Supplemental Digital Content is available in the text
Keywords: antibiotic-associated colitis, children, hematochezia, Klebsiella oxytoca, proctocolitis
Abstract
Diseases causing hematochezia range from benign to potentially life-threatening. Systematic pediatric data on the causes of hematochezia are scarce. We studied the underlying causes and long-term outcome of hematochezia in children. We further investigated the relevance of antibiotic-associated hemorrhagic colitis in children, especially if caused by Klebsiella oxytoca.
Infants, children, and adolescents with hematochezia were recruited prospectively. Patients were grouped according to age (<1 year, 1–5 years, 6–13 years, >14 years). In addition to routine diagnostics, K oxytoca stool culture and toxin analysis was performed. We collected data on history, laboratory findings, microbiological diagnostic, imaging, final diagnosis, and long-term outcome.
We included 221 patients (female 46%; age 0–19 years). In 98 (44%), hematochezia was caused by infectious diseases. Endoscopy was performed in 30 patients (13.6%). No patient died due to the underlying cause of hematochezia. The most common diagnoses according to age were food protein-induced proctocolitis in infants, bacterial colitis in young children, and inflammatory bowel disease in children and adolescents. Seventeen (7.7%) had a positive stool culture for K oxytoca. Antibiotic-associated colitis was diagnosed in 12 (5%) patients: 2 caused by K oxytoca and 2 by Clostridium difficile; in the remaining 8 patients, no known pathobiont was identified.
Infections were the most common cause of hematochezia in this study. In most patients, invasive diagnostic procedures were not necessary. Antibiotic-associated hemorrhagic colitis caused by K oxytoca was an uncommon diagnosis in our cohort. Antibiotic-associated colitis with hematochezia might be caused by pathobionts other than C difficile or K oxytoca.
1. Introduction
Hematochezia in children and adolescents is a troubling sign for parents, and also for physicians. The spectrum of underlying diseases is diverse and ranges from benign to potentially life-threatening.[1,2] However, its epidemiologic properties have not been studied adequately in children. Only few larger series about causes of hematochezia in pediatric patients exist.[3–10] This prospective study aimed at elucidating the range of disorders causing hematochezia in pediatric patients across all age groups.
A second aim was to investigate the frequency of antibiotic-associated colitis (AAC) presenting with hematochezia in the pediatric population. Antibiotic treatment alters the ecology of the normal intestinal microbiome, which might result in AAC (Clostridium difficile infection [CDI]).[11,12] Extensive antibiotic-associated growth of the bacterium C difficile is widely known as a cause of AAC.[13] Recently Klebsiella oxytoca has been discussed as a novel enteric pathobiont causing a distinct form of AAC, termed antibiotic-associated hemorrhagic colitis (AAHC).[11,14] Our study group was able to identify a toxin produced by K oxytoca as the low-molecular-weight compound tilivalline, which is a unique enterotoxin only found in K oxytoca.[14] In contrast to colitis induced by C difficile, AAHC is usually segmental and predominately located in the right colon, characterized by bloody diarrhea and acute abdominal pain.[11] In children, only few case reports about AAHC caused by K oxytoca exist.[15,16] Currently, no studies about preconditions, incidence, and age pattern of AAHC in pediatric population are available.
2. Material and methods
2.1. Patient cohort and inclusion criteria
From May 2011 to December 2012, pediatric patients aged 0 to 19 years with bloody stool were prospectively recruited at 5 Austrian (nonsurgical) pediatric hospitals. Each hospital had at least 1 pediatric gastroenterologist in charge of patient ascertainment. Patient ascertainment was supported by continuous dissemination of study information among hospital staff, direct referral of children with hematochezia to pediatric gastroenterologists, and by daily clinical staff meetings. Hematochezia (blood per rectum, in or separate from stool) was confirmed by hospital staff (visual stool inspection). A guaiac test (Haemoccult, Beckman Coulter, Germany) was mandatory when hematochezia could not be reliably confirmed by visual inspection.
As a second inclusion criterion and in addition to routine stool diagnostics, a stool culture for K oxytoca was performed. Pediatric patients from oncology wards were not included in our study. After inclusion decisions regarding evaluation and treatment of patients were not influenced by the study. For all patients in this study long-term outcome was documented either by chart review, and if chart review did not give conclusive information, by telephone interview. The time from initial diagnosis to determination of long-term outcome was documented. In all, 237 patients with hematochezia were invited to participate; 15 patients were excluded because hematochezia could not be confirmed, and in 1 case no stool culture for K oxytoca was performed. None of the patients refused to participate in the study. As a control group for infants with suspected food protein-induced proctocolitis (FPIP) we screened 33 otherwise healthy infants (aged 1–6 months) hospitalized for nongastrointestinal (GI) diseases for K oxytoca. The local ethics committee of the Medical University Graz approved our study (23–270 ex 10/11). The study was registered in an international clinical study registry (UMIN clinical trial registry; R000008499).
2.2. Clinical data analysis
Medical history, clinical course, and final diagnosis were documented for all patients. The following clinical and laboratory parameters were assessed: age at onset of symptoms; sex; history; signs and symptoms; medication history; laboratory results (white blood cell count, C-reactive protein [CRP]); stool diagnostics (Campylobacter, Salmonella, Shigella, Yersinia, K oxytoca, C difficile, Adenovirus, Norovirus, and Rotavirus); the need for blood transfusions; imaging studies (ultrasound, colonoscopy).
2.3. Definitions
Patients were grouped into the following groups: infants (<1 year), young children (1–5 years), children (6–13 years), and adolescents (14–19 years).[17]
Antibiotic-associated colitis was diagnosed in patients with bloody diarrhea during or up to 14 days after completion of antibiotic therapy. Patients positive for C difficile toxin and with positive C difficile stool culture were diagnosed with CDI. A positive K oxytoca stool culture, a positive toxin assay and toxin PCR,[11] and the documentation of colitis by sonography were required for the diagnosis of AAHC. Depending on history, clinical symptoms, microbiological findings, imaging results, and the clinical course, we arbitrarily defined the causal association of a given diagnosis and a positive stool culture for K oxytoca as likely, possible, and unlikely.
Infants not appearing sick (aged 1–6 months) with blood-streaked stools and cessation of hematochezia after change of nutrition to either extensively hydrolyzed formula or a cow-milk protein-free nutrition of the infant's mother were diagnosed with suspected FPIP.[18]
The diagnosis “hematochezia with signs of GI infection, infectious work-up negative” was made in patients with obvious signs of a GI infection (fever, abdominal pain, diarrhea, and/or vomiting), an uneventful clinical course and full recovery, but no pathogen detection. The diagnosis “hematochezia without GI infection” was made in patients without clinical signs of GI infection, whose clinical and infectiological work-up was unremarkable and in whom hematochezia ceased spontaneously. As a surrogate for long-term outcome we documented the time from initial inclusion to the final chart review (including follow-up visits of inpatient or outpatient visits, related or unrelated to hematochezia).
2.4. Microbiological diagnostics and analysis of K oxytoca strains
Stool culture was performed for Salmonella, Shigella, Yersinia, Campylobacter, EHEC (Shiga toxin PCR, in case of a positive test result conventional culture for EHEC), and additionally for K oxytoca. To detect C difficile, a combination of GDH and Toxin A/B EIA test (Innsbruck, Wels, Leoben, Oberwart; Alere C. Diff Quik Chek Complete, Alere, Austria; ImmunoCard, Szabo Scandic, Austria) or a Toxin A/B PCR (Graz) was followed by toxigenic culture if positive. Rota, Adeno, and Norovirus were detected by enzyme-linked immunosorbent assay (ELISA). K oxytoca species were identified using the API 20E or VITEK test (bioMerieux, Marcy l’Etoile, France).
2.5. K oxytoca toxin analysis (classical assay/PCR)
We have previously shown that K oxytoca strains causing AAHC produce a cytotoxin (tilivalline).[11,19] The toxin-producing K oxytoca strains possess a pathogenicity island (PAI) encoding genes necessary for tilivalline production.[14] Analysis of toxin production was performed using classical cell culture toxin assay and PCR as previously described (detailed description see Schneditz et al).[14,19,20]
2.6. Statistics
For statistical analysis we used IBM SPSS Statistics 21 (V 21.000, SPSS Inc., Chicago, IL). The Fisher exact test was used to analyze associations between groups. A P value <0.01 was considered statistically significant.
3. Results
3.1. Patient characteristics/clinical features
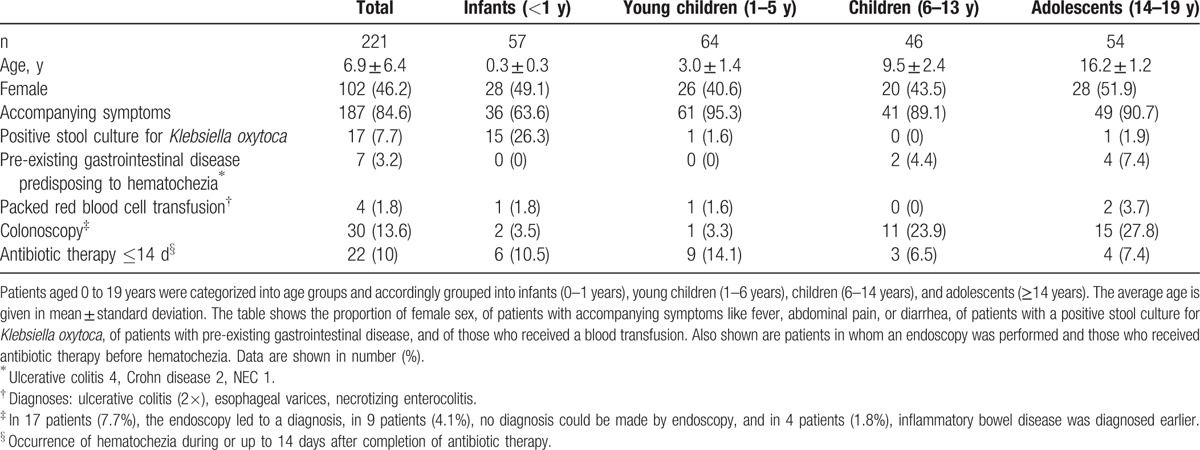
In all, 221 pediatric patients (female, n = 102, 46%) were included in this study (Table 1). All included patients were inpatients at study inclusion. Hematochezia was confirmed by visual inspection only in 143 patients (64,7%), and by visual inspection and positive guaiac test in 78 patients (35,3%). In 2 patients hematochezia can be interpreted as an incidental finding by an underlying coagulation disorder (1 disseminated intravascular coagulation,1 immune thrombocytopenia). Furthermore, in 7 patients an underlying GI condition potentially causing hematochezia was already present at time of study inclusion (1 necrotizing enterocolitis [NEC], 6 inflammatory bowel disease [IBD]). Thirty patients (13.6%) had a pre-existing chronic condition (10 patients with a chronic GI disease, 20 patients with a chronic non-GI disease). Long-term outcome (related or unrelated to hematochezia) was documented in all patients (mean 24 months, SD ± 7.5 months). In 4 patients (1.8%) a packed red blood cell transfusion was administered (Table 1). Colonoscopy was performed in 30 (14.0%) patients. In 17 of these 30 patients endoscopy led to a diagnosis, in 11/30 colonoscopy and histology remained inconclusive, and in 2/30 the diagnosis was already known (repeated ileocolonoscopy in 2 ulcerative colitis [UC] patients). Twenty-seven (90.0%) of the colonoscopies were performed in children and adolescents, 3 in infants and young children (1 infant: signs of GI infection, infectious work-up negative; 1 infant: hematochezia without definitive diagnosis; 1 young child: connective tissue disease/vasculitis). Twenty-two patients (10%) had a positive history of ongoing antibiotic therapy or termination of antibiotic therapy within 14 days of onset of bloody diarrhea. AAC as the underlying cause of hematochezia was determined in 12 of these patients. In the remainder hematochezia was caused by other underlying diseases or pathogens (eg, IBD, Campylobacter, NEC, esophageal varices, disseminated intravascular coagulation).
Table 1.
Patient characteristics categorized by age group.
3.2. Frequency of underlying diagnoses in pediatric patients presenting with hematochezia
Infectious diseases were a common cause of hematochezia in all age groups (Fig. 1, Table 2). Campylobacter enterocolitis was the most frequent diagnosis (n = 48, 21.8%) followed by signs of GI infection, infectious work-up negative (n = 31, 14.0%), and Salmonella enterocolitis (n = 29, 13.1%). IBD (n = 21, 9.5%; 13 ulcerative colitis, 8 Crohn disease), hematochezia without definite diagnosis (n = 20, 9.0%), and suspected FPIP (n = 19, 8.6%) occurred with comparable frequency. Norovirus enterocolitis-associated hematochezia was found in 10 patients (5.4%). Anal fissure (n = 5, 2.3%) and intussusception (n = 5, 2.3%) were uncommon diagnoses in our patient cohort. IBD was not diagnosed in infants and young children, intussusception (n = 5, 2.3%) was only diagnosed in these 2 age groups. Diseases diagnosed only twice or once in our patient cohort are listed in Table 3.
Figure 1.
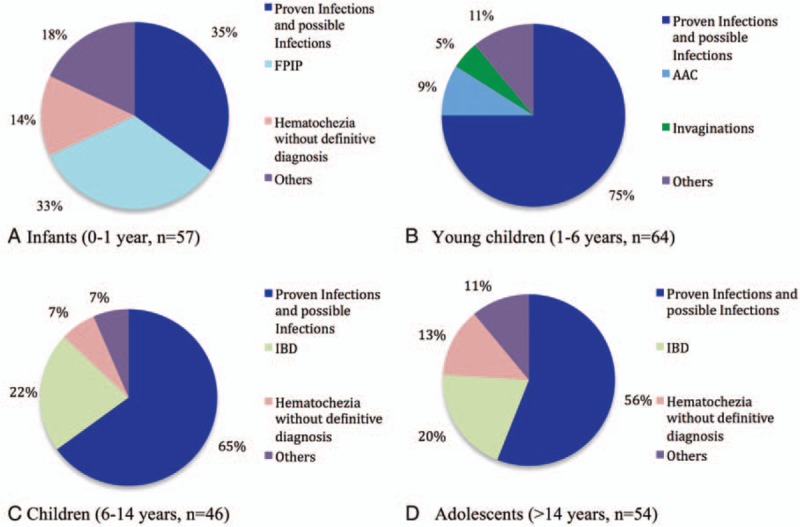
The 3 most common diagnoses versus less common diagnoses (Others, diseases diagnosed twice or once in the respective age group) in infants, young children, children, and adolescents with the leading symptom of hematochezia. Patients with infectious causes were pooled together (infectious diseases listed in order of frequency: Campylobacter; signs of GI infection, infectious work-up negative; Salmonella; norovirus; AAHC; CDI; Shigella; adenovirus; rotavirus; EHEC; hantavirus). AAC = antibiotic-associated colitis, AAHC = antibiotic-associated hemorrhagic colitis, CDI = Clostridium difficile infection, EHEC = entero-hemorrhagic Escherichia coli, FPIP = food protein-induced proctocolitis, IBD = inflammatory bowel disease.
Table 2.
Relative frequency of different diagnoses in children and adolescents (0–19 years) with the leading symptom of hematochezia.
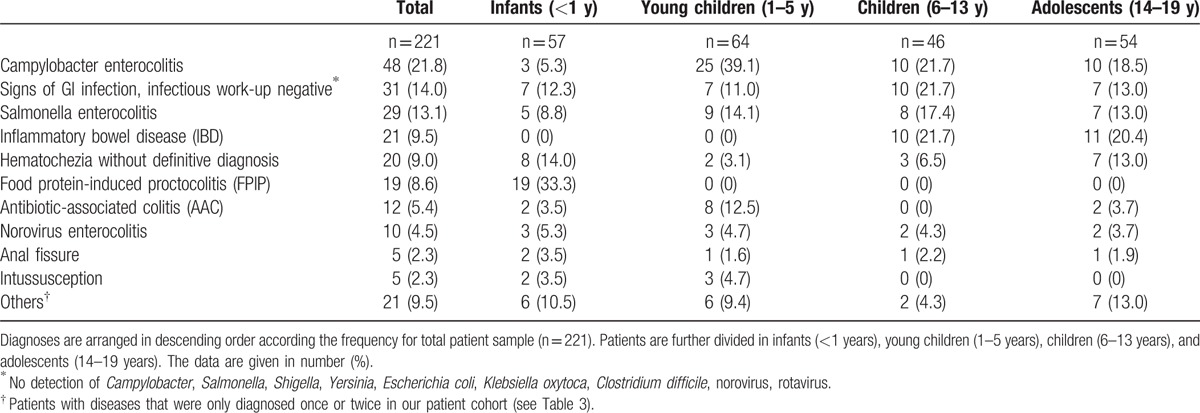
Table 3.
Other diseases in our patient cohort with leading symptom of hematochezia.

3.3. Infants (<1 year)
Suspected FPIP was diagnosed in 19 infants (33.3%). In 9 (47.7%) of the patients diagnosed with suspected FPIP, the stool culture for K oxytoca was positive. When comparing these 19 infants with suspected FPIP with 33 healthy control infants (17 breastfeeding difficulties, 10 mother–child interaction problems, 6 inconsolable crying under observation), only 4 (12%) had a positive stool culture for K oxytoca (P < .01). Other frequent diagnoses in infants were hematochezia without GI infection (n = 8, 14%) and hematochezia with signs of GI infection, infectious work-up negative (n = 7, 12.3%). Salmonella enterocolitis (n = 5, 8.8%) and Campylobacter enterocolitis (n = 3, 5.3%) were less frequent in infants compared with other age groups. NEC (n = 2, 3.5%) and AAHC (n = 2, 3.5%) were diagnosed in infants only.
3.4. Young children (1–5 years)
The most frequent cause of hematochezia in patients aged between 1 and 5 years was Campylobacter enterocolitis (n = 25, 39.1%), followed by Salmonella enterocolitis (n = 9, 14.1%), AAC (n = 8, 12.5%), and hematochezia with signs of GI infection (infectious work-up negative) (n = 7, 11.0%). In other age groups AAC was an uncommon diagnosis (n ≤ 2).
3.5. Children (6–13 years)
Campylobacter enterocolitis, hematochezia with signs GI infection (infectious work-up negative), IBD (each n = 10, 21.7%), and Salmonella enterocolitis (n = 8, 17.4%) were the most frequent diagnoses in children between 6 and 13 years of age. No patient of this age group was diagnosed with AAC.
3.6. Adolescents (14–19 years)
Inflammatory bowel disease (n = 11, 20.4%) (UC n = 7, 12.7% or Crohn disease n = 4, 7.4%) and Campylobacter enterocolitis (n = 10, 18.5%) were the most frequent diagnoses in patients >14 years, followed by hematochezia with signs of GI infection (infectious work-up negative), Salmonella enterocolitis, and hematochezia without GI infection (each n = 7, 13%). Within our patient cohort, only 1 adolescent was diagnosed with hemorrhoids.
3.7. Patients with AAC
The diagnosis of AAC was made in 12 patients (5.4%, male-to-female ratio 2:1; 2 infants, 8 young children, 2 adolescents) (Supplemental Digital Content [SDC] 1: Table 4). Two infants fulfilled the criteria for AAHC including a positive stool culture for K oxytoca and toxin detection (<1% of total patient cohort with hematochezia; 16.7% of AAC patients). Two young children (16.7% of AAC patients) were diagnosed with CDI. In 8/12 patients (66.7%) with the clinical diagnosis of AAC, neither C difficile nor K oxytoca could be detected (6 young children, 2 adolescents). Differences in symptoms between patients with AAHC and CDI could not be detected. In 9/12 (75%) patients, hematochezia had started during antibiotic therapy. The duration of initial antibiotic intake until start of bloody stools averaged 6.6 days. The triggering antibiotic treatments was heterogeneous; however, all patients had been treated with beta-lactam antibiotics. In addition to hematochezia, diarrhea, abdominal pain, vomiting, and fever were common symptoms. After termination or change of antibiotic therapy hematochezia resolved in all cases. In none of the patients diagnosed with AAC colonoscopy was performed.
3.8. Patients with positive K oxytoca stool culture
In 17 (7.7%) of all patients with hematochezia (female n = 6, 35.3%; male n = 11, 64.7%), the stool culture for K oxytoca was positive (SDC 2: Table 5). Of these patients, 15 (88.2%) were infants. Among patients with a positive stool culture for K oxytoca, 9 (52.9%) were diagnosed with suspected FPIP, 2 (11.8%) with AAHC, and the remaining 6 (29.4%) with diseases diagnosed only once in our patient cohort (SDC 2: numbers 13–17). In 1 case, due to a complex history and postoperative course after heart surgery, a definitive diagnosis as the cause of hematochezia could not be made (SDC 2: number 12). Seven (41.2%) patients with positive K oxytoca stool culture had received antibiotic therapy before the onset of hematochezia. K oxytoca strains could be retained for further analysis in 15 (88.2%) cases. In 11 of these 15 K oxytoca strains, toxin production was demonstrated by classical toxin assay and PCR assay. In view of patient history, clinical course, and K oxytoca toxin result, a possible causal association of K oxytoca and hematochezia was defined as likely in both cases of AAHC, as possible in all patients with suspected FPIP, and also in patient number 12 and 13 because of medical history, and as unlikely in the remaining patients.
4. Discussion
Although hematochezia is a sign frequently encountered in clinical practice, there are only a few prospective studies about the frequency, age distribution, and differential diagnoses causing bloody stools in pediatric patients.[3–10,21] Our study includes 221 pediatric patients (0–19 years), which were recruited in a (nonsurgical) pediatric setting. An even distribution of patients across age groups in our cohort allows comparison of the frequency of diagnoses according to age. Surprisingly, there are only few prospective studies investigating causes of hematochezia in the pediatric population with a comparable number of patients.[3,5,8–10] Most reports used very different definitions for pediatric age groups, differed in the way patients were recruited, in how clinical diagnoses were defined, or in how clinical data were acquired and documented. Furthermore, most studies have an uneven distribution of patients according to pediatric age groups. Taken together, these facts make comparisons between these series, including our own, difficult, if not impossible.
In our patient cohort, GI infection was the most common cause of hematochezia across all age groups. Cucchiara et al,[7] Yachha et al,[4], and Teach and Fleisher[6] described infectious colitis as 1 of the most common causes of hematochezia. Spencer,[5] Uno et al,[21] and Moravej[9] did not mention this illness, although some of these studies recruited patients in a nonsurgical setting. Some diagnoses causing hematochezia (eg, suspected FPIP, IBD) are correlated to certain age groups, which is reflected in our results. In our cohort, suspected FPIP was 1 of the most common diagnoses in infants (33.3%). Teach et al,[6] Cucchiara et al,[7] and Yaccha et al[4] diagnosed suspected FPIP in between 2.2% and 16.4% of their pediatric patient cohort. Studies that recruited in a pediatric surgical setting did not mention this diagnosis.[3,5,21,22] In contrast, IBD was a frequent diagnosis in children and adolescents. Apart from de Ridder et al, none of the other studies about causes of hematochezia mentioned IBD in a comparable number of patients.[3,4,6,7,9,10,21] This might be explained by younger patient cohorts[3,4,7,10,21] and/or high proportions of infants and young children in these studies.[3,4,6,7,9,10,21] Two studies from a surgical department observed intussusception as 1 of the most common diagnoses.[6,21] Studies from pediatric departments (ie, nonsurgical setting), in accordance to our results, did not mention this condition.[4,7–10] These examples underline the need for exact definitions of clinical settings (eg, nonsurgical vs surgical), patient cohort, age groups, and diagnoses for future studies in pediatric patients with hematochezia. Our observations and attempts to compare with existing literature raise the question if current reviews on the causes of hematochezia in children need to be revised according to patient cohort and age.[23–27]
Life-threatening diseases such as intussusception, NEC, or esophageal varices were uncommon diagnoses within our study cohort. None of our patients died due to the underlying cause of hematochezia. In general, our observations suggest that hematochezia in nonsurgical pediatric patients is not a life-threatening symptom in the majority of cases. It is currently unclear whether and when invasive diagnostic procedures (eg, endoscopy) are necessary in pediatric patients with hematochezia. Several studies using endoscopy in pediatric patients with hematochezia identified polyps as 1 of the most frequent diagnoses.[3,7,8,10,28,29] In our pediatric internistic patient cohort, polyps were identified as the cause of hematochezia only twice. It is unlikely that our restrictive use of endoscopy led to underdiagnosing polyps, because hematochezia does not spontaneously resolve in these patients. GI infection, the most frequent cause of hematochezia in our cohort, and suspected FPIP, the most common diagnosis in infants, do not require acute invasive diagnostic.[30,31] It might therefore be concluded that in a nonsurgical pediatric setting, endoscopy should be used restrictively in patients with hematochezia, especially in infants and young children.
Antibiotic-associated diarrhea constitutes 1 of the most frequent side effects of antimicrobial therapy with variable clinical presentation, ranging from mild self-limiting diarrhea to severe AAC.[32–34] AAC was assumed to be mainly caused by infection with toxin-producing C difficile strains.[35] Previously, our study group has shown that AAHC, a distinct form of AAC where C difficile is absent, is caused by K oxytoca.[11] Whereas AAC due to C difficile goes along with bloody diarrhea in only 14% of patients,[36] hematochezia is a main feature in all patients with K oxytoca-associated AAHC.[20] In our cohort, AAC with bloody diarrhea was diagnosed in 5.4% of patients; most of these patients were young children. AAHC caused by K oxytoca in pediatric patients is largely unexplored. Currently, only 3 case reports are available in the literature.[15,16] Within our study cohort, only 2 infants fulfilled the clinical presentation of AAHC (bloody diarrhea, acute abdominal pain after antibiotic treatment, detection of toxin-producing K oxytoca). We conclude that AAHC caused by K oxytoca in pediatric patients is an uncommon diagnosis, comparable with the frequency of Shigellosis or EHEC infection in northern Europe.[37,38] Nonetheless, AAHC should be considered as a differential diagnosis in pediatric patients with hematochezia and a history of antibiotic treatment. Thus, by including K oxytoca into routine stool diagnostics, unnecessary invasive diagnostics and/or drug treatment could be avoided in children.
A surprising observation was the presence of AAC in the absence of known bacterial or viral enteropathogens (including K oxytoca and C difficile) in 8 children accounting for 67% of all AAC cases. Although the occurrence of AAC in the absence of established enteropathogens has been observed in previous studies with adults[11] and children,[32,39] this finding is usually not discussed by the authors. We therefore want to point out that apart from AAC due to K oxytoca or C difficile, there is a syndrome of colitis due to antibiotics not caused by these pathogens.[40,41] This entity, which we term AAC of unknown origin (AACU), needs to be investigated in future studies because neither pathogenesis nor clinical, endoscopic, and histologic features have been described in detail so far. Alterations in the intestinal microbiome by antibiotics leading to dysbiosis and overgrowth of pathobionts,[40,42] which in return results in colitis, seems to be an attractive hypothesis for the pathogenesis of AACU. This hypothesis has to be evaluated in larger patient cohort with AAC in children and also in adults.
Most of our patients with a positive stool culture of K oxytoca were infants. In our study, more than 25% of all infants with hematochezia were positive for K oxytoca. In the other age groups, stool positivity for K oxytoca was uncommon or not present. Currently, there are no published data about the presence of K oxytoca in stool of healthy children. In our study, K oxytoca was found in 12% of healthy infants. Högenauer et al[11] tested stool samples from healthy adults for K oxytoca: only 1.6% were positive. Similar to reports of high colonization rates of C difficile in infants,[43] our results suggest that colonization rates of K oxytoca might be higher in infants compared with other age groups. Further investigations with larger pediatric cohorts including all age groups are necessary to make an assertion about the natural enteric colonization with K oxytoca in the pediatric population. Most of the infants in our cohort of patients positive for K oxytoca were suspected to have FPIP. Our data on K oxytoca positivity in healthy infants make this observation statistically significant. The relevance of this observation has to be further investigated.
Our study has several limitations: First, we termed a large patient group “hematochezia with signs of GI infection, infectious work-up negative” (refer “Definitions” subsection above). Whereas from a clinical point of view, these patients were interpreted as having acute GI infection, routine infectious work-up was negative. We cannot rule out that these patients had rare infectious diseases that we did not test for (eg, non-STEC Escherichia coli pathotypes, rare enteric viruses) or even noninfectious diseases (eg, toxic exposure). Second, at study initiation, due to our previous experience with AAHC and K oxytoca in children and also in adults, we decided not to include oncology ward patients. Therefore, this patient group and their possible causes of hematochezia (eg, typhlitis, graft-vs-host disease) are not represented in our study. Third, we did not routinely test for Entamoeba histolytica in our patients, a disease that often causes hematochezia. This disease is very uncommon in Austria. Since we did not interfere with clinical decision-making, it was optional for clinicians to order testing for E histolytica, given, for example, a travel history or contact with an index patient. However, in our study cohort, no test for E histolytica was ordered. Given these points to consider, we argue that it is unlikely that E histolytica infection was common in our cohort or would significantly change our results. Fourth, there is no clear definition of the time frame for a causal relationship of a previous antibiotic therapy with the occurrence of AAC. The risk for CDI is highest after 1 month, but is still increased even 3 months after antibiotic therapy[44]. As we assessed previous antibiotic therapy only within 14 days before the occurrence of hematochezia, it is possible that we underestimated cases of antibiotic-associated colitis in our study. Finally, since we only report on children in Austria, our data have to be interpreted with caution in other geographic regions.
5. Conclusions
In conclusion, our large nonsurgical pediatric cohort provides important information about the frequency of different diseases causing hematochezia across pediatric age groups. The frequency of AAHC caused by K oxytoca, at least for the northern European regions, is comparable with rare GI infections such as Shigellosis or EHEC infection. Our data support the conclusion that AAC presenting with hematochezia, apart from C difficile and K oxytoca, might be caused by other yet unidentified pathobionts.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AAC = antibiotic-associated colitis, AACU = antibiotic-associated colitis of unknown origin, AAHC = antibiotic-associated hemorrhagic colitis, CDI = Clostridium difficile infection, FPIP = food protein-induced proctocolitis, GI = gastrointestinal, IBD = inflammatory bowel disease, NEC = necrotizing enterocolitis, UC = ulcerative colitis.
Funding: This prospective multicenter study was supported by the nonprofit organization for pediatric patients INVITA. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors have no conflict of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bakarat M, El-Kady Z, Mostafa M, et al. Antibiotic-associated bloody diarrhea in infants: clinical, endoscopic, and histopathologic profiles. J Pediatr Enterol Nutr 2011;52:60–4. [DOI] [PubMed] [Google Scholar]
- [2].Friedlander J, Mamula P. Wyllie R, Hyams JS, Kay M. Gastrointestinal hemorrhage. Pediatric Gastrointestinal and Liver Disease 4th ed.Philadelphia, PA: Saunders, Elsevier; 2011. 146–53. [Google Scholar]
- [3].Mandhan P. Sigmoidoscopy in children with chronic lower gastrointestinal bleeding. J Paediatr Child Health 2004;40:365–8. [DOI] [PubMed] [Google Scholar]
- [4].Yachha SK, Khanduri A, Sharma BC, et al. Gastrointestinal bleeding in children. J Gastroenterol Hepatol 1996;11:903–7. [PubMed] [Google Scholar]
- [5].Spencer R. Gastrointestinal hemorrhage in infancy and childhood: 476 cases. Surgery 1964;55:718–34. [PubMed] [Google Scholar]
- [6].Teach SJ, Fleisher GR. Rectal bleeding in the pediatric emergency department. Ann Emerg Med 1994;23:1252–8. [DOI] [PubMed] [Google Scholar]
- [7].Cucchiara S, Guandalini S, Staiano A, et al. Sigmoidoscopy, colonoscopy, and radiology in the evaluation of children with rectal bleeding. J Pediatr Gastroenterol Nutr 1983;2:667–71. [DOI] [PubMed] [Google Scholar]
- [8].de Ridder L, van Lingen AV, Taminiau JA, et al. Rectal bleeding in children: endoscopic evaluation revisited. Eur J Gastroenterol Hepatol 2007;19:317–20. [DOI] [PubMed] [Google Scholar]
- [9].Moravej H, Dehghani SM, Hamideh N, et al. Lower gastrointestinal bleeding in children: experiences from referral center in southern Iran. J Comprehensive Pediatr 2013;3:115–8. [Google Scholar]
- [10].Motamed F, Najafi M, Khodadad A, et al. Colonoscopic findings in children with lower gastrointestinal bleeding. Govaresh 2008;13:54–7. [Google Scholar]
- [11].Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med 2006;355:2418–26. [DOI] [PubMed] [Google Scholar]
- [12].McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis 1998;16:292–307. [DOI] [PubMed] [Google Scholar]
- [13].Kelly CP, LaMont JT. Clostridium difficile: more difficult than ever. N Engl J Med 2008;359:1932–40. [DOI] [PubMed] [Google Scholar]
- [14].Schneditz G, Rentner J, Roier S, et al. Enterotoxicity of a nonribosomal peptide causes antibiotic-associated colitis. Proc Natl Acad Sci U S A 2014;111:13181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hoffmann KM, Deutschmann A, Weitzer C, et al. Antibiotic-associated hemorrhagic colitis caused by cytotoxin-producing Klebsiella oxytoca. Pediatrics 2010;125:e960–3. [DOI] [PubMed] [Google Scholar]
- [16].Shinjoh M, Iwata S, Takahashi T. Klebsiella oxytoca-positive, penicillin-associated hemorrhagic enterocolitis in children. Pediatr Int 2010;52:132–3. [DOI] [PubMed] [Google Scholar]
- [17].Kerbl R, Kurz R, Roos R, et al. Checkliste Pädiatrie. Stuttgart, Germany: Thieme; 2011. [Google Scholar]
- [18].Lake AM. Food-induced eosinophilic proctocolitis. J Pediatr Gastroenterol Nutr 2000;30(suppl):S58–60. [DOI] [PubMed] [Google Scholar]
- [19].Joainig MM, Gorkiewicz G, Leitner E, et al. Cytotoxic effects of Klebsiella oxytoca strains isolated from patients with antibiotic-associated hemorrhagic colitis or other diseases caused by infections and from healthy subjects. J Clin Microbiol 2010;48:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zollner-Schwetz I, Hogenauer C, Joainig M, et al. Role of Klebsiella oxytoca in antibiotic-associated diarrhea. Clin Infect Dis 2008;47:e74–8. [DOI] [PubMed] [Google Scholar]
- [21].Uno T, Harada Y, Kimura T, et al. Investigation of melena and hematochezia as the chief complaint of gastrointestinal bleeding in pediatric surgical patients. Acta Paediatr Jpn 1994;36:268–71. [DOI] [PubMed] [Google Scholar]
- [22].Sherman NJ, Clatworthy HW., Jr Gastrointestinal bleeding in neonates: a study of 94 cases. Surgery 1967;62:614–9. [PubMed] [Google Scholar]
- [23].Treem WR. Gastrointestinal bleeding in children. Gastrointest Endosc Clin N Am 1994;4:75–97. [PubMed] [Google Scholar]
- [24].Vinton NE. Gastrointestinal bleeding in infancy and childhood. Gastroenterol Clin North Am 1994;23:93–122. [PubMed] [Google Scholar]
- [25].Roy CC. Pediatric Clinical Gastroenterology. St. Louis, MO: Mosby; 1995. [Google Scholar]
- [26].MacMillan JA, Oski FA. Oskis Pediatrics. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- [27].Fleisher GR. Textbook of Pediatric Emergency Medicine. Baltimore, MD: Williams & Wilkins; 1993. [Google Scholar]
- [28].Quak SH, Prabhakaran K. Colonoscopy in children with bleeding per rectum. Singapore Med J 1990;31:454–6. [PubMed] [Google Scholar]
- [29].El-Mouzan MI, Abdullah AM. Yield of colonoscopy in children with rectal bleeding. Saudi Med J 2004;25:998–1001. [PubMed] [Google Scholar]
- [30].Speer C, Gahr M. Pädiatrie. Berlin, Heidelberg, Germany: Springer; 2013. [Google Scholar]
- [31].Rodeck B, Zimmer K. Pädiatrische Gastroenterologie, Hepatologie und Ernährung. Berlin, Heidelberg: Springer; 2013. [Google Scholar]
- [32].Kato S, Ebina K, Ozawa A, et al. Antibiotic-associated hemorrhagic colitis without Clostridium difficile toxin in children. J Pediatr 1995;126:1008–10. [DOI] [PubMed] [Google Scholar]
- [33].Kim J, Smathers SA, Prasad P, et al. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001–2006. Pediatrics 2008;122:1266–70. [DOI] [PubMed] [Google Scholar]
- [34].McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol 2008;3:563–78. [DOI] [PubMed] [Google Scholar]
- [35].Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002;346:334–9. [DOI] [PubMed] [Google Scholar]
- [36].Hoegenauer C, Hinterleitner T. Scheld WL, Hammer SM, Hughes JM. Klebsiella oxytoca as a cause of antibiotic-associated colitis. Emerging Infections 8. Washington, DC: ASM Press; 2008. 293–311. [Google Scholar]
- [37].Aboutaleb N, Kuijper EJ, van Dissel JT. Emerging infectious colitis. Curr Opin Gastroenterol 2014;30:106–15. [DOI] [PubMed] [Google Scholar]
- [38].Institut für Hygiene MuU. Resistenzbericht. Graz: Institut für Hygiene, Mikrobiologie und Umweltmedizin, Labor für Medizinische Bakteriologie und Mykologie; 2013. [Google Scholar]
- [39].Barakat M, El-Kady Z, Mostafa M, et al. Antibiotic-associated bloody diarrhea in infants: clinical, endoscopic, and histopathologic profiles. J Pediatr Gastroenterol Nutr 2011;52:60–4. [DOI] [PubMed] [Google Scholar]
- [40].Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008;6:e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gorkiewicz G. Nosocomial and antibiotic-associated diarrhoea caused by organisms other than Clostridium difficile. Int J Antimicrob Agents 2009;33(suppl 1):S37–41. [DOI] [PubMed] [Google Scholar]
- [42].Stecher B, Maier L, Hardt WD. ‘Blooming’ in the gut: how dysbiosis might contribute to pathogen evolution. Nat Rev Microbiol 2013;11:277–84. [DOI] [PubMed] [Google Scholar]
- [43].Faust SN, Wilcox MH, Banaszkiewicz A, et al. Lack of evidence for an unmet need to treat Clostridium difficile infection in infants aged <2 years: expert recommendations on how to address this issue. Clin Infect Dis 2015;60:912–8. [DOI] [PubMed] [Google Scholar]
- [44].Hensgens MP, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012;67:742–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


