Abstract
The aim of this study is to determine the contribution of neck and chest 99mTc-pertechnetate scan to the management of postoperative patients with suspicious metastatic differentiated thyroid cancer (DTC), particularly to the prediction of response to radioiodine (131I) therapy. Just before 131I administration, a total of 184 postoperative DTC patients with stimulated serum thyroglobulin (ssTg) >10 ng/mL were enrolled to undergo neck and chest 99mTc-pertechnetate scan, which were directly compared with post-therapeutic 131I scan to determine the concordance of site and number of metastatic lesions. The percentage changes in ssTg between 99mTc-pertechnetate-avid group and 99mTc-pertechnetate-nonavid group were compared, and the response to 131I in both groups was analyzed according to the nature of 99mTc-pertechnetate avidity as well. The percentages of concordance between 99mTc-pertechnetate and 131I scan in detecting metastases were 65.7% and 26.0% in per-patient and per-site analyses with low unweighted kappa, respectively. 99mTc-pertechnetate scan led to a change in therapeutic decision making in 19/184 (10.3%) patients. In 72 patients with 131I-avid metastases, the ssTg in 99mTc-pertechnetate-avid group (n = 13) decreased significantly compared with that in 99mTc-pertechnetate-nonavid group (n = 59) (median: −81.56% vs −48.14%; Z = −4.276, P = .000). The difference of therapeutic response between 99mTc-pertechnetate-avid group and 99mTc-pertechnetate-nonavid group was statistically significant (χ2 = 8.4; P = .03). Although the consistency between 99mTc-pertechnetate scan before 131I administration and post-therapy 131I scan in detecting metastases is low, identifying metastases in postoperative DTC patients with elevated ssTg via 99mTc-pertechnetate scan prior to 131I therapy provides incremental value for therapeutic decision making. Notably, patients with 99mTc-pertechnetate-avid metastases may be more prone to benefit from 131I therapy than those with 99mTc-pertechnetate-nonavid metastases.
Keywords: 99mTc-pertechnetate, differentiated thyroid cancer, radioiodine, SPECT/CT, thyroglobulin
1. Introduction
Differentiated thyroid cancers (DTCs) are typically iodine-avid and can be effectively treated with radioiodine (131I) when persistent/recurrent or metastatic disease are not amenable to surgery.[1–4] In this context, a diagnostic 131I whole body scan (Dx-WBS) may be useful to detect iodine-avid metastases before 131I therapy, allowing a more precise selection of therapeutic candidate and appropriate activity of 131I. If metastases are detected in Dx-WBS, then the prescribed 131I activity can be increased to maximize therapeutic success at first 131I treatment.[5–7]
However, specialists who favor post-therapeutic 131I whole body scan (Rx-WBS) argue that scans performed after therapeutic activity of 131I administration are more sensitive for disease detection and avoid possible stunning of thyroid remnants and distant metastases from thyroid cancer.[8–12] Until now the real mechanism of stunning effect has not been known. Some researchers also found molecular-level evidence that the stunning effect exists even at a low activity of 131I, which further hampered the use of Dx-WBS before 131I therapy.[13] Rx-WBS possibly neglect sufficient radiation activity to metastatic lesions during the first course of 131I therapy since disease was not determined timely.[14]
99mTc-pertechnetate has been widely applied to evaluate thyroid diseases because of its ideal imaging characteristics and favorable price and dosimetry. Pertechnetate-avid metastatic lesions from DTC have been described by several case reports.[15–18] However, only 3 comparative studies with 131I can be retrieved, mainly focusing on the diagnostic efficiency of 99mTc-pertechnetate scan for thyroid remnant.[19–21] To date, the value of 99mTc-pertechnetate scan (planar scan combined with SPECT/CT [single photon emission computed tomography/computed tomography]) in the management of DTC patients has not been adequately assessed. The relationship between the therapeutic response to 131I and the avidity of metastatic lesions for 99mTc-pertechnetate is still unknown. Here, we therefore, designed a dedicated study to determine the contribution of neck and chest 99mTc-pertechnetate scan to the management of postoperative DTC patients with suspicious metastatic lesions.
2. Subjects and methods
2.1. Study design and population
This study was approved by the ethics board of Shanghai Jiao Tong University Affiliated Sixth People's Hospital before its initiation (No. 2014-–0013). All participants were informed of details of the study with the information sheet and had signed the consent form prior to their inclusion in the study.
We prospectively evaluated 184 consecutive patients with DTC who were considered to have suspicious metastatic disease after total or nearly total thyroidectomy (≥4 weeks) based on elevated stimulated serum thyroglobulin (ssTg) levels >10 ng/mL, with thyroid-stimulating hormone (TSH) level >30 mIU/mL. All patients were confirmed with lymph node (LN) metastasis by the pathology of thyroidectomy. Patients with a history of malignant tumor other than DTC were excluded from the study. Thyroxine withdrawal and low-iodine diet for at least 4 week were required before blood samples were drawn and 131I administered. Serum TSH, Tg, and Tg-antibody levels were measured using a chemiluminescent immunoassay system (Immulite, Diagnostic Products Corp., Los Angeles, CA). Empirically fixed dose of 131I was given at 5.5 GBq for patients with local or suspected disease and 7.4 GBq for patients with distant metastases. All patients underwent neck and chest 99mTc-pertechnetate planar scan with SPECT/CT just before 131I administration or switch to reoperation.
2.2. Imaging protocol and image analyses
99mTc-pertechnetate planar scan in neck and chest was performed 15 min after intravenous injection of 99mTc-pertechnetate (370 MBq). The neck and chest SPECT/CT images were obtained using a hybrid SPECT/CT imaging system (Millennium VG and Hawkeye, GE Healthcare). The Rx-WBS and SPECT/CT were carried out 5 days after administration of 131I with the same technique equipped with a high-energy collimator. The detailed parameters have been described previously by our team.[22]
Two experienced nuclear medicine physicians, who were aware of the results of all relevant examinations, laboratory investigations, and imaging studies, evaluated the 99mTc-pertechnetate scans and came to an agreement by consensus. The Rx-WBS with SPECT/CT was reviewed by other 2 nuclear medicine physicians who did not know the previous pretreatment pertechnetate scans. Foci of radioactivity identified on planar imaging were further evaluated on SPECT/CT and classified according to their location and nature as positive scan, which was defined as anatomical finding from CT with increased radiotracer uptake higher than surrounding background excluding thyroid remnant or physiological distribution; equivocal scan, which included the following situations: abnormal anatomical localization from CT without unnatural increased radiotracer uptake or increased radiotracer uptake without corresponded anatomical finding from CT excluding thyroid remnant; and neither increased radiotracer uptake nor unnatural anatomical foci from CT excluding thyroid remnant were considered as negative scan.
Concordant rate of per-patient and per-lesion basis was analyzed and the strength of agreement was obtained using unweighted kappa statistical analysis.
2.3. Assessment during follow-up
Nearly 6 months after initial 131I therapy, patients who had 131I-avid foci in the post-therapeutic 131I scan received the second course of 131I treatment, and ssTg levels were also obtained. The ssTg before the first and second course of 131I treatment was marked as ssTg1 and ssTg2, respectively. The range of interval between ssTg1 and ssTg2 was 171 to 207 days (median: 187 days). The percentage changes in ssTg were calculated as (ssTg2 − ssTg1)/ssTg1. Patients experienced reassessment of therapeutic response to 131I with TSH suppressive therapy 6 to 12 months after the second course of 131I therapy. CT or F-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) was conducted if persistent or newly identified evidence was suspected.
According to the therapeutic response assessment system proposed by 2015 American Thyroid Association guidelines, we analyzed the clinical data obtained during follow-up and divided all patients who experienced at least 2 dose of 131I therapy into 4 categories: excellent response (ER), indeterminate response (IDR), biochemical incomplete response (BIR), and structural incomplete response (SIR) during follow-up.[2]
2.4. Statistical analyses
All statistical analyses were performed using a statistical software program (SPSS, version 16.0; SPSS, Inc Chicago, IL). The parameters such as age at diagnosis, sex, subtype of DTC, 131I activity, ssTg level, and the percentage change in ssTg were analyzed by univariate analysis and confirmed by the χ2 test or Wilcoxon rank sum tests or Student t test. The correlation of the percentage changes in ssTg between pertechnetate-avid group and pertechnetate-nonavid group were evaluated using the Spearman rank correlation. Fisher exact test was used to test the statistical significance of categorical data. All P values reported were 2-sided, and a P value of less than .05 was considered significant.
3. Results
3.1. Patient demographic and baseline characteristics
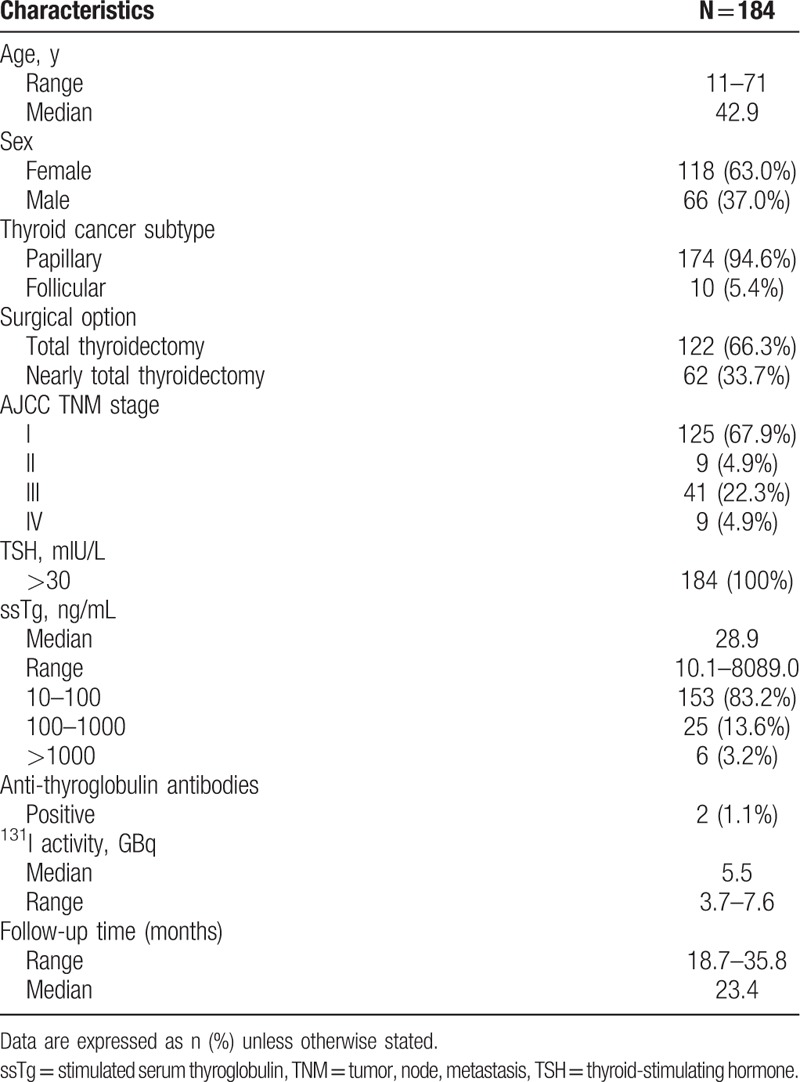
Patient demographic and baseline characteristics are summarized in Table 1. Seventy-four (33.2%) of 184 patients experienced 131I therapy 2 times or more. The duration of follow-up was a minimum of 18.7 months, and a maximum of 35.8 months after the first 131I therapy. Tg levels are between 10.1 and 8089.0 ng/mL (median 28.9 ng/mL).
Table 1.
Patient demographic and baseline characteristics.
3.2. Findings in 99mTc-pertechnetate planar scans combined with SPECT/CT
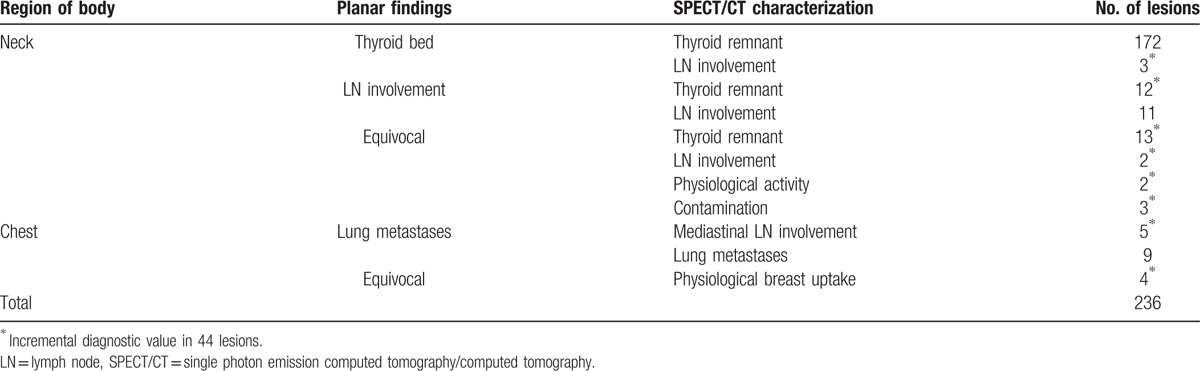
In the 184 patients, 236 foci avid for 99mTc-pertechnetate were observed on planar scans. 99mTc-pertechnetate SPECT/CT led to a revision of the original diagnosis based on planar imaging in 44 of 236 foci (18.6%) (Table 2). In particular, 12 of 23 lesions considered to be LN and 22 of 24 lesions considered to be indeterminate were clearly reclassified as benign. Three lesions classified as thyroid remnant and 2 equivocal lesions by planar scan had been reclassified as LNs. Furthermore, 99mTc-pertechnetate SPECT/CT allowed the identification of 5 mediastinal LNs misinterpreted as metastatic lung nodes.
Table 2.
Additional value of neck and chest 99mTc-pertechnetate SPECT/CT in precise diagnosis.
Besides, there were 151 equivocal foci of 68 patients on 99mTc-pertechnetate SPECT/CT distributed to 90 foci in LN, 56 foci in lung nodules, and 5 foci in bone lesions.
3.3. Comparison of 99mTc-pertechnetate and 131I scan
Final diagnostic criteria for metastases from DTC were based on pathological findings, serum Tg levels, and other imaging modalities, such as 131I scan, high-resolution ultrasonography, CT and MRI, and by correlation with clinical follow-up. Seventy-four patients (74/184, 33.2%) showed 131I-avid metastases in 131I scans, including 55 patients with only LN metastases, 5 patients with only lung metastases, and 9 patients with LN and lung metastases concurrently. Besides, 6 patients were confirmed with cervical nodal metastases by surgical pathology. There were a total of 7 patients (4 with LN metastases and 3 lung metastases) with negative metastatic foci in 131I scan diagnosed by serum Tg levels, high-resolution ultrasonography, CT, and clinical follow-up.
3.4. Per-patient analysis
For the per-patient analysis, data on the relationship between 99mTc-pertechnetate and 131I scan were compared. After exclusion of 6 patients with operable lesions identified by 99mTc-pertechnetate scan from the 184 patients in total, 13/178 (7.3%) patients showed tracer-avid metastases in both 99mTc-pertechnetate and 131I scans, 104/178 (58.4%) showed negative metastases in both imaging, and 61/178 (34.3%) showed metastases with 131I uptake but without pertechnetate uptake. The overall percentage of concordance was 65.7% (117/178). The unweighted kappa was 0.309. A total of 176 patients had identified thyroid remnants by both 99mTc-pertechnetate and 131I scan, and the remaining 2 patients who underwent total thyroidectomy did not show residual thyroid in both imaging.
In cervical LN, positive, equivocal, and negative findings by both 99mTc-pertechnetate and 131I scans were found in 11, 8, and 106 patients, respectively. Negative pertechnetate but positive or equivocal 131I findings were revealed in 27 and 26 patients, respectively. Thus, the percentage of concordance was 70.2% (125/178) with the unweighted kappa of 0.257. In lung, positive, equivocal, and negative findings by both 99mTc-pertechnetate and 131I scans were found in 2, 4, and 160 patients. There were 12 patients who showed negative (n = 8)/equivocal (n = 4) 99mTc-pertechnetate but positive 131I scan. So, the percentage of concordance was 93.2% (166/178) with the unweighted kappa of 0.518. In bone, there were only 5 patients who were defined as positive on 131I scan but equivocal on 99mTc-pertechnetate scan, yielding a percentage of concordance of 0% with the unweighted kappa of 0.
3.5. Per-site analysis
There were a total of 30 positive metastatic foci on 99mTc-pertechnetate scan. Of these, 21 foci were located in LNs (Fig. 1), 9 foci in lung nodules (Fig. 2). Altogether, there were 151 equivocal foci on 99mTc-pertechnetate scan clarified as 90 foci in LN, 56 foci in lung, and 5 foci in bone.
Figure 1.
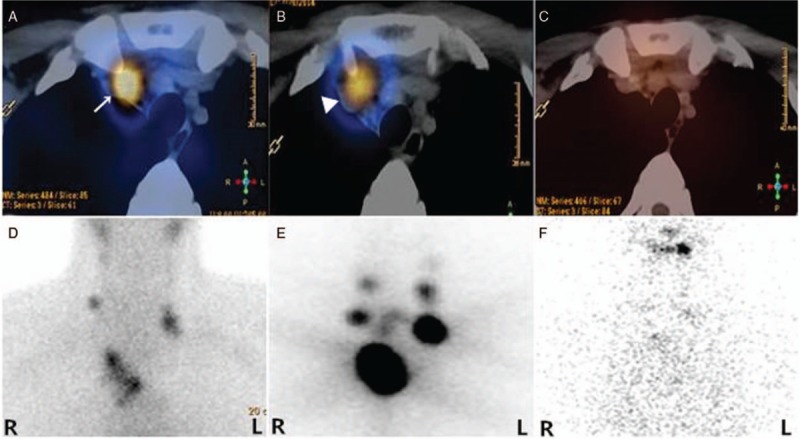
131I-avid PTC metastatic LNs accumulating 99mTc-pertechnetate. A 30-year-old male with PTC presented with suspicious metastatic disease 2 months after total thyroidectomy with elevated ssTg of 349 ng/mL and TSH of 107.83 mU/L. Transaxial image of 99mTc-pertechnetate SPECT/CT (A; arrow) and planar image (D) before the initial administration of 5.55 MBq (150 mCi) 131I followed by 131I SPECT/CT (B; arrowhead) and planar imaging (E) showed radiotracer-avid LNs in the neck and superior mediastinum. ssTg decreased to 4.48 ng/mL with TSH of 54.0 mU/L just before the third course of 131I therapy. Decreased uptake of 131I in LNs in the neck and superior mediastinum was revealed by the SPECT/CT (C) and planar image (F). LN = lymph nodes, PTC = papillary thyroid carcinoma, ssTg = stimulated serum thyroglobulin, TSH = thyroid-stimulating hormone.
Figure 2.
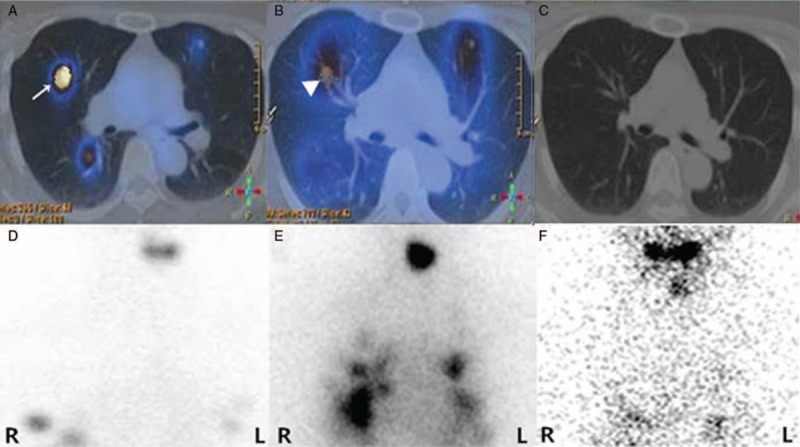
131I-avid PTC metastatic pulmonary lesions accumulating 99mTc-pertechnetate. A 50-year-old female patient with PTC presented with suspected metastatic disease in the lungs 1 month after total thyroidectomy with ssTg of 500.0 ng/mL and TSH of 77.45 mU/L. 99mTc-pertechnetate SPECT/CT (A; arrow) and planar image (D) before 131I therapy showed pulmonary nodules with obviously increased 99mTc-pertechnetate uptake. 131I SPECT/CT (B; arrowhead) and planar image (E) 5 days after the initial administration of 7.4 MBq (200 mCi) 131I correspondingly showed the pulmonary nodules with increased 131I accumulation. Six months later, ssTg decreased to 13.78 ng/mL with TSH of 37 mU/L just before the second course of 131I administration (7.4 MBq, 200 mCi), and the pulmonary nodules were not found on 131I SPECT/CT (C) and planar image (F). PTC = papillary thyroid carcinoma.
There were 164 131I-avid metastatic lesions revealed by 131I scan, including all the aforementioned 30 lesions with 99mTc-pertechnetate uptake. Positive 131I scan but equivocal 99mTc-pertechnetate scan were found in 134/164 (81.1%) lesions, with 79/134 (59.0%) foci located in LN, 50/134 (37.3%) foci located in lung, and 5/134 (3.7%) located in bone. The remaining 17 (10.7%) foci showed equivocal findings in 131I scan, including 11 foci located in LN and 6 foci located in lung.
With regard to per-site analysis, the overall percentage of concordance between 99mTc-pertechnetate and 131I scan was 26.0% (47/181) with the unweighted kappa of 0.027. The percentages of concordance in LN, lung, and bone subgroups were 28.8%, 23.1%, and 0%, respectively. The unweighted kappas in LN, lung, and bone subgroups were 0.072, 0.032, and 0, respectively.
3.6. Impact of 99mTc-pertechnetate scan on management
The information obtained at the time of 99mTc-pertechnetate scan led to a change in management decision in 19/184 (10.3%) patients. In 6/184 (3.3%) patients, 99mTc-pertechnetate SPECT/CT showed bulky and/or multiple residual cervical nodal metastases nonavid for 99mTc-pertechnetate with surgical indication, and they were then referred back to surgery for reoperative neck dissection prior to 131I therapy. Besides, 99mTc-pertechnetate SPECT/CT detected pertechnetate-nonavid regional lung and bone metastases in 4 and 5 patients, respectively, who were then given 7.4 GBq of 131I in consideration with their high Tg levels (range: 182.0–8089.0 ng/mL). 99mTc-pertechnetate scan detected pertechnetate-avid regional lung metastases in 4/184 (2.2%) patients. Accordingly, the identification of distant metastases by 99mTc-pertechnetate scan resulted in an increase of prescribed 131I activity to 7.4 GBq in the 4 patients previously recommended for 5.5 GBq.
3.7. Association between the avidity of 99mTc-pertechnetate and the therapeutic response
Following the exclusion of 2 anti-Tg antibody positive patients from the 74 patients who experienced 131I therapy 2 or more times, the parameters such as age at diagnosis, sex, subtype of DTC, 131I activity, ssTg level between pertechnetate-avid group (n = 13), and pertechnetate-nonavid group (n = 59) were analyzed, and no differences were observed. The percentage changes in ssTg levels between pertechnetate-avid group and pertechnetate-nonavid group were compared (Table 3). As a result, ssTg decreased 81.56% ± 31.22% (median ± interquartile range) in pertechnetate-avid group and 48.14% ± 44.70% (median ± interquartile range) in pertechnetate-nonavid group with statistical significance (Z = −4.276; P = .000) and correlation (r = 0.506; P = .000).
Table 3.
Comparison between 99mTc-pertechnetate-avid group and 99mTc-pertechnetate-nonavid group.
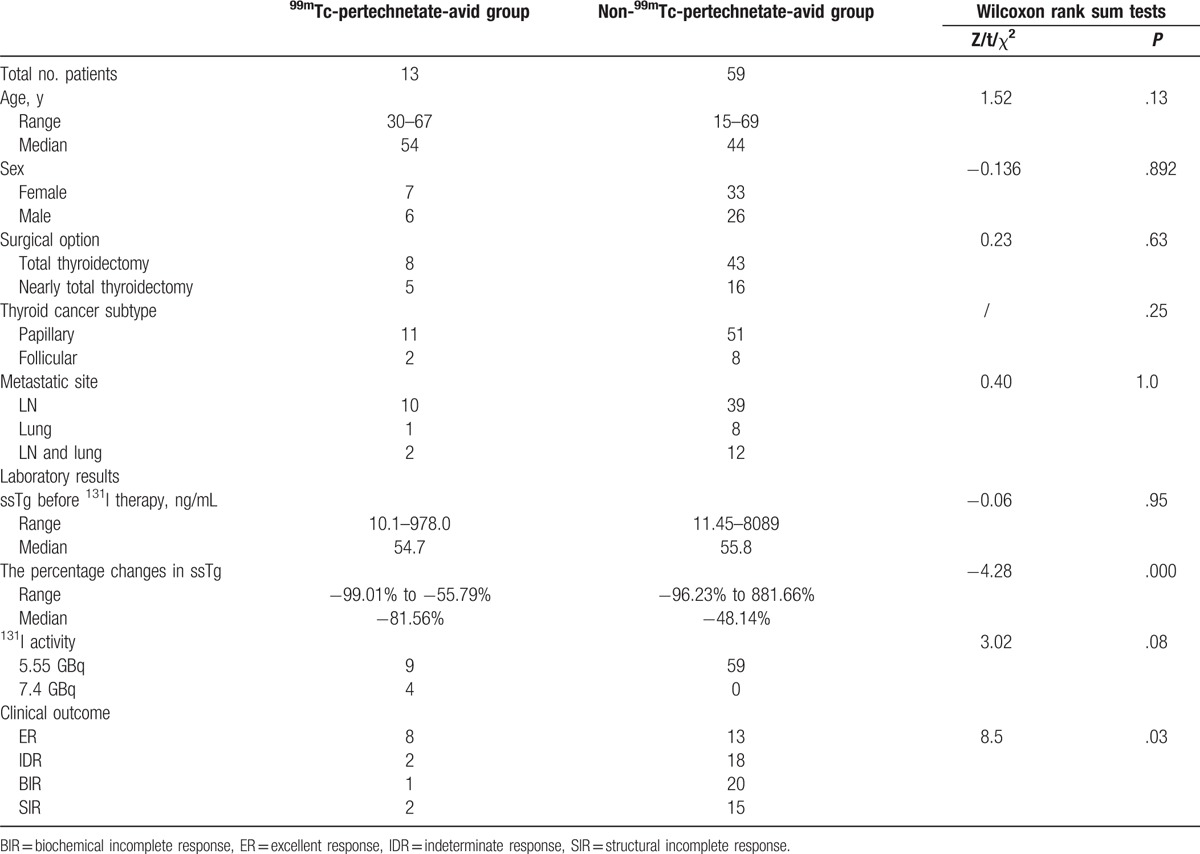
During the follow-up with a median duration of 13.4 months after the first 131I therapy, the proportion of ER, IDR, BIR, and SIR in 99mTc-pertechnetate-avid group amounted to 61.5% (8/13), 15.4% (2/13), 7.7% (1/13), and 15.4% (2/13), respectively. In 99mTc-pertechnetate-nonavid group, 21.3% (13/61), 29.5% (18/61), 32.8% (20/61), and 24.6% (15/61) of patients were categorized to ER, IDR, BIR, and SIR, respectively. The difference of therapeutic response between 99mTc-pertechnetate-avid group and 99mTc-pertechnetate-nonavid group was statistically significant (χ2 = 8.4; P = .03).
4. Discussion
The assessment of postoperative disease status has vital role in the management of advanced DTC patients.[2,23] The likelihood of identifying either loco-regional recurrent/persistent disease or distant metastases on the Rx-WBS increases as either the suppressed or ssTg values rise above 5 to 10 ng/mL.[24,25] However, the localization of metastatic DTC lesions commonly falls back on imaging modalities. Although ultrasonography is very sensitive for the identification of cervical nodal compartments metastases, the specificity is low and the prescribed regions are limited, which impedes its use in the identification of distant metastases.[26,27] Meanwhile, controversies on the utility of Dx-WBS in guiding 131I therapeutic decision making still exist, and predictors on the outcome of 131I therapy of DTC are unavailable at present.[2]
99mTc-pertechnetate is a monovalent anion similar to iodide and can be accumulated by sodium/iodide symporter located in the membrane of thyroidal cytes. Theoretically, visualization of metastatic deposits on pertechnetate scans can hint at their strong capacity to concentrate 131I and therefore assure the potential to respond to 131I therapy.[17] However, there is limited literature describing the value of pertechnetate scans in detecting metastatic disease, and its clinical implication has not been fully assessed.[16–18,20] Our group demonstrated that the information obtained at the time of 99mTc-pertechnetate scans led to a change in management decision in 19/184 (10.3%) patients, including referral for resection (n = 6) or alternating the prescribed 131I dose (n = 13). More importantly, during analysis of association between avidity of 99mTc-pertechnetate and the response of 131I therapy in 72 patients who had 131I-avid foci in the Rx-SPECT/CT, we found that ssTg in pertechnetate-avid group decreased more significantly than in pertechnetate-nonavid group. Furthermore, the difference of therapeutic response between the 2 groups was also statistically significant. It suggests that in patients with 131I-avid metastases, more favorable therapeutic response to 131I therapy may be obtained in pertechnetate-avid group compared with pertechnetate-nonavid group. This can be explained by that 99mTc-pertechnetate-avid lesions may concentrate more 131I than those nonavid for 99mTc-pertechnetate, yielding a higher radiation dose.[22,28] Besides, our study also demonstrates that the percentages of concordance between 99mTc-pertechnetate and 131I scan in detecting metastases were as low as 65.7% and 26.0% in per-patient and per-site analysis, respectively, indicating that 99mTc-pertechnetate scan cannot be regarded as an attractive alternative for evaluating suspicious metastases in postsurgical DTC patients. Several factors may be involved in the discrepancy between 99mTc-pertechnetate and 131I scan. 99mTc-pertechnetate can be concentrated by thyroid follicular cells, but do not undergo the organification process, which might cause insufficient visualization because of lower accumulation and target/background ratio and much shorter deposit time compared with 131I.[22,28,29]
Our findings on the diagnostic value of 99mTc-pertechnetate scan are inconsistent with study by Chantadisai et al, which demonstrated that preablative 99mTc-pertechnetate planar scan with neck and chest SPECT/CT has good correlation with post-therapeutic 131I planar scan with SPECT/CT per-patient basis.[20] However, our group reports a relatively lower overall patient-based or lesion-based percentage of concordance. The possible explanation is that our study focused mainly on metastatic lesions, but Chantadisai et al cared mainly about thyroid remnant (92.2%). Other studies have also revealed that postoperative 99mTc-pertechnetate scan has a high positive predictive value to detect remnant tissue in DTC patients in the literature.[19,21,29–31] Their studies were limited to scan of the thyroid bed, so no comments can be made about the value of 99mTc-pertechnetate scan in detecting extrathyroidal or metastatic sites. Additionally, a low sensitivity of 99mTc-pertechnetate scan compared with diagnostic 131I WBS in detecting extrathyroidal and metastatic disease was report in 1990 with an obvious limitation of very small sample size as 5.[32] Different from the previous reports, our study focused on the value of 99mTc-pertechnetate neck and chest planar scan combined with SPECT/CT in the management of patients with suspicious metastatic DTCs post-total or near total thyroidectomy in a much larger size of sample.
The pertechnetate avidity of metastatic deposits beyond thyroid bed determined by SPECT can provide features of lesions and has incremental value over planar scan in increasing diagnostic accuracy, reducing pitfalls, and yielding a specific diagnosis. Meanwhile, the CT component of hybrid SPECT/CT can be used to determine the location and size of metastatic lesions without radiotracer uptake.[22] Therefore, proceeding with a 99mTc-pertechnetate planar scan combined with SPECT/CT may lead to cost savings and streamlined management decisions in patients with suspicious metastases.
Inevitably, there were some limitations in our study. We did not compare 99mTc-pertechnetate scan to diagnostic scan using low activity 131I in this series directly. Besides, the value of 99mTc-pertechnetate scan in patients with metastases in other sites beyond neck and chest is still unknown. Finally, we admit that assessing the outcome of therapeutic interventions requires prolonged follow-up in this relatively indolent malignancy, studies with longer follow-ups are still needed.
In conclusion, although the consistency of 99mTc-pertechnetate scan before 131I administration and 131I scan post-131I therapy in detecting metastases is low, identifying metastases via 99mTc-pertechnetate scan prior to 131I therapy has incremental value in making therapeutic decision. Notably, patients with 99mTc-pertechnetate-avid lesions may be more prone to benefit from 131I therapy than those with 99mTc-pertechnetate-nonavid lesions.
Footnotes
Abbreviations: 131I = radioiodine, BIR = biochemical incomplete response, DTC = differentiated thyroid cancer, Dx-WBS = diagnostic radioiodine whole body scan, ER = excellent response, IDR = indeterminate response, LN = lymph node, MRI = magnetic resonance imaging, PET/CT = positron emission tomography/computed tomography, Rx-WBS = post-therapeutic radioiodine whole body scan, SIR = structural incomplete response, SPECT/CT = single photon emission computed tomography/computed tomography, ssTg = stimulated serum thyroglobulin, TSH = thyroid-stimulating hormone.
ML, LC, and QL are the co-first authors. ZL and LC contributed equally.
This study was sponsored by the National Natural Science Foundation of China (81271609 and 81671711) and the Shanghai Rising-Star Program (12QH1401600).
The authors have no conflicts of interest to disclose.
References
- [1].Lin JD, Hsueh C, Chao TC. Long-term follow-up of the therapeutic outcomes for papillary thyroid carcinoma with distant metastasis. Medicine 2015;94:e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Haugen BRM, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheng L, Liu M, Ruan M, et al. Challenges and strategies on radioiodine treatment for differentiated thyroid carcinoma. Hell J Nucl Med 2016;19:23–32. [DOI] [PubMed] [Google Scholar]
- [4].Fagin JA, Wells SA., Jr Biologic and clinical perspectives on thyroid cancer. N Engl J Med 2016;375:1054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Avram AM, Esfandiari NH, Wong KK. Preablation 131-I scans with SPECT/CT contribute to thyroid cancer risk stratification and 131-I therapy planning. J Clin Endocrinol Metab 2015;100:1895–902. [DOI] [PubMed] [Google Scholar]
- [6].Wong KK, Sisson JC, Koral KF, et al. Staging of differentiated thyroid carcinoma using diagnostic I-131 SPECT/CT. Am J Roentgenol 2010;195:730–6. [DOI] [PubMed] [Google Scholar]
- [7].Avram AM, Fig LM, Frey KA, et al. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: what is the impact on staging? J Clin Endocrinol Metab 2013;98:1163–71. [DOI] [PubMed] [Google Scholar]
- [8].Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006;154:787–803. [DOI] [PubMed] [Google Scholar]
- [9].Travagli JP, Cailleux AF, Ricard M, et al. Combination of radioiodine (I-131) and probe-guided surgery for persistent or recurrent thyroid carcinoma. J Clin Endocrinol Metab 1998;83:2675–80. [DOI] [PubMed] [Google Scholar]
- [10].Sisson JC, Avram AM, Lawson SA, et al. The so-called stunning of thyroid tissue. J Nucl Med 2006;47:1406–12. [PubMed] [Google Scholar]
- [11].Muratet JP, Giraud P, Daver A, et al. Predicting the efficacy of first iodine-131 treatment in differentiated thyroid carcinoma. J Nucl Med 1997;38:1362–8. [PubMed] [Google Scholar]
- [12].Hilditch TE, Dempsey MF, Bolster AA, et al. Self-stunning in thyroid ablation: evidence from comparative studies of diagnostic 131I and 123I. Eur J Nucl Med Mol Imaging 2002;29:783–8. [DOI] [PubMed] [Google Scholar]
- [13].Lassmann M, Luster M, Hanscheid H, et al. Impact of I-131 diagnostic activities on the biokinetics of thyroid remnants. J Nucl Med 2004;45:619–25. [PubMed] [Google Scholar]
- [14].Van Nostrand D, Aiken M, Atkins F, et al. The utility of radioiodine scans prior to iodine-131 ablation in patients with well-differentiated thyroid cancer. Thyroid 2009;19:849–55. [DOI] [PubMed] [Google Scholar]
- [15].Scott GC, Meier DA, Dickinson CZ. Cervical lymph node metastasis of thyroid papillary carcinoma imaged with fluorine-18-FDG, technetium-99m-pertechnetate and iodine-131-sodium iodide. J Nucl Med 1995;36:1843–5. [PubMed] [Google Scholar]
- [16].Carr HA, Temple TE, Staab EV. Early visualization of Tc-99m pertechnetate in metastatic thyroid cancer in a patient with Graves’ disease. J Nucl Med 1971;12:40–2. [PubMed] [Google Scholar]
- [17].Verma N, Singh-Wadhwa S, Arvela OM. Metastatic thyroid cancer visualized on technetium pertechnetate and iodine-131 scintigraphy. Clin Nucl Med 2002;27:610. [DOI] [PubMed] [Google Scholar]
- [18].Vieras F. Preoperative scintigraphic detection of cervical metastases from thyroid carcinoma with technetium-99m pertechnetate. Clin Nucl Med 1985;10:567–9. [DOI] [PubMed] [Google Scholar]
- [19].Khammash NF, Halkar RK, Abdel-Dayem HM. The use of technetium-99m pertechnetate in postoperative thyroid carcinoma. A comparative study with iodine-131. Clin Nucl Med 1988;13:17–22. [DOI] [PubMed] [Google Scholar]
- [20].Chantadisai M, Kingpetch K. Usefulness of (99m) Tc-pertechnetate whole body scan with neck and chest SPECT/CT for detection of post-surgical thyroid remnant and metastasis in differentiated thyroid cancer patients. Ann Nucl Med 2014;28:674–82. [DOI] [PubMed] [Google Scholar]
- [21].Ozdemir D, Cuhaci FN, Ozdemir E, et al. The role of postoperative Tc-99m pertechnetate scintigraphy in estimation of remnant mass and prediction of successful ablation in patients with differentiated thyroid cancer. Nucl Med Commun 2016;37:640–5. [DOI] [PubMed] [Google Scholar]
- [22].Chen L, Luo Q, Shen Y, et al. Incremental value of I-131 SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med 2008;49:1952–7. [DOI] [PubMed] [Google Scholar]
- [23].Gao X, Zhang X, Zhang Y, et al. Is papillary thyroid microcarcinoma an indolent tumor? A retrospective study on 280 cases treated with radioiodine. Medicine 2016;95:e5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oyen WJ, Verhagen C, Saris E, et al. Follow-up regimen of differentiated thyroid carcinoma in thyroidectomized patients after thyroid hormone withdrawal. J Nucl Med 2000;41:643–6. [PubMed] [Google Scholar]
- [25].de Rosário PW, Guimarães VC, Maia FF, et al. Thyroglobulin before ablation and correlation with posttreatment scanning. Laryngoscope 2005;115:264–7. [DOI] [PubMed] [Google Scholar]
- [26].Gu WJ, Yan HX, Luo YK, et al. Characterization of papillary thyroid microcarcinomas using sonographic features in malignant papillary thyroid cancer: a retrospective analysis. Medicine 2015;94:e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qiu ZL, Luo QY. Coexistant iodine-negative pleural metastasis with iodine-positive lung and bone metastases in a patient with differentiated thyroid cancer. Clin Nucl Med 2009;34:836–7. [DOI] [PubMed] [Google Scholar]
- [28].Ahn BC. Radioiodine SPET/CT guided needle aspiration as a useful technique for recurrence surveillance in a thyroidectomized differentiated thyroid cancer patient with negative US and serum Tg and positive Tg of the lymph node aspirate. Hell J Nucl Med 2013;16:142–3. [PubMed] [Google Scholar]
- [29].Kueh SS, Roach PJ, Schembri GP. Role of Tc-99m pertechnetate for remnant scintigraphy post-thyroidectomy. Clin Nucl Med 2010;35:671–4. [DOI] [PubMed] [Google Scholar]
- [30].Jung JS, Lee SM, Kim SJ, et al. Prediction of the success of thyroid remnant ablation using preablative 99mTc pertechnetate scintigraphy and postablative dual I-131 scintigraphy. Nucl Med Commun 2015;36:38–44. [DOI] [PubMed] [Google Scholar]
- [31].Tsai CJ, Cheng CY, Shen DH, et al. Tc-99m imaging in thyroidectomized differentiated thyroid cancer patients immediately before I-131 treatment. Nucl Med Commun 2016;37:182–7. [DOI] [PubMed] [Google Scholar]
- [32].Campbell CM, Khafagi FA. Insensitivity of Tc-99m pertechnetate for detecting metastases of differentiated thyroid carcinoma. Clin Nucl Med 1990;15:1–4. [PubMed] [Google Scholar]


