Abstract
Rationale:
Tyrosine kinase inhibitors (TKIs) are known to have greater efficacy in epidermal growth factor receptor (EGFR) mutation nonsmall cell lung cancer (NSCLC). However, about 10% of EGFR wild-type (wt) patients respond to TKIs.
Patient concerns:
Several strategies to increase the efficacy of TKIs in wt NSCLC are the subjects of ongoing investigations. One of them is combining EGFR TKI with intercalated chemotherapy.
Diagnoses:
We describe a patient with EGFR wt NSCLC, who was found with ovarian and lung metastasis, was treated with pemetrexed and intercalated icotinib.
Interventions:
In this case, we reported the successful long-term maintenance treatment of a patient with EGFR wt NSCLC with pemetrexed and Icotinib. The patient (40-year-old female) was found with ovarian masses and lung masses. Pathological, immunohistochemical, and amplification refractory mutation system (ARMS) assay examinations of ovarian specimen suggested the expression of metastatic lung adenocarcinoma with wt EGFR. After failure treatment with paclitaxel-carboplatin, the patient received 4 cycles of pemetrexed plus platinum with intercalated icotinib and then remained on pemetrexed and icotinib.
Outcomes:
A partial response was achieved after the treatment. The patient's condition had remained stable on pemetrexed and icotinib for more than 20 months, with no evidence of progression.
Lessons:
To our knowledge, this is the first report using the long-term maintenance treatment with pemetrexed and intercalated icotinib in EGFR wt patient. The therapeutic strategies warrant further exploration in selected populations of NSCLC.
Keywords: EGFR wild-type, icotinib, NSCLC, ovarian metastasis, pemetrexed
Authors have no conflicts of interest to disclose.
1. Introduction
Lung cancer, a leading cause of cancer-related death worldwide, is often diagnosed at advanced stages. The most common histological subtype of lung cancer is adenocarcinoma. After reports of clinical trials and clinical guidelines,[1–4] the use of the tyrosine kinase inhibitors (TKIs) is now common practice for first-line treatment of patients with sensitizing epidermal growth factor receptor (EGFR) mutations. Beyond first-line treatment, in particular for patients with wild-type (wt) EGFR who have received first-line chemotherapy, recommendations regarding the potential benefits of TKIs are less clear.[5]
Unfortunately, the majority of lung cancer patients have a wt phenotype; therefore, the treatment of this molecular subgroup represents a relevant issue. TKIs target the tyrosine kinase domain of EGFR, inhibiting down-stream signaling processes for growth and proliferation, and mutations in the EGFR gene can affect the behavior of the receptor and its response to inhibitors. At present, all indirect data suggest a superiority of chemotherapy over TKIs in all settings in patients with EGFR wt disease, at least for progression-free survival (PFS).[6–8] Nevertheless, clinical trials on the application of TKIs in the EGFR wt lung cancer did not stop.
Chemotherapy combination with TKIs compared with chemotherapy alone cannot improve survival. By contrast, preclinical data show that sequential administration of TKIs after chemotherapy might be effective. Some clinical trials have proved this modality.[9,10]
The present case report features a rare case of lung adenocarcinoma with metastasis to the ovary and describes the clinicopathologic characteristics, diagnostic challenges, and selection of appropriate and adequate treatment.
2. Case report
A 40-year-old Chinese woman was hospitalized at the emergency department in June 2013. She complained of wheezing and tightness in her chest. The patient had no history of smoking or radiation. No evidence of lymphoadenopathy or breast nodules was noted on physical examination. A chest computed tomography (CT) scan revealed infiltration in the left upper lobe and large pericardial effusion (Fig. 1A1, B1, and C1). A pericardiocentesis was performed. Exfoliative cell examination of pericardial effusion found a small amount of adenocarcinoma cells. To distinguish the primary lesions from the metastatic ones, a total-body fluorodeoxyglucose–positron emission tomography (PET)/CT imaging was performed, which revealed radioactive uptake in the left upper lobe and in her neck, clavicle area, mediastinal lymph nodes. PET/CT also revealed a 4 cm solid mass arising from the left adnexa. Considering lung samples were difficult to obtain, to further clarify the diagnosis, a left adnexa puncture was carried out.
Figure 1.
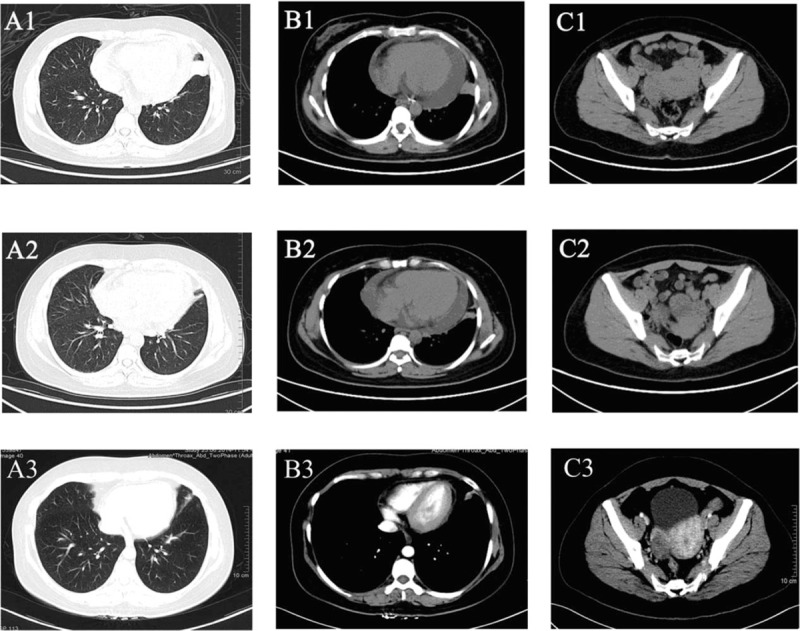
CT images of the patient (A1, B1, and C1) CT scans when diagnosed. A1, Thoracic CT scan, a solid inhomogeneous parenchymal lung tissue in left upper lobe with irregular shape and pleural projections. B1, Thoracic CT scan (soft-tissue window), a solid inhomogeneous parenchymal lung tissue in the left upper lobe and large pericardial effusion. C1, Abdominal-pelvic CT scan, a left ovarian complex mass with necrotic colliquative central area and solid peripheral area. A2, B2, and C2, CT scans after 4 cycles of treatment. A2, The volume of the solid inhomogeneous parenchymal lung tissue in left upper lobe was significantly reduced compared with A1. B2, The solid inhomogeneous parenchymal lung tissue in the left upper lobe shrinked and pericardial effusion decreased than B1. C2, A left ovarian complex mass shrinked than C2. A3, B3, and C3, CT scans after1year maintenance therapy. The cancer mass were stable and pericardial effusion has almost disappeared compared with A2, B2, and C2. CT = computed tomography.
The clinical course and pathological features supported the diagnosis of bronchioloalveolar carcinoma (BAC) with ovarian metastasis (Fig. 2A). To confirm the same, an immunohistochemical staining was performed. The tumor cells showed marked nuclear transcription termination factor, RNA polymerase I, napsin A, and Cytokeratin-7 staining (Fig. 2B–D) but negative caudal type homcoboxtranscription factor 2, CK20, Villin, estrogen receptor, and progesterone receptor staining. Thus, this immunohistochemical staining profile for the present case confirmed the diagnosis of BAC with ovarian metastasis. An EGFR mutation analysis by ARMS PCR assay was performed using ABI7500 system which showed a wt status both in ovarian tissue (Fig. 3) and serum (data not shown).
Figure 2.
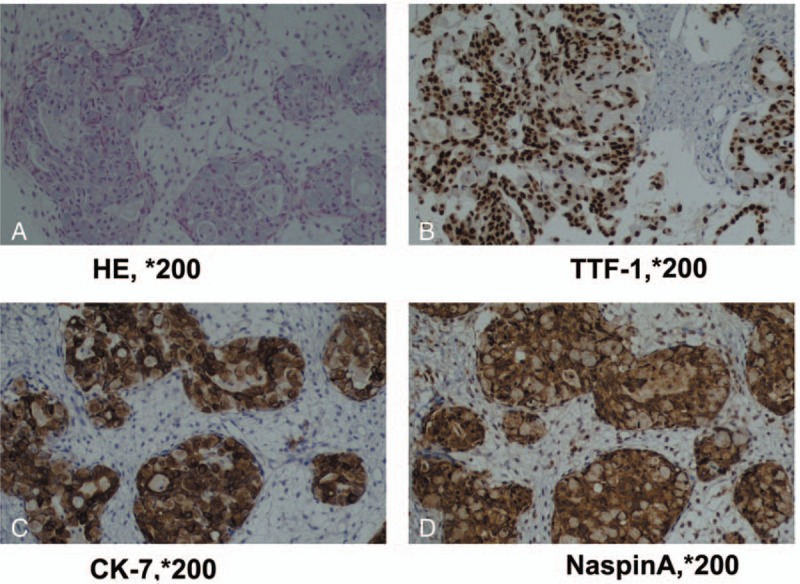
Histopathological findings in the case (A) in high power field, tumor cells contain cuboidal to low-columnar cells, including hyperchromasia, pleomorphism, and prominent nucleoli (H&E, ×200). B, The tumor cells express TTF-1, indicating that they are metastasized from lung (TTF-1, ×200). C, The tumor cells express CK-7(CK-7, ×200). D, The tumor cells express NaspinA (NaspinA, ×200). CK-7 = cytokeratin-7, TTF-1 = transcription termination factor, RNA polymerase I.
Figure 3.
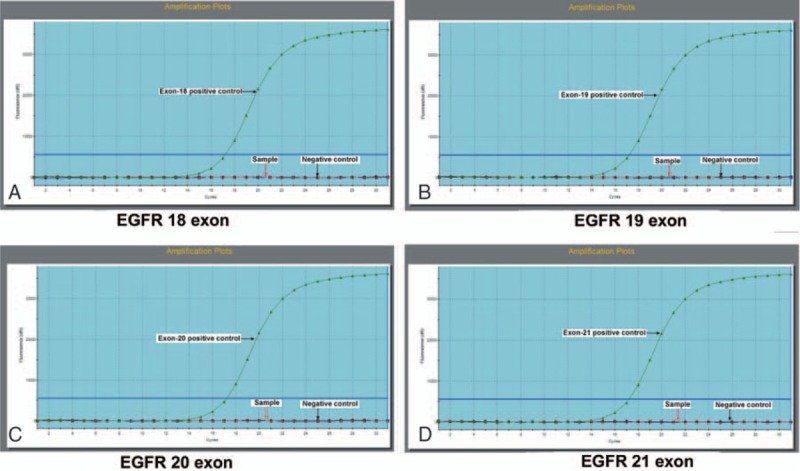
EGFR mutation analysis EGFR mutation status was detected by amplification refractory mutation system (ARMS) in ovarian tissue. A, EGFR exon 18. B, EGFR exon 19. C, EGFR exon 20. D, EGFR exon 21. They all had wild-type status. (The horizontal arrows represent positive control which presents the amplification curve, the red vertical arrow represents the lung cancer tissue sample, and the blue vertical arrow represents negative control which has no amplification curve). EGFR = epidermal growth factor receptor.
The patient received 1 cycle of paclitaxel (150 mg/m2 on day 1, intravenously, 21 d/cycle) plus platinum (carboplatin 5×area under the curve on day 2, intravenously, 21 d/cycle) in June 29, 2013. Unfortunately, the woman came to emergency department again because of wheezing and tightness in her chest after 2 weeks. B ultrasound revealed large pericardial effusion. A pericardiocentesis was performed again.
Considering the rapid progress of the disease we had to change the treatment regime. The patient started the second cycle of chemotherapy in July 20, 2013. The regime was pemetrexed (500 mg/m2 on day 1, intravenously, 21 d/cycle) plus platinum (carboplatin 5 × area under the curve on day 2, intravenously, 21 d/cycle) with intercalated icotinib (125 mg tid on days 8–20, orally; chemotherapy plus icotinib). The good news was that the chest symptom of the patient improved obviously after chemotherapy. Another 3 cycles were administered every 3 weeks (August–October 2013). No adverse events (AEs) were reported.
A CT scan was carried out in November 2013, which revealed a partial response after 4 cycles of treatment (Fig. 1A2, B2, and C2). The symptoms of wheezing and tightness almost disappeared. Due to residual disease, clinical benefit, and good PS, chemotherapy was continued. The treatment of her lung cancer was followed by a maintenance therapy of pemetrexed (500 mg/m2 on day 1, intravenously, 28 d/cycle) and icotinib (125 mg tid on days 8–27, orally; chemotherapy plus icotinib). With regular follow-up imaging, she was remained on maintenance therapy until the time of this report (20 months). Her condition and cancer mass were stable (Fig. 1A3, B3, and C3), pericardial effusion has almost disappeared, and she continued the therapy.
3. Discussion
Due to differences in survival rates and treatment modalities, the differentiation between primary and secondary ovarian malignancy is important. Despite some difficulties, we diagnosed a rare case of lung adenocarcinoma with metastasis to the ovary. In fact, how to treat this disease gives us more difficulties.
Platinum doublet chemotherapy is the current standard strategy for first-line treatment in patients with EGFR wt. In this case, the patient received paclitaxel plus platinum at first. After rapid progress of the disease, pemetrexed plus platinum was hence used as the second-line therapy. In addition, icotinib had also been inserted into the chemotherapy. Then, she was remained on maintenance therapy by pemetrexed and icotinib. This was a new treatment strategy.
Mok et al reported the findings of a phase III FASTACT-2 trial, which showed that intercalated chemotherapy (gemcitabine plus platinum) and erlotinib significantly prolonged PFS in patients with advanced nonsmall cell lung cancer (NSCLC) in an unselected population of East Asian patients.[10] In the present case, we applied a similar approach, but the application of drugs was different with Mok, and the patient then remained on pemetrexed and icotinib. Icotinib is a new oral human EGFR TKI. A China company (Betta Pharmaceuticals Co, Ltd, Hangzhou, China) developed the orally EGFR-TKI named icotinib hydrochloride (Conmana). The large, randomized, head-to-head, phase III clinical trial (ICOGEN) demonstrated that icotinib has equivalent efficacy and better tolerability to gefitinib in patients with NSCLC.[11] Another report described 38 advanced lung adenocarcinoma patients with unknown EGFR gene status in China, which was divided into 2 subgroup including patients treated with first-line pemetrexed-based chemotherapy, and subsequently treated with icotinib as second-line or maintenance therapy. In that study, the authors revealed that patients treated with sequential therapy of first-line pemetrexed followed by icotinib seems to have a better survival.[12] Base on those studies and our case report, intercalated chemotherapy and TKIs may be served as a good choice for patients with advanced NSCLC with EGFR wt. However, a review from the United States believed that there is no benefit of using EGFR TKIs alone or in combination in EGFR wt NSCLC.[13] Addition of an EGFR TKI (erlotinib or gefitinib) to first-line combination chemotherapies has failed to improve survival in unselected patients with advanced NSCLC in several large, randomized, controlled studies.[14,15] A recent study unveiled that cytotoxic synergism of pemetrexed and TKI in human NSCLC cells was schedule-dependent.[16] They concluded that continuous administration of an EGFR TKI-induced G1 arrest of tumor cells could obviate the effects of subsequent pulse administration of cytotoxic agents, which may give rise to the negative effect of administration of TKI in combination with conventional chemotherapy.[16] This negative interaction can be avoided by removing erlotinib from the cell culture medium for a sufficient interval before exposure to pemetrexed.[16] That study suggests interrupting erlotinib before the subsequent pemetrexed treatment may be served as effective sequence for combined treatment with those agents. Further efforts are needed to understand the potential reasons for benefit or lack of benefit with these agents.
As we all know, the majority of patients with NSCLC are EGFR wt. Maintenance and second-line therapy with EGFR TKIs are recommended for patients with EGFR mutation-positive status. However, the guidelines allow erlotinib treatment in patients with EGFR wt status who progress after first- or second-line chemotherapy if they have not previously received any EGFR TKIs.[5] As maintenance treatment, Vale et al[17] found that TKIs offer a modest improvement in median PFS compared with no active treatment of approximately 3 weeks for patients with wt EGFR.
Based on retrospective and heterogeneous case series, the prognosis of malignant pericardial effusion in lung cancer is generally considered to be poor with a median survival which does not exceed 100 days and a 1-year survival generally lower than 10%.[18] Although this is a malignant pericardial effusion patient with EGFR wt, she still benefited from the intercalated regimen and maintenance therapy. The patient's condition had remained stable for more than 20 months. In FASTACT-2 trial, patients with EGFR wt NSCLC did not benefit from this intercalated regimen.[10] But, this patient had obtained a better effect. Intercalated strategy may delay or prevent the onset of acquired resistance. Another explanation is that the sequential approach might have avoided the G1 arrest by icotinib, thus optimizing the cell-cycle phase-dependent activity of chemotherapy.[16]
Many studies indicated that there was a subgroup of EGFR wt patient who obtained a clinical benefit from TKI treatment, which suggests that factors other than EGFR mutation may lead to TKI sensitivity in a small number of patients. Other biological mechanisms, such as EGFR amplification, EGFR expression or phosphorylation, EGFR-ligand expression, or other biomarkers, may play a role in the EGFR pathway.[19]
An analysis of 3 phase III trials confirmed that pemetrexed could improve survival of patients with nonsquamous NSCLC in first-line, second-line, and maintenance therapy.[20] In this case, the patient remained on pemetrexed and icotinib. Although we did not know which drugs play a major role, we believed that the combination scheme might have greater prospects.
Regardless of the diagnosis or treatment, this case has its unique features. The patient did not show any typical clinical and gross features of metastasis, but histopathologic features and immunohistochemical results led us to make the right diagnosis. Although this is an EGFR wt patient, she still benefited from the intercalated regimen and maintenance therapy. Further research is required to understand the potential biomarkers for benefit or lack of benefit with these agents.
Footnotes
Abbreviations: ARMS = amplification refractory mutation system, BAC = bronchioloalveolar carcinoma, EGFR = epidermal growth factor receptor, NSCLC = nonsmall cell lung cancer, PFS = progression-free survival, TKIs = tyrosine kinase inhibitors.
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration.
TX, HW, and SJ contributed equally to this work.
This study was supported by the National Natural Science Foundation of China (81301898, 81602071), Natural Science Foundation of Jiangsu Province for Youth (BK20161066).
References
- [1].Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase iii, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in asia (IPASS). J Clin Oncol 2011;29:2866–74. [DOI] [PubMed] [Google Scholar]
- [2].Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- [3].Zhou CC, Wu YL, Chen GY, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- [4].Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- [5].Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:56–64. [DOI] [PubMed] [Google Scholar]
- [6].Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New Engl J Med 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- [7].Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–8. [DOI] [PubMed] [Google Scholar]
- [8].Janne PA, Wang XF, Socinski MA, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol 2012;30:2063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou T, Zheng L, Hu ZH, et al. The effectiveness of RECIST on survival in patients with NSCLC receiving chemotherapy with or without target agents as first-line treatment. Sci Rep 2015;5:7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777–86. [DOI] [PubMed] [Google Scholar]
- [11].Shi YK, Zhang L, Liu XQ, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953–61. [DOI] [PubMed] [Google Scholar]
- [12].Zheng Y, Fang W, Deng J, et al. Sequential treatment of icotinib after first-line pemetrexed in advanced lung adenocarcinoma with unknown EGFR gene status. J Thorac Dis 2014;6:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stinchcombe TE. The use of EGFR tyrosine kinase inhibitors in EGFR wild-type non-small-cell lung cancer. Curr Treat Option Oncol 2016;17:18. [DOI] [PubMed] [Google Scholar]
- [14].Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol 2004;22:777–84. [DOI] [PubMed] [Google Scholar]
- [15].Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J Clin Oncol 2004;22:785–94. [DOI] [PubMed] [Google Scholar]
- [16].Li T, Ling YH, Goldman ID, et al. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res 2007;13:3413–22. [DOI] [PubMed] [Google Scholar]
- [17].Vale CL, Burdett S, Fisher DJ, et al. Should tyrosine kinase inhibitors be considered for advanced non-small-cell lung cancer patients with wild type EGFR? Two systematic reviews and meta-analyses of randomized trials. Clin Lung Cancer 2015;16:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rousseau-Bussac G, Crequit P, Alifano M, et al. Management of malignant pericardial effusion in lung cancer. Rev Mal Respir 2014;31:746–53. [DOI] [PubMed] [Google Scholar]
- [19].Laurie SA, Goss GD. Role of epidermal growth factor receptor inhibitors in epidermal growth factor receptor wild-type non-small-cell lung cancer. J Clin Oncol 2013;31:1061–9. [DOI] [PubMed] [Google Scholar]
- [20].Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase iii trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64–70. [DOI] [PubMed] [Google Scholar]


