Abstract
This was a prospective comparative study.
The aim of this study was to compare the clinical and radiologic outcomes of patients treated with cortico/cancellous composite allograft or autoiliac bone graft in anterior cervical discectomy and fusion.
Several methods have been developed to fuse the cervical spine for treatment of cervical spondylosis. Cortico/cancellous composite allograft might be another alternative.
A total of 46 patients who underwent surgery for treatment of cervical spondylosis were evaluated between September 2010 and January 2015. The duration of operation, blood loss, perioperative complications, neck disability index (NDI), visual analogue scale (VAS), and fusion rates were compared between the 2 groups.
There were no significant differences in clinical or radiologic outcomes between the patients treated with cortico/cancellous composite allograft and those treated with autoiliac bone graft. The 2 groups showed similar improvements in clinical symptoms and fusion rates. Although not statistically significant, the subsidence rate was lower in the cortico/cancellous composite group.
Cortico/cancellous composite allograft is an effective alternative to conventional allograft or autograft in anterior cervical discectomy and fusion.
Keywords: anterior cervical discectomy and fusion, cortico/cancellous composite allograft, patient outcome
1. Introduction
Anterior cervical discectomy and fusion (ACDF) is a basic surgery used to cure degenerative cervical disease.[1,2] After disc removal, fusion can be performed using either auto- or allogenic bone. If the surgeon chooses to fuse the cervical spine with an anterior approach, there are several options for the material to be inserted into the intervertebral disc space. Although autologous iliac bone graft has been considered superior for cervical arthrodesis,[3,4] donor site morbidity is problematic.[5] To overcome this issue, a variety of other materials have been used as substitutes for autoiliac bone grafts in ACDF,[6,7] including allogenic strut bone graft. The diversity of materials used is a reflection of the uncertainty regarding effectiveness and outcomes. The purpose of this study was to compare the clinical and radiologic outcomes between autoiliac bone grafts and cortico/cancellous composite allografts. The primary outcome measure was evidence of fusion and graft subsidence. Secondary outcome measures included adverse events, pain, and neck disability scores.[8]
2. Materials and methods
2.1. Subjects
This study included 46 patients who underwent surgery for symptomatic cervical spondylosis between September 2010 and January 2015. Inclusion criteria were as follows: less than 3 levels of cervical degenerative disc disease as demonstrated by plain radiography and magnetic resonance imaging, and persistent radiculopathy and/or myelopathy symptoms despite conservative treatment. We prospectively enrolled the patients who met the inclusion criteria and observed clinical outcomes up to 2 years after surgical treatment. All patients provided informed consent and underwent ACDF with an allograft cage or autoiliac bone graft. Cortico/cancellous composite allograft (Corner stone L-ASR; Medtronic, Minnesota) or autoiliac tricortical bone graft was randomly implanted in each patient. Initially, a total of 71 participants were included in the study, but 19 were excluded due to cervical radiculopathy or myelopathy with trauma or tumor. Three patients who underwent previous operative treatment were also excluded. Consequently, we excluded 25 patients, and 3 additional patients were lost to follow-up. A power analysis was conducted to determine the minimum sample size required for 90% power, showing that a sample size of 21 participants in each group was sufficient.
2.2. Graft materials
2.2.1. Cortico/cancellous composite allograft
Freeze dried, fully machined (capital D shape) allograft bone was used. Two cortical lateral walls and cancellous center were combined together by medial/lateral parallel cortical bone pins. Cortical portion provides structural support and cancellous portion provides scaffold for bone in-growth (available size: 7–13 mm heights x 14 mm width x 11 mm depth).
2.2.2. Autoiliac tricortical bone
Full-thickness tricortical grafts, which include the iliac crest, were harvested from the anterior ilium. After stripping the outer and inner table muscles, entire thickness of the ilium was exposed, and oscillating saw or osteotome was used to remove full-thickness tricortical grafts. We trimmed the graft with capital D shape according to preoperative computed tomography (CT) measurement, similar to the shape and size of L-ASR cage.
2.3. Operative techniques
All operations were performed by the same surgeon (JYH) using a standard anterior approach. In most cases, we used surgical microscope. Decompression of the targeted nerve was performed, after removal of the herniated nucleus pulposus. Once the posterior longitudinal ligament was opened, osteophytes and remnant discs were removed with drill and punch. If the origin and exit portions of nerve root were completely decompressed, cartilaginous endplates were gently abraded with curette, preserving bony endplates. The appropriate size for the graft material was determined by intraoperative evaluation using a trial cage. Graft materials were inserted into the disc space using an impactor. After implantation of the graft material, anterior plating was performed to ensure primary stabilization. Patients were allowed to sit up and walk on the first postoperative day, with a rigid cervical collar in place.
2.4. Outcome assessment
Patients received check-ups during outpatient clinic visits at 1, 3, 6, and 12 months postoperation and annually thereafter. Neurologic function and presence of complications were assessed at each visit. The preoperative and postoperative Neck Disability Index (NDI) and visual analogue scale (VAS) score were recorded. An independent observer who was not present during surgery interviewed each patient and evaluated the clinical results. In addition, a qualitative assessment of fusion was made at the 6-, 12-, and 24-month evaluations. We performed a CT scan as well as plain radiographs. Bone fusion was evaluated by the following: less than 2 mm of interspinous motion on dynamic radiographs; no radiolucent gap; and evident bone bridging between graft and end plate.[9] We also used CT images to assess the quality of fusion according to the Bridwell classification system.[8] Significant graft subsidence was defined as a difference >3 mm in disc height, according to the calculation between immediate postoperative radiographs and those taken at the final follow-up.[10]
2.5. Statistical methods
In order to compare pre- and postoperative outcomes between groups, t tests, and analysis of variance (ANOVA) were used for the analysis. In addition, Chi-square test was used to compare demographics between the 2 groups. Statistical analysis was performed with SAS survey procedures (version 9.3; SAS Institute, Cary, NC) in a manner that reflected sampling weights. P values less than .05 were considered statistically significant.
3. Results
This study analyzed 46 patients (31 male, 15 female) who underwent ACDF with a cortico/cancellous composite allograft cage or autoiliac tricortical bone graft. The mean age of the composite allograft population was 52.48 ± 4.12 years, and 19 (76%) of the patients were male. The mean age of the autoiliac bone graft population was 60.08 ± 4.01 years, and 12 (52%) of the patients were male. The mean follow-up period was 22.7 ± 2.2 months in the allograft group and 20.8 ± 3.1 months in the autograft group. There were no significant differences in demographics or intraoperative factors between the 2 groups (P > .05) (Table 1).
Table 1.
Comparison of patient characteristics between those treated with cortico/cancellous composite allograft or tricortical autograft.

3.1. Clinical outcomes
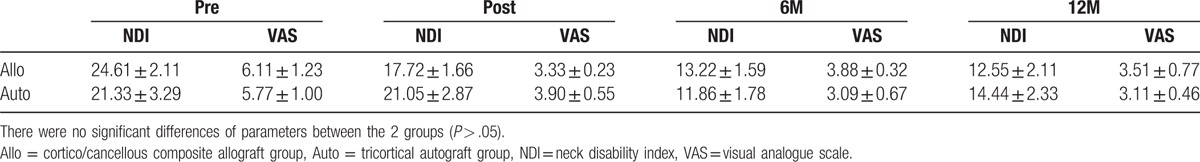
Table 2 summarizes the clinical outcomes of both groups. Overall neck and arm pain decreased significantly, with mean preoperative VAS scores of 6.11 ± 1.23 in the composite allograft group and 5.77 ± 1.00 in the autoiliac bone graft group. These scores decreased to 3.51 ± 0.77 and 3.11 ± 0.46, respectively, at 1-year postsurgery (P < .05). Neck discomfort also decreased significantly; mean preoperative NDI scores decreased from 24.61 ± 2.11 to 12.55 ± 2.11 in the composite allograft group and from 21.33 ± 3.29 to 14.44 ± 2.33 in the autoiliac bone graft group at the 1-year follow-up (P < .05). No neurologic complications were noted in either group. There was 1 case of prolonged dysphasia in the autograft group, but it was resolved at 3 months postoperation. We found 1 case of screw breakage in the allograft group at 9 months postsurgery, but the patient showed no symptoms.
Table 2.
Comparison of clinical outcomes between the 2 groups.
3.2. Radiologic outcomes
There was no significant difference in fusion status between the treatment groups (P > .05). The mean interspinous gap on flexion-extension was 1.04 ± 0.33 mm in the composite allograft group and 0.88 ± 0.13 mm in the autoiliac bone graft group (P > .05). On the basis of our definition of instability (a difference >2 mm), 2 cases in the composite allograft group and 1 case in the autograft group were identified, concordant with CT findings. In terms of subsidence, final follow-up radiographs showed a mean total segment height of 7.98 ± 2.33 mm in the composite allograft group and 8.06 ± 1.57 mm in the autograft group, with no significant difference between groups (Table 3). The mean difference between the 2 sequential radiographs was 2.34 ± 0.33 mm in the composite allograft group and 2.09 ± 0.15 mm in the autograft group (P > .05). Figures 1 and 2 show solid union of grafted bone at the final follow-up.
Table 3.
Comparison of radiologic outcomes between the 2 groups.
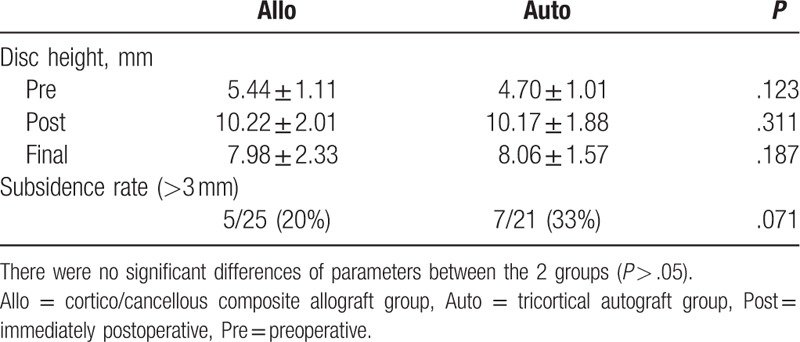
Figure 1.

Lateral plain radiographs of a 59-year-old male patient who underwent ACDF from C5 to C7. The image shows solid fusion of grafted cortical/cancellous composite allograft with minimal subsidence.
Figure 2.

Final follow-up CT from the same patient showing a complete bony bridge between the donor and recipient bone.
4. Discussion
Selection of an appropriate graft material is imperative to achieve successful bone fusion and an optimal clinical outcome with ACDF.[11] Autologous iliac bone grafts are one of the best adjuvant materials for bony union and are important for spinal fusion. Although the gold standard material for cervical fusion is autologous bone from the iliac crest, many complications have been reported with autograft substrates.[12] Iliac donor site complications include pain, neurovascular injury, avulsion fracture of the ASIS, hematoma, infection, herniation of abdominal contents, gait disturbance, cosmetic deformity, violation of the sacroiliac joint, and ureteral injury.[13] As a result, cages have been widely used as an alternative to autologous iliac bone grafts because they avoid autograft harvest-related complications.[14] The ideal bone replacement material should be osteo-inductive or conductive, nonpathogenic, minimally antigenic, and mechanically stable. Compared with autografts, allografts show delayed vascularization and remodeling of the fusion mass. Allogenous bone has limited osteo-inductive properties and carries the risk of subsidence due to delayed union or nonunion. Currently, several modified allograft cages have been introduced to enhance union rate and structural stability, including cortico/cancellous composite allograft. In this study, we compared the outcomes of 2 different graft groups to determine the efficacy of cortico/cancellous composite allografts.
We found similar clinical outcomes between patients treated with cortico/cancellous composite allograft or tri-cortical autoiliac bone. No statistically meaningful differences were identified between the 2 groups using the NDI and VAS pain scales. The fusion rates of the 2 groups were also similar on plain radiographs and CT. However, the subsidence rate in the cortico/cancellous composite allograft group was slightly lower than that of the autoiliac bone group, and the cortico/cancellous composite allograft group had less donor site morbidity and a shorter operative time. Cortico/cancellous composite allograft is a freeze-dried bone cage composed of cortical lateral walls with a cancellous center. The cortical portion provides structural support for the disc space, while the cancellous portion provides a scaffold for bone in-growth that can minimize graft subsidence with an enhanced fusion rate. Our results suggest that cortico/cancellous composite allograft can be a good alternative to traditional autograft. However, there were also shortcomings of cortico/cancellous composite allograft, including 1 case of screw breakage. On serial plain radiographs, we found that the time needed to achieve fusion in the cortico/cancellous composite allograft group was longer than that of the autoiliac bone group. We found that, in cases of multilevel fusion, delayed union could result in hardware breakage. However, further study is needed to confirm a high complication rate of multilevel fusion with cortico/cancellous composite allograft. The high cost of this graft was problematic for some patients, and economic outcomes, such as cost per improved outcome or cost per quality-adjusted life year, should be examined in the future.
There are limitations to our study. First, long-term follow-up is needed for better comparison of the 2 groups. In addition, although the mean fused level between the 2 groups was not significantly different, we did not account for the influence of level of fusion on outcome.[15] Second, although we prospectively gathered patient data, we could not randomize all variables. In addition, the investigator was not blinded to the treatment received, which could have affected the study results. Third, although we performed power analysis to determine the appropriate number of subjects, we could not confirm the incidence of subsidence in the 2 groups due to the relatively small number of study groups. In addition, we could not detect any significant differences in NDI scores, VAS scores, or fusion rates between groups, possibly due to our sample size. Further comparison of various parameters is needed to confirm the efficacy of allograft. However, we found good clinical and radiological results compared with those of conventional tricortical autograft group with up to 2 years of follow-up, and our results suggest that cortico/cancellous composite allograft is a good alternative.
5. Conclusion
This study demonstrated similar clinical and radiologic outcomes between patients treated with cortico/cancellous composite allograft or autograft for ACDF, with a decreased subsidence rate in the cortico/cancellous composite allograft group. Cortico/cancellous composite allograft cages can be an alternative graft material for ACDF.
Footnotes
Abbreviations: ACDF = anterior discectomy and fusion, CT = computed tomography, NDI = neck disability index, VAS = visual analogue scale.
JHP and YKB contributed equally to the manuscript as first authors.
The authors report no conflicts of interest.
References
- [1].Cunningham MR, Hershman S, Bendo J, et al. Systematic review of cohort studies comparing surgical treatments for cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2010;35:537–43. [DOI] [PubMed] [Google Scholar]
- [2].Matz PG, Ryken TC, Groff MW, et al. Techniques for anterior cervical decompression for radiculopathy. J Neurosurg Spine 2009;11:183–97. [DOI] [PubMed] [Google Scholar]
- [3].Jacobs WC, Anderson PG, Limbeek J, et al. Single or double-level anterior interbody fusion techniques for cervical degenerative disc disease. Cochrane Database Syst 2004;CD004958. [DOI] [PubMed] [Google Scholar]
- [4].Lee CH, Hyun SJ, Kim MJ, et al. Comparative analysis of 3 different construct systems for single-level anterior cervical discectomy and fusion: stand-alone cage, iliac graft plus plate augmentation, and cage plus plating. J Spinal Disord Tech 2013;26:112–8. [DOI] [PubMed] [Google Scholar]
- [5].Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2013;28:134–9. [DOI] [PubMed] [Google Scholar]
- [6].Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J 2009;18:449–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wigfield CC, Nelson RJ. Nonautologous interbody fusion materials in cervical spine surgery: how strong is the evidence to justify their use. Spine (Phila Pa 1976) 2001;26:687–94. [DOI] [PubMed] [Google Scholar]
- [8].Bridwell KH, Lenke LG, Mc Enery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine 1995;20:1410–8. [PubMed] [Google Scholar]
- [9].Thome C, Krauss JK, Zevgaridis D, et al. A prospective clinical comparison of rectangular titanium cages and iliac crest autografts in anterior cervical discectomy and fusion. Neurosurg Rev 2004;27:34–41. [DOI] [PubMed] [Google Scholar]
- [10].Zhou J, Li X, Dong J. Three-level anterior cervical discectomy and fusion with self-locking stand-alone polyetheretherketone cages. J Clin Neurosci 2011;18:1505–9. [DOI] [PubMed] [Google Scholar]
- [11].Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anteriorcervical discectomy and fusion with rigid plate fixation. Spine J 2003;3:451–9. [DOI] [PubMed] [Google Scholar]
- [12].Scheufler KM, Diesing D. Use of bone graft replacement in spinal fusions. Orthopade 2015;44:146–53. [DOI] [PubMed] [Google Scholar]
- [13].Ebraheim NA, Elgafy H, Xu R, et al. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg 2001;9:210–8. [DOI] [PubMed] [Google Scholar]
- [14].Hacker RJ, Cauthen JC, Gilbert TJ, et al. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine (Phila Pa 1976) 2000;25:2646–54. discussion 2655. [DOI] [PubMed] [Google Scholar]
- [15].Kim SY, Park KS, SS Jung. An early comparative analysis of the use of autograft versus allograft in anterior cervical discectomy and fusion. Korean J Spine 2012;9:142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


