Abstract
Epidemiological studies were inconsistent on the association between soy food intake and risk of gastric cancer (GC). This study aimed to determine the role of soy food intake in the development of GC.
A systematic search was conducted in PubMed and Web of Science to identify all relevant studies. Study-specific relative risks (RRs) and 95% confidence intervals (CIs) were pooled using a random-effects model, and the dose–response relationship between soy food intake and GC risk was also assessed.
Thirteen prospective studies were identified with a total of 517,106 participants and 5800 cases. Among 11 types of soy food, high intake of total soy food (the highest vs the lowest category: RR: 0.78, 95% CI: 0.62–0.98) and nonfermented soy food (RR: 0.63, 95% CI: 0.50–0.79) were inversely associated with GC risk, while high intake of miso soup was associated with the risk in male (RR: 1.17, 95% CI: 1.02–1.36). In dose–response meta-analysis, total soy food intake (0–150 g/day) showed no significant association with GC risk, while high intake of nonfermented soy food was inversely related, especially an intake of more than 100 g/day. In male, miso soup intake (1–5 cups/day) was significantly associated with GC risk.
High intake of nonfermented soy food might reduce the risk of GC, while miso soup intake might increase the risk in male.
Keywords: dose–response, gastric cancer, meta-analysis, soy food intake
1. Introduction
Gastric cancer (GC) is one of the most common cancers around the world, with an estimated 951,600 cases and 723,100 deaths per year.[1] Dietary factors have been reported to play an important role in the development of GC.[2] The decreasing incidence of GC in development countries may partly contribute to the wide use of refrigeration, availability of fresh fruit and vegetables, and decreased intake of salted or preserved food.[3] It is necessary to identify the potential protective or risk factors in diet, and prevent GC from the source. Soy food is a good source of isoflavones, which are antioxidants known to reduce GC risk.[4] However, the Asian cohort still suffers from a higher GC risk than other cohorts, although they have a high intake of soy food. Previous meta-analyses have focused on this, but reached inconsistent results.[5–7] In Woo et al study,[6] high intake of soy food was inversely associated with GC risk (odds ratio [OR] and 95% confidence interval [CI]: 0.32 [0.25–0.40] for soybean, 0.56 [0.45–0.71] for tofu, and 0.67 [0.46–0.98] for soy milk). However, in Tse and Eslick study,[7] no significant association was found between soy intake and GC risk (OR: 0.94, 95% CI: 0.85–1.05). The inconsistency may contribute to the inappropriate pooling of case–control and prospective studies, and different subtypes of soy food. Therefore, we conducted a dose–response meta-analysis of prospective studies to determine the role of soy food intake in the development of GC.
2. Materials and methods
2.1. Search strategy
The databases of PubMed and Web of Science were searched for relevant studies published up to May 8, 2017, using the keywords including: (“diet∗” OR “soy” OR “soybean” OR “bean” OR “legume” OR “tofu” OR “miso” OR “natto”) AND (“gastric” OR “stomach” OR “upper gastrointestinal tract”) AND (“cancer” OR “carcinoma” OR “tumor” OR “neoplasm”). Studies in languages other than English or Chinese were excluded. Moreover, we also reviewed the references of related studies and reviews for undetected studies. This study was approved by the ethics committee of Chongqing the Seventh People's Hospital.
2.2. Study selection and exclusion
Two authors (WKG and YYL) reviewed the studies independently. The inclusion criteria were as follows: prospective cohort study; contained at least 3 categories of soy food intake; evaluated the association between soy food intake and GC risk; and presented relative risk (RR), OR, or hazard ratio estimates with 95% CI. The exclusion criteria were as follows: abstracts without full texts, reviews, case reports, pediatric, and animal studies.
2.3. Data extraction and quality assessment
Two authors (WK-G and YY-L) extracted the data by a standardized collection form. All differences were resolved by discussion. In each study, the following information was extracted: first author, publication year, study area, interview time, follow-up deadline, number of participants and cases, soy food type, and adjusted factors. The Newcastle–Ottawa Scale was used to assess the methodological quality of included studies.[8]
2.4. Statistical analysis
As the incidence of GC was less than 10%, OR and hazard ratio could be roughly regarded as the RR in this study.[9] To evaluate the risk of high intake of soy food, we pooled the risk estimates for the highest versus lowest intake categories. Furthermore, we also evaluated the risks according to gender. A random-effects model was used as the pooling method, which considers both within-study and between-study variation. The heterogeneity between studies was estimated by Q test and I2 statistic, and I2 > 50% represented substantial heterogeneity.[10] The Egger test was used to detect publication bias.[11]
In dose–response meta-analysis, the assigned dose in each category was defined as the mean intake. If the mean intake per category was unavailable, we chose the midpoint of the upper and lower boundaries in each category as the assigned dose. For open-ended lower categories, we defined the lowest boundary as zero. For open-ended upper categories, the midpoint of the category was set as 1.5 times the lower boundary.[12] Groups were regarded in equal size or follow-up when cohort size or person-year per category was unavailable, and the case number per category was obtained by the method of Bekkering et al.[13] When the intake was measured as milliliters (mL), the data were converted to cups by dividing the mean intake by 240 (1 cup ≈ 240 mL). Then, 2-stage random-effects dose–response meta-analysis was conducted to examine linear relationship between soy food intake and GC risk. In the 1st stage, the method by Greenland and Longnecker[14] and Orsini et al[15] (generalized least-square regression) was used to calculate the correlation within each study. Second, study-specific estimates were combined by using a random-effects meta-analysis. Nonlinear dose–response relationship was modeled by using restricted cubic splines with 3 knots at 10%, 50%, and 90% percentiles of the distribution.[16] The Wald test was chosen to evaluate linear or nonlinear trends.[17]
All statistical analyses were performed with STATA version 12.0 software (StataCorp, College Station, TX) and R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). P values < .05 were considered statistically significant.
3. Results
3.1. Study characteristics
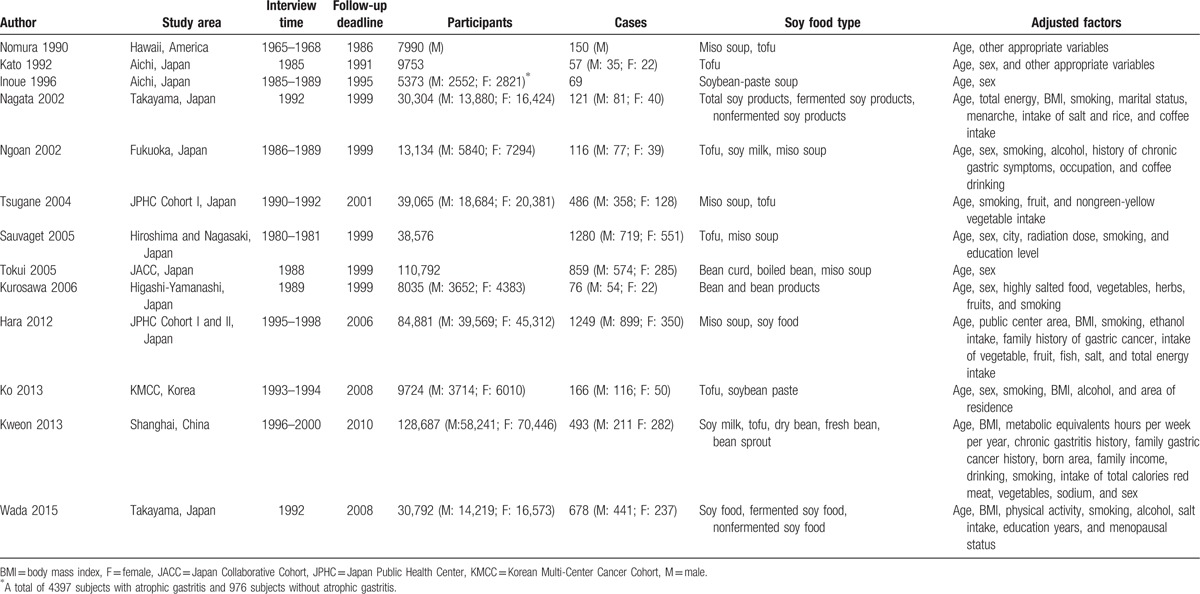
The search strategy resulted in 4998 records: 2674 from PubMed, 2313 from Web of Science, and 11 through other sources. After excluding duplicated and irrelevant records, 13 studies were included in this meta-analysis with a total of 517,106 participants and 5800 cases (Table 1).[18–30] Ten studies were conducted in Japan, and the remaining 3 were performed in America, China, and Korea. Eight studies reported the results by gender, while 1 was based on the subjects with and without atrophic gastritis, respectively. Food frequency questionnaires (FFQs) were used to measure soy food intake in all studies, which contained various food items and intake frequency. A total of 11 types of soy food were included into the analysis. In quality assessment, the included studies had an average score of 7.69.
Table 1.
Characteristics of included studies.
3.2. Soy food intake and GC risk
High intake of total soy food showed an inverse association with GC risk (the highest vs the lowest category: RR: 0.78, 95% CI: 0.62–0.98) (Fig. 1). Fermented soy food intake was not significantly associated with GC risk (RR: 0.92, 95% CI: 0.75–1.14), including the subtypes of miso soup (RR: 1.09, 95% CI: 0.96–1.24) and soybean paste (RR: 2.01, 95% CI: 0.52–8.50). Nonfermented soy food intake was inversely associated with GC risk (RR: 0.63, 95% CI: 0.50–0.79), but no significant association was found among the subtypes of fresh bean (RR: 1.03, 95% CI: 0.79–1.34), dry bean (RR: 0.82, 95% CI: 0.65–1.03), boiled bean (RR: 0.90, 95% CI: 0.63–1.28), bean sprout (RR: 0.98, 95% CI: 0.76–1.27), soy milk (RR: 0.90, 95% CI: 0.71–1.12), and tofu (RR: 0.86, 95% CI: 0.68–1.09).
Figure 1.
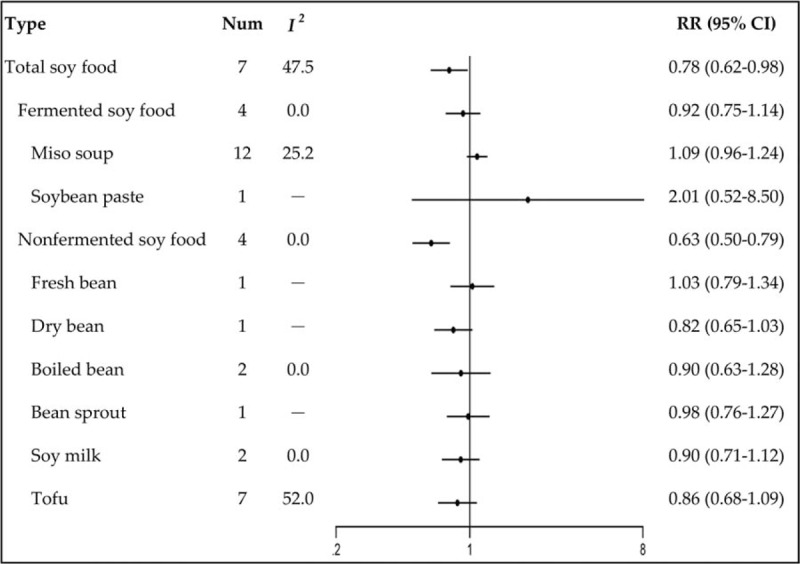
Relative risks of gastric cancer for soy food intake.
3.3. Soy food intake and GC risk according to gender
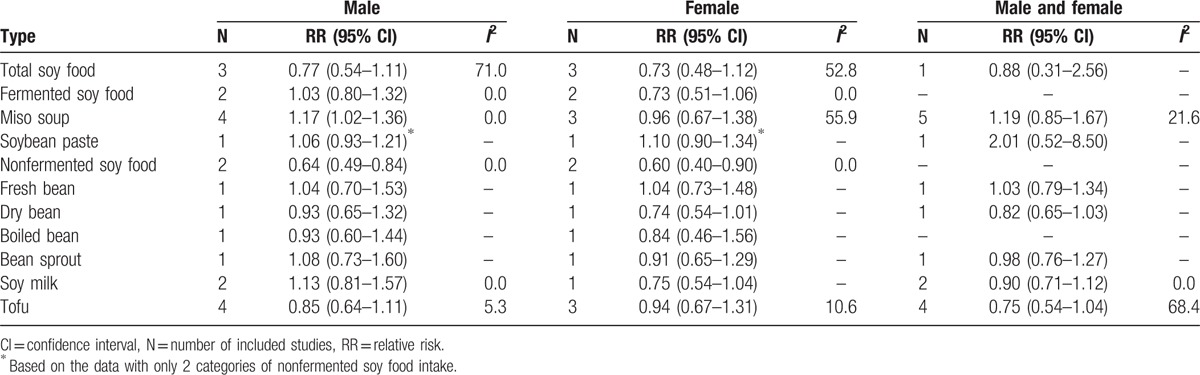
High intake of miso soup was associated with GC risk in male (the highest vs the lowest category: RR: 1.17, 95% CI: 1.02–1.36) (Table 2). Nonfermented soy food intake was inversely associated with the risk in both male and female (RR: 0.64, 95% CI: 0.49–0.84; RR: 0.60, 95% CI: 0.40–0.90). The other types of soy food showed no significant association with GC risk neither in male or female.
Table 2.
Relative risks of gastric cancer for soy food intake according to gender.
3.4. Dose–response meta-analysis
We also evaluated the dose–response relationship between total soy food and nonfermented food intake and GC risk, as well as miso soup intake and the risk in male. All of these showed a nonlinear relationship with GC risk (P for nonlinearity = .013 for total soy food, .001 for nonfermented soy food, and .022 for miso soup in male). The RRs (95% CI) for 50, 100, and 150 g/day intake of total soy food were 1.05 (0.90–1.23), 1.03 (0.81–1.31), and 0.89 (0.72–1.09), while it was 0.87 (0.66–1.14), 0.72 (0.50–1.02), and 0.57 (0.41–0.79) for nonfermented soy food (Figs. 2 and 3). In male, the RRs (95% CI) for 1, 3, and 5 cups/day intake of miso soup were 1.14 (1.00–1.29), 1.29 (1.06–1.56), and 1.31 (1.06–1.62) (Fig. 4). Thus, the dose–response analysis suggested no significant association between total soy food intake (0–150 g/day) and GC risk, while high intake of nonfermented soy food was inversely related, especially an intake of more than 100 g/day. In male, miso soup intake (1–5 cups/day) was significantly associated with GC risk.
Figure 2.
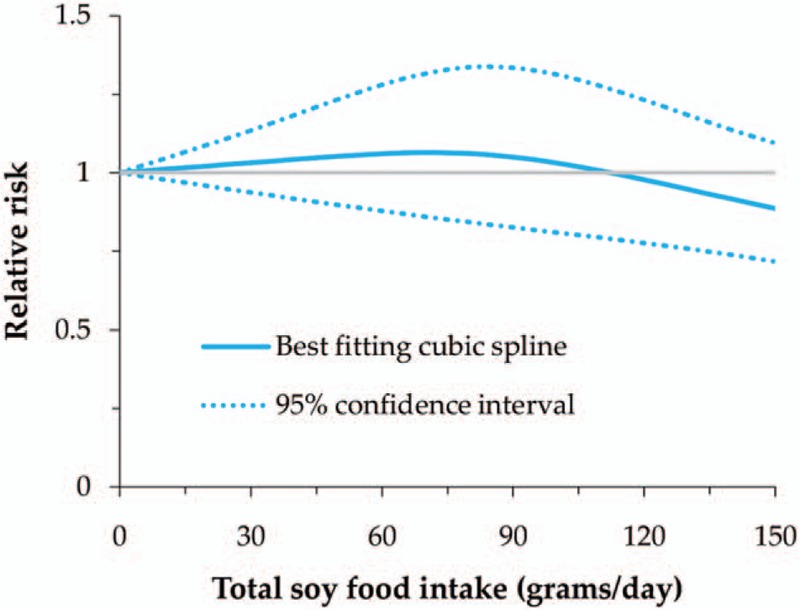
Nonlinear dose–response meta-analysis of total soy food intake and gastric cancer risk.
Figure 3.
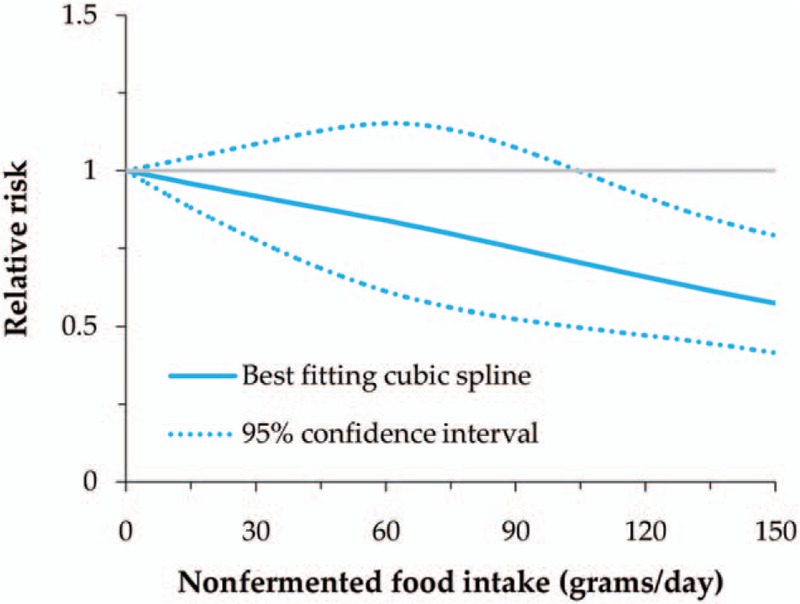
Nonlinear dose–response meta-analysis of nonfermented soy food intake and gastric cancer risk.
Figure 4.
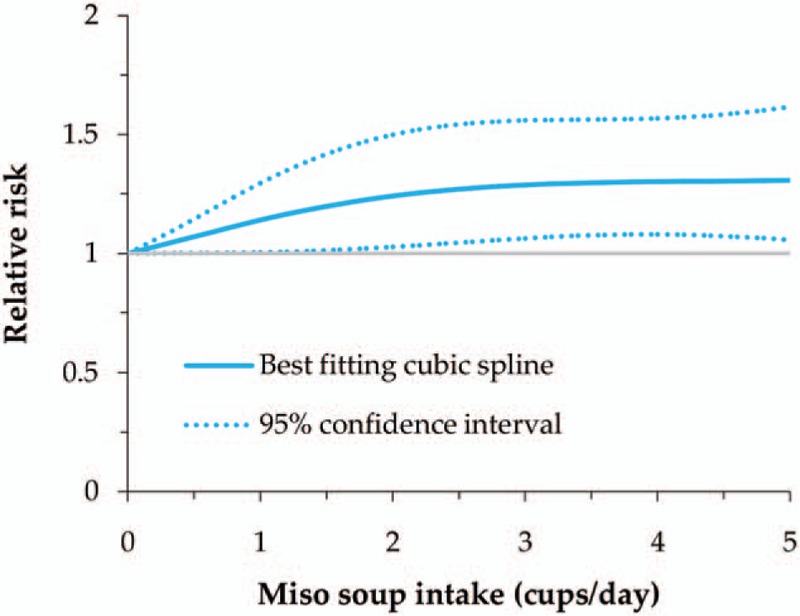
Nonlinear dose–response meta-analysis of miso soup intake and gastric cancer risk in male.
3.5. Publication bias
For the pooled analyses with more than 4 included studies, we conducted an Egger test to detect the publication bias. No obvious publication bias was found in total soy food (P = .108), fermented soy food (P = .344), miso soup (P = .177), nonfermented soy food (P = .072), and tofu (P = .405).
4. Discussion
In recent years, soy food has received considerable attention for its potential role in reducing cancer risk, which contains a number of anticarcinogenic phytochemicals including phytosterols, phenolic acids, and protease inhibitors.[31] Moreover, soy food is the main of source of isoflavones, which have a limited distribution in nature.[32] Isoflavones are regarded as phytoestrogens for the similar structure and metabolism to mammalian estrogens. They could prevent hormonally mediated cancers by acting on estrogen receptors and regulating body estrogen levels.[33] Furthermore, they could also act as antioxidants and prevent cancers by inhibiting angoiogeneis, topoisomerase, and tyrosine kinase.[34] The meta-analysis of Tse and Eslick[7] has indicated an association between isoflavone intake and risk of gastrointestinal cancer. Several meta-analyses also focused on soy food intake and GC risk, but the results were inconsistent for the inappropriate pooling of case–control and prospective studies, and different subtypes of soy food.[5–7] Therefore, we conducted a dose–response meta-analysis of prospective studies to determine the role of soy food intake in the development of GC.
In our study, we found an inverse association between total soy food intake and GC risk, but no significant association was found in male or female, as well as in the subsequent dose–response analysis. As a result, we did not suggest a protective role of total soy food in the development of GC, which was consistent with the meta-analysis of Tse et al[7] (OR: 0.94, 95% CI: 0.85–1.05). Furthermore, nonfermented soy food intake was inversely associated with GC risk, while miso soup intake was associated with the risk in male. Both of these were validated in the dose–response analysis. Thus, we thought that high intake of nonfermented soy food might reduce the risk of GC, while high intake of miso soup might increase the risk in male.
As one kind of fermented food, miso soup is a popular soup style in Japan, and 99% Japanese consume it several times a week or day. This is consistent with the high incidence of GC in Japan. However, fermentation is basically the similar process to digestion, which results in the release of the sugar molecule from the isoflavone glycoside, leaving an isoflavone aglycone. Therefore, fermented food is considerably to have a stronger antitumor effect than nonfermented food. This was controversial with our study, which might attribute to the increase of the carcinogen of nitrite in the storage process. The protective effect of isoflavones might be weakened by the increasing nitrite in fermented food. In the meta-analysis of Kim et al[5] based on Japanese and Korean populations, fermented soy food intake was not significantly associated with GC risk in prospective studies (OR: 1.12, 95% CI: 0.88–1.41), but the study did not focus on the subtypes. In the meta-analysis of Tse et al,[7] dietary isoflavone intake showed no significant association with GC risk (OR: 0.67, 95% CI: 0.45–1.02). In our study, we also found no significant association between fermented soy food intake and GC risk. However, the fermented food of miso food showed a significant association with GC risk in male. We thought that some other ingredients in miso soup might also play a role in the development of GC. For example, high concentrations of salt in miso soup could also increase the risk of GC.[6,26] Moreover, the same factor might play different roles in the development of GC between male and female, just like the significant difference in the incidence of GC.
As for nonfermented soy food, Kim et al also reported an inverse association with GC risk (OR: 0.64, 95% CI: 0.54–0.77). However, we found no subtypes showed a significant association. In the meta-analysis of Woo et al[6] based on Korean population, high intake of tofu and soy milk was inversely associated with GC risk (RR: 0.32, 95% CI: 0.25–0.40; RR: 0.67, 95% CI: 0.46–0.98), but the results were based on a small number of case–control studies. In our study, most subtypes showed a protective effect in GC, although it was not significant. Thus, considering the group effect, we suggested an intake of multiple subtypes of nonfermented soy food.
This meta-analysis study has several strengths. First, to our knowledge, this is the first dose–response meta-analysis to identify the association between soy food intake and GC risk. Second, the included studies were prospective designed with at least 3 categories of exposure, which demonstrated a higher quality than those with 2 categories or case–control studies. Third, the subtypes of soy food were also analyzed, respectively, as well as by gender. There were also a few limitations in this study. First, the number of included studies was small in some subtypes of soy food. Second, not all potential risk factors were adjusted in each study, like high-temperature intake.[35] Third, all included studies ignored the effects of cooking style and high temperature, which could cause significant losses of some antitumor nutrients in soy food (eg, phenolic acid, benzoic group, cinnamic group, and isoflavones).[36,37]
5. Conclusions
In conclusion, high intake of nonfermented soy food might reduce the risk of GC, while miso soup intake might increase the risk in male.
Footnotes
Abbreviations: CI = confidence interval, GC = gastric cancer, OR = odds ratio, RR = relative risk.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- [2].Fang X, Wei J, He X, et al. Landscape of dietary factors associated with risk of gastric cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer 2015;51:2820–32. [DOI] [PubMed] [Google Scholar]
- [3].Thrumurthy SG, Chaudry MA, Hochhauser D, et al. The diagnosis and management of gastric cancer. BMJ 2013;347:f6367. [DOI] [PubMed] [Google Scholar]
- [4].Wu AH, Yang D, Pike MC. A meta-analysis of soyfoods and risk of stomach cancer: the problem of potential confounders. Cancer Epidemiol Biomarkers Prev 2000;9:1051–8. [PubMed] [Google Scholar]
- [5].Kim J, Kang M, Lee JS, et al. Fermented and non-fermented soy food consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci 2011;102:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Woo HD, Park S, Oh K, et al. Diet and cancer risk in the Korean population: a meta-analysis. Asian Pac J Cancer Prev 2014;15:8509–19. [DOI] [PubMed] [Google Scholar]
- [7].Tse G, Eslick GD. Soy and isoflavone consumption and risk of gastrointestinal cancer: a systematic review and meta-analysis. Eur J Nutr 2016;55:63–73. [DOI] [PubMed] [Google Scholar]
- [8].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. Available online: http://www.ohri.ca. [Google Scholar]
- [9].Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- [10].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analysis. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rong Y, Chen L, Zhu T, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ 2013;346:e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bekkering GE, Harris RJ, Thomas S, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? Am J Epidemiol 2008;167:1017–26. [DOI] [PubMed] [Google Scholar]
- [14].Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- [15].Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40–57. [Google Scholar]
- [16].Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Orsini N. Multivariate dose-response meta-analysis: an update on glst. In Nordic and Baltic Users Group Meeting, Stockholm, Sweden, 2013. [Google Scholar]
- [18].Nomura A, Grove JS, Stemmermann GN, et al. A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res 1990;50:627–31. [PubMed] [Google Scholar]
- [19].Kato I, Tominaga S, Matsumoto K. A prospective study of stomach cancer among a rural Japanese population: a 6-year survey. Jpn J Cancer Res 1992;83:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Inoue M, Tajima K, Kobayashi S, et al. Protective factor against progression from atrophic gastritis to gastric cancer – data from a cohort study in Japan. Int J Cancer 1996;66:309–14. [DOI] [PubMed] [Google Scholar]
- [21].Nagata C, Takatsuka N, Kawakami N, et al. A prospective cohort study of soy product intake and stomach cancer death. Br J Cancer 2002;87:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ngoan LT, Mizoue T, Fujino Y, et al. Dietary factors and stomach cancer mortality. Br J Cancer 2002;87:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tsugane S, Sasazuki S, Kobayashi M, et al. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer 2004;90:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sauvaget C, Lagarde F, Nagano J, et al. Lifestyle factors, radiation and gastric cancer in atomic-bomb survivors (Japan). Cancer Causes Control 2005;16:773–80. [DOI] [PubMed] [Google Scholar]
- [25].Tokui N, Yoshimura T, Fujino Y, et al. Dietary habits and stomach cancer risk in the JACC Study. J Epidemiol 2005;15:S98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kurosawa M, Kikuchi S, Xu J, et al. Highly salted food and mountain herbs elevate the risk for stomach cancer death in a rural area of Japan. J Gastroenterol Hepatol 2006;21:681–6. [DOI] [PubMed] [Google Scholar]
- [27].Hara A, Sasazuki S, Inoue M, et al. Isoflavone intake and risk of gastric cancer: a population-based prospective cohort study in Japan. Am J Clin Nutr 2012;95:147–54. [DOI] [PubMed] [Google Scholar]
- [28].Ko KP, Park SK, Yang JJ, et al. Intake of soy products and other foods and gastric cancer risk: a prospective study. J Epidemiol 2013;23:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kweon SS, Shu XO, Xiang Y, et al. Intake of specific nonfermented soy foods may be inversely associated with risk of distal gastric cancer in a Chinese population. J Nutr 2013;143:1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wada K, Tsuji M, Tamura T, et al. Soy isoflavone intake and stomach cancer risk in Japan: from the Takayama study. Int J Cancer 2015;137:885–92. [DOI] [PubMed] [Google Scholar]
- [31].Kietsiriroje N, Kwankaew J, Kitpakornsanti S, et al. Effect of phytosterols and inulin-enriched soymilk on LDL-cholesterol in Thai subjects: a double-blinded randomized controlled trial. Lipids Health Dis 2015;14:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baglia ML, Gu K, Zhang X, et al. Soy isoflavone intake and bone mineral density in breast cancer survivors. Cancer Causes Control 2015;26:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marinho DS, Calió ML, Santos MA, et al. Evaluation of the isoflavones and estrogen effects on the rat adrenal. Gynecol Endocrinol 2017;28:1–5. [DOI] [PubMed] [Google Scholar]
- [34].Mizushina Y, Shiomi K, Kuriyama I, et al. Inhibitory effects of a major soy isoflavone, genistein, on human DNA topoisomerase II activity and cancer cell proliferation. Int J Oncol 2013;43:1117–24. [DOI] [PubMed] [Google Scholar]
- [35].Deandrea S, Foschi R, Galeone C, et al. Is temperature an effect modifier of the association between green tea intake and gastric cancer risk? Eur J Cancer Prev 2010;19:18–22. [DOI] [PubMed] [Google Scholar]
- [36].Kumari S, Chang SK. Effect of cooking on isoflavones, phenolic acids, and antioxidant activity in sprouts of prosoy soybean (glycine max). J Food Sci 2016;81:C1679–91. [DOI] [PubMed] [Google Scholar]
- [37].Zhang Y, Chang SK. Isoflavone profiles and kinetic changes during ultra-high temperature processing of soymilk. J Food Sci 2016;81:C593–9. [DOI] [PubMed] [Google Scholar]


