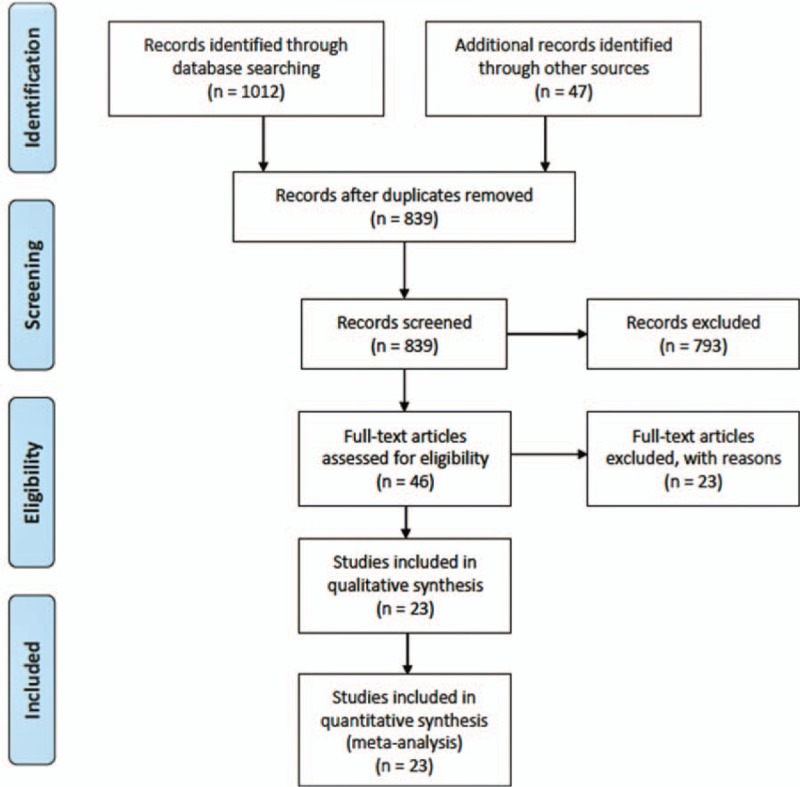
A flowchart of the study screening and selection process.
Figure 2.
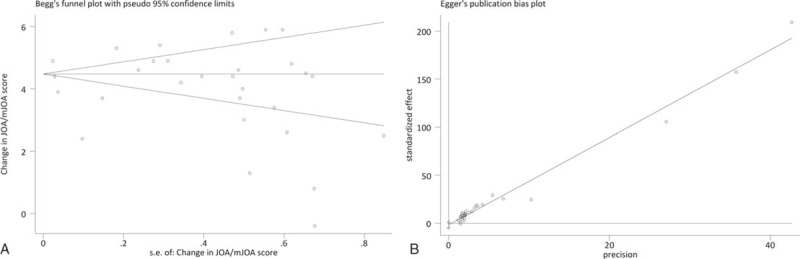
Graphs showing the outcomes of publication bias tests indicative of the absence of a significant publication bias. (A) Begg test's funnel plot, and (B) Egger test precision plot. Both tests were subjected to the meta-analysis of the change in JOA score at latest follow-up.
Overall, surgical interventions significantly (P < .00001) improved JOA score at the latest follow-up (Fig. 3). Anterior surgical approaches involving corpectomy and/or discectomy were associated with an effect size of 4.80 [4.10–5.50], whereas laminectomy and laminoplasty were associated with an improvement of the JOA score of 3.57 [2.39–4.75] and 3.99 [3.65–4.32], respectively. In the subgroup analyses, JOA scores after surgery [4.21 (3.1–5.31)] and at last follow-up [4.04 (3.75–4.32)] were not significantly different.
Figure 3.
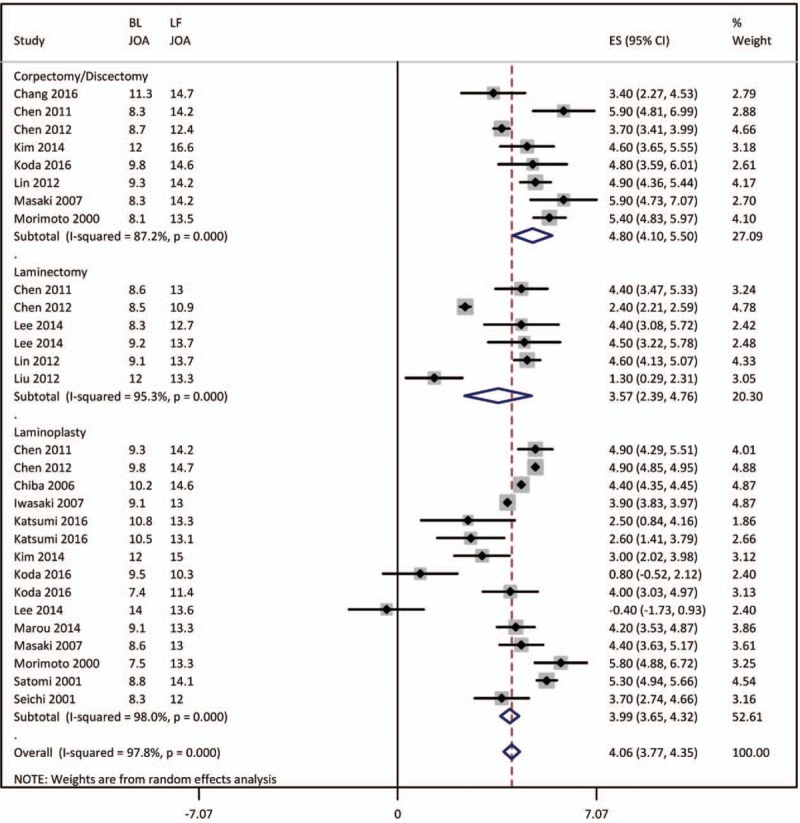
Forest graph showing the overall effect size of the meta-analysis of the improvement in JOA score at the latest follow-up along with subgroup effect sizes.
In the meta-regression analyses, preoperative JOA score was significantly inversely related to the change in JOA score at latest follow-up (coefficient: −0.605; P < .00001). Independently, the year of publication was significantly inversely associated with the change in JOA score at latest follow-up (coefficient: −0.13141; P = .009). But in the multivariate meta-regression by including preoperative JOA score and follow-up duration, the year of publication had no significant association, but preoperative JOA was still significantly associated with the change in JOA score at latest follow-up. However, the year of publication was also negatively correlated with follow-up duration and the number of subjects in a study (correlation coefficients: −0.444; P = .007 and −0.51; P = .001, respectively).
None of the other explanatory variables tested, including follow-up duration, sample size, age, gender, number of levels involved, surgery duration, and blood volume loss during surgery, were significantly associated with the change in JOA score at the latest follow-up.
Surgical interventions did not significantly affect cervical lordosis at latest follow-up (Fig. 4). Anterior surgical approaches involving corpectomy and/or discectomy were associated with an effect size of the change in lordosis of −0.21 [−5.28 to 4.86]; P = .935 degrees, whereas laminectomy and laminoplasty were associated with a change in lordosis of −1.27 [−6.63 to 3.82]; P = .625 and −1.89 [−5.42 to 1.63]; P = .293 degrees, respectively.
Figure 4.
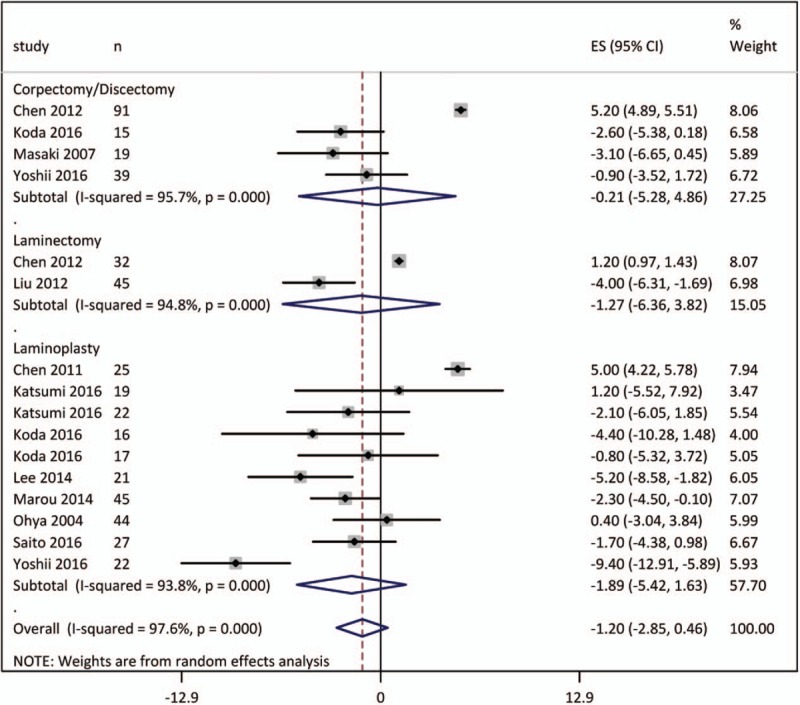
Forest graph showing the overall effect size of the meta-analysis of the change lordosis angle at the latest follow-up along with subgroup effect sizes.
In the meta-regression analyses, none of the other explanatory variables tested, including follow-up duration, sample size, age, gender, number of levels involved, preoperative JOA score, preoperative lordosis, surgery duration, and blood volume loss during surgery, were significantly associated with the change in lordosis at the latest follow-up.
4. Discussion
Surgical interventions for the treatment of cervical spondylosis due to OPLL are found to be associated with significantly improved JOA score at latest follow-up (average 55 months), which was significantly inversely associated with baseline (preoperative) JOA score. The number of levels involved in surgery, although had a negative relationship, but were not significantly associated with the change in JOA score at the latest follow-up (coefficient: −0.282; P = .245). Similarly, preoperative lordosis was also negatively but not statistically significantly (coefficient: −3.587; P = .165) associated with the change in JOA score at latest follow-up. Moreover, surgery did not significantly affect the cervical lordosis.
These results show that surgical interventions were associated with more improvement in patients with relatively lower preoperative JOA score suggesting that the capacity of improvement in JOA score was less in patients with better preoperative JOA score. Surgical interventions are also found to be beneficial irrespective of the number of cervical levels or the follow-up duration, although a nonsignificant negative relationship has been observed for both these explanatory variables, which make it necessary to confirm these results in larger datasets in future.
Majority of the included studies of this meta-analysis reported JOA outcomes at latest follow-up and there was no significant difference between JOA scores after surgery (studies which reported) and at last follow-up [4.21 (3.1–5.31) vs 4.04 (3.75–4.32)]. Also, there was no evidence of change of JOA score with increasing duration of follow-up as seen in the meta-regression analyses [coefficient 0.088 (−0.086 to 0.262); P = .293].
Our results also suggest that in recent years, there is a trend toward a preference of earlier surgical treatment to cervical spondylosis due to OPLL, as the year of publication (2000 through 2016) was significantly inversely associated with the change in JOA score at latest follow-up (more recent years are associated with less change in JOA score). This trend may be due to advancement in the surgical techniques and better prognosis in recent years. But in the multivariate meta-regression with preoperative JOA score, study population size, and follow-up duration, only preoperative JOA was significantly inversely associated with the change in JOA score at latest follow-up. Moreover, more recent studies are also relatively smaller, as the year of publication was negatively correlated with follow-up duration and the number of subjects in a study.
In the present study, age was also not found to be associated with the change in JOA score in the meta-regression analysis. Subgroup analysis by stratifying JOA scores as over and under median age subgroups also did not reveal a meaningful difference. However, majority of the patients in this sample population were below 60 years of age. Only 2 studies recruited relatively older patients (Maruo et al;[31] age 66.9 ± 8.6 years; change in JOA 4.2 ± 2.3 and Saito et al;[36] age 67.1 ± 8.2 years; change in JOA: 3.9 ± 1.8), but outcomes were not much different from those of others.
We have also found that there was no notable change in lordosis after surgery. Lordosis of the cervical spine has clinical implications,[42] as moderate postoperative cervical lordotic curvature is reported to be associated with better surgical outcomes.[43,44] Therefore, reattainment of cervical lordosis is also considered as an important outcome of surgery that have preventive benefits for possible compression of nervous tissue and consequent injury.[45] For successful cervical laminectomy or laminoplasty, it is thought that the presence of preoperative lordosis angle of at least 10° and the preservation of at least 50% facet joints is necessary.[46,47] This factor may explain the relatively lower improvement in JOA score observed in the present study in laminectomy and laminoplasty subjects, as overall preoperative lordosis angle in this patient population was 5.34 [95% CI: 4.13–6.54] degrees.
In several regions of the world, OPLL is recognized as an important pathological factor for cervical compressive myelopathy.[48,49] Several unique pathological characteristics of OPLL make the choice of surgical approach debatable. When OPLL affects multilevel cervical spine, anterior decompression involving corpectomy or discectomy and fusion yields direct decompression of the spinal cord,[50,51] enables reconstruction of the lordotic alignment,[52] prevents delayed neurologic deterioration,[53] and permits easier achievement of cervical stability with instrumented arthrodesis.[54,55] However, there are also some disadvantages of surgeries involving multilevel corpectomy/discectomy such as increased incidence of adjacent-segment degeneration, graft dislodgment, and complications, including neck stiffness, hoarseness, dysphagia, and pseudomeningocele.[56–58]
Alternatively, a posterior approach such as laminoplasty can also cope with multi-segmental OPLL and comorbid developmental spinal canal stenosis. Nevertheless, there can be limited surgical outcomes after laminoplasty with risk of kyphotic cervical alignment,[59,60] the spinal canal occupation,[61,62] reossification,[62–64] and hypermobility of the cervical spine. [65,66] Laminoplasty has been advocated because of its preservation of neck ROM compared with laminectomy with fusion. However, OPLL is different from other etiological factors of myelopathy with respect to neck ROM that may incite further progression of OPLL.[67] In general, laminoplasty results in long-term decrease in ROM due to unintended autofusion along the lateral margins of the laminoplasty.[4] It is suggested that progression of OPLL is stimulated by the dynamic factors and ROM stabilization may reduce the progression of OPLL.[22,68] Because posterior instrumented fusion avoids postoperative cervical instability, it can be a better alternative.[20]
Among the limitations of the present study, high statistical heterogeneity and less availability of associational data are important considerations. Data regarding ethnicity, education, weight, height, and body mass index (BMI) of the subjects were not sufficiently available in the respective research articles that could further provide associational findings. Moreover, in the included studies of this meta-analysis, only 4 studies measured and reported NDI scores and none of these studies provided Nurick Scale score and therefore it was not possible to have a comparative account of these outcome variables along with JOA score.
5. Conclusion
Surgical interventions for the management of cervical spondylosis due to OPLL significantly improves JOA score without affecting cervical lordosis, as observed at the latest follow-up. The change in JOA score was significantly inversely associated with preoperative JOA score. There may be a trend toward earlier treatment for cervical spondylosis due to OPLL in more recent years that may be attributed to improved diagnostic and surgical techniques.
Acknowledgment
We acknowledge the support and input from The First Affiliated Hospital of Kunming Medical University and Mindong Hospital Affiliated to Fujian Medical University.
Footnotes
Abbreviations: CI = confidence interval, CT = computed tomography, JOA = Japanese Orthopedic Association, MeSH = medical subject heading, OPLL = ossification of posterior longitudinal ligament, ROM = range of motion.
DW conceptualized and designed the study; CZL and HY extracted and analyzed data, and drafted the manuscript; HL and NC provided technical support in the analyses and writing. All authors agree to be accountable for all aspects of the work.
Ethical approval and informed consents were not required because we neither collected patients’ information nor influenced the patient care.
The authors report no conflicts of interest.
References
- [1].Matsunaga S, Sakou T. Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine (Phila Pa 1976) 2012;37:E309–14. [DOI] [PubMed] [Google Scholar]
- [2].Fujimori T, Watabe T, Iwamoto Y, et al. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: results of whole spine CT scans in 1500 Japanese patients. Spine (Phila Pa 1976) 2016;41:1668–76. [DOI] [PubMed] [Google Scholar]
- [3].Pham MH, Attenello FJ, Lucas J, et al. Conservative management of ossification of the posterior longitudinal ligament. A review. Neurosurg Focus 2011;30:E2. [DOI] [PubMed] [Google Scholar]
- [4].Fragen KM, Cox JB, Hoh DJ. Does ossification of the posterior longitudinal ligament progress after laminoplasty? Radiographic and clinical evidence of ossification of the posterior longitudinal ligament lesion growth and the risk factors for late neurologic deterioration. J Neurosurg Spine 2007;17:512–24. [DOI] [PubMed] [Google Scholar]
- [5].Ijiri K, Sakou T, Taketomi E, et al. Epidemiological Study of Ossification of Posterior Longitudinal Ligament in Utah. Investigation Committee 1995 report on the ossification of the spinal ligaments of the Japanese Ministry of Public Health and Welfare. 1996;Tokyo, Japan: Springer–Verlag, 24–5. [Google Scholar]
- [6].Ren Y, Liu ZZ, Feng J, et al. Association of a BMP9 haplotype with ossification of the posterior longitudinal ligament (OPLL) in a Chinese population. PLoS One 2012;7:e40587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ren Y, Feng J, Liu ZZ, et al. A new haplotype in BMP4 implicated in ossification of the posterior longitudinal ligament (OPLL) in a Chinese population. J Orthop Res 2012;30:748–56. [DOI] [PubMed] [Google Scholar]
- [8].Tanaka T, Ikari K, Furushima K, et al. Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet 2003;73:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nakajima M, Takahashi A, Tsuji T, et al. Genetic Study Group of Investigation Committee on Ossification of the Spinal Ligaments, Ikegawa S. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat Genet 2014;46:1012–6. [DOI] [PubMed] [Google Scholar]
- [10].Sohn S, Chung CK. Increased bone mineral density and decreased prevalence of osteoporosis in cervical ossification of the posterior longitudinal ligament: a case-control study. Calcif Tissue Int 2013;92:28–34. [DOI] [PubMed] [Google Scholar]
- [11].An HS, Al-Shihabi L, Kurd M. Surgical treatment for ossification of the posterior longitudinal ligament in the cervical spine. J Am Acad Orthop Surg 2014;22:420–9. [DOI] [PubMed] [Google Scholar]
- [12].Epstein N. Anterior approaches to cervical spondylosis and ossification of the posterior longitudinal ligament: review of operative technique and assessment of 65 multilevel circumferential procedures. Surg Neurol 2001;55:313–24. [DOI] [PubMed] [Google Scholar]
- [13].Mizuno J, Nakagawa H. Ossified posterior longitudinal ligament: management strategies and outcomes. Spine J 2006;6:282–8. [DOI] [PubMed] [Google Scholar]
- [14].Shinomiya K, Okamoto A, Kamikozuru M, et al. An analysis of failures in primary cervical anterior spinal cord decompression and fusion. J Spinal Disord 1993;6:277–88. [DOI] [PubMed] [Google Scholar]
- [15].Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg 2002;96(Suppl 2):180–9. [PubMed] [Google Scholar]
- [16].Matsunaga S, Sakou T, Taketomi E, et al. Clinical course of patients with ossification of the posterior longitudinal ligament: a minimum 10-year cohort study. J Neurosurg 2004;100(Suppl 3):245–8. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.3. Available at: http://handbook.cochrane.org/, accessed Jun 8, 2017. [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [19].Chang HC, Tu TH, Chang HK, et al. Hybrid corpectomy and disc arthroplasty for cervical spondylotic myelopathy caused by ossification of posterior longitudinal ligament and disc herniation. World Neurosurg 2016;95:22–30. [DOI] [PubMed] [Google Scholar]
- [20].Chen Y, Guo Y, Lu X, et al. Surgical strategy for multilevel severe ossification of posterior longitudinal ligament in the cervical spine. J Spinal Disord Tech 2011;24:24–30. [DOI] [PubMed] [Google Scholar]
- [21].Chen Y, Liu X, Chen D, et al. Surgical strategy for ossification of the posterior longitudinal ligament in the cervical spine. Orthopedics 2012;35:e1231–7. [DOI] [PubMed] [Google Scholar]
- [22].Chiba K, Ogawa Y, Ishii K, et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy: average 14-year follow-up study. Spine (Phila Pa 1976) 2006;31:2998–3005. [DOI] [PubMed] [Google Scholar]
- [23].Houten JK, Cooper PR. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery 2003;52:1081–7. [PubMed] [Google Scholar]
- [24].Iwasaki M1, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 1: clinical results and limitations of laminoplasty. Spine (Phila Pa 1976) 2007;32:647–53. [DOI] [PubMed] [Google Scholar]
- [25].Katsumi K, Izumi T, Ito T, et al. Posterior instrumented fusion suppresses the progression of ossification of the posterior longitudinal ligament: a comparison of laminoplasty with and without instrumented fusion by three-dimensional analysis. Eur Spine J 2016;25:1634–40. [DOI] [PubMed] [Google Scholar]
- [26].Kim B, Yoon DH, Shin HC, et al. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament. Spine J 2015;15:875–84. [DOI] [PubMed] [Google Scholar]
- [27].Koda M, Mochizuki M, Konishi H, et al. Comparison of clinical outcomes between laminoplasty, posterior decompression with instrumented fusion, and anterior decompression with fusion for K-line (-) cervical ossification of the posterior longitudinal ligament. Eur Spine J 2016;25:2294–301. [DOI] [PubMed] [Google Scholar]
- [28].Lee CH, Jahng TA, Hyun SJ, et al. Expansive laminoplasty versus laminectomy alone versus laminectomy and fusion for cervical ossification of the posterior longitudinal ligament: is there a difference in the clinical outcome and sagittal alignment? Clin Spine Surg 2016;29:E9–15. [DOI] [PubMed] [Google Scholar]
- [29].Lin D, Ding Z, Lian K, et al. Cervical ossification of the posterior longitudinal ligament: anterior versus posterior approach. Indian J Orthop 2012;46:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu B, Ma W, Zhu F, et al. Comparison between anterior and posterior decompression for cervical spondylotic myelopathy: subjective evaluation and cost analysis. Orthop Surg 2012;4:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maruo K, Moriyama T, Tachibana T, et al. The impact of dynamic factors on surgical outcomes after double-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine 2014;21:938–43. [DOI] [PubMed] [Google Scholar]
- [32].Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech 2007;20:7–13. [DOI] [PubMed] [Google Scholar]
- [33].Morimoto T, Uranishi R, Nakase H, et al. Extensive cervical laminoplasty for patients with long segment OPLL in the cervical spine: an alternative to the anterior approach. J Clin Neurosci 2000;7:217–322. [DOI] [PubMed] [Google Scholar]
- [34].Ogawa Y, Toyama Y, Chiba K, et al. Long-term results of expansive open-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine 2004;1:168–74. [DOI] [PubMed] [Google Scholar]
- [35].Ohya J, Oshima Y, Oka H, et al. Patient satisfaction with posterior decompression surgery for cervical ossification of the posterior longitudinal ligament: prognostic radiographic factors and patient-reported outcomes for the effectiveness of surgical treatment. World Neurosurg 2016;96:272–9. [DOI] [PubMed] [Google Scholar]
- [36].Saito J, Maki S, Kamiya K, et al. Outcome of posterior decompression with instrumented fusion surgery for K-line (-) cervical ossification of the longitudinal ligament. J Clin Neurosci 2016;32:57–60. [DOI] [PubMed] [Google Scholar]
- [37].Sakai K, Okawa A, Takahashi M, et al. Five-year follow-up evaluation of surgical treatment for cervical myelopathy caused by ossification of the posterior longitudinal ligament: a prospective comparative study of anterior decompression and fusion with floating method versus laminoplasty. Spine (Phila Pa 1976) 2012;37:367–76. [DOI] [PubMed] [Google Scholar]
- [38].Satomi K, Ogawa J, Ishii Y, et al. Short-term complications and long-term results of expansive open-door laminoplasty for cervical stenotic myelopathy. Spine J 2001;1:26–30. [DOI] [PubMed] [Google Scholar]
- [39].Seichi A, Takeshita K, Ohishi I, et al. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine (Phila Pa 1976) 2001;26:479–87. [DOI] [PubMed] [Google Scholar]
- [40].Yoshii T, Sakai K, Hirai T, et al. Anterior decompression with fusion versus posterior decompression with fusion for massive cervical ossification of the posterior longitudinal ligament with a ≥50% canal occupying ratio: a multicenter retrospective study. Spine J 2016;16:1351–7. [DOI] [PubMed] [Google Scholar]
- [41].Zhang T, Guo Y, Hu N, et al. Segmental subtotal corpectomy and reconstruction with titanium cage and anterior plate for multilevel ossification of the posterior longitudinal ligament. Orthopedics 2016;39:e1140–6. [DOI] [PubMed] [Google Scholar]
- [42].Kawakami M, Tamaki T, Yoshida M, et al. Axial symptoms and cervical alignments after cervical anterior spinal fusion for patients with cervical myelopathy. J Spinal Disord 1999;12:50–6. [PubMed] [Google Scholar]
- [43].Baba H, Uchida K, Maezawa Y, et al. Lordotic alignment and posterior migration of the spinal cord following en bloc open-door laminoplasty for cervical myelopathy: a magnetic resonance imaging study. J Neurol 1996;85:626–32. [DOI] [PubMed] [Google Scholar]
- [44].Stein J. Failure of magnetic resonance imaging to reveal the cause of a progressive cervical myelopathy related to postoperative spinal deformity: a case report. Am J Phys Med Rehabil 1997;76:73–5. [DOI] [PubMed] [Google Scholar]
- [45].Harrison DE, Cailliet R, Harrison DD, et al. A new 3-point bending traction method for restoring cervical lordosis and cervical manipulation: a nonrandomized clinical controlled trial. Arch Phys Med Rehabil 2002;83:447–53. [DOI] [PubMed] [Google Scholar]
- [46].McAllister BD, Rebholz BJ, Wang JC. Is posterior fusion necessary with laminectomy in the cervical spine? Surg Neurol Int 2012;3(Suppl 3):S225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Epstein NE. Laminectomy for cervical myelopathy. Spinal Cord 2003;41:317–27. [DOI] [PubMed] [Google Scholar]
- [48].Fujimori T, Le H, Hu SS, et al. Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: a CT-based study. Spine (Phila Pa 1976) 2015;40:E394–403. [DOI] [PubMed] [Google Scholar]
- [49].Kalb S, Martirosyan NL, Perez-Orribo L, et al. Analysis of demographics, risk factors, clinical presentation, and surgical treatment modalities for the ossified posterior longitudinal ligament. Neurosurg Focus 2011;30:E11. [DOI] [PubMed] [Google Scholar]
- [50].Matz PG, Pritchard PR, Hadley MN. Anterior cervical approach for the treatment of cervical myelopathy. Neurosurgery 2007;60(1 Supp1 1):S64–70. [DOI] [PubMed] [Google Scholar]
- [51].Sakai K, Okawa A, Takahashi M, et al. Five-year follow-up evaluation of surgical treatment for cervical myelopathy caused by ossification of the posterior longitudinal ligament: a prospective comparative study of anterior decompression and fusion with floating method versus laminoplasty. Spine (Phila Pa 1976) 2012;37:367–76. [DOI] [PubMed] [Google Scholar]
- [52].Liu K, Shi J, Jia L, et al. Surgical technique: hemilaminectomy and unilateral lateral mass fixation for cervical ossification of the posterior longitudinal ligament. Clin Orthop Relat Res 2013;471:2219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yonenobu K, Okada K, Fuji T, et al. Causes of neurologic deterioration following surgical treatment of cervical myelopathy. Spine (Phila Pa 1976) 1986;11:818–23. [DOI] [PubMed] [Google Scholar]
- [54].Liu X, Min S, Zhang H, et al. Anterior corpectomy versus posterior laminoplasty for multilevel cervical myelopathy: a systematic review and meta-analysis. Eur Spine J 2014;23:362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fraser JF, Hartl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine 2007;6:298–303. [DOI] [PubMed] [Google Scholar]
- [56].Cardoso MJ, Koski TR, Ganju A, et al. Approach-related complications after decompression for cervical ossification of the posterior longitudinal ligament. Neurosurg Focus 2011;30:E12. [DOI] [PubMed] [Google Scholar]
- [57].Kimura A, Seichi A, Hoshino Y, et al. Perioperative complications of anterior cervical decompression with fusion in patients with ossification of the posterior longitudinal ligament: a retrospective, multiinstitutional study. J Orthop Sci 2012;17:667–72. [DOI] [PubMed] [Google Scholar]
- [58].Xu R, Bydon M, Macki M, et al. Adjacent segment disease after anterior cervical discectomy and fusion: clinical outcomes after first repeat surgery versus second repeat surgery. Spine (Phila Pa 1976) 2014;39:120–6. [DOI] [PubMed] [Google Scholar]
- [59].Liu H, Li Y, Chen Y, et al. Cervical curvature, spinal cord MRIT2 signal, and occupying ratio impact surgical approach selection in patients with ossification of the posterior longitudinal ligament. Eur Spine J 2013;22:1480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Matsumoto M, Chiba K, Toyama Y. Surgical treatment of ossification of the posterior longitudinal ligament and its outcomes: posterior surgery by laminoplasty. Spine (Phila Pa 1976) 2012;37:E303–8. [DOI] [PubMed] [Google Scholar]
- [61].Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament, part 1: clinical results and limitations of laminoplasty. Spine (PhilaPa 1976) 2007;32:647–53. [DOI] [PubMed] [Google Scholar]
- [62].Kim B, Yoon DH, Shin HC, et al. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament. Spine J 2015;15:875–84. [DOI] [PubMed] [Google Scholar]
- [63].Kawaguchi Y, Kanamori M, Ishihara H, et al. Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am 2001;83:1798–802. [DOI] [PubMed] [Google Scholar]
- [64].Tokuhashi Y, Ajiro Y, Umezawa N. A patient with two resurgeries for delayed myelopathy due to progression of ossification of the posterior longitudinal ligaments after cervical laminoplasty. Spine (Phila Pa 1976) 2009;34:E101–5. [DOI] [PubMed] [Google Scholar]
- [65].Li H, Jiang LS, Dai LY. A review of prognostic factors for surgical outcome of ossification of the posterior longitudinal ligament of cervical spine. Eur Spine J 2008;17:1277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Maruo K, Moriyama T, Tachibana T, et al. The impact of dynamic factors on surgical outcomes after double-door laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine 2014;21:938–43. [DOI] [PubMed] [Google Scholar]
- [67].Singhatanadgige W, Limthongkul W, Valone F, 3rd, et al. Outcomes following laminoplasty or laminectomy and fusion in patients with myelopathy caused by ossification of the posterior longitudinal ligament: a systematic review. Global Spine J 2016;6:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fujimori T, Iwasaki M, Nagamoto Y, et al. Three-dimensional measurement of growth of ossification of the posterior longitudinal ligament. J Neurosurg Spine 2012;16:289–895. [DOI] [PubMed] [Google Scholar]


