Abstract
Rationale:
Luminal subtype breast cancer, accounting for 70 to 80% of all breast cancers, has been reported to be associated with good prognosis. However, for the patients with large mass or worse mass position, omental flap transplantation may provide a new option for breast reconstruction.
Patient concerns:
Ten patients (6 luminal B1, 2 luminal B2, 2 luminal A), were enrolled into the study, between January 23, 2015 and August 22, 2016. The mean age was 34.6 ± 6.96 (24-44) years old. Immunohistochemistry demonstrated that the tumor cells were positive for estrogen receptor and progestrone receptor.
Diagnoses:
According to the clinicopathological features, diagnosis of breast cancer patients were made.
Interventions:
Breast-conserving surgery, laparoscopic greater omentum harvest and vascular anas-tomosis were carried out orderly. Postoperative operative results, cosmetic outcomes, complications, as well as blood supply were investigated for surgery evaluation. Reasonable chemotherapy and irradia-tion were adopted to patients according to the pathological condition.
Outcomes:
We successfully accomplished breast reconstruction by omental flap transplantation, ex-cept one failed case because of the necrosis of omentum and changed to fat transplantation. The volumes and symmetry of breasts were all satisfied. The blood supply was detected to be fluent. Only one case of slight hematoma and another case of one distant metastasis were observed during fol-low-up period. No arm mordities or arm movement restriction occurred after surgery. Moreover, radia-tion therapy and chemotherapy had no clear effects on the reconstructed breast.
Lessons:
Immediate breast reconstruction surgery by transplanting omental flap for luminal breast cancer patients can be considered successful based on the excellent clinic outcome.
Keywords: breast-conserving surgery, immediate breast reconstruction, luminal breast cancer, omental flap
1. Introduction
Breast cancer is recently one of the commonest cancers in China, with more than 1.6 million people being diagnosed and 1.2 million people dying each year, accounting for 12.2% of all newly diagnosed breast cancers and 9.6% of all breast cancer-related deaths worldwide.[1] Recently, gene expression profiling has reshaped our understanding of breast cancer, characterizing breast cancer as 4 main intrinsic molecular subtypes: human epidermal growth factor receptor 2-enriched, basal-like, luminal A, and luminal B subtypes.[2] Luminal subtype breast cancer, accounting for 70% to 80% of all breast cancers, has been reported to be associated with a very good prognosis.[3] However, more than 1/2 of ethnic Chinese breast cancer patients experienced high-level depression due to the poor cosmetic results, which may lead to poor treatment outcome and undesirable quality of life.
Despite the significant advances in early detection, medical therapy, and multidisciplinary rehabilitation for breast cancer, surgical procedure still remains the general first-line treatment.[4] Breast-conserving therapy, mastectomy, and mastectomy with breast reconstruction (immediate or delayed) are available to breast cancer patients. As reported, breast conservative surgery along with beast reconstruct techniques is now considered as an essential component in making up the breast cosmetic deficiency, especially for the patients in early stage.[5] Meanwhile, immediate autologous breast reconstruction has been proved to achieve better psychosocial well-being, esthetic outcome, and patient/physician satisfaction.[6] But the decision of which donor tissue to use in reconstruction may have an impact on a subsequent reconstruction. Autologous fat grafting, where the patient's own fat is harvested using a liposuction technique and then transplanted into the breast, was demonstrated with plenty of advantages when compared with other biomaterials.[7] Bilateral simultaneous breast reconstruction with transverse musculocutaneous gracilis flaps displayed relative shorter operating time, similar complication rates, and high patient satisfaction levels.[8] Similarly, inferior dermal flap, serratus anterior fascia, and the superficial pectoral fascia flaps can be served as an excellent single-stage reconstruction option for women with ptotic breast.[9,10] The superior gluteal artery perforator flap is also a possible choice for autologous breast reconstruction and autologous skin can be provided for inadequate skin coverage.[11,12] Despite the promising application, we have to notice that not all patients are ideal candidates, clinicians should take inadequate volume of donor sites into consideration.[13] Beyond that it is hard to guarantee the survival of implanted tissue, the following morbidity and cancer recurrence may be triggered after the pedicled transplantation.
The abdomen has been long applied as the preferred donor site in breast reconstruction.[14] The greater omentum, an original visceral flap possessing unique properties of localizing and healing inflammatory processes, offers great possibilities to repair the complex defects.[15] Pedicled greater omentum graft was originally served to repair recurrent urinary fistulae after kidney transplantation, yet with some inevitable boundedness and compliance, such as incisional hernia and even necrosis.[16] Besides, breast reconstruction by pedicled greater omentum can lengthen the subcutaneous tunnel. The migration of omentum in abdominal may significantly reduce the utilization efficiency of omentum. Hence, omental flap transplantation may be available, avoiding the above deficiencies. As reported, free omental flap is a safe procedure with minimal donor-site morbidity and deformity, and may be served as additional option for breast reconstruction.[17] But the prognosis was not taken into consideration, as well as the compatibility of free omentum after reconstruction.
Presently, we asserted that free omentum transplantation for breast reconstruction might be more available for the luminal breast cancer patients with good prognosis. So far, no exploration has been proceeded according to the subtype of patients. To investigate the availability of the reconstructive strategy to luminal breast cancer patients, we reported 10 cases of breast reconstruction with omental flap. Operative results, cosmetic outcomes, complications, and blood supply were all evaluated as research endpoints.
2. Methods
2.1. Patients
Ten patients (6 luminal B1, 2 luminal B2, 2 luminal A) were enrolled into the study between January 23, 2015 and August 22, 2016. The baseline characteristics were listed in Table 1. The mean age was 34.6 ± 6.96 (24–44) years old. Inclusion criteria: age 18 to 60 years old; diagnosed with luminal subtypes; patients rejected to use prosthesis for breast reconstruction; and patients with high demand for breast appearance. Before surgery, all appropriate breast reconstruction options were offered and discussed with patients. In addition, we had sufficient communication with the recipient about the risk, benefit of surgery, and possible complications. The final decision was voluntary and the participants could change their choice at any time before surgery. Enrolled patients had strong demand for surgery and their family gave written consent, we also achieved the approval from the ethics committee of Xi-Jing Hospital.
Table 1.
Patients’ characteristics.
2.2. Procedure
Breast-conserving surgery, laparoscopic greater omentum harvest, and vascular anastomosis were carried out orderly. The omentum was harvested laparoscopically, following breast-conserving surgery (Fig. 1). With the removal of resected omentum, ice protection fluid was used to pure into free omentum from right gastric omentum artery. The right arteriovenous vein of stomach omentum was dissected carefully under microscope. Before transplantation, front latissimus dorsi, as well as the right thoracic dorsal artery and accompanying vein were all separated. Greater omentum was filled to shape the reconstructed breast, when vascular anastomosis between right stomach omentum arteriovenous and right thoracic dorsal arteriovenous done (Fig. 2).
Figure 1.
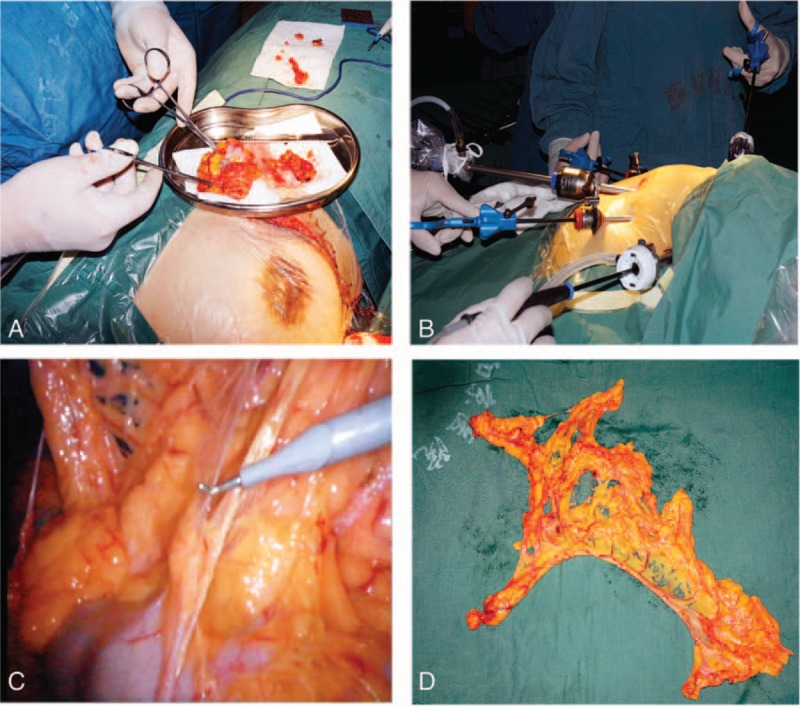
Breast-conserving surgery and laparoscopic-guided free of greater omentum. (A) Mastectomy and the dissected tumor. (B) Laparoscopic abdominal operation for the greater omentum isolation. (C) The harvest of greater omentum under laparoscope. (D) The whole omental flap.
Figure 2.
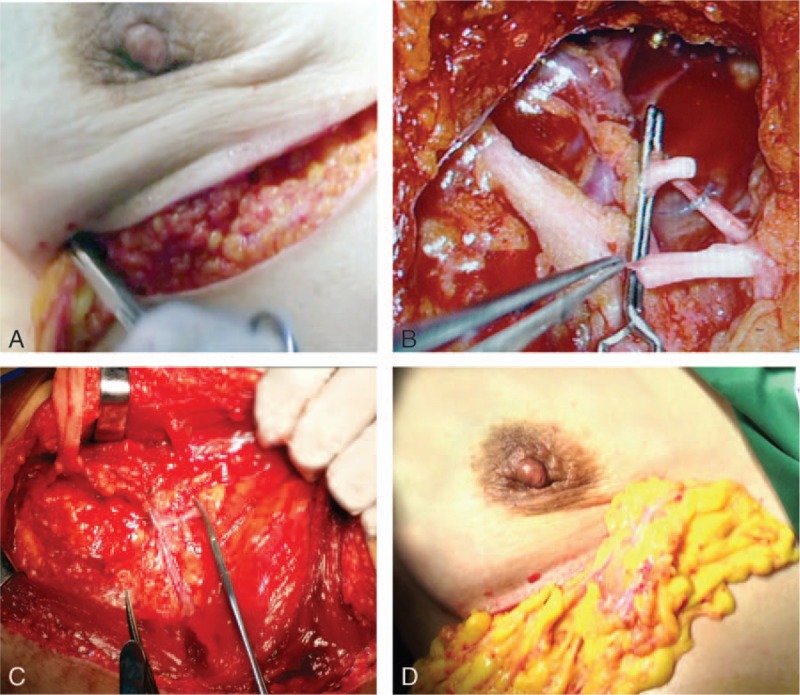
Auto-transplantation of free omentum. (A) Transplantation of free omentum to the position of vascular anastomosis. (B and C) End-to-end vascular anastomosis. (D) The padding of deficient breast after vascular anastomosis.
2.3. Postoperative evaluation and treatment
Postoperatively, the dynamic oxygen pressure curve and CDUA outcomes were used to monitor the oxygen partial pressure and blood supply. Cosmetic results were evaluated according to the Harris criteria. Four-point scale was formulated to evaluate the cosmetic score as excellent (size and shape of reconstructed breast are identical to the original breast); good (deformity of the reconstructed breast involved <1/4 of the original breast; fair (deformity of the reconstructed breast involves <1/4 to 1/2 of the original breast); and poor (breast deformity involves >1/2 of the original breast). Furthermore, the operating time, postoperative hospital days, complications, and blood loss were all investigated for therapeutic assessment.
Following the National Comprehensive Cancer Network guidelines, irradiation was proceeded to all patients after surgery, to effectively inhibit the cancer. Moreover, we adopt chemotherapy for patients after surgery (Table 2).
Table 2.
Operative results of patients after surgery.
2.4. Blood supply measurement
The transplanted tissue failure is mainly the result of impaired blood supply. Transcutaneous oxygen tension measurement (TcPO2) as well as the CDUA is beneficial for blood supply detection. The standard value of ideal blood supply was 40 mm Hg.
3. Results
3.1. Operative results
As shown in Table 2, the total operation time was 373.5 ± 59.35 (295–465) min. The average blood loss was 108.0 ± 54.53 (30–200) mL. All patients were negative in pathological detection of margin. Postoperatively, the patients were discharged approximately 7.70 ± 4.72 (4–20) days.
3.2. Cosmetic results
Successful breast reconstruction includes the creation of a natural breast mound in addition to achieving maximal symmetry of both breasts. Here, we successfully accomplished 9 cases with good satisfaction. Only 1 case was suspended because of the intraoperative necrosis of free omentum and changed to fat transplantation. As shown in Fig. 3A and B, the overall and regional breast volumes were recovered well. Pre- and postoperative volume evaluation of breast mound displayed desired volume persistence. Symmetry characteristics were satisfactory. No phenomenon of invagination and abnormal secretion were found in bilacteral nipple. Outwardly, the reconstruct breast looks even more artistic than the contralateral breast. Postoperatively, incision on the donor site and acceptor site was inconspicuous and recovered well. The incision on axilla was covert, masking the esthetically displeasing new scars and no deformity was found in axilla (Fig. 3C and D). Most patients were satisfied with reconstructed breast, the cosmetic scores were all up to good level (Table 2).
Figure 3.
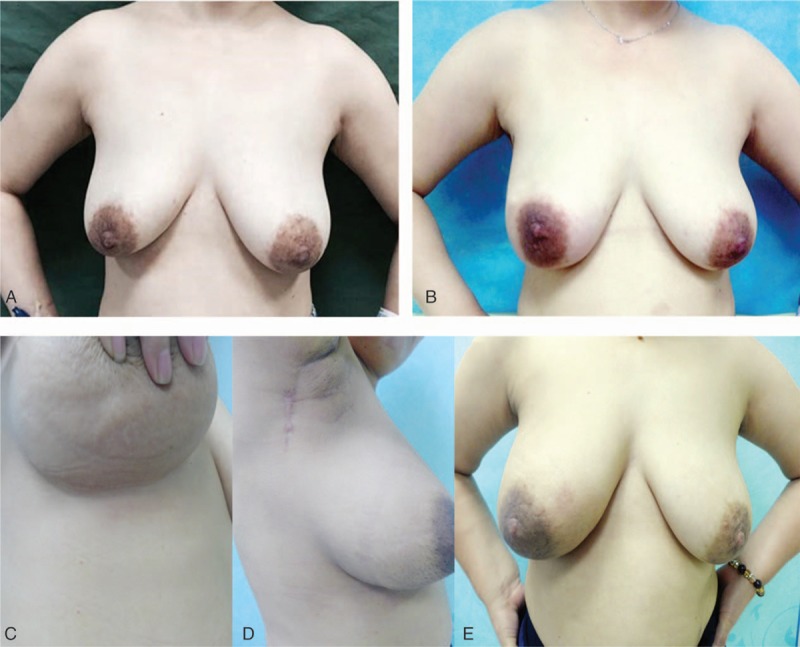
Postoperative recovery and wound healing of reconstructed breast. (A) The patient before surgery. (B) The size and shape of the reconstructed breast are almost the same as those of original breast. (C) The scars on the breast are not obvious. (D) The scars on the axillar are covert and no axillary deformity occurred in the affected upper limb. (E) No size reduction was found in reconstructed breast after chemotherapy.
3.3. Complications
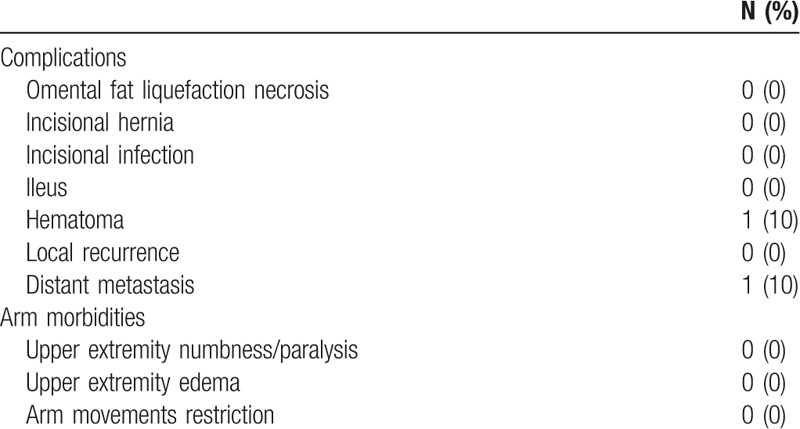
Postoperative complications significantly affect the life quality of patients. At present, the abdominal organs are minimally affected, and normal oral feeding can be restored 6 h after the surgery. Uneventful follow-up showed no abdominal complication in donor site, the surface skin displayed no swelling. Major complications, such as infection, or necrosis, were not experienced on the healthy symmetrized breasts. One patient was observed with slight hematoma and another patient was found with distant metastasis. Similarly, no arm morbidities occurred after surgery, like numbness, paralysis, upper extremity edema, and arm movement restriction (Table 3). After radiation therapy and chemotherapy, no size reduction was noted in reconstructed breast (Fig. 3E). In addition, postoperative blood supply measurement indicated favorable blood supply.
Table 3.
Complications after surgery.
4. Discussion
Luminal breast cancer can be separated at least into 2 subgroups: luminal A and luminal B.[18] Luminal A tumors were characterized with the highest expression of estrogen-related and low expression of proliferation-related genes. Luminal B cancers showed lower expression of estrogen receptor as well as low expression of progesterone receptor genes and higher expression of proliferation cluster genes.[19] Luminal breast cancer has generally been regarded with more favorable outcome and better response to anticancer therapy. Hence, it is of greater significance to improve the life quality of this part of population. In our study, 10 luminal breast cancer patients were proceeded with breast-conserving surgery and reconstructed with free omentum, achieving satisfactory outcomes.
Adequate blood supply is essential to the end-to-end vascular anastomosis.[20] Prior to all, the vascular selection was critical to accomplish vascular anatomosis. As is known to all, the famous vessel around breast includes internal thoracic artery, thoracoacromial artery, and thoracic dorsal artery. Selecting thoracic dorsal artery as recipient vessel for vascular anastomosis possessed many additional benefits, being associated with secluded location, stable anatomy, available size, and the similar caliber to right artery of stomach omentum. By contrast, the unreliable location of deep internal thoracic artery and thoracoacromial artery indicated the bigger injure during vascular anastomosis, which most probably contributed to an obvious incision. Besides, visualization of blood supply was essential to reconstructed breast. Color Doppler ultrasonography, a widely available, noninvasive, low-cost diagnostic modality was used before and after surgery.[21] The dynamic oxygen pressure outcomes further support the success of anastomosis.
Postoperative complications have been proved inversely to patient's satisfaction.[22] Necrosis is one of the most significant concerns in autologous breast reconstruction, which created unsightly scarring, producing contour irregularities, and deforming the breast mound.[23] It has been reported that surgical delay method was helpful to prevent partial necrosis. Tissue necrosis may be susceptible to the appreciate treatment of omentum flap.[24] Besides, free transplantation of omentum could easily result in thrombosis than conventional transplantation. Hypertonic citrate adenine (HC-A) solution containing citrate and adenine, widely used in isolated kidney preservation, has been proved to achieve ideal efficacy and safety.[25] In this serious of operation, ice HC-A solution was served as protection fluid to omentum vessel, extending the ischemia time. Other complications, such as incisional hernia, delayed wound healing, incisional infection has never been observed in our study, suggesting the established applicability of omentum.[26–28]
The great omentum is of great interest in the context of metabolic disease where adipose tissue exhibits inflammatory changes.[29] During the transplantation of greater omentum, the unavoidable period of ischemia may induce enhanced up-regulation of inflammatory mediators. Nelson et al summarized that compellingly local immunotherapy approaches have emerged in tumor immunology, which was drove by natural suppressive/protective factors in the tissue environment.[30] It is most likely that omentum transplantation was not merely an auto-graft, but also an important immunotherapeutic option, which prevented the recurrence of tumor after surgery.
Nevertheless, we recognized that this procedure has some shortcomings. First of all, a relative large achievement of transferred greater omentum may result in the enlargement of abdominal incision, which fluctuates the acceptance of patients. So, we made a new hypothesis that the hybrid operation, combining prosthesis and the greater omentum transplantation, may ameliorate the authenticity and sags of the reconstructed breast. Meanwhile, another main challenge of omental flap grafting is the volume assessment, because no effective technique can be used to evaluate the omentum capacity for breast plastic surgery and adhesion condition of greater omentum. Abdominal and pelvic computed tomography may be a reliable test to evaluate recurrent sarcoma which is not suitable for this operation.[31] Except for this, abdominal laparoscope observation must be the most direct approach, which required abundant profession and high laparoscopic skill. In addition, the interferences’ result from postreconstructive chemotherapy/radiotherapy was still need further exploration. With the further popularization of our surgery, the prospective and larger investigations are warranted for the establishment of appropriate guidelines.
In conclusion, immediate breast reconstruction by transplanting omental flap can be considered successful based on the excellent clinic outcome, providing an optional strategy for breast cancer patients.
Acknowledgments
The authors thank the administration stuff of both departments for their efficient participation in the project. The authors also thank the patients who volunteered to participate in this study and the staff members of the study sites who cared for them.
Footnotes
Abbreviation: HC-A = hypertonic citrate adenine.
This work is the result of cooperation between the Department of Burn and Skin Surgery, and the Department of Digestive Surgery.
NL, ZZ, and JL have contributed equally to this work.
This work was funded by the National Science Foundation of China (Nos 81572917 and 81472598) and Wujieping Foundation (320.6750.13292).
The authors have no conflicts of interest to disclose.
References
- [1].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [2].Ades F, Zardavas D, Bozovic-Spasojevic I, et al. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives A B. J Clin Oncol 2014;32:2794–803. [DOI] [PubMed] [Google Scholar]
- [3].Franchet C, Duprez-Paumier R, Lacroix-Triki M. Cancer du sein luminal et apport des classifications intrinsèques moléculaires: comment identifier les tumeurs luminales A et B en 2015? Bull Cancer 2015;102:S34–46. [DOI] [PubMed] [Google Scholar]
- [4].Matsen CB, Neumayer LA. Breast cancer: a review for the general surgeon. JAMA Surg 2013;148:971–9. [DOI] [PubMed] [Google Scholar]
- [5].Adimulam G, Challa VR, Dhar A, et al. Assessment of cosmetic outcome of oncoplastic breast conservation surgery in women with early breast cancer: a prospective cohort study. Indian J Cancer 2014;51:58–62. [DOI] [PubMed] [Google Scholar]
- [6].Eltahir Y, Werners LL, Dreise MM, et al. Which breast is the best? Successful autologous or alloplastic breast reconstruction: patient-reported quality-of-life outcomes. Plast Reconstr Surg 2015;135:43–50. [DOI] [PubMed] [Google Scholar]
- [7].Matrai Z, Pesthy P, Gulyas G, et al. Autologous fat transplantation in the modern reconstructive surgery of breast cancer. Orv Hetil 2012;153:1816–31. [DOI] [PubMed] [Google Scholar]
- [8].Bodin F, Schohn T, Dissaux C, et al. Bilateral simultaneous breast reconstruction with transverse musculocutaneous gracilis flaps. J Plast Reconstr Aesthet Surg 2015;68:e1–6. [DOI] [PubMed] [Google Scholar]
- [9].King IC, Harvey JR, Bhaskar P. One-stage breast reconstruction using the inferior dermal flap, implant, and free nipple graft. Aesthet Plast Surg 2014;38:358–64. [DOI] [PubMed] [Google Scholar]
- [10].Alani HA, Balalaa N. Complete tissue expander coverage by musculo-fascial flaps in immediate breast mound reconstruction after mastectomy. J Plast Surg Hand Surg 2013;47:399–404. [DOI] [PubMed] [Google Scholar]
- [11].Baumeister S, Werdin F, Peek A. The sGAP flap: rare exception or second choice in autologous breast reconstruction? J Reconstr Microsurg 2010;26:251–8. [DOI] [PubMed] [Google Scholar]
- [12].Dutra AK, Andrade WP, Carvalho SM, et al. Immediate breast reconstruction using autologous skin graft associated with breast implant. J Plast Reconstr Aesthet Surg 2012;65:187–94. [DOI] [PubMed] [Google Scholar]
- [13].Weichman KE, Broer PN, Tanna N, et al. The role of autologous fat grafting in secondary microsurgical breast reconstruction. Ann Plast Surg 2013;71:24–30. [DOI] [PubMed] [Google Scholar]
- [14].Roostaeian J, Yoon AP, Sanchez IS, et al. The effect of prior abdominal surgery on abdominally based free flaps in breast reconstruction. Plast Reconstr Surg 2014;133:e247–55. [DOI] [PubMed] [Google Scholar]
- [15].Micheau P. The greater omentum. Its role in reconstructive plastic surgery. Ann Chir Plast Esthet 1995;40:192–207. [PubMed] [Google Scholar]
- [16].Ye J, Li Q, Liu R, et al. Pedicled greater omentum graft: a new technique to repair recurrent urinary fistulae after kidney transplantation. Cell Biochem Biophys 2012;62:69–72. [DOI] [PubMed] [Google Scholar]
- [17].Marzouk K, Lawen J, Alwayn I, et al. The impact of vascular anastomosis time on early kidney transplant outcomes. Transplant Res 2013;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 2003;100:10393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shimada Y, Okumura T, Nagata T, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus 2011;8:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lomonte C, Meola M, Petrucci I, et al. The key role of color Doppler ultrasound in the work-up of hemodialysis vascular access. Semin Dial 2015;28:211–5. [DOI] [PubMed] [Google Scholar]
- [22].Craggs B, Vanmierlo B, Zeltzer A, et al. Donor-site morbidity following harvest of the transverse myocutaneous gracilis flap for breast reconstruction. Plast Reconstr Surg 2014;134:682e–91e. [DOI] [PubMed] [Google Scholar]
- [23].Nykiel M, Sayid Z, Wong R, et al. Management of mastectomy skin flap necrosis in autologous breast reconstruction. Ann Plast Surg 2014;72(suppl 1):S31–4. [DOI] [PubMed] [Google Scholar]
- [24].Zhu H, Xie Y, Xie F, et al. Prevention of necrosis of adjacent expanded flaps by surgical delay. Ann Plast Surg 2014;73:525–30. [DOI] [PubMed] [Google Scholar]
- [25].Sui M, Zhang L, Yang J, et al. A new HC-A II solution for kidney preservation: a multi-center randomized controlled trial in China. Ann Transplant 2014;19:614–20. [DOI] [PubMed] [Google Scholar]
- [26].Pinell-White XA, Kapadia SM, Losken A. The management of abdominal contour defects following TRAM flap breast reconstruction. Aesthet Surg J 2014;34:264–71. [DOI] [PubMed] [Google Scholar]
- [27].Nelson JA, Chung CU, Fischer JP, et al. Wound healing complications after autologous breast reconstruction: a model to predict risk. J Plast Reconstr Aesthet Surg 2015;68:531–9. [DOI] [PubMed] [Google Scholar]
- [28].Olsen MA, Lefta M, Dietz JR, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg 2008;207:326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Carlow DA, Gold MR, Ziltener HJ. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J Immunol (Baltimore Md: 1950) 2009;183:1155–65. [DOI] [PubMed] [Google Scholar]
- [30].Nelson D, Fisher S, Robinson B. “The Trojan Horse” approach to tumor immunotherapy: targeting the tumor microenvironment. J Immunol Res 2014;2014:789069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pestieau SR, Jelinek JS, Sugarbaker PH. Abdominal and pelvic CT for detection and volume assessment of peritoneal sarcomatosis. Tumori 2002;88:209–14. [DOI] [PubMed] [Google Scholar]


