Abstract
This study aimed to investigate the correlation between quantitative hepatitis B e antigen (HBeAg) and hepatitis B virus (HBV) DNA levels, and to determine whether semiquantitative measurement of HBeAg can indicate the extent of HBV replication in HBeAg-positive subjects in the immune tolerant phase.
A cross-sectional, community-based survey was carried out in 12 communities of 2 counties in Zhejiang Province, China. A panel of 788 HBeAg-positive subjects was divided into 4 groups according to HBV DNA level.
Groups I (n = 111), II (n = 91), III (n = 124), and IV (n = 462) had HBV DNA levels below 103 copies/mL (PCR undetectable), between 103 and 105 copies/mL (PCR detectable), between 105 and 2 × 107 copies/mL (hybridization detectable), and >2 × 107 copies/mL, respectively. The HBeAg level correlated well with the HBV DNA level (R2 = 0.658; P < .01) on a log scale. The average HBeAg level in group IV was significantly higher than those in the other 3 groups, and the best HBeAg cut-off value for differentiating group IV from the other 3 groups was 768 S/CO, with a sensitivity of 94.4% and specificity of 91.1%.
Semiquantification of HBeAg could indicate a relative HBV DNA level in HBeAg-positive subjects in the immune tolerant phase.
Keywords: hepatitis B e antigen, hepatitis B virus deoxyribonucleic acid, immune tolerant phase of HBV infection, serological test
1. Introduction
Hepatitis B virus (HBV) infection poses a significant challenge to public health worldwide. It is estimated that 240 million people are chronically infected with HBV, and chronic HBV infection is a major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC), resulting in approximately 778,000 deaths each year.[1,2] Chronic HBV infection consists of different clinical manifestations, and a majority of subjects (70%–90%) are so-called hepatitis B surface antigen (HBsAg) asymptomatic carriers who are HBsAg positive for more than 6 months with a normal serum alanine aminotransferase (ALT) level or normal liver histological findings.[3,4] Many of these subjects are said to be in the immune tolerant phase because they are found to be hepatitis B e antigen (HBeAg) positive and have a high level of hepatitis B virus deoxyribonucleic acid (HBV DNA); however, they show no evidence of liver injury.
China is a highly endemic country for HBV infection,[5] and approximately 93 million people in China are chronically infected with HBV.[6] Approximately 15% to 40% of subjects with chronic HBV infection develop severe liver disease during their lifetime. A recent study demonstrated that the mortality rate among chronic HBV infected subjects is 70% higher than that among the general population due to HBV-related complications.[7,8] Although de Franchis et al reported that inactive HBV carriers who are HBeAg negative with low or no viremia carry a low risk for developing severe liver disease over a 10-year period,[4] Chon et al reported that more than half of asymptomatic carriers have active chronic liver disease.[9]
HBV DNA is a quantitative virologic marker reflecting HBV replication level. A number of studies have found that the HBV DNA level is related to the extent of liver injury and liver fibrosis severity, and it can be used as an independent factor predicting response to antiviral treatment.[10–14] Quantitative assaying of HBV requires expensive equipment and a contamination-free facility, and it cannot be routinely done in hospitals serving rural communities where more than half of the population of China lives. Thus, there is a clinical need for an alternative assay that is simple and can be routinely performed in community hospitals.
HBeAg is a serologic marker associated with high level of viral replication and infectivity. In general, HBeAg positivity correlates with a high level of HBV DNA.[15–17] However, HBeAg is still qualitatively assayed despite advances in quantitative detection of HBsAg.[18] Furthermore, a truly quantitative relationship between HBeAg and HBV DNA levels remains to be established. In this study, we conducted a large-scale community-based screening of HBV-infected individuals by random collection of blood samples from the entire populations of 12 villages. Then we investigated the relationship between semiquantitative HBeAg and HBV DNA levels and determined whether quantification of HBeAg could serve as a useful marker indicating the relative HBV DNA level in asymptomatic HBeAg-positive carriers.
2. Materials and methods
2.1. Study site
This study was carried out in the northern region of Zhejiang Province, which is located in the southern region of the Yangtze River Delta in southeast China.
2.2. Study population and sampling strategy
Individuals who were HBsAg positive for more than 6 months, but also persistently had normal levels of serum ALT (tested every 3–6 months), and a normal liver background on regular ultrasound examination (every 6 months) for at least the preceding 24 months, were enrolled. Subjects who were HBeAg positive and also had a high level of HBV DNA (> 2 × 107 copies/mL) were considered to be in the immune tolerant phase. Field work was conducted from March 2012 to October 2014. The participants were permanent residents in the northern region of Zhejiang Province, including both rural and urban areas. The target study population was selected from the list of residents using the random multistage cluster sampling approach. First, 2 municipalities with different landscapes (plain vs coastland) were chosen randomly. Next, Shaoxing (plain) and Yuhuan Island (coastal), representing 1 county/city (equivalent to county) from each municipality, were selected. Towns/districts in each of the 2 selected counties were divided into 3 economic levels. One town/district was randomly selected to represent each level. Finally, 2 villages were selected to represent each selected town/district. A total of 12 villages were included in 2 selected counties. HBV infection was screened in all residents of the 12 villages.
Subjects were enrolled in this study if they were HBsAg-positive for longer than 6 months, also had a normal serum ALT level, and had normal or near normal liver histological findings. Subjects were excluded if they had received antiviral therapy (interferon or lamivudine); were positive for antibodies to human immunodeficiency virus (HIV), hepatitis C virus (HCV), or hepatitis D virus (HDV); or had been diagnosed with other liver diseases.
We established a list of HBeAg-positive subjects who were considered in the immune tolerant phase from among the participants. All selected carriers were divided into 4 groups according to HBV DNA level. Groups I (n = 111), II (n = 91), III (n = 124), and IV (n = 462) had HBV DNA levels <103 (PCR undetectable), between 103 and 105 (PCR detectable), between 105 and 2 × 107 copies/mL (hybridization detectable), and >2 × 107 copies/mL, respectively. The demographic data for all subjects were collected.
2.3. Serological testing
A 5 mL blood sample was collected from each participant following strict hygiene and safety guidelines. Blood samples were kept in a cold container and immediately delivered to Adicon Clinical Laboratories, Inc (Shanghai, China) for serum separation and indefinite storage at −30°C. Sample processing and serology was performed by the Central Laboratory (Hangzhou, China).
A commercial HBsAg enzyme immunoassay kit (Acon Biotech Co, Hangzhou, China) was used to assess HBsAg status. A microparticle enzyme immunoassay kit (Abbott, Chicago, IL) was used to assess HBeAg titer. Briefly, HBeAg in the sample first bound to anti-HBe antibodies coated on the microparticles, and then the bound HBeAg was detected upon addition of anti-HBe antibodies conjugated to alkaline phosphatase. The HBeAg levels were evaluated using ratios of sample to cut-off values (S/CO), and HBeAg positivity was suggested if the S/CO was ≥1.0. Verification of test results was carried out by randomly retesting 5% of the specimens using the same kit. Only samples that were positive on both tests were considered reactive. For the purpose of analysis, HBsAg positivity was considered evidence of HBV infection.
HBV DNA levels were determined using a hybridization capture kit (Digene, Gaithersburg, MD). HBV DNA-negative samples in a hybridization array were further analyzed by qPCR determination of HBV DNA level (7300; Applied Biosystems, Inc, Foster City, CA). The lower detection limit of HBV DNA of this qPCR was 103 copies/mL.
The Architect C8000 automated biochemistry analyzer (Abbott Laboratories, Abbott Park, IL) was used to measure ALT levels. An ALT value >38 IU/L was considered abnormal.
2.4. Ethics statement
This study was performed in accordance with international guidelines on use of human subjects in medical research and approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University College of Medicine. All participants provided informed consent.
2.5. Statistical analysis
Data were processed using SPSS software version 19.0 (SPSS, Inc, Chicago, IL). Pearson correlation was used to evaluate the correlation between HBeAg and HBV DNA levels on a log scale. Receiver operating characteristic (ROC) curve analysis was used to determine the best cut-off level. Categorical data were analyzed using the chi-squared test and Mann-Whitney test when appropriate. A P value <.05 was considered statistically significant.
3. Results
A total of 130,152 serum samples were tested, and 9325 (7.17%) were HBsAg positive. Subjects were excluded if they had elevated ALT; had received antiviral therapy (interferon or lamivudine); were positive for antibodies to HIV, HCV, or HDV; or had been diagnosed with another liver disease. A total of 788 subjects with HBeAg-positive, including 412 men and 376 women, with an average age of 41.83 ± 13.14 years (range, 3–90 years), were finally included in this study. Detailed clinical features including HBV DNA and HBeAg levels in the 4 groups are presented in Table 1. Among all HBeAg-positive asymptomatic carriers, more than half (58.6%) had an HBV DNA level >2 × 107 copies/mL. The average age in group IV was 39.10 ± 13.16 years, which was significantly younger than those of the other groups (P < .01).
Table 1.
Clinical features, HBV DNA levels, and HBeAg titers in the 4 groups.
The average log HBeAg (S/CO) values were 0.89 ± 0.85 for group I, 1.13 ± 0.97 for group II, 1.66 ± 0.97 for group III, and 3.08 ± 0.20 for group IV. There was no significant difference in the HBeAg level between groups I and II (P > .05), but significant differences were noted between groups I and III, groups I and IV, groups II and III, groups II and IV, and groups III and IV (P < .01). The HBeAg S/CO ratio in group IV was significantly greater than those in the other groups; and the HBeAg S/CO ratio in group I was comparable to that in group II, but significantly lower than those in groups III and IV.
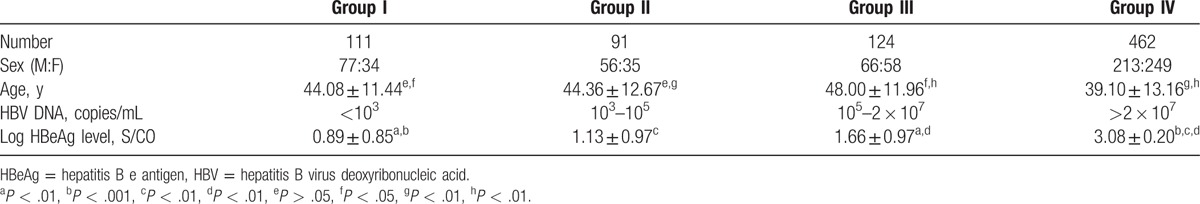
Because the average HBV DNA level in group I was <103 copies/mL, for the purpose of statistical analysis, the log HBV DNA level in group I was assigned to be 2. Figure 1 shows the highly significant quantitative correlation between HBeAg and HBV DNA levels on the log scale (R2 = 0.658; P < .01).
Figure 1.
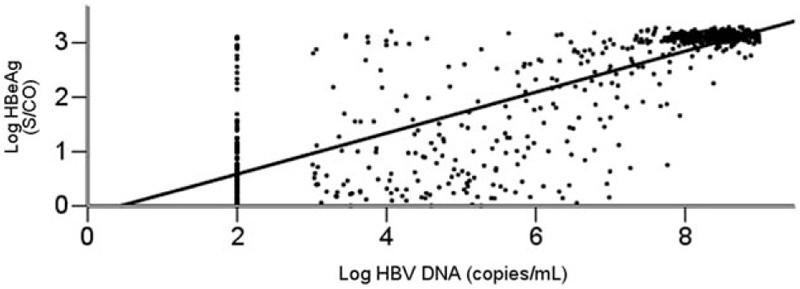
Correlation between HBeAg and HBV DNA levels. The horizontal axis indicates the log HBV DNA (copies/mL). The vertical axis represents the log HBeAg (S/CO). HBeAg = hepatitis B e antigen, HBV DNA= hepatitis B virus deoxyribonucleic acid.
Based on ROC curves, the best cut-off value of HBeAg that differentiated group IV from the other groups was 768 S/CO with 94.4% sensitivity and 91.1% specificity (Fig. 2).
Figure 2.
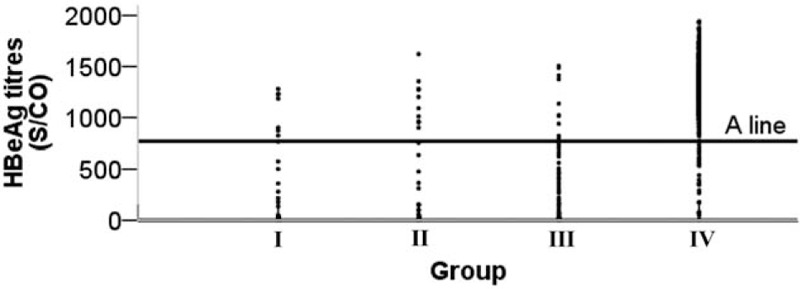
The best cut-off value for the HBeAg level that differentiated group IV from the other groups (A line): 768 S/CO, sensitivity 94.4%, specificity 91.1%. HBeAg = hepatitis B e antigen.
4. Discussion
In this study, we examined the correlation between semiquantitative HBeAg and HBV DNA levels in a large number of HBeAg-positive HBV carriers who had a normal ALT level. We also investigated whether quantification of HBeAg can indicate the relative HBV DNA level.
We divided all subjects into 4 groups using arbitrary HBV DNA levels to assess any correlation between HBeAg and HBV DNA levels. Because both HBeAg and HBV DNA assays in this study were quantitative, the correlative analysis revealed interesting findings that would not have been observed if HBeAg levels had not been quantified.
One finding was the very wide range of HBeAg concentrations among HBeAg-positive carriers, with HBeAg levels varying over 3 orders of magnitude. Traditionally, individuals who are HBeAg positive are seen when at a phase with a high level of HBV replication and when the virus is highly infectious.[19,20] This is not completely accurate in view of new findings in this study, because the HBeAg level varied as much as 1000-fold among HBeAg-positive individuals, and a low level of HBeAg, though positive, most likely suggested a low level of HBV DNA. Conceivably, subjects with low levels of HBeAg can relatively easily achieve HBeAg loss or seroconversion to anti-HBe positive. Presumably, a higher percentage of those subjects, if treated with antivirals, will experience HBeAg loss.[21] Thus, HBeAg quantification is of clinical value for predicting HBeAg loss or seroconversion and should be performed in place of the current qualitative HBeAg assays.
Another finding was the strong correlation between semiquantitative HBeAg and HBV DNA levels, and this correlation appeared to be tighter at the highest HBeAg levels. Our results suggested that a high HBeAg level effectively indicates the relative HBV DNA level. As tested in this study, an HBeAg level of 768 S/CO can effectively cover 94% of carriers who have HBV DNA >2 × 107 copies/mL. Our results are consistent with those of previous studies,[22–24] and a clinical utility of this finding is that we can use high HBeAg levels to estimate corresponding HBV DNA levels without qPCR detection of HBV DNA. This is particularly useful in the setting of rural hospitals where quantitative analysis of HBV DNA levels cannot be performed.
Finally, we noted that more than half of the HBV-infected individuals in this cohort (58.6%) had an HBV DNA level >2 × 107 copies/mL. These subjects were considerably younger than the subjects in other groups. It appears that there is a correlation between HBV replication levels, as indicated by HBV DNA, HBeAg levels, and aging. As chronic HBV-infected subjects age, HBV replication likely slows down.[25] Thus, a subject younger than 40 years old is more likely to have a high level of HBeAg, which is highly likely to be accompanied by a high level of HBV DNA. Therefore, age can be used to estimate the likelihood of a high HBV DNA level if it cannot be tested in a community hospital.
We were surprised that low HBV DNA levels were detected in a number of subjects who had a high level of HBeAg, suggesting a possible dissociation between HBeAg and HBV DNA levels. We do not understand why this dissociation occurred despite strong correlation between the 2 markers in the majority of samples. HBV DNA replication can be inhibited by a strong immune response.[26] We believe that a kinetic study of HBeAg and HBV DNA levels in the future could shed light on this issue.
There are a few limitations in this study. First, our HBeAg assay was only semiquantitative, not an absolute quantitative assay. Second, this was a cross-sectional study, and a kinetic study would provide kinetic data for both HBeAg and HBV DNA over time. Third, no HBV-infected subjects in the immune clearance phase were included. Our future studies will address these limitations.
In summary, our study showed that the HBeAg concentration varied by as much as 3 orders of magnitude from individual to individual. The HBeAg level, particularly high levels of HBeAg, correlated well with a high level of HBV DNA. Thus, in rural hospitals where HBV DNA levels cannot be quantitatively determined, semiquantitative HBeAg measurement can be used to indicate the relative HBV DNA level. In addition, semiquantitative HBeAg analysis is useful for predicting HBeAg loss or seroconversion.
Acknowledgments
We thank Drs Jing Yang, Ping Zhang, and Wei-Hang Ma of the Bureau of Health of Zhejiang Province, and the members of the Bureaus of Health of each county of Zhejiang Province, for supporting the data collection.
Footnotes
Abbreviations: ALT = alanine aminotransferase, Anti-HBeAb = hepatitis B e antibody, Anti-HBsAb = hepatitis B surface antibody, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HBV DNA = hepatitis B virus deoxyribonucleic acid, ROC = receiver operating characteristic.
This study was supported by grants from the Mega-Project for National Science and Technology Development under the “12th Five-Year Plan of China” (2013ZX10004904 and 2014ZX10004008), the Medical & Health Technology Program of Zhejiang Province (grant No. 2016KYA079), and the Open Foundation of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital of Medical College, Zhejiang University (grant No. 2017KF10).
The authors report no conflicts of interest.
References
- [1].World Health Organization. WHO Guidelines of Hepatitis B 2015. WHO; 2015. [Google Scholar]
- [2].Jazayeri MS, Basuni AA, Cooksley G, et al. B virus genotypes, core gene variability and ethnicity in the Pacific region. J Hepatol 2004;41:139–46. [DOI] [PubMed] [Google Scholar]
- [3].Hoofnagle JH, Shafritz DA, Popper H. Chronic type B hepatitis and the “healthy” HBsAg carrier state. Hepatology 1987;7:758–63. [DOI] [PubMed] [Google Scholar]
- [4].de Franchis R, Meucci G, Vecchi M, et al. The natural history of asymptomatic hepatitis B surface antigen carriers. Ann Intern Med 1993;11:191–4. [DOI] [PubMed] [Google Scholar]
- [5].Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China: declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550–7. [DOI] [PubMed] [Google Scholar]
- [6].Lu FM, Zhuang H. Prevention of hepatitis B in China: achievements and challenges. Chin Med J 2009;122:2925–7. [PubMed] [Google Scholar]
- [7].Lok AS. Chronic hepatitis B. N Engl J Med 2002;346:1682–3. [DOI] [PubMed] [Google Scholar]
- [8].Montuclard C, Hamza S, Rollot F, et al. Causes of death in people with chronic HBV infection: a population-based cohort study. J Hepatol 2015;62:1265–71. [DOI] [PubMed] [Google Scholar]
- [9].Chon CY, Han KH, Lee KS, et al. Peritoneoscopic liver biopsy findings in asymptomatic chronic HBsAg carriers with normal liver function tests and no hepatomegaly. Yonsei Med J 1996;37:295–301. [DOI] [PubMed] [Google Scholar]
- [10].Gerken G, Gomes J, Lampertico P, et al. Clinical evaluation and applications of the Amplicor HBV Monitor test, a quantitative HBV DNA PCR assay. J Virol Methods 1998;74:155–65. [DOI] [PubMed] [Google Scholar]
- [11].Mommeja-Marin H, Mondou E, Blum MR, et al. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology 2003;37:1309–19. [DOI] [PubMed] [Google Scholar]
- [12].Sanai FM, Helmy A, Bzeizi KI, et al. Discriminant value of serum HBV DNA levels as predictors of liver fibrosis in chronic hepatitis B. J Viral Hepat 2011;18:e217–25. [DOI] [PubMed] [Google Scholar]
- [13].Nagata I, Colucci G, Gregorio GV, et al. The role of HBV DNA quantitative PCR in monitoring the response to interferon treatment in chronic hepatitis B virus infection. J Hepatol 1999;30:965–9. [DOI] [PubMed] [Google Scholar]
- [14].Xu Y, Wu XN, Shi YW, et al. Baseline hepatitis B virus DNA level is a promising factor for predicting the 3rd month virological response to entecavir therapy: a study of strict defined hepatitis B virus induced cirrhosis. Chin Med J 2015;128:1867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jardi R, Buti M, Rodriguez-Frias F, et al. The value of quantitative detection of HBV-DNA amplified by PCR in the study of hepatitis B infection. J Hepatol 1996;24:680–5. [DOI] [PubMed] [Google Scholar]
- [16].Rabbi FJ, Rezwan MK, Shirin T. HBeAg/anti-HBe, alanine aminotransferase and HBV DNA levels in HBsAg positive chronic carriers. Bangladesh Med Res Counc Bull 2008;34:39–43. [DOI] [PubMed] [Google Scholar]
- [17].Hasan KN, Rumi MA, Hasanat MA, et al. Chronic carriers of hepatitis B virus in Bangladesh: a comparative analysis of HBV-DNA, HBeAg/anti-HBe, and liver function tests. Southeast Asian J Trop Med Public Health 2002;33:110–7. [PubMed] [Google Scholar]
- [18].Alghamdi A, Aref N, El-Hazmi M, et al. Correlation between hepatitis B surface antigen titers and HBV DNA levels. Saudi J Gastroenterol 2013;19:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009;373:582–92. [DOI] [PubMed] [Google Scholar]
- [20].Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48:335–52. [DOI] [PubMed] [Google Scholar]
- [21].Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003;348:808–16. [DOI] [PubMed] [Google Scholar]
- [22].Gowans EJ. Relationship between HBeAg and HBV DNA in subjects with acute and persistent hepatitis B infection. Med J Aust 1986;145:439–41. [DOI] [PubMed] [Google Scholar]
- [23].Ljunggren KK, Nordenfelt E, Kidd A. Correlation of HBeAg/anti-HBe, ALT levels, and HBV DNA PCR results in HBsAg-positive subjects. J Med Virol 1993;39:297–302. [DOI] [PubMed] [Google Scholar]
- [24].Niederau C, Heintges T, Lange S, et al. Long-term follow-up of HBeAg-positive subjects treated with interferon alfa for chronic hepatitis B. N Engl J Med 1996;334:1422–7. [DOI] [PubMed] [Google Scholar]
- [25].Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 2003;38:1075–86. [DOI] [PubMed] [Google Scholar]
- [26].Du DW, Jia ZS, Li GY, et al. HBV DNA vaccine with adjuvant cytokines induced specific immune responses against HBV infection. World J Gastroenterol 2003;9:108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


