Abstract
Rationale:
Pleomorphic liposarcoma (PLS) is a rare and aggressive malignant tumor, and both radiation and conventional cytotoxic chemotherapy remain controversial for metastatic or unresectable disease.
Patient Concerns:
We presented an 81-year-old Chinese woman with advanced PLS who received apatinib after failure chemotherapy.
Diagnoses:
The patient was diagnosed as having PLS by biopsy.
Interventions: After a failed chemotherapy, apatinib started to be taken orally 425 mg per day.
Outcomes:
This patient achieved 3-month progression-free survival (PFS) and a higher quality of life. Meanwhile, this patient suffered grade 2 hypertension and grade 3 hand–foot syndrome (HFS).
Lessons: In this case, apatinib presented good efficacy and safety to treat PLS. Randomized clinical studies are required to confirm the efficacy and safety of apatinib in the treatment of PLS.
Keywords: apatinib, pleomorphic liposarcoma, targeted therapy, TKI, VEGFR-2
1. Introduction
Liposarcoma (LPS) is a rare cancer of high recurrence rate and low response to existing treatment.[1] Recurrence and metastasis tumors are associated with high mortality. There are 3 different subtypes that are widely diverse in clinicopathological and molecular characteristics: well-differentiated/dedifferentiated (WD/DD) liposarcoma, myxoid/round-cell liposarcoma, and pleomorphic liposarcoma (PLS).[2]
PLS represents approximately 5% to 15% of liposarcomas.[3] PLS is the less frequent type with complex genomic gains and losses, which are also seen in poorly differentiated sarcoma. It is much more aggressive than other LPS subtypes and highly resistant to conventional treatment.[4,5]
Apatinib mesylate is a new inhibitor of VEGFR-2 tyrosine kinase targeting the intracellular adenosine triphosphate (ATP) binding site of the receptor, also one of the latest oral antiangiogenic agents with encouraging preclinical and clinical data in the treatment of a variety of solid tumors. In 2014, apatinib was approved by China State Food and Drug Administration for the treatment of patients with metastatic gastric cancer who receive 2 or more lines of prior chemotherapy.
We report a case of chemoresistant PLS, in which the patient obtained 3-month progression-free survival (PFS) by orally taking antiangiogenic drug apatinib. To the best of our knowledge, this is the first case of PLS of the scalp that responded to apatinib.
2. Case presentation
We report a case of an 81-year-old woman that was referred to our observation for a progressive volumetric increase of the abdomen. On September 10, 2015, CT showed a large lobulated, heterogeneously enhancing mass lesion in the pelvic cavity. The diameter of the mass was 11 cm × 10 cm. There were cystic and necrotic areas in the mass (Fig. 1A). Biopsy confirmed PLS. Immunohistochemical staining results were S-100 (+), CD68 (–), myoglobin (–), CK7 (–), CK20 (–), and villin (+) (Fig. 2). Because of poor general condition and elderly, the patient did not agree with the use of anthracyclines or ifosfamide, but signed a consent form to be treated with other types of chemotherapeutics. Anthracycline-based regimens did not result in an improved response rate compared to nonanthracycline-based therapies in advanced PLS.[6] Therefore, based on the results of clinical trials,[7,8] we selected docetaxel and gemcitabine. On September 25, 2015, the patient received intraperitoneal perfusion chemotherapy, cisplatin 100 mg. On October 25, 2015, the patient received 100 mg cisplatin intraperitoneal perfusion chemotherapy again. At the same time, docetaxel 100 mg was used for systemic chemotherapy. After treatment, her ascites and tumor volume continued to increase (Fig. 1B). Based on RECIST 1.1 criteria, the patient was evaluated as disease progression (PD). We changed the treatment into the intraperitoneal perfusion of docetaxel and gemcitabine systemic chemotherapy. Docetaxel 100 mg was used for intraperitoneal perfusion chemotherapy on December 21, 2015. On the same day, gemcitabine 1.4 g was infused via intravenous drip, and on December 21, the same dose of gemcitabine was administered again. But, the ascites was still increasing, and her ECOG performance status (PS) score increased rapidly to 4 together with anorexia, nausea, and vomiting after a series of treatment.
Figure 1.
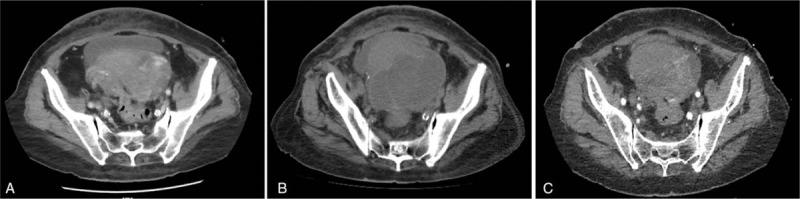
(A) CT showed a large lobulated, heterogeneously enhancing mass lesion in the pelvic cavity. The diameter of the mass was 11 cm × 10 cm. (B) After 2 cycles of chemotherapy, CT scan showed that the tumor volume increased slightly. (C) Two months after apatinib was orally taken, CT scan showed that the tumor volume decreased compared with that in (B). CT = computed tomography.
Figure 2.
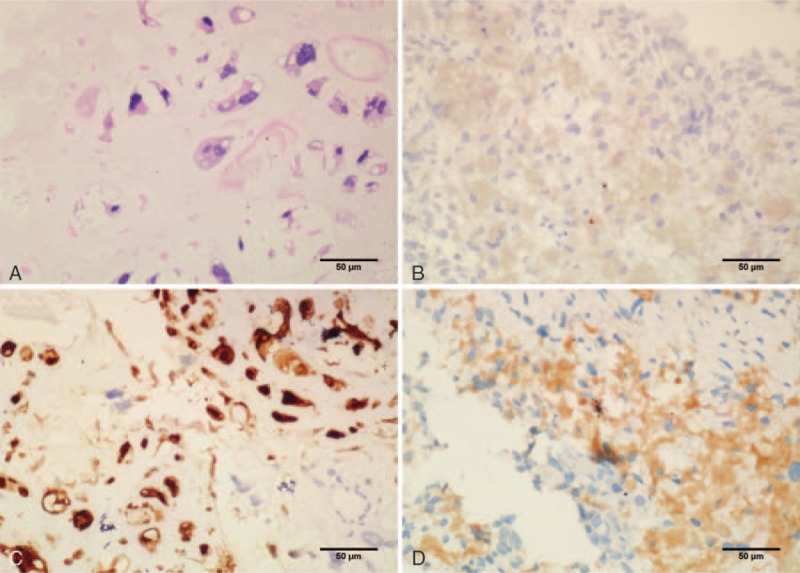
The patient was initially diagnosed with PLS by fine-needle aspiration. Hematoxylin and eosin stain revealed pleomorphic spindle cells, round cells, and special-shaped lipoblasts (A). The section showed negative staining for myoglobin (B), positive staining for S-100 (C), and villin (D) (400× magnification). PLS = pleomorphic liposarcoma.
The study was approved by Ethics Committee of Ji’nan Central Hospital Affiliated to Shandong University and written informed consent was obtained from patient. Apatinib started to be taken orally 425 mg per day from February 15, 2016. One week later, the symptoms of abdominal distension, nausea, and vomiting were gradually alleviated. The PS score reduced to 3. Then, reviewed through CT scan, we found tumor slightly shrank and the inner liquid-density area was enlarged, which was considered as necrosis. This case was evaluated as partial remission (PR) (Fig. 1C). However, in the course of taking apatinib, the patients suffered hypertension, and the highest blood pressure was 170/110 mm Hg. We controlled blood pressure by antihypertensive drug. Valsartan (a type I angiotensin II receptor antagonist) 80 mg once a day was used. Three days later, blood pressure dropped to 130/80 mm Hg. After 2 months of follow-up, the disease of the patient was stable, but she presented grade 3 hand–foot syndrome (HFS) with the hand and foot blister and perianal ulcers. Apatinib was reduced to 250 mg per day and moisturizing ointment was used. After 1 week, HFS of the patient fell to grade 2. This patient stopped oral apatinib on May 6 because of aphagia and died on May 18, 2016.
3. Discussion
PLS pathologically exhibits large, multivacuolated pleomorphic lipoblasts.[9] Complete surgical resection is the only means of local disease treatment. Radiation and conventional cytotoxic chemotherapy remain controversial for metastatic or unresectable LPS.[10]
In this case, the drugs we used included docetaxel, gemcitabine, and cisplatin, but all were invalid. The use of apatinib achieved a 3-month PFS.
Conventional chemotherapy is of low efficiency, so new drugs and target therapy might be good options. For example, eribulin and pazopanib are new treatment options for the patients with metastatic STS.[11,12] Eribulin mesylate was reported to have selective activity in LPS.[11] However, the adverse effects of eribulin were severe. In a phase III trial, treatment emergent adverse events occurred in 224 (99%) of 226 patients who received eribulin. Grade 3/4 adverse events were 152 (67%) who received eribulin.[13] The Food and Drug Administration (FDA) approved pazopanib as second-line chemotherapy for the treatment of patients with advanced nonlipogenic STS, but still not yet for LPS.[12] In addition, eribulin and pazopanib have not been approved for clinical use in China.
A majority of STS have been discovered to have the high expression level of proangiogenic growth factors that contribute tumor angiogenesis, growth, and progression. VEGF expression was observed in 68% of PLS, and microvessel density was especially higher in liposarcoma,[14] which are effective indicators of antiangiogenic drugs. Meanwhile, antiangiogenic targeted drugs such as sunitinib, sorafenib, and pazopanib all appeared to demonstrate acceptable antitumor activity in liposarcomas.[15–17] Moreover, several articles reported that apatinib had a good effect in angiosarcoma[18] and round cell liposarcoma.[19] Furthermore, owing to old age and poor PS, the patient could not tolerate to continue chemotherapy. Therefore, apatinib monotherapy was then used to control the disease.
In a number of study, apatinib is generally well tolerated by patients. The most frequently observed drug-related adverse events were hypertension, proteinuria, and HFS.[20] In the previous reports, the most serious complication was gastrointestinal hemorrhage, but the incidence was very low.[21]
Although obtained some experience in this case, we should have more explorations in terms of treatment options. For example, combination approaches, such as apatinib combined with radiotherapy or tyrosine kinase inhibitor (TKI) or cyclin-dependent kinases (CDK) inhibitors, may be an attractive potential therapy paradigms. Besides, to find biomarkers to predict drug efficacy is also one of the challenges with antiangiogenic inhibitors such as apatinib. A study of biomarkers in breast cancer patients treated with apatinib showed that hypertension was independent predictive factors for improved PFS and clinical benefit rate.[22] Predictive biomarkers could discriminate patients who are most likely to be sensitive to apatinib and avoid exposure to useless toxic medicine. Thus, additional studies on biomarkers may be useful in predicting personalized therapeutic response.
4. Conclusions
In this case report, apatinib presented good efficacy and safety to treat PLS. It is possible that apatinib might be an appropriate choice of advanced PLS. Whether it can be used in the first line, or whether it can be used together with other chemotherapeutic agents, requires a large number of randomized clinical trials to confirm.
Footnotes
Abbreviations: ATP = adenosine triphosphate, CDK = cyclin-dependent kinases, CT = computed tomography, ECOG = Eastern Cooperative Oncology Group, FDA = Food and Drug Administration, HFS = hand–foot syndrome, MLS = myxoid/round-cell liposarcoma, PD = disease progression, PFS = progression-free survival, PLS = pleomorphic liposarcoma, PR = partial remission, PS = performance status, RECIST = Response Evaluation Criteria in Solid Tumors, STS = soft tissue sarcomas, TKI = tyrosine kinase inhibitor, VEGF = vascular endothelial growth factor, VEGFR-2 = vascular endothelial growth factor receptor-2, WD/DD = well-differentiated/dedifferentiated.
This work was supported by grants from the National Science Foundation for Young Scientists of China (Grant No. 81402195).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Steen S, Stephenson G. Current treatment of soft tissue sarcoma. Proc (Bayl Univ Med Cent) 2008;21:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fletcher CDM, Brdige JA, Hogendoorn PCW, et al. World Health Organization Classification of Tumours of Soft Tissue and Bone. 4th edLyon, France: nIARC Press; 2013. [Google Scholar]
- [3].Azumi N, Curtis J, Kempson RL, et al. Atypical and malignant neoplasms showing lipomatous differentiation. A study of 111 cases. Am J Surg Pathol 1987;11:161–83. [DOI] [PubMed] [Google Scholar]
- [4].Hornick JL, Bosenberg MW, Mentzel T, et al. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol 2004;28:1257–67. [DOI] [PubMed] [Google Scholar]
- [5].Ghadimi MP, Liu P, Peng T, et al. Pleomorphic liposarcoma: clinical observations and molecular variables. Cancer 2011;117:5359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Italiano A, Garbay D, Cioffı A, et al. Advanced pleomorphic liposarcomas:clinical outcome and impact of chemotherapy. Ann Oncol 2012;23:2205–6. [DOI] [PubMed] [Google Scholar]
- [7].Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;20:2824–31. [DOI] [PubMed] [Google Scholar]
- [8].Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J Clin Oncol 2007;25:2755–63. [DOI] [PubMed] [Google Scholar]
- [9].Enzinger FM, Weiss SWA, Liposarcoma SW, et al. Soft Tissue Tumors. 3rd edn1995;St. Louis, MO: Mosby, 431–66. [Google Scholar]
- [10].Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer 2010;46:72–83. [DOI] [PubMed] [Google Scholar]
- [11].Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes. Lancet Oncol 2011;12:1045–52. [DOI] [PubMed] [Google Scholar]
- [12].Ranieri G, Mammì M, Donato Di Paola E, et al. Pazopanib a tyrosine kinase inhibitor with strong anti-angiogenetic activity: a new treatment for metastatic soft tissue sarcoma. Crit Rev Oncol Hematol 2014;89:322–9. [DOI] [PubMed] [Google Scholar]
- [13].Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma:a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629–37. [DOI] [PubMed] [Google Scholar]
- [14].Baneth V, Raica M, Cimpean AM. Assessment of angiogenesis in softtissue tumors. Rom J Morphol Embryol 2005;46:323–7. [PubMed] [Google Scholar]
- [15].Mahmood ST, Agresta S, Vigil CE, et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer 2011;129:1963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Canter RJ, Borys D, Olusanya A, et al. Phase I trial of neoadjuvant conformal radiotherapy plus sorafenib for patients with locally advanced soft tissue sarcoma of the extremity. Ann Surg Oncol 2014;21:1616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yoo KH, Kim HS, Lee SJ, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer 2015;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ji G, Hong L, Yang P. Successful treatment of angiosarcoma of the scalp with apatinib: a case report. Onco Targets Ther 2016;9:4989–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dong M, Bi J, Liu X. Significant partial response of metastatic intra-abdominal and pelvic round cell liposarcoma to a small-molecule VEGFR-2 tyrosine kinase inhibitor apatinib: A case report. Medicine (Baltimore) 2016;95:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015;9:6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li XF, Tan YN, Cao Y, et al. A case report of gastrointestinal hemorrhage and perforation during apatinib treatment of gastric cancer. Medicine (Baltimore) 2015;94:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 2014;143:141–51. [DOI] [PubMed] [Google Scholar]


